Clinical Analysis of Persistent Subretinal Fluid after Pars Plana Vitrectomy in Macula with Diabetic Tractional Retinal Detachment
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Measurement of Systemic Parameters
2.3. Surgical Technique
2.4. Statistical Analyses
3. Results
3.1. Demographics of Patients
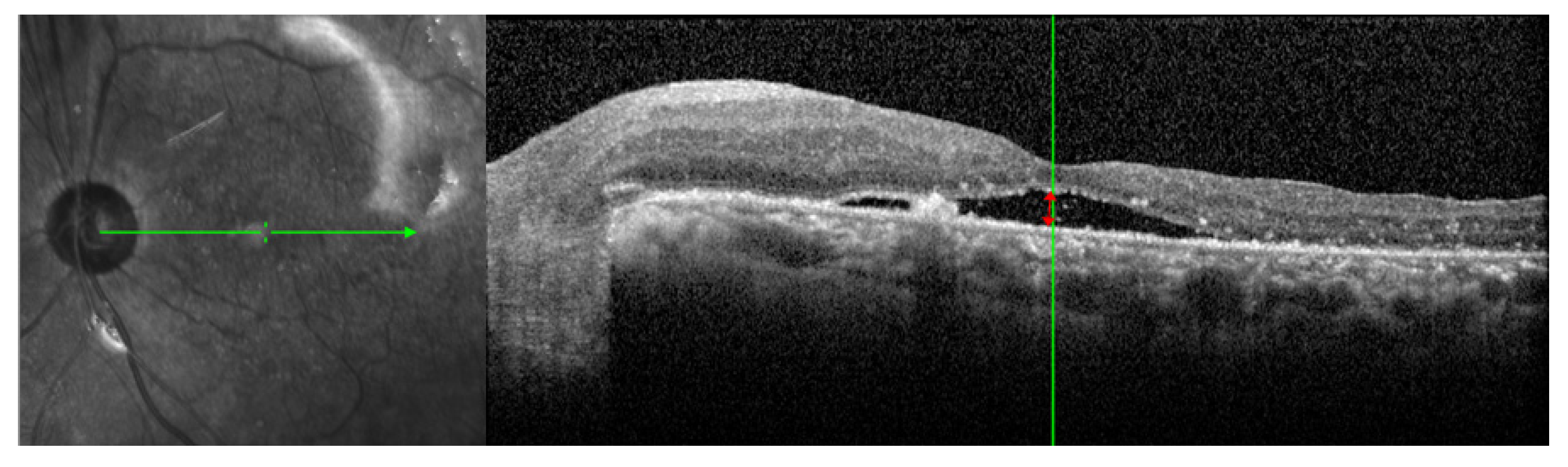
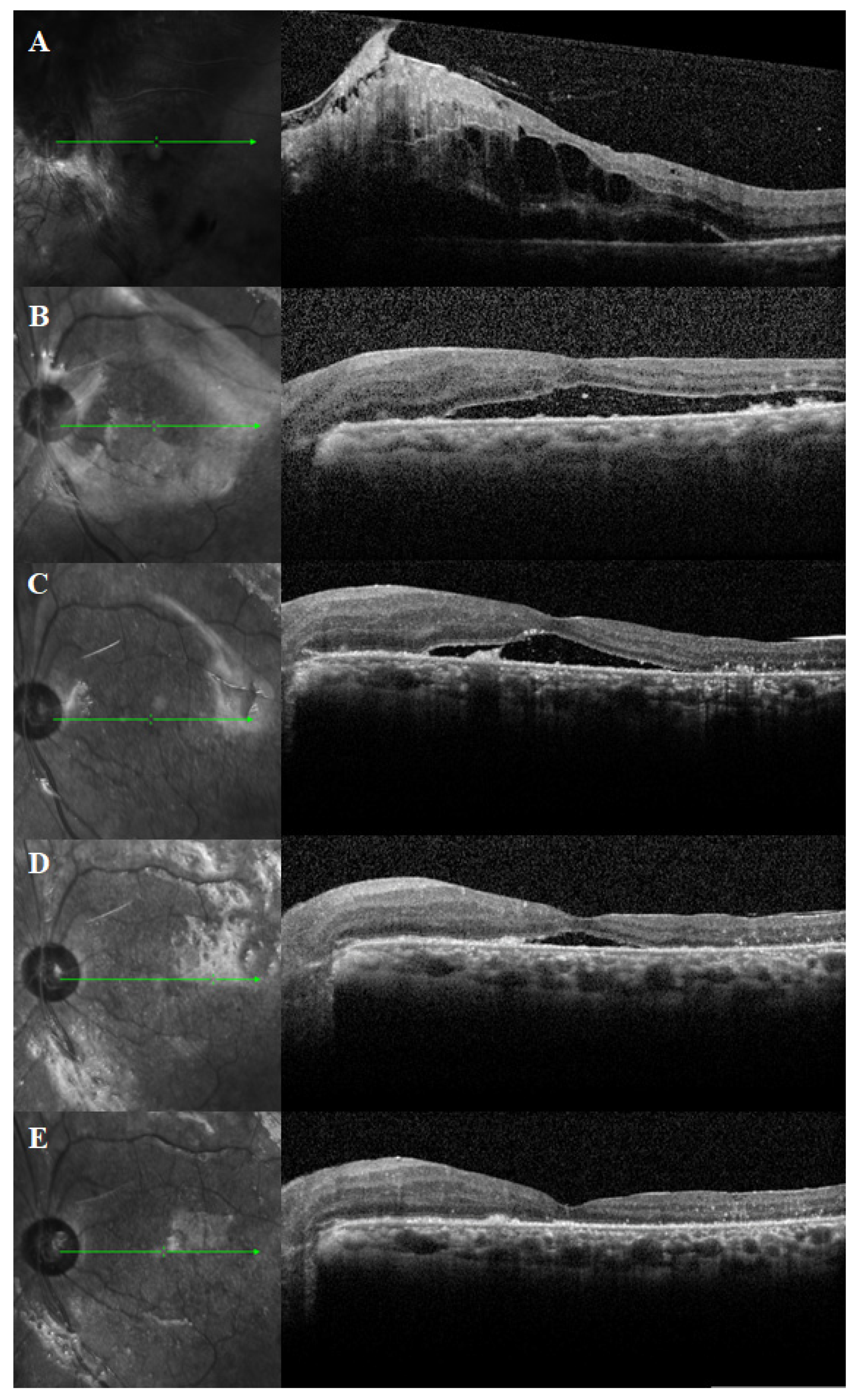
3.2. Duration of PSF and SRFH
3.3. Best-Corrected Visual Acuity
3.4. Clinical Factors Associated with PSF Duration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chew, E.Y.; Ferris, F.L., 3rd; Csaky, K.G.; Murphy, R.P.; Agron, E.; Thompson, D.J.; Reed, G.F.; Schachat, A.P. The long-term effects of laser photocoagulation treatment in patients with diabetic retinopathy: The early treatment diabetic retinopathy follow-up study. Ophthalmology 2003, 110, 1683–1689. [Google Scholar] [CrossRef]
- Writing Committee for the Diabetic Retinopathy Clinical Research Network; Gross, J.G.; Glassman, A.R.; Jampol, L.M.; Inusah, S.; Aiello, L.P.; Antoszyk, A.N.; Baker, C.W.; Berger, B.B.; Bressler, N.M.; et al. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA 2015, 314, 2137–2146. [Google Scholar] [CrossRef]
- Hagimura, N.; Iida, T.; Suto, K.; Kishi, S. Persistent foveal retinal detachment after successful rhegmatogenous retinal detachment surgery. Am. J. Ophthalmol. 2002, 133, 516–520. [Google Scholar] [CrossRef]
- Ricker, L.J.; Noordzij, L.J.; Goezinne, F.; Cals, D.W.; Berendschot, T.T.; Liem, A.T.; Hendrikse, F.; La Heij, E.C. Persistent subfoveal fluid and increased preoperative foveal thickness impair visual outcome after macula-off retinal detachment repair. Retina 2011, 31, 1505–1512. [Google Scholar] [CrossRef]
- Tee, J.J.; Veckeneer, M.; Laidlaw, D.A. Persistent subfoveolar fluid following retinal detachment surgery: An SD-OCT guided study on the incidence, aetiological associations, and natural history. Eye 2016, 30, 481–487. [Google Scholar] [CrossRef]
- Veckeneer, M.; Derycke, L.; Lindstedt, E.W.; van Meurs, J.; Cornelissen, M.; Bracke, M.; Van Aken, E. Persistent subretinal fluid after surgery for rhegmatogenous retinal detachment: Hypothesis and review. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2012, 250, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Barzideh, N.; Johnson, T.M. Subfoveal fluid resolves slowly after pars plana vitrectomy for tractional retinal detachment secondary to proliferative diabetic retinopathy. Retina 2007, 27, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Karimov, M.I.; Gasymov, E.M.; Aliyeva, I.J.; Akhundova, L.A.; Rustambayova, G.R.; Aliyev, K.D. An optical coherence tomography study of residual subfoveal fluid after successful pars plana vitrectomy in patients with diabetic tractional macular detachment. Eye 2018, 32, 1472–1477. [Google Scholar] [CrossRef]
- Long, K.; Meng, Y.; Chen, J.; Luo, J. Multifactor analysis of delayed absorption of subretinal fluid after scleral buckling surgery. BMC Ophthalmol. 2021, 21, 86. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Sharma, T.; Fong, A.; Lai, T.Y.; Lee, V.; Das, S.; Lam, D. Surgical treatment for diabetic vitreoretinal diseases: A review. Clin. Exp. Ophthalmol. 2016, 44, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Meredith, T.A.; Kaplan, H.J.; Aaberg, T.M. Pars plana vitrectomy techniques for relief of epiretinal traction by membrane segmentation. Am. J. Ophthalmol. 1980, 89, 408–413. [Google Scholar] [CrossRef]
- Tao, Y.; Jiang, Y.R.; Li, X.X.; Gao, L.; Jonas, J.B. Long-term results of vitrectomy without endotamponade in proliferative diabetic retinopathy with tractional retinal detachment. Retina 2010, 30, 447–451. [Google Scholar] [CrossRef]
- Williams, D.F.; Williams, G.A.; Hartz, A.; Mieler, W.F.; Abrams, G.W.; Aaberg, T.M. Results of vitrectomy for diabetic traction retinal detachments using the en bloc excision technique. Ophthalmology 1989, 96, 752–758. [Google Scholar] [CrossRef]
- Abrams, G.W.; Azen, S.P.; McCuen, B.W., 2nd; Flynn, H.W., Jr.; Lai, M.Y.; Ryan, S.J. Vitrectomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy: Results of additional and long-term follow-up. Silicone Study report 11. Arch. Ophthalmol. 1997, 115, 335–344. [Google Scholar] [CrossRef]
- Qamar, R.M.; Saleem, M.I.; Saleem, M.F. The outcomes of pars plana vitrectomy without endotamponade for tractional retinal detachment secondary to proliferative diabetic retinopathy. Int. J. Ophthalmol. 2013, 6, 671–674. [Google Scholar] [CrossRef][Green Version]
- Jung, B.J.; Jeon, S.; Lee, K.; Baek, J.; Lee, W.K. Internal Limiting Membrane Peeling for Persistent Submacular Fluid after Successful Repair of Diabetic Tractional Retinal Detachment. J. Ophthalmol. 2019, 2019, 8074960. [Google Scholar] [CrossRef]
- Marmor, M.F. Control of subretinal fluid: Experimental and clinical studies. Eye 1990, 4 Pt 2, 340–344. [Google Scholar] [CrossRef]
- Jain, A.; Saxena, S.; Khanna, V.K.; Shukla, R.K.; Meyer, C.H. Status of serum VEGF and ICAM-1 and its association with external limiting membrane and inner segment-outer segment junction disruption in type 2 diabetes mellitus. Mol. Vis. 2013, 19, 1760–1768. [Google Scholar] [PubMed]
- Koo, N.K.; Kim, Y.C. Resolution of macular edema after systemic treatment with furosemide. Korean J. Ophthalmol. KJO 2012, 26, 312–315. [Google Scholar] [CrossRef]
- Tsai, M.J.; Hsieh, Y.T.; Shen, E.P.; Peng, Y.J. Systemic Associations with Residual Subretinal Fluid after Ranibizumab in Diabetic Macular Edema. J. Ophthalmol. 2017, 2017, 4834201. [Google Scholar] [CrossRef] [PubMed]
- Man, R.E.; Sasongko, M.B.; Wang, J.J.; MacIsaac, R.; Wong, T.Y.; Sabanayagam, C.; Lamoureux, E.L. The Association of Estimated Glomerular Filtration Rate with Diabetic Retinopathy and Macular Edema. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4810–4816. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Cheng, C.K.; Wang, Y.C. Association of Body Fluid Expansion with Optical Coherence Tomography Measurements in Diabetic Retinopathy and Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3606–3612. [Google Scholar] [CrossRef] [PubMed]
- Takamura, Y.; Matsumura, T.; Ohkoshi, K.; Takei, T.; Ishikawa, K.; Shimura, M.; Ueda, T.; Sugimoto, M.; Hirano, T.; Takayama, K.; et al. Functional and anatomical changes in diabetic macular edema after hemodialysis initiation: One-year follow-up multicenter study. Sci. Rep. 2020, 10, 7788. [Google Scholar] [CrossRef]

| Characteristics | Value |
|---|---|
| Number of eyes, n (%) | |
| OD | 14 (35.0%) |
| OS | 26 (65.0%) |
| Sex, n (%) | |
| Male | 23 (57.5%) |
| Female | 17 (42.5%) |
| Mean age, years | 49.9 ± 12.0 |
| Mean duration between diagnosis and surgery, days | 31.1 ± 32.8 |
| Mean follow up periods, months | 27.3 ± 18.2 |
| Duration of PSF, months | 4.4 ± 4.7 |
| Systemic characteristics | |
| Duration of diabetes mellitus, years | 9.2 ± 6.7 |
| HbA1c, % | 9.3 ± 2.3 |
| Hypertension, n (%) | 15 (37.5%) |
| Chronic kidney disease, n (%) | 10 (25.0%) |
| BUN, mg/dL | 24.7 ± 11.2 |
| Creatinine, mg/dL | 1.4 ± 0.9 |
| eGFR, mL/min/1.73 m2 | 65.7 ± 29.2 |
| Ocular characteristics | |
| Panretinal photocoagulation prior to vitrectomy, n (%) | 34 (85.0%) |
| Anti-VEGF therapy prior to vitrectomy, n (%) | 14 (35.0%) |
| Preoperative intravitreal bevacizumab injection, n (%) | 33 (82.5%) |
| Phacovitrectomy, n (%) | 32 (80.0%) |
| Internal SRF drainage during vitrectomy, n (%) | 23 (57.5%) |
| ILM peeling during vitrectomy, n (%) | 9 (22.5%) |
| Intraocular SO tamponade, n (%) | 33 (82.5%) |
| Parameters | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | CI (95%) | p-Value | Odds Ratio | CI (95%) | p-Value | |
| Male sex | 0.099 | −2.993 to 3.192 | 0.949 | |||
| Age at TRD diagnosis | −0.013 | −0.142 to 0.115 | 0.836 | |||
| Duration of DM | −0.032 | −0.265 to 0.200 | 0.779 | |||
| Hemoglobin A1c | −0.123 | −0.884 to 0.638 | 0.745 | |||
| CKD on hemodialysis | −1.456 | −4.954 to 2.042 | 0.405 | |||
| BUN | 0.157 | 0.029 to 0.286 | 0.018 | −0.094 | −0.343 to 0.156 | 0.452 |
| Creatinine | 2.386 | 0.810 to 3.962 | 0.004 | 1.017 | −2.392 to 4.427 | 0.549 |
| eGFR | −0.086 | −0.131 to −0.042 | <0.001 | −0.089 | −0.170 to −0.009 | 0.030 |
| Axial length | −0.429 | −2.361 to 1.504 | 0.656 | |||
| PRP prior to vitrectomy | −0.775 | −5.049 to 3.499 | 0.716 | |||
| Anti-VEGF therapy prior to vitrectomy | 1.315 | −1.861 to 4.491 | 0.407 | |||
| Preoperative intravitreal bevacizumab injection | 2.655 | −1.273 to 6.583 | 0.179 | |||
| Phacovitrectomy | −0.276 | −3.538 to 2.987 | 0.865 | |||
| Internal SRF drainage | −1.445 | −5.063 to 2.199 | 0.430 | |||
| ILM peeling during vitrectomy | 1.852 | −1.758 to 5.462 | 0.306 | |||
| Intraocular SO tamponade | −1.591 | −5.377 to 2.195 | 0.400 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.-K.; Shin, J.-P. Clinical Analysis of Persistent Subretinal Fluid after Pars Plana Vitrectomy in Macula with Diabetic Tractional Retinal Detachment. J. Clin. Med. 2021, 10, 5929. https://doi.org/10.3390/jcm10245929
Kang Y-K, Shin J-P. Clinical Analysis of Persistent Subretinal Fluid after Pars Plana Vitrectomy in Macula with Diabetic Tractional Retinal Detachment. Journal of Clinical Medicine. 2021; 10(24):5929. https://doi.org/10.3390/jcm10245929
Chicago/Turabian StyleKang, Yong-Koo, and Jae-Pil Shin. 2021. "Clinical Analysis of Persistent Subretinal Fluid after Pars Plana Vitrectomy in Macula with Diabetic Tractional Retinal Detachment" Journal of Clinical Medicine 10, no. 24: 5929. https://doi.org/10.3390/jcm10245929
APA StyleKang, Y.-K., & Shin, J.-P. (2021). Clinical Analysis of Persistent Subretinal Fluid after Pars Plana Vitrectomy in Macula with Diabetic Tractional Retinal Detachment. Journal of Clinical Medicine, 10(24), 5929. https://doi.org/10.3390/jcm10245929






