Abstract
All the 560 glaucomatous eyes of 375 Japanese subjects (181 men, 194 women; mean age ± standard deviation, 76.0 ± 13.2 years) who underwent microhook ab interno trabeculotomy (µLOT) alone (159 eyes, 28%) or combined µLOT and cataract surgery (401 eyes, 72%) performed by one surgeon at Matsue Red Cross Hospital between May 2015 and March 2018 to control intraocular pressure (IOP) were retrospectively assessed. Preoperative and postoperative IOPs, numbers of antiglaucoma medications, the logarithm of the minimum angle of resolution visual acuity (logMAR VA), anterior chamber (AC) flare, visual field mean deviation (MD), and corneal endothelial cell density (CECD) were compared up to 36 months. Surgical complications and required interventions were described. The duration of the follow-up was 405 ± 327 (range, 2–1326) days. The mean preoperative IOP (20.2 ± 7.0 mmHg) and number of antiglaucoma medications (2.8 ± 1.1) decreased to 13.9 ± 4.5 mmHg (31% reduction, p < 0.0001) and 2.5 ± 1.0 (11% reduction, p < 0.0001), respectively, at the final visit. After combined surgery, compared with preoperatively, the final VA improved 0.11 logMAR (p < 0.0001), AC flare increased 4.5 photon counts/msec (p = 0.0011), MD improved 0.6 decibel (p < 0.0001), and the CECD decreased 6% (p < 0.0001). Layered hyphema (172 eyes, 31%) and hyphema washout (26 eyes, 5%) were the most common postoperative complication and intervention, respectively. At the final visit, 379 (69%) eyes achieved successful IOP control of ≤18 mmHg and ≥20% IOP reduction, and 349 (64%) eyes achieved successful IOP control of ≤15 mmHg and ≥20% IOP reduction. Older age, steroid-induced glaucoma, developmental glaucoma, and the absence of postoperative complications were associated with lower final IOP; exfoliation glaucoma, other types of glaucoma, and higher preoperative IOP were associated with higher final IOP. µLOT has a significant IOP-lowering potential in patients with glaucoma, and improves visual function when combined with cataract surgery.
1. Introduction
The intraocular pressure (IOP) in adults and children with glaucoma is reduced by trabeculotomy (LOT), which alleviates the resistance to aqueous flow by cleaving the trabecular meshwork (TM) and inner walls of the Schlemm’s canal [1,2,3]. The absence of a bleb in LOT reduces the likelihood of vision-threatening complications, such as a flat anterior chamber (AC), bleb leaks/infections, hypotony maculopathy, and choroidal detachment. These can develop following trabeculectomy in which antifibrotic agents are used [1,4].
The ab externo approach has been used to perform LOT in combination with metal trabeculotomes that incise a third of the meshwork [1,2,3], or with 5-0 and 6-0 polypropylene sutures, and a microcatheter that incises the full 360 degrees of the meshwork [5,6]. Surgeons have also reported using ab interno approaches with LOT techniques [7,8]. In 2015, we treated both eyes of one patient with steroid-induced glaucoma with a novel ab interno LOT procedure, which we referred to as microhook trabeculotomy (µLOT) [9]. As a result of the substantial reduction in IOP and less ocular surface invasiveness, we performed the procedure in other cases and reported our early postoperative results and safety profile in an initial case series [10,11]. We achieved a 43% IOP decrease, from the preoperative value of 25.9 to 14.7 mmHg postoperatively, with µLOT alone during the final 6 month evaluation [10]; when µLOT was combined with cataract surgery, we achieved a 28% decrease, i.e., from 16.4 to 11.8 mmHg postoperatively, at the final 9.5 month examination [11]. In the current study, we report the midterm surgical results and safety profile of µLOT in 560 consecutive eyes; the study included all cases in which µLOT was performed after the first case [9].
2. Materials and Methods
2.1. Methods
This retrospective observational case series included 560 consecutive glaucomatous eyes of 375 Japanese subjects (181 men, 194 women; mean age ± standard deviation (SD), 76.0 ± 13.2 years) who underwent µLOT performed by one surgeon (M.T.) at Matsue Red Cross Hospital between May 2015 and March 2018 to control the IOP. Preoperatively, the possible risks and benefits of µLOT, cataract surgery, and other possible glaucoma surgeries were explained to the patients, and the patients who chose µLOT alone or combined µLOT and cataract surgery underwent surgery. The study adhered to the tenets of the Declaration of Helsinki; the institutional review board (IRB) of Matsue Red Cross Hospital reviewed and approved the research (IRB No. 261). Preoperatively, all subjects provided written informed consent for surgery and use of the clinical data regarding the glaucoma treatment obtained during the follow-up periods. The patients’ demographic data and surgical procedures are summarized in Table 1. The mean follow-up was 405 days in this dataset.

Table 1.
Demographic Patient Data
2.2. Surgical Procedure
µLOT was performed as described previously [10,11]. Three specifically designed microhooks for µLOT, i.e., straight (M-2215S), right-angled (M-2215R), and left-angled (M-2215L) (all from Inami & Co., Ltd., Tokyo, Japan), were used [12]. When the combined procedure was performed, phacoemulsification cataract surgery was performed before µLOT; the cataract surgery was performed through a 2.2 mm wide clear corneal incision created at the 9 to 10 o’clock position (i.e., temporal incision for the right eye and nasal incision for the left eye) and a corneal port created at the 2 to 3 o’clock position. A one-piece soft-acrylic intraocular lens (IOL) was inserted through the same clear corneal incision; the Vivinex iSert XY1 IOL (Hoya, Tokyo, Japan) was used in most cases, and the AcrySof IQ IOL (Alcon Japan, Tokyo, Japan) and Tecnis OptiBlue IOL (AMO Japan, Tokyo, Japan) in others. After IOL implantation, standard sub-Tenon anesthesia was induced using 2% lidocaine (in most earlier cases) or intracameral anesthesia using 1% lidocaine (in most later cases). A viscoelastic material (1% sodium hyaluronate, Opegan Hi, Santen Pharmaceutical, Osaka, Japan) was also injected into the AC to widen the angle. Using a Swan-Jacob gonioprism lens (Ocular Instruments, Bellevue, WA, USA) to observe the angle, a microhook was inserted into the AC through the corneal incision. The tip of the microhook was then inserted into the Schlemm’s canal and moved circumferentially to incise the inner wall of Schlemm’s canal and TM over 3 clock hours. Using the same procedure, LOT was performed in the opposite angle using a microhook that was inserted through the corneal port. To improve the operability in most cases, a straight hook was used to incise the nasal angle and the right-angled and left-angled hooks were used to incise the temporal angle. After the viscoelastic material was aspirated, the corneal incision and port were closed by corneal stromal hydration. At the end of surgery, 1.65 mg of dexamethasone sodium phosphate (Decadron, Aspen, Japan, Tokyo, Japan) was injected subconjunctivally and 0.3% ofloxacin ointment (Tarivid, Santen Pharmaceutical) was applied. Finally, 1.5% levofloxacin (Nipro, Osaka, Japan) and 0.1% betamethasone (Sanbetason, Santen Pharmaceutical) were applied topically four times daily for 3 to 4 weeks (i.e., 1 bottle/eye), postoperatively in all cases. Topical non-steroidal anti-inflammatory drugs were not used routinely.
2.3. Measurements
The clinical parameters, including age, sex, glaucoma type, lens status, ocular surgical history, surgical procedure (i.e., µLOT alone or combined µLOT and cataract surgery), preoperative and postoperative best-corrected visual acuities (BCVA), IOP, number of antiglaucoma medications, AC flare measured using the FM-600 laser flare meter (Kowa, Nagoya, Japan), corneal endothelial cell density (CECD) measured using the EM-3000 specular microscope (Tomey, Nagoya, Japan), visual field mean deviation (MD) (central 30-2 program, Humphrey Visual Field Analyzer, Carl Zeiss Meditec, Dublin, CA, USA), and duration of postoperative follow-up were collected from the medical charts. The decimal BCVA was converted to the logarithm of the minimum angle of resolution VA. Counting fingers, hand motions, light perception, and no light perception were regarded as decimal VAs of 0.0025, 0.002, 0.0016, and 0.0013, respectively [13]. The IOP was measured using Goldmann applanation tonometry except for that measured on postoperative day 3 using the iCARE rebound tonometer (M.E. Technica, Tokyo, Japan). The site at which the LOT procedure was performed (i.e., nasal or temporal angle or both) and the extent, perioperative complications, simultaneous procedures other than regular cataract surgery, interventions for complications, and additional glaucoma surgeries performed were recorded by reviewing the medical and surgical records.
2.4. Statistical Analysis
The preoperative and final IOPs, medications, BCVA, AC flare, MD, and CECD were compared using the paired t-test. Successful IOP control was assessed by the fixed-point analysis and the survival curve analysis. For the fixed-point analysis, success was defined as a postoperative IOP of 18 mmHg or less and an IOP reduction of 20% or more compared with the preoperative value, or a postoperative IOP of 15 mmHg or less and IOP reduction of 20% or more compared with the preoperative value at the final visit. For survival curve analysis, the uncensored date was defined as the postoperative period of later than 90 days and the day when the IOP exceeded 18 mmHg (definitions A and C) or 15 mmHg (definitions B and D), IOP reductions of less than 20% (definitions A and B) or those that exceeded the baseline IOP (definitions C and D) with use of antiglaucoma medications, additional glaucoma surgery (all definitions), or loss of light perception (all definitions); the cases other than those that were uncensored were regarded as censored cases at the final visit. The cumulative incidence of additional glaucoma surgery after µLOT was analyzed by survival curve analysis; the uncensored date was defined as the day additional glaucoma surgery was performed, and the cases other than uncensored cases were regarded as censored cases at the final visit. To adjust for possible biases derived from the inclusion of both eyes of a patient and for differences in follow-up periods, the preoperative IOP and IOPs measured on day 3, weeks 1 to 2, and months 1 (3–5 weeks), 3 (2–4 months), 6 (5–7 months), 9 (8–10 months), 12 (11–14 months), 18 (15–21 months), 24 (22–27 months), 30 (28–33 months), and 36 (34–39 months) were compared using a mixed-effects regression model in which each patient’s identification number was regarded as a random effect and the time period as a fixed effect; this was followed by the t-test for the post-hoc comparison between groups. Postoperative changes in the number of antiglaucoma medications, BCVA, AC flare, MD, and CECD were also assessed using the mixed-effects regression model. In addition to the analyses in all eyes, the analyses were performed separately in eyes treated with µLOT alone or combined µLOT and cataract surgery separately. To assess the possible factors affecting the postoperative IOP, multiple regression analyses were performed; for the analyses, the IOP recorded at the final visit was a dependent variable, and age, gender, glaucoma types, surgical procedure, preoperative lens status, LOT extent, preoperative IOP, preoperative number of medications, and presence of postoperative complications were independent variables. All continuous data were expressed as the mean ± SD. All statistical analyses were performed using the JMP version 11.0 statistical software (SAS Institute, Inc., Cary, NC, USA). p < 0.05 was considered significant.
3. Results
Table 1 summarizes the patient data. Primary open-angle glaucoma (POAG) (57%) was the most frequent glaucoma type in this case series, followed by exfoliation glaucoma (20%), primary angle-closure glaucoma (PACG) including mixed-mechanism glaucoma (13%), steroid-induced glaucoma (3%), developmental glaucoma (3%), and others including secondary glaucoma due to uveitis or various causes (4%). µLOT was performed as an initial ocular surgery in 428 (76%) eyes. Among the 79 (14%) eyes with a history of previous cataract surgery, 47 eyes (8%) had no history of glaucoma surgery; thus, µLOT was performed as an initial glaucoma surgery in 475 (85%) eyes. µLOT was performed as a solo procedure in 159 (28%) eyes and combined procedure in 401 (72%) eyes; half of the eyes treated with the solo procedure were pseudophakic. µLOT was performed on both the nasal and temporal sides in 512 (92%) eyes, only on the nasal side in 24 (4%) eyes, and only on the temporal side in 24 (4%) eyes. The LOT extent was 6.9 h when µLOT was performed on both sides and 3.8 h and 3.6 h, respectively, when µLOT was performed only on the nasal or the temporal side. The duration of the follow-up was 405 ± 327 (range, 2–1326) days.
With the mixed-effects regression model, the postoperative changes in IOP were significant in the entire dataset, and in eyes treated with µLOT alone or combined µLOT (p < 0.0001 for each model) (Table 2). Compared with the preoperative data, in all eyes and eyes treated with µLOT alone or the combined procedure, the IOP reductions were significant (p < 0.0001–0.0072) at every time point up to 36 months postoperatively; the reductions in IOPs were 6.3 (31%) mmHg, 6.9 (31%) mmHg, and 6.0 (31%) mmHg in each group at the final visit, respectively. The postoperative changes in the number of antiglaucoma medications were significant in the entire dataset and in eyes treated with µLOT alone or combined µLOT (p < 0.0001 for each model) (Table 3). Compared with preoperatively, in the total dataset, the reductions in the number of antiglaucoma medications were significant up to 24 months (p < 0.0001–0.0191), but were not significant at 20 months and later, for up to 36 months postoperatively (p = 0.0918–0.2612). In all the eyes and eyes treated with µLOT alone or the combined procedure, the respective reductions in medications were 0.3 (11%), 0.5 (15%), and 0.3 (11%) in each group at the final visit; excluding 12 eyes for which data were missing, 534 (97%) eyes used at least one antiglaucoma medication at the final visit.

Table 2.
Preoperative and Postoperative Intraocular Pressures (mmHg) Compared by Mixed-Effects Regression Model.

Table 3.
Preoperative and Postoperative Medications Compared by Mixed-Effects Regression Model.
At the final visit, as assessed by the fixed-point success rate analysis, 379 (69%) eyes achieved successful IOP control of 18 mmHg or less and IOP reductions of 20% or more, and 349 (64%) eyes achieved successful IOP control of 15 mmHg or less and 20% IOP reductions of 20% or more. By life-table analysis, with antiglaucoma medication use, the success rates of IOP control of 18 mmHg or lower and IOP reductions of 20% or more, were 44.6% and 32.1% at postoperative years 1 and 2, respectively (Figure 1a), and the rates of 15 mmHg or lower and IOP reductions of 20% or more were 36.9% and 24.7% at postoperative years 1 and 2, respectively (Figure 1b). With the less demanding definitions of success, with antiglaucoma medication use, the success rates of IOP control of 18 mmHg or less and not exceeding the preoperative IOP were 69.1% and 58.0% at postoperative years 1 and 2, respectively (Figure 1c), and the rates of 15 mmHg or lower and not exceeding the preoperative IOP were 53.6% and 40.1% at postoperative years 1 and 2, respectively (Figure 1d).
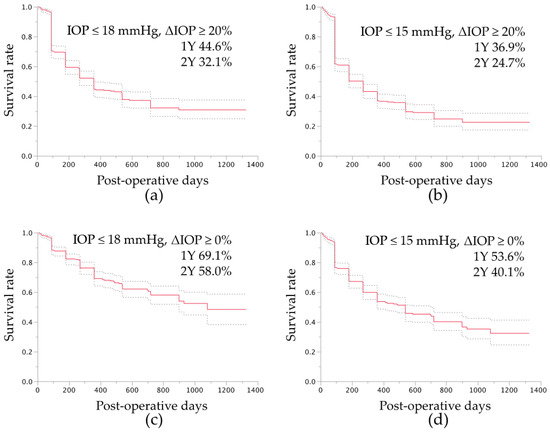
Figure 1.
Success rate of intraocular pressure (IOP) control after microhook trabeculotomy by survival curve analysis. For the survival curve analysis, the uncensored date was defined as the postoperative period of later than 90 days and the day when the IOP level exceeded 18 mmHg (a,c) or 15 mmHg (b,d), IOP reduction of less than 20% (a,b), or exceeding the baseline IOP (c,d) with antiglaucoma medication, additional glaucoma surgery (a–d), or loss of light perception (a–d); the cases other than those that were uncensored were regarded as censored cases at the final visit. The dotted lines indicate the ranges of the 95% confidence intervals of the survival analysis. Y, years.
Intraoperative complications and additional procedures were recorded in 24 (4%) eyes and 36 (6%) eyes, respectively (Table 4); most complications and additional procedures were related to the cataract surgery. The postoperative complications developed and interventions required were in 239 (43%) eyes and 63 (11%) eyes, respectively (Table 5). The most common postoperative complications and interventions other than additional glaucoma surgery were layered hyphema in 172 (30%) eyes and hyphema washout in 26 (5%), respectively. Additional glaucoma surgery was required in 57 (10%) eyes; the procedures included Ahmed Glaucoma Valve implantation in 21 (3%) eyes, trabeculectomy in 18 (3%) eyes, Ex-PRESS shunt in 13 (2%) eyes, µLOT in four (<1%) eyes, and goniosynechialysis and selective laser trabeculoplasty in one (<1%) eye each. The cumulative incidence rates of additional glaucoma surgery are shown in Figure 2. Additional surgeries were performed at 303 ± 264 days (range, 8–968 days) after the µLOT procedure.

Table 4.
Intraoperative Complications and Interventions

Table 5.
Postoperative Complications and Interventions
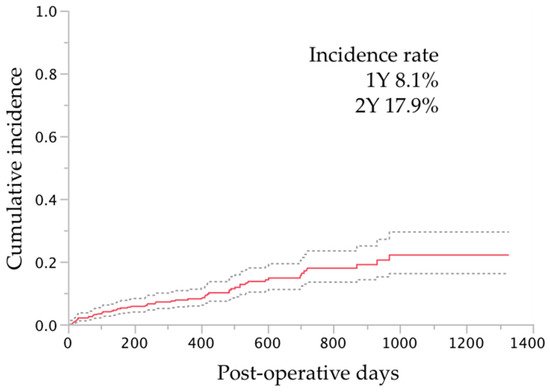
Figure 2.
The cumulative incidence of additional glaucoma surgery after microhook trabeculotomy by survival curve analysis. For the survival curve analysis, the uncensored date was defined as the day when additional glaucoma surgery was performed; the cases other than those that were uncensored were regarded as censored at the final visit. The dotted lines indicate the ranges of the 95% confidence intervals of the survival analysis. Y, years.
In all eyes, compared with preoperatively, significantly better BCVA values (Table 6), higher AC flare values (Table 7), better visual field MD (Table 8), and fewer CECD (Table 9) were observed at the final visit (p < 0.0001–0.0011); these significant differences were observed in the combined surgery group (p < 0.0001–0.0004) but not in the µLOT-alone group (p = 0.1568–0.9069).

Table 6.
Preoperative and Postoperative Best-Corrected Visual Acuity (Logarithm of the Minimum Angle of Resolution) Compared by Mixed-Effects Regression Model.

Table 7.
Preoperative and Postoperative Anterior Chamber Flare (Photon Counts/msec) Compared by Mixed-Effects Regression Model.

Table 8.
Preoperative and Postoperative Visual Field MD (dB) Compared by Mixed-Effects Regression Model.

Table 9.
Preoperative and Postoperative CECD (cells/mm2) Compared by Mixed-Effects Regression Model.
Finally, the possible factors associated with the postoperative IOP were assessed by multiple regression analyses (Table 10). Among the factors included in the model, older age, steroid-induced glaucoma, developmental glaucoma, and absence of postoperative complications were associated with lower final IOPs, and exfoliation glaucoma, other types of glaucoma (most cases were uveitic glaucoma with various etiology), and higher preoperative IOP were associated with higher final IOPs. Gender, solo or combined surgery, lens status, extent of trabeculotomy, and number of preoperative medications were not detected as a significant factor.

Table 10.
Assessment of Factors Associated with Postoperative Intraocular Pressure Levels.
4. Discussion
This study included all 560 eyes treated with µLOT between the time when the procedure was developed in 2015 and March 2018. In the current cases, marked IOP reductions were achieved after the LOT procedure during the early to midterm postoperative period for up to 3 years in eyes with various glaucoma types. This agrees with the previous results after ab externo 120-degree LOT for POAG [1,2,3,14], exfoliation glaucoma [14,15], and PACG [16]. In eyes with POAG, the respective postoperative IOP and percentages of IOP reduction were, respectively, 15.4 mmHg and 13% 17 months after cataract surgery alone was performed [17], 16.1 mmHg and 24% 12 months after the ab externo 120-degree LOT procedure combined with cataract surgery [2,3], and 14.1 mmHg and 41% 12 months after phacotrabeculectomy in which mitomycin C was used [18]. Therefore, µLOT with/without cataract surgery achieved a 31% postoperative IOP reduction with medication at the final visit, comparable to or exceeding the reductions achieved with cataract surgery alone and ab externo 120-degree LOT combined with cataract surgery [2,3], and was less effective than phacotrabeculectomy with mitomycin C. However, this difference should be clarified in a comparative study. With medication use, about two-thirds of the current eyes achieved successful IOP control below 15 mmHg at the final visit by fixed-point analysis, and half of the cases achieved 15 mmHg 1 year postoperatively by life-table analyses (Figure 1d); thus, µLOT seems effective for normalizing the IOP at least during the early postoperative period, but its effect was not sufficiently powerful in cases that required target IOPs lower than normal or in cases in which medications had to stop.
In the current study, the surgeon determined the site at which LOT was performed, i.e., either in the temporal, nasal, or both angles, although in most (92%) cases LOT was performed in both angles. A perfusion study of autopsy eyes reported that incisions in the TM for 1, 4, and 12 clock hours eliminated 30%, 44%, and 51%, respectively, of outflow resistance, at a perfusion pressure of 7 mmHg, and 30%, 56%, and 72%, respectively, of outflow resistance at a perfusion pressure of 25 mmHg [19], indicating that different extents of LOT can result in different degrees of IOP reduction. Accordingly, it would be interesting to compare between IOP reductions with angle incisions on both sides and one side, and the correlations between the extent of the incisions and IOP reductions after µLOT. The current multivariate analyses showed that the extent of LOT (range, 2–10 clock hours) was not associated with the final postoperative IOP (Table 10). Previously, neither the optical coherence tomography (OCT)-detected extent of LOT opening after Trabectome (range; 0–160 degrees) [20], nor the extent of LOT during suture LOT (S-LOT) (range, 150–320 degrees) [21], was associated with the postoperative IOP. Other studies have reported greater IOP reductions with goniotomy performed using a Kahook Dual Blade (KDB) (extent of about 90 degrees) than with single iStent trabecular bypass implantation (lumen, 120 µm) [22,23,24]. Evidence suggests that goniotomy exceeding one quadrant might exert a clinically detectable maximal IOP reduction, but the possible correlation between the extent of µLOT and its efficacy is inconclusive and should be investigated further.
Various complications developed perioperatively (Table 4 and Table 5), although most resolved spontaneously or were treated with relatively minor interventions, such as washout of the hyphema. Macular edema (ME) seen on OCT has been reported to range from 4% to 11% after modern cataract surgery [25], and 4.3% after trabeculectomy [26]. In the Ahmed Baerveldt Comparison Study, cystoid ME was reported in 10 (3.6%) of 276 eyes within 3 months postoperatively [27], and in 13 (4.7%) eyes after 3 months for up to 5 years postoperatively [28]. Accordingly, the incidence of ME (22 eyes, 4%) in this case series was equivalent to that of cataract surgery or filtration surgeries. The absence of the use of topical non-steroidal anti-inflammatory drugs may be associated with the incidence of ME, although the association was undetermined. In cases with combined surgery, no severe complications associated with cataract surgery developed, and the VA (Table 6) or visual field (Table 8) improved significantly at the final visit compared to the preoperative values. Thus, simultaneous cataract surgery with µLOT resulted in visual function recovery in eyes with glaucoma with visually relevant cataracts. A transient IOP spike was reported in 15.2% to 29% after ab externo LOT [2,15,29,30], 28% to 33% after S-LOT [31,32], 5.4% after Trabectome [33], and 5.7% after KDB [34]. Thus, the incidence of an IOP spike after µLOT (6%) seems lower than ab externo LOT or ab interno LOT with a wider incision, and equivalent to other ab interno goniotomy procedures. In advanced cases, a postoperative IOP spike is potentially vision-threatening. Although some surgeons have reported the clinical relevance of performing Trabectome for advanced glaucoma [35], we recommend that the decision to perform µLOT should be considered carefully in glaucomatous eyes with advanced visual field defects. We observed increased AC flare after µLOT (Table 7), which returned to the preoperative level by 6 months postoperatively. As reported previously, postoperative increases in AC flare after µLOT might last longer than filtration surgery, such as Ex-PRESS shunt [36]. The loss of CECD was reported to be 6.5% 1 year after cataract surgery alone in eyes with glaucoma [37], 6.3% 2 years after trabeculectomy monotherapy [38], and 2.4% 1 year after Trabectome (combined surgery, 47%) [39]. In our cases, the rates of losses of CECD were 0% and 6% after monotherapy and combined surgery, respectively (Table 9); thus, µLOT itself seems to have minimal impact on the surgical loss of CECD. In the current case series, persistent hypotony subsequent to ciliochoroidal detachment (CCD) developed in four eyes. As discussed previously [40], increased uveoscleral outflow due to LOT [41] or the creation of a cyclodialysis cleft by traction of the pectinate ligament [40] can be a mechanism of CCD development. Akagi et al. reported that a CCD detected by anterior-segment OCT developed in 14 (42%) of 33 eyes 3 days after a Trabectome procedure; the CCD persisted in four eyes (12%) at 1 month and resolved by 3 months [42]. Sato et al. reported that CCDs detected by anterior-segment OCT developed in 21 (48%) of 44 eyes within 7 days after S-LOT; the CCDs resolved in 19 eyes within 1 month and in two eyes by 3 months [43]. Cyclodialysis and hypotony maculopathy were reported in one case after KDB [44]. Thus, CCD itself is not rare after goniotomy/LOT, and although rare, hypotony might persist after ab interno goniotomy/LOT.
Intraoperatively, the incisional depth can be controlled by monitoring the tip of the hook through the TM, based on the resistance. This allows surgeons to make a selective incision of the TM/inner wall of the Schlemm’s canal with minimal damage to the outer wall of the Schlemm’s canal; incising the inner wall without damaging the outer wall of the Schlemm’s canal may be difficult when using a straight knife. µLOT seems to be an easier procedure than traditional goniotomy. Conjunctival and scleral sparing with the ab interno technique, short surgical time, moderate IOP reduction, and no bleb-related complications fulfill the conditions of minimally invasive glaucoma surgery [45,46], as with the recent techniques of ab interno LOT/trabeculectomy and gonio-bypass surgeries, such as the Trabectome [33], iStent [47], gonioscopy-assisted transluminal LOT/S-LOT [7,8], ab interno canaloplasty [48], and KDB [49,50]. Because of the minimally invasive characteristics of the surgery, the µLOT can be performed at the time of the surgery for visually significant cataracts in glaucoma eyes, and this can explain the inclusion of eyes with low preoperative IOP in this dataset. The low surgical cost because of no requirement for expensive devices and the use of reusable hooks are other advantages of our procedure; thus, µLOT can be a suitable procedure, especially in areas/countries with resource-poor settings.
The limitations of the current study included the absence of a control group, the retrospective design, the short mean follow-up, and the inclusion of eyes with various glaucoma types and previous ocular surgeries. Large numbers of lost follow-up are another limitation for the implication of surgical efficacy in this study; however, we believe that including all 560 eyes into the analyses might have merit to provide unbiased information regarding the adverse events of this surgical procedure. Based on the multivariate analyses (Table 10), among the glaucoma types, steroid-induced glaucoma and developmental glaucoma are especially good candidates for µLOT, which agreed with previous studies of ab externo LOT [51,52]. The inclusion of both eyes of a patient, various follow-up periods, and missing data may also have introduced bias, although we minimized this by using a mixed-effects regression model. We believe that the current results show that µLOT is worth further evaluation in a comparative study of other surgeries, such as cataract surgery alone or other TM surgeries.
5. Conclusions
In summary, the mean preoperative intraocular pressure (IOP) (20.2 mmHg) and number of antiglaucoma medications (2.8) decreased 31% (13.9 mmHg) and 11% (2.5), respectively, at the mean final visit of 405 days postoperatively. In conclusion, µLOT has a significant IOP-lowering potential in patients with glaucoma, and improves visual function when combined with cataract surgery.
Author Contributions
Conceptualization, M.T.; methodology, M.T.; formal analysis, M.T.; investigation, M.T., K.S., A.T., K.H., K.M. and Y.M.; data curation, M.T., K.S., A.T., K.H., K.M. and Y.M.; writing—original draft preparation, M.T.; writing—review and editing, K.S., A.T., K.H., K.M. and Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study adhered to the tenets of the Declaration of Helsinki; the institutional review board (IRB) of Matsue Red Cross Hospital reviewed and approved the research (IRB No. 261; May 19, 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is fully available upon reasonable request to corresponding author.
Conflicts of Interest
The microhooks used were co-developed by Masaki Tanito, and Inami & Co., Ltd. (Tokyo, Japan). Tanito receives royalties from Inami & Co., Ltd. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. Other authors report no conflicts of interest in this work.
References
- Chihara, E.; Nishida, A.; Kodo, M.; Yoshimura, N.; Matsumura, M.; Yamamoto, M.; Tsukada, T. Trabeculotomy ab externo: An alternative treatment in adult patients with primary open-angle glaucoma. Ophthalmic Surg. 1993, 24, 735–739. [Google Scholar]
- Tanito, M.; Ohira, A.; Chihara, E. Surgical outcome of combined trabeculotomy and cataract surgery. J. Glaucoma 2001, 10, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Ohira, A.; Chihara, E. Factors leading to reduced intraocular pressure after combined trabeculotomy and cataract surgery. J. Glaucoma 2002, 11, 3–9. [Google Scholar] [PubMed]
- Kashiwagi, K.; Kogure, S.; Mabuchi, F.; Chiba, T.; Yamamoto, T.; Kuwayama, Y.; Araie, M. Change in visual acuity and associated risk factors after trabeculectomy with adjunctive mitomycin C. Acta Ophthalmol. 2016, 94, e561–e570. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.D.; Lynch, M.G. 360 degrees trabeculotomy for primary congenital glaucoma. Arch. Ophthalmol. 1995, 113, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.; Nitta, T.; Shinmei, Y.; Aoyagi, M.; Nitta, A.; Ohno, S.; Ishida, S.; Yoshida, K. Reduction of intraocular pressure using a modified 360-degree suture trabeculotomy technique in primary and secondary open-angle glaucoma: A pilot study. J. Glaucoma 2012, 21, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Grover, D.S.; Godfrey, D.G.; Smith, O.; Feuer, W.J.; Montes de Oca, I.; Fellman, R.L. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: Technique report and preliminary results. Ophthalmology 2014, 121, 855–861. [Google Scholar] [PubMed]
- Sato, T.; Hirata, A.; Mizoguchi, T. Outcomes of 360 degrees suture trabeculotomy with deep sclerectomy combined with cataract surgery for primary open angle glaucoma and coexisting cataract. Clin. Ophthalmol. 2014, 8, 1301–1310. [Google Scholar] [CrossRef]
- Tanito, M.; Sano, I.; Ikeda, Y.; Fujihara, E. Microhook ab interno trabeculotomy, a novel minimally invasive glaucoma surgery, in eyes with open-angle glaucoma with scleral thinning. Acta Ophthalmol. 2016, 94, e371–e372. [Google Scholar] [CrossRef]
- Tanito, M.; Sano, I.; Ikeda, Y.; Fujihara, E. Short-term results of microhook ab interno trabeculotomy, a novel minimally invasive glaucoma surgery in Japanese eyes: Initial case series. Acta Ophthalmol. 2017, 95, e354–e360. [Google Scholar] [PubMed]
- Tanito, M.; Ikeda, Y.; Fujihara, E. Effectiveness and safety of combined cataract surgery and microhook ab interno trabeculotomy in Japanese eyes with glaucoma: Report of an initial case series. Jpn. J. Ophthalmol. 2017, 61, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M. Microhook ab interno trabeculotomy, a novel minimally invasive glaucoma surgery. Clin. Ophthalmol. 2018, 12, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Fishman, G.A.; Anderson, R.J.; Tozatti, M.S.; Heckenlively, J.R.; Weleber, R.G.; Edwards, A.O.; Brown, J., Jr. Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology 1999, 106, 1780–1785. [Google Scholar] [CrossRef]
- Hara, K.; Takai, Y.; Tanito, M. Outcomes after Combined Deep Sclerectomy and Trabeculotomy to Treat Primary Open-Angle Glaucoma and Exfoliation Glaucoma. Shimane J. Med. Sci. 2019, 35, 43–52. [Google Scholar]
- Honjo, M.; Tanihara, H.; Inatani, M.; Honda, Y.; Ogino, N.; Ueno, S.; Negi, A.; Ichioka, H.; Mizoguchi, T.; Matsumura, M.; et al. Phacoemulsification, intraocular lens implantation, and trabeculotomy to treat pseudoexfoliation syndrome. J. Cataract. Refract. Surg. 1998, 24, 781–786. [Google Scholar] [CrossRef]
- Tanihara, H.; Negi, A.; Akimoto, M.; Nagata, M. Long-term results of non-filtering surgery for the treatment of primary angle-closure glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 1995, 233, 563–567. [Google Scholar] [CrossRef]
- Chen, P.P.; Lin, S.C.; Junk, A.K.; Radhakrishnan, S.; Singh, K.; Chen, T.C. The Effect of Phacoemulsification on Intraocular Pressure in Glaucoma Patients: A Report by the American Academy of Ophthalmology. Ophthalmology 2015, 122, 1294–1307. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kobayashi, K. Randomized comparison of the intraocular pressure-lowering effect of phacoviscocanalostomy and phacotrabeculectomy. Ophthalmology 2007, 114, 909–914. [Google Scholar] [CrossRef]
- Rosenquist, R.; Epstein, D.; Melamed, S.; Johnson, M.; Grant, W.M. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr. Eye Res. 1989, 8, 1233–1240. [Google Scholar] [CrossRef]
- Wecker, T.; Anton, A.; Neuburger, M.; Jordan, J.F.; van Oterendorp, C. Trabeculotomy opening size and IOP reduction after Trabectome® surgery. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1643–1650. [Google Scholar] [CrossRef]
- Manabe, S.I.; Sawaguchi, S.; Hayashi, K. The effect of the extent of the incision in the Schlemm canal on the surgical outcomes of suture trabeculotomy for open-angle glaucoma. Jpn. J. Ophthalmol. 2017, 61, 99–104. [Google Scholar] [CrossRef]
- Dorairaj, S.K.; Kahook, M.Y.; Williamson, B.K.; Seibold, L.K.; ElMallah, M.K.; Singh, I.P. A multicenter retrospective comparison of goniotomy versus trabecular bypass device implantation in glaucoma patients undergoing cataract extraction. Clin. Ophthalmol. 2018, 12, 791–797. [Google Scholar] [CrossRef] [PubMed]
- ElMallah, M.K.; Seibold, L.K.; Kahook, M.Y.; Williamson, B.K.; Singh, I.P.; Dorairaj, S.K. 12-Month Retrospective Comparison of Kahook Dual Blade Excisional Goniotomy with Istent Trabecular Bypass Device Implantation in Glaucomatous Eyes at the Time of Cataract Surgery. Adv. Ther. 2019, 36, 2515–2527. [Google Scholar] [CrossRef]
- Lee, D.; King, J.; Thomsen, S.; Hirabayashi, M.; An, J. Comparison Of Surgical Outcomes Between Excisional Goniotomy Using The Kahook Dual Blade And iStent Trabecular Micro-Bypass Stent In Combination With Phacoemulsification. Clin. Ophthalmol. 2019, 13, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Patel, S.; Baumrind, B.; Johnson, K.; Levinsohn, D.; Marcus, E.; Tannen, B.; Roy, M.; Bhagat, N.; Zarbin, M. Management of pseudophakic cystoid macular edema. Surv. Ophthalmol. 2015, 60, 123–137. [Google Scholar] [CrossRef]
- Manabe, K.; Matsuoka, Y.; Tanito, M. Incidence of macular edema development after filtration surgery. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1343–1345. [Google Scholar] [CrossRef]
- Budenz, D.L.; Barton, K.; Feuer, W.J.; Schiffman, J.; Costa, V.P.; Godfrey, D.G.; Buys, Y.M. Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology 2011, 118, 443–452. [Google Scholar] [CrossRef]
- Budenz, D.L.; Feuer, W.J.; Barton, K.; Schiffman, J.; Costa, V.P.; Godfrey, D.G.; Buys, Y.M. Postoperative Complications in the Ahmed Baerveldt Comparison Study During Five Years of Follow-up. Am. J. Ophthalmol. 2016, 163, 75–82.e3. [Google Scholar] [CrossRef]
- Inatani, M.; Tanihara, H.; Muto, T.; Honjo, M.; Okazaki, K.; Kido, N.; Honda, Y. Transient intraocular pressure elevation after trabeculotomy and its occurrence with phacoemulsification and intraocular lens implantation. Jpn. J. Ophthalmol. 2001, 45, 288–292. [Google Scholar] [CrossRef]
- Tanihara, H.; Honjo, M.; Inatani, M.; Honda, Y.; Ogino, N.; Ueno, S.; Negi, A.; Ichioka, H.; Mizoguchi, T.; Matsumura, M.; et al. Trabeculotomy combined with phacoemulsification and implantation of an intraocular lens for the treatment of primary open-angle glaucoma and coexisting cataract. Ophthalmic Surg. Lasers 1997, 28, 810–817. [Google Scholar]
- Sato, T.; Kawaji, T.; Hirata, A.; Mizoguchi, T. 360-degree suture trabeculotomy ab interno to treat open-angle glaucoma: 2-year outcomes. Clin. Ophthalmol. 2018, 12, 915–923. [Google Scholar] [CrossRef]
- Sato, T.; Kawaji, T.; Hirata, A.; Mizoguchi, T. 360-degree suture trabeculotomy ab interno with phacoemulsification in open-angle glaucoma and coexisting cataract: A pilot study. BMJ Open Ophthalmol. 2018, 3, e000159. [Google Scholar] [CrossRef] [PubMed]
- Minckler, D.S.; Baerveldt, G.; Alfaro, M.R.; Francis, B.A. Clinical results with the Trabectome for treatment of open-angle glaucoma. Ophthalmology 2005, 112, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Berdahl, J.P.; Gallardo, M.J.; ElMallah, M.K.; Williamson, B.K.; Kahook, M.Y.; Mahootchi, A.; Rappaport, L.A.; Lazcano-Gomez, G.S.; Díaz-Robles, D.; Dorairaj, S.K. Six-Month Outcomes of Goniotomy Performed with the Kahook Dual Blade as a Stand-Alone Glaucoma Procedure. Adv. Ther. 2018, 35, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Bhatt, A.; Schmutz, M.; Mosaed, S. Trabectome outcomes across the spectrum of glaucoma disease severity. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1703–1710. [Google Scholar] [CrossRef]
- Tanito, M.; Manabe, K.; Mochiji, M.; Takai, Y.; Matsuoka, Y. Comparison of anterior chamber flare among different glaucoma surgeries. Clin. Ophthalmol. 2019, 13, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Chihara, E.; Umemoto, M.; Tanito, M. Preservation of corneal endothelium after pars plana tube insertion of the Ahmed glaucoma valve. Jpn. J. Ophthalmol. 2012, 56, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, K.; Nitta, E.; Ukegawa, K.; Sato, S.; Kiuchi, Y. Effect of trabeculectomy on corneal endothelial cell loss. Br. J. Ophthalmol. 2020, 104, 376–380. [Google Scholar] [CrossRef]
- Kasahara, M.; Shoji, N.; Matsumura, K. The Influence of Trabectome Surgery on Corneal Endothelial Cells. J. Glaucoma 2019, 28, 150–153. [Google Scholar] [CrossRef]
- Ishida, A.; Mochiji, M.; Manabe, K.; Matsuoka, Y.; Tanito, M. Persistent Hypotony and Annular Ciliochoroidal Detachment after Microhook Ab Interno Trabeculotomy. J. Glaucoma 2020. [Google Scholar] [CrossRef]
- Amari, Y.; Hamanaka, T.; Futa, R. Pathologic investigation failure of trabeculotomy. J. Glaucoma 2015, 24, 316–322. [Google Scholar] [CrossRef]
- Akagi, T.; Nakano, E.; Nakanishi, H.; Uji, A.; Yoshimura, N. Transient Ciliochoroidal Detachment After Ab Interno Trabeculotomy for Open-Angle Glaucoma: A Prospective Anterior-Segment Optical Coherence Tomography Study. JAMA Ophthalmol. 2016, 134, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kawaji, T.; Hirata, A. Transient ciliochoroidal detachment after 360-degree suture trabeculotomy ab interno for open-angle glaucoma: 12-month follow-up. Eye 2019, 33, 1081–1089. [Google Scholar] [CrossRef]
- Shue, A.; Levine, R.M.; Gallousis, G.M.; Teng, C.C. Cyclodialysis Cleft Associated with Kahook Dual Blade Goniotomy. J. Curr. Glaucoma Pract. 2019, 13, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.I. MIGS and the FDA: What’s in a Name? Ophthalmology 2015, 122, 1737–1739. [Google Scholar] [CrossRef] [PubMed]
- Kahook, M.Y.; Seibold, L.K.; SooHoo, J.R.; Mansouri, K.; Sharaawy, T. A nuanced approach to the surgical management of glaucoma. Middle East. Afr. J. Ophthalmol. 2015, 22, 1. [Google Scholar] [CrossRef]
- Malvankar-Mehta, M.S.; Chen, Y.N.; Iordanous, Y.; Wang, W.W.; Costella, J.; Hutnik, C.M. iStent as a Solo Procedure for Glaucoma Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0128146. [Google Scholar] [CrossRef]
- Khaimi, M.A. Canaloplasty: A Minimally Invasive and Maximally Effective Glaucoma Treatment. J. Ophthalmol. 2015, 2015, 485065. [Google Scholar] [CrossRef]
- Seibold, L.K.; Soohoo, J.R.; Ammar, D.A.; Kahook, M.Y. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am. J. Ophthalmol. 2013, 155, 524–529.e2. [Google Scholar] [CrossRef]
- SooHoo, J.R.; Seibold, L.K.; Kahook, M.Y. Ab interno trabeculectomy in the adult patient. Middle East. Afr. J. Ophthalmol. 2015, 22, 25–29. [Google Scholar] [CrossRef]
- Ikeda, H.; Ishigooka, H.; Muto, T.; Tanihara, H.; Nagata, M. Long-term outcome of trabeculotomy for the treatment of developmental glaucoma. Arch. Ophthalmol. 2004, 122, 1122–1128. [Google Scholar] [CrossRef]
- Iwao, K.; Inatani, M.; Tanihara, H. Success rates of trabeculotomy for steroid-induced glaucoma: A comparative, multicenter, retrospective cohort study. Am. J. Ophthalmol. 2011, 151, 1047–1056.e1. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).