Abstract
High-density lipoprotein (HDL) functional traits have emerged as relevant elements that may explain HDL antiatherogenic capacity better than HDL cholesterol levels. These properties have been improved in several lifestyle intervention trials. The aim of this systematic review is to summarize the results of such trials of the most commonly used dietary modifications (fatty acids, cholesterol, antioxidants, alcohol, and calorie restriction) and physical activity. Articles were screened from the Medline database until March 2021, and 118 randomized controlled trials were selected. Results from HDL functions and associated functional components were extracted, including cholesterol efflux capacity, cholesteryl ester transfer protein, lecithin-cholesterol acyltransferase, HDL antioxidant capacity, HDL oxidation status, paraoxonase-1 activity, HDL anti-inflammatory and endothelial protection capacity, HDL-associated phospholipase A2, HDL-associated serum amyloid A, and HDL-alpha-1-antitrypsin. In mainly short-term clinical trials, the consumption of monounsaturated and polyunsaturated fatty acids (particularly omega-3 in fish), and dietary antioxidants showed benefits to HDL functionality, especially in subjects with cardiovascular risk factors. In this regard, antioxidant-rich dietary patterns were able to improve HDL function in both healthy individuals and subjects at high cardiovascular risk. In addition, in randomized trial assays performed mainly in healthy individuals, reverse cholesterol transport with ethanol in moderate quantities enhanced HDL function. Nevertheless, the evidence summarized was of unclear quality and short-term nature and presented heterogeneity in lifestyle modifications, trial designs, and biochemical techniques for the assessment of HDL functions. Such findings should therefore be interpreted with caution. Large-scale, long-term, randomized, controlled trials in different populations and individuals with diverse pathologies are warranted.
1. Introduction
Low concentrations of high-density lipoprotein (HDL) cholesterol (HDL-C) have been linked with greater incidence of coronary heart disease in epidemiological studies [1,2]. Nevertheless, experimental and genetic studies have questioned the therapeutic utility of raising HDL-C levels. Pharmacological interventions aimed at increasing HDL-C concentrations (fibrates, niacin, statins, inhibitors of the cholesteryl ester transfer protein –CETP–) have failed to reduce the incidence of cardiovascular disease (CVD) [1,3]. In addition, Mendelian randomization studies have reported that presenting genetic predisposition to high levels of HDL-C is not linked to lower CVD risk [4,5], although recent results show a likely causal association with medium-size HDL-C [6].
On the other hand, HDL functions and associated functional components have been shown to be independently associated with lower CVD incidence [7] and stand as promising alternative biomarkers to explain the HDL atheroprotective role. The most studied HDL atheroprotective function is reverse cholesterol transport (Figure 1), which can be measured in vitro by the cholesterol efflux capacity (CEC) technique. It evaluates the ability of HDLs to remove cholesterol excess from cells and is measured in macrophage-derived cell cultures incubated with radio-labelled or fluorescent-labelled cholesterol [8]. HDLs can also be linked with enzymes related to HDL reverse cholesterol transport, such as lecithin cholesterol acyltransferase (LCAT), involved in cholesterol esterification, or cholesteryl ester transfer protein (CETP), crucial for cholesterol removal to the liver (see Figure 1) [8]. The second HDL atheroprotective function is antioxidant capacity, the ability to prevent LDL oxidation [9]. HDL carries antioxidant enzymes capable of degrading oxidized lipids, mainly paraoxonase-1 (PON1) and phospholipase A2 (LpPLA2) [9]. The global antioxidant capacity of HDLs can be measured in vitro by techniques such as the HDL oxidative/inflammatory index (HOII) [10]. On the contrary, HDLs could become dysfunctional after oxidation of their components. The oxidative status of HDLs can be evaluated by measuring the content of malondialdehyde (a lipid peroxide) [11]. A third HDL function is the capacity to modulate inflammatory responses. HDLs are potentially able to decrease expression of endothelial adhesion proteins and chemokines [12]. However, HDLs can also carry on their surface pro-inflammatory proteins related to HDL dysfunctionality, such as serum amyloid A (SAA) and alpha-1-antitrypsin [13]. Finally, this lipoprotein could also present a protective effect on the endothelial layer of the arteries [12]. A healthy endothelium maintains a proper permeability and regulates vascular tone, helping to prevent atherosclerosis. In this regard, HDLs have shown the capacity to improve the release of nitric oxide, a potent vasodilator secreted by the endothelium. Nitric-oxide production can be evaluated in vitro in cellular cultures of endothelial cells [12].
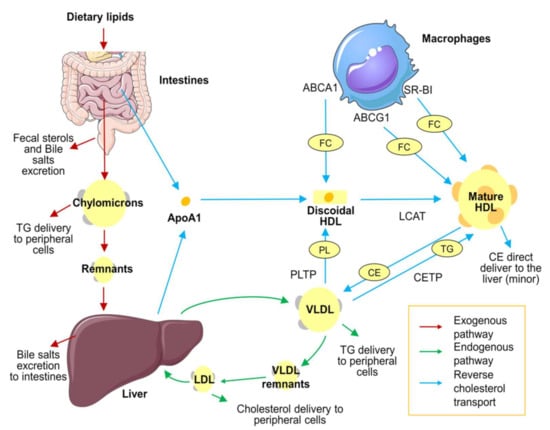
Figure 1.
Lipoprotein metabolism overview. Lipid distribution in the body occurs in three different pathways. First, the exogenous pathway (red arrows): The absorption of dietary triglycerides, free cholesterol, and cholesteryl esters occurs in the small intestine. In enterocytes, dietary lipids are packed in chylomicrons and diffused to the bloodstream. Chylomicrons diffuse triglycerides to peripheral cells, and their remnants are therefore cleared in the liver. Second, the endogenous pathway (green arrows): Triglycerides and cholesterol synthetized in the liver are recirculated in the bloodstream packed in VLDL. VLDL transports triglycerides to peripheral cells. VLDL remnants are transported to the liver, where remaining triglycerides are removed by hepatic lipase action and become LDL. LDL transports cholesterol to peripheral tissues and is eventually cleared by the liver. Finally, HDLs are responsible for reverse cholesterol transport (blue arrows): ApoA1 is synthetized in the liver and enterocytes and released as a lipid-free monomer. Then it incorporates phospholipids by action of PLTP from VLDL. Lipid-free ApoA1 is able to collect free cholesterol of peripheral cells, such macrophages, through the ABCA1 receptor. The accumulation of phospholipids and free cholesterol results in the formation of discoidal HDL. Free cholesterol is transformed to cholesteryl esters and continuously internalized in the HDL core by LCAT enzymes, forming the mature form of HDL. Mature HDL continues to pick up cholesterol through ABCG1 and SR-BI receptors. Finally, the accumulated cholesterol can be transported back to the liver, mainly in an indirect way (exchanging cholesteryl esters for triglycerides with VLDL through CETP activity) or, in a minor proportion, directly through hepatic receptors. The figure was produced using Servier Medical Art (http://smart.servier.com/ accessed on 6 December 2021). ABCA1: ATP-binding cassette transporter A1. ABCG1: ATP-binding cassette transporter G1. ApoA1: Apolipoprotein A1. CE: cholesterol esters. CETP: cholesteryl ester transfer protein. FC: free cholesterol. HDL: high-density lipoprotein. LCAT: lecithin cholesterol acyltransferase. LDL: low-density lipoprotein. PL: phospholipid. PLTP: phospholipid transfer protein. TG: triglycerides. VLDL: very low-density lipoprotein.
In a recent meta-analysis, high CEC values have been inversely related to CVD incidence [7]. Furthermore, CVD incidence has been linked to HDL antioxidant/anti-inflammatory properties and HDL-related endothelial protection in individual prospective studies in a general population and one at elevated cardiovascular disease risk [14,15,16]. There is a wide range of lifestyle intervention studies regarding other HDL functions. They include interventions on several HDL functions with different dietary modifications (intake of monounsaturated, polyunsaturated, saturated, and trans fatty acids–MUFAs, PUFAs, SFAs, and TFAs, respectively–cholesterol, antioxidant vitamins, phenolic compounds and other minor compounds, ethanol, and calorie restriction) and physical activity. The aim of this systematic review is to summarize all the evidence of the effects of dietary modifications and physical activity on HDL functions.
2. Materials and Methods
2.1. Search Strategy
To identify relevant trials, we searched the electronic database PubMed, looking for articles published until 10 March 2021. We performed twelve searches, one for each HDL function or HDL-associated functional component terms; (1) CEC activity; (2) CETP activity; (3) lecithin cholesterol acyl transferase (LCAT) activity; (4) HDL antioxidant capacity; (5) HDL oxidation status; (6) PON1 activity; (7) HDL anti-inflammatory and endothelial protection properties; (8) HDL-associated phospholipase A2; (9) HDL-associated SAA; (10) HDL sphingosine-1-phosphate content; (11) HDL-alpha-1-antitrypsin; and (12) HDL-associated complement proteins. The exact search terms in each case are displayed in the Supplementary Material (Supplementary File S1).
The literature search was developed by two authors (A.S. and A.H.), with a standardized strategy. The articles were first filtered according to title, then abstract, and finally full text content. The bibliography of each selected article was also reviewed to find additional references. Any disagreement between the two authors was resolved by a third author (M.F.).
2.2. Study Selection, Inclusion, and Exclusion Criteria
Studies included were randomized controlled trials (RCTs) with any lifestyle intervention in humans that modified HDL functional traits (including whole diets, individual dietary components or near-dietary dose supplements, and physical activity). Postprandial studies, interventions of less than a week, studies with fewer than ten participants, and studies that used pharmacological therapies or high-dose supplements were discarded. The search was limited to articles written in English.
2.3. Data Extraction
The following information was extracted from each article: author, year, country, number of participants and characteristics (basal disease), type of intervention (randomization, control group, dose, and duration), parameter measured, and study outcomes. The study outcomes were quite heterogeneous due to the diversity of methodologies employed to measure HDL functions. All methodologies are stated in the beginning of each section of the review.
To facilitate analyses, lifestyle interventions were sub-grouped into five categories: dietary lipid interventions, antioxidant-rich interventions, ethanol, physical activity and calorie-restriction interventions, and other lifestyle interventions.
2.4. Risk of Bias Assessment
The risk of bias of each RCT was assessed by two authors (A.S. and A.H.) with the Cochrane Collaboration Risk of Bias Tool [17]. This instrument includes seven domains: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; and (7) other potential biases. We individually assessed and classified each domain as low, high, or unclear risk of bias (when the information provided was insufficiently clear). The overall risk of an article was evaluated as low when the majority of the domains corresponded to low risk and no key domain was high-risk; as high if one or more key domains were classified as high-risk; and unclear when the majority of the key domains were categorized as unclear risk of bias.
3. Results
3.1. Study Selection and Description
We obtained 12,796 results from all twelve searches combined. After screening the primary search and excluding duplicates, 815 articles were selected. Following screening by abstract, 263 were considered adequate for full-text review. Finally, after examining the full text, 88 articles were excluded for not following a randomized design, 18 for using pharmacological therapies in combination with lifestyle interventions, 24 for not studing any pre-specified HDL function technique, eight for employing vitamins at considerably high dosage, and seven for being postprandial interventions, shorter than a week, or including fewer than 10 participants. Finally, a total of 118 RCTs were selected for a qualitative synthesis (Figure 2).
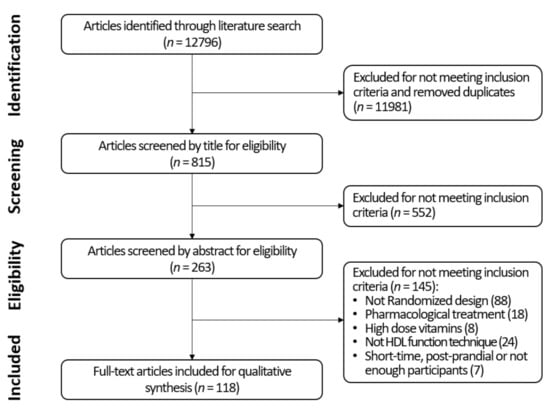
Figure 2.
Flow diagram of study selection.
Cholesterol efflux was studied in 37 of the trials, HDL CETP activity or mass in 53, LCAT activity in 32, antioxidant properties in six, HDL oxidation in six, PON1 activity in 45, HDL anti-inflammatory and endothelial protection properties in nine, HDL-associated phospholipase A2 in one, HDL-bound SAA in two, and HDL-alpha-1-antitrypsin in one article. No results were found for HDL sphingosne-1-phosphate and HDL-associated complement proteins.
3.2. Characteristics of Studies Included
All studies were RCTs (with a parallel group or crossover design) and included a total of 5645 participants. Most were short-term interventions, ranging from 7 days to 6 months. Only 10 RCTs (8.4% of the total) [18,19,20,21,22,23,24,25,26,27,28] lasted from 6 to 12 months. In addition, sample sizes were generally modest. With respect to number of participants, 73.4% of the studies included 50 or fewer subjects, 17.8% had between 51 and 100, and 8.4% (ten studies) include more than 100 subjects [19,20,21,22,25,28,29,30,31,32]. Due to the nature of lifestyle interventions, most of the studies were not blinded to participants.
3.3. Quality Assessment
From 118 RCTs, 28.0% presented a low risk of bias, 17.8% a high risk, and 54.2% an unclear risk (Figure 3). Unclear risk was mainly caused by the absence of detailed information in the study protocols. Individual bias evaluation of the articles is available in Supplemental Materials (Table S1).
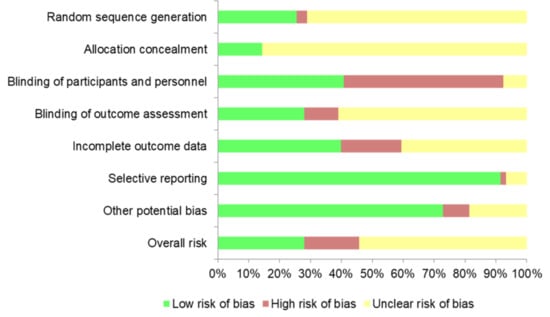
Figure 3.
Overall risk of bias across studies.
3.4. Dietary Lipids and HDL Function
3.4.1. Monounsaturated Fatty Acids (MUFA): Oleic-Acid-Rich Oils
To determine the effect of MUFA interventions on HDL functions, nine studies were selected (Table 1). All of them assessed the effect of MUFAs from olive oil, canola oil, and peanut oil, compared to saturated fats and PUFAs.

Table 1.
Studies with monounsaturated fatty acids (MUFA): oleic-acid-rich oil.
In a 4-week RCT, MUFA intake (olive oil) was associated with a 4.7% increase in CEC values compared to an equivalent fat-calorie intake from cheese [33]. Similarly, an RCT with a 4-week consumption of canola oil (59% MUFAs) or a high-oleic canola oil (72% MUFAs) increased CEC by 39.1% and 33.6%, respectively, relative to baseline levels but not when compared to three interventions rich in PUFAs [29]. In contrast, an 8-week oleic-acid-rich diet did not improve CEC relative to a linoleic-acid-rich diet [34].
In one RCT, MUFA intake was associated with decreased CETP activity relative to SFA-rich and TFA-rich diets of 6 weeks [35]. However, no significant effects were reported in another RCT comparing a 35-day diet rich in canola oil (49% MUFAs) to diets rich in palm oil (50% SFAs), soybean oil (44% PUFAs), and partially hydrogenated soybean oil (13% TFAs) [36].
A MUFA-rich 6-week RCT based on the consumption of peanut oil increased serum LCAT activity relative to diets rich in rapeseed oil (also MUFA-rich) and dairy fats (from butter and cream, SFA-rich) [37]. In contrast, LCAT activity was not modified by a MUFA-rich dietary intervention compared to two interventions rich in PUFA oils during a period of 2 weeks [38]. HDL particles did not present higher levels of LCAT on their surface after a 32-day MUFA-rich diet intervention, compared to a high carbohydrate diet [39].
Finally, in relation to oxidative-stress-related properties, a MUFA-based supplemented intervention was not linked to changes in PON1 paraoxonase activity compared to supplements of EPA and DHA (2 g/day) during a 6-week period [40]. Nevertheless, overall HDL oxidation was decreased relative to an 8-week linoleic-rich diet [34].
From the nine studies regarding MUFA intervention, five reported no effect on HDL function biomarkers at all (four of them, however, were compared with PUFA interventions), two observed some effect in healthy volunteers, and two more found some effect in obese and metabolic syndrome patients.
3.4.2. Polyunsaturated Fatty Acids: Vegetable Oils and Nuts
Twenty-one RCTs evaluated the effect of vegetable oils, linoleic-rich nuts, and linolenic fatty acids on HDL functions (Table 2). Seven assessed the effects of vegetable PUFAs against SFA and TFA interventions, six against other PUFA types and MUFAs, and seven compared them to low-fat diets and PUFA-free diets.

Table 2.
Studies with polyunsaturated fatty acids (PUFA): vegetable oils and nuts.
Regarding cholesterol metabolism, the consumption of almonds (43 g/day for 6 weeks) increased non-ABCA1 CEC compared to an isocaloric muffin without PUFAs in normal-weight participants with non-significant results for global and ABCA1 CEC [41]. A 4-week intervention with corn and safflower oils (both rich in linoleic acid) and an intervention with flaxseed oil (rich in linolenic acid) incremented CEC values relative to baseline [29]. Another RCT with two servings/day of pistachios for 4 weeks was associated with CEC improvements relative to a subgroup of participants with low C-reactive protein levels consuming one serving/day [42]. However, another four RCTs assessing vegetal PUFA intake did not report associations with changes in CEC relative to MUFA-rich diets [34,43], nor SFA- or TFA-rich diets [44,45].
Regarding CETP, a flaxseed-oil intervention (α-linolenic acid, 5.5 g/day for 12 weeks) decreased CETP activity relative to the control diet with corn oil [46]. A safflower-oil-rich diet for 6 weeks (50% of total dietary fat, rich in linoleic acid) decreased CETP from HDLs to apolipoprotein B-containing lipoproteins in relation to a butter-rich diet [47]. A 17-day linoleic-acid-rich diet (8% of total energy) decreased CETP activity relative to a TFA-rich diet [48]. An 8-week baru-almond-enriched diet (30.13% of fat as linoleic acid) decreased plasma CETP levels [49]. However, other trials did not report any changes in CETP activity or concentrations after an intervention with pistachios [50], as well as two PUFA-rich diets, relative to SFA-rich dietary arms [51,52].
LCAT activity increased after a 6-week diet complemented with 60 mL/day of sunflower oil (rich in linoleic acid) compared to a low-erucic-acid rapeseed-oil diet (rich in MUFAs) and a diet rich in dairy fats (SFAs) [37]. Nevertheless, LCAT activity in plasma was decreased in relation to baseline values after 2 weeks of 60 g/day of flaxseed oil (rich in α-linolenic acid) [38], although no changes were reported in a similar trial [53].
Finally, when assessing HDL antioxidant properties, the consumption of a walnut-paste-enriched meat (20% walnut paste, 750 g/week) increased PON1 paraoxonase activity relative to the control intervention with a low-fat diet [54], as well as relative to baseline in two similar RCTs with the same walnut intake [55,56]. Intake of linoleic-acid-rich safflower oil (4.5 g/day for 4-weeks) was also associated with increases in PON arylesterase activity relative to trans-conjugated linoleic-acid intervention in an RCT [57].
Briefly, in healthy individuals, no effect of a PUFA intervention (vegetable oils and nuts) on HDL function was observed in four out of seven studies, while in three studies, some effect was reported. From 14 studies in subjects with cardiovascular risk-related pathologies, 10 demonstrated some effect, while no benefit was reported in four.
3.4.3. Polyunsaturated Fatty Acids: Fish, Eicosapentaenoic and Docosahexaenoic Acids (EPA, DHA)
The effect of fish fatty acids (eicosapentaenoic and docosahexaenoic acids (EPA, DHA)) on HDL functions was evaluated in eleven RCTs (Table 3). Three interventions made comparisons with other PUFAs, and eight were supplements compared with placebos or non-supplemented interventions.

Table 3.
Studies with polyunsaturated fatty acids (PUFA): fish, eicosapentaenoic and docosahexaenoic acids (EPA, DHA).
An intervention with a DHA-enriched canola-oil smoothie (5.8% DHA content) was associated with a 55% increment in CEC values, relative to baseline, in metabolic syndrome patients [29]. However, a trial studying the effect of dietary fatty fish (1 g/day EPA + DHA) compared to lean fish or with camelina oil (high in linolenic) did not show effects on CEC [58]. Additionally, higher doses of EPA + DHA in supplements (four capsules of 1.88 g EPA + 1.48 g of DHA per day) did not result in effects on CEC compared to a placebo [59].
Cholesteryl ester transference from HDLs to apolipoprotein B-containing lipoproteins decreased relative to baseline after an EPA + DHA supplement (4 g/day) [60]. In contrast, CETP levels did not change in an RCT with an EPA + DHA supplement (four capsules 1.88 g EPA + 1.48 g DHA per day) [59]. LCAT activity was also decreased relative to baseline values in an RCT after a fish-oil supplement (3.8 g/day) [53], whilst the circulating levels of the enzyme did not change in RCTs assessing an EPA + DHA supplement [59], and in lower doses in an EPA/DHA-enriched milk (131.25 mg EPA and 243.75 mg DHA) [61].
With respect to HDL antioxidant properties, PON1 activity was significantly increased relative to a placebo control in RCTs with fish-oil supplements (1 g/day) [62] and EPA (2 g/day). PON1 circulating levels also incremented in RCTs with 2 g/day EPA supplements [63], compared to the control, and with EPA/DHA-enriched milk (relative to baseline) [61]. However, no significant differences in PON1 levels were found in an RCT studying a low-dose supplement of DHA (500 mg/day) [64] and after the consumption of 2 g/day purified EPA and DHA relative to olive oil [40]. The global antioxidant capacity of HDLs was evaluated in an intervention with EPA + DHA supplementation (4 g/day), which was associated with a deleterious effect on the HDL inflammatory index in heart failure patients compared to lesser doses of EPA + DHA (1 g/day) and placebo treatments [65].
From the 11 studies of patients with cardiovascular risk-related pathologies, rheumatoid arthritis or heart failure, seven showed some effect on HDL function after a fish, EPA, or DHA intervention, whilst four reported no effects.
3.4.4. Saturated (SFA) and Trans Fatty Acids (TFA)
Sixteen RCTs evaluated the effect of SFAs and TFAs on HDL functions (Table 4). Among them, twelve were compared with PUFAs and MUFAs.

Table 4.
Studies with saturated (SFA) and trans fatty acids (TFA).
Regarding CEC, a diet containing 12.5% SFAs from butter improved CEC values (+4.3%) relative to a cheese-rich isocaloric diet or a carbohydrate-rich diet [33], whilst no differences relative to baseline were observed after a 4-week intervention with palm-oil-rich and TFA-rich (hydrogenated soybean oil) diets in healthy individuals [44].
Increases in CETP activity and mass were reported in an RCT with a palmitic-acid-enriched diet (45% total fat content) [35], compared with MUFA-rich dietary patterns. Rises in CETP activity were also found in RCTs comparing a butter-rich diet with a linoleic-acid-rich intervention [47] and a lauric-acid-rich diet (relative to baseline) [66]. In contrast, no effects were observed in two short-term RCTs after consumption of palm-oil-rich diets relative to two PUFA-rich diets [36,51] and in a general saturated-fat-rich diet relative to a PUFA-rich diet [52]. Another RCT comparing the effect of 70 g/day saturated medium-chain fatty acids (caprylic and capric acids) with a high-MUFA intervention (70 g/day) did not report changes in CETP activity [67].
Intake of TFAs was associated with an increase in CETP activity when a 17-day TFA-rich dietary intervention (8% trans fats) was compared with stearic and linoleic-acid-rich diets [48]. In a similar manner, a 5-week margarine-rich diet intervention also incremented CETP activity relative to butter-rich and semiliquid margarine-rich diets [68], and a marginally significant increase was reported after 5 weeks high-TFA margarine consumption in older hypercholesterolemic women [69]. In comparison, no effects on CETP were observed after a 5-week consumption of partially hydrogenated soybean oil in hypercholesterolemic individuals [36,70] and another TFA-rich intervention [71].
Regarding LCAT activity, 6-week SFA-rich diets (from butter and cream) decreased LCAT activity relative to sunflower and peanut oils (rich in linoleic and oleic acids) [37]. However, no effects were reported after a 5-week TFA-rich dietary modification (based on partially hydrogenated soybean oil with 3% TFAs) [70].
Finally, when referring to HDL antioxidant properties, a 4-weekTFA-rich intervention (9.3% total fat in TFA from margarine) decreased PON1 paraoxonase activity relative to a SFA-rich diet [72]. However, no effects were reported after a 5-week consumption of a partially hydrogenated soybean oil (13% total fats as TFAs) or palm oil (50% total fats as SFAs) [36].
From 16 studies with SFA and TFA interventions, eight were conducted with healthy volunteers, and some detrimental effect on HDL function was reported in four of them. In addition, in subjects with dyslipidemia or obesity, some effect was observed in four studies.
3.4.5. Dietary Cholesterol
The effects of dietary cholesterol, provided mainly by egg intake, on HDL functions were studied in 15 RCTs (Table 5).

Table 5.
Studies with dietary cholesterol.
Consuming two eggs/day significantly incremented CEC relative to control interventions (a low-fat diet with the same weight of yolk-free eggs) after 24 days and 4 weeks, respectively [73,74], and a similar effect relative to baseline values was described after a 12-week intervention with three eggs/day [75].
Three RCTs increased CETP activity after the consumption of one egg/day (64 mg/day) per 1 month in cholesterol-sensitive participants (individuals whose plasma total and HDL-C levels increased after egg consumption) in relation to non-egg consumption [76,77] and relative to baseline levels [78]. Increases in CETP mass (also parallel to increases in HDL-C levels) were observed in a high-cholesterol diet (320 mg/1000 kcal), relative to a low-cholesterol diet (80 mg/1000 kcal) [79] and after increasing egg consumption in men [80] but not in women [81]. Conversely, no effects were reported in four RCTs based on the consumption of three eggs/day for 12 weeks in metabolic syndrome patients [82], after two eggs/day for 4 weeks in overweight postmenopausal women [74], in heathy young participants [83], and after a one egg/day intervention for 4 weeks in postmenopausal women [84].
LCAT activity incremented parallel to increases in cholesterol intake. In one RCT, it augmented in interventions based on one and two eggs/day for a month (relative to lower egg intake) in healthy young individuals [85], three eggs/day for 12 weeks in carbohydrate-restricted diets in metabolic syndrome and excess-weight patients (relative to baseline levels) [82,86], and one egg/day for 1 month in dietary-cholesterol-sensitive individuals [77,78]. No effects were found in a 4-week two-egg intervention [74,83] and in a one-egg intervention after a month [84].
Finally, in some RCTs, the consumption of eggs did not modify HDL antioxidant capacity or PON10-circulating activity [74,87]. It was, however, associated with HDLs with greater lipid hydroperoxide content (more oxidized) and pro-inflammatory proteins (serum amyloid A) [87].
Briefly, from the 11 studies of healthy subjects, eight of them reported some effect. Four more studies were performed with subjects with some pathology (overweight/obese, metabolic syndrome), and they all observed some effect on HDL function after an egg-intake intervention.
3.5. Antioxidants and HDL Function
3.5.1. Antioxidant Nutrients and Antioxidant-Rich Foods
The capacity of polyphenols, carotenoids, and antioxidant vitamins in foods and supplements to improve HDL functions was assessed in 26 RCTs (Table 6).

Table 6.
Studies with antioxidant nutrients and antioxidant-rich foods.
A 3-week intervention with a virgin olive oil naturally rich in phenolic compounds (25 mL/day, raw) increased CEC by 3% relative to the control intervention (a refined olive oil) [88], and a functional virgin olive oil, further enriched with thyme polyphenols (25 mL/day, consumed raw) also augmented CEC relative to a standard virgin olive oil after 3 weeks [89]. Supplements of anthocyanins (320 mg/day) for 12 and 24 weeks were associated with significant increases in CEC (by 18–20%) relative to placebo [25,31]. However, a 3-week intervention based on an anthocyanin-rich grape powder (60 g/day) did not lead to changes in metabolic syndrome patients [90].
CETP activity was also investigated in several short-term antioxidant-based RCTs. Lycopene supplementation (70 mg/week) for 12 weeks was associated with decreased CETP activity relative to a placebo [91]. A similar effect was described after 12-week anthocyanin supplementation (320 mg/day) [31] and the consumption of a lyophilized grape powder (6 g) for 4 weeks [92]. However, no effects were observed after a 6-week intake of one cup of raisins per day [93]. The use of French-press coffee (high in diterpenes, such as cafestol and kahweol) increased CETP activity compared to a control filtered coffee (less diterpene content) [94]. Only two studies, one assessing the effects of phenol-enriched functional virgin olive oils and the other, a dietary intervention with vegetables, berries, and apples, reported no changes in CETP activity [95,96].
Finally, LCAT activity also appears to improve after antioxidant-rich interventions. High intake of lycopene (such as a lycopene-rich diet 224 to 350 mg/week or supplementation of 70 mg/week for 12 weeks) was associated with increased LCAT activity relative to baseline [91]. A phenolic-compound-rich, functional virgin olive oil also augmented LCAT mass relative to the control intervention (with standard virgin olive oil). It induced the changes in HDL composition that are usually associated with LCAT function (relative decreases in free cholesterol in the lipoprotein) [95]. Two interventions with diterpenes from coffee (cafestol and kahweol) showed decreased LCAT activity relative to baseline levels [94,97]. In contrast, an anthocyanin supplement and a dietary intervention with vegetables, berries, and apples were not related to improvements in the function of this enzyme [31,96].
In controlled trials, dietary antioxidants have also been associated with beneficial effects on HDLs by incrementing content and making HDLs more oxidation-resistant. Two interventions with natural and functional virgin olive oils also increased HDL content of dietary antioxidants (olive-oil phenolic compound metabolites, β-cryptoxanthin, and lutein) relative to the control interventions [88,89]. Indeed, as many as 17 clinical trials have focused on the promotion of PON1 antioxidant activities. Regarding carotenoids, high intake of lycopene (lycopene-rich diet 224 to 350 mg/week or supplementation of 70 mg/week) increased PON1 arylesterase activity relative to the control [91], and a supplement of astaxanthin (4 mg) for 3 months increased PON1 diazoxonase activity relative to baseline [98]. However, two trials with tomato juice (lycopene-rich 37–47 mg/day) for 2 and 8 weeks did not show differences relative to control interventions (water and carrot juice) [99,100]. In relation to phenolic compounds, two anthocyanin-based interventions (anthocyanin supplementation of 320 mg/d for 24 weeks, barberry juice 200 mL/day for 8 weeks) increased PON1 paraoxonase activity and concentration, respectively [25,101], and a 6-month intervention with a pomegranate extract (1 g/day) incremented PON1 lactonase activity relative to a placebo [24]. In contrast, a 3-week intervention with grape powder (60 g/day) did not result in significant differences in PON1 activity [90]. In three 3-week RCTs, two phenolic-compound-rich oils (virgin olive and argan oils) significantly increased PON1 paraoxonase and arylesterase activities [102]. Moreover, two virgin olive oils (one natural and the other enriched with olive phenolic compounds) promoted PON1 paraoxonase- and lactonase-specific activities [103], and another virgin olive oil enriched with thyme polyphenols boosted arylesterase activity [95], all relative to baseline values. With regard to other phenolic-compound-rich interventions in RCTs, powdered ginger (3 g/day for 3 month) increased PON1 arylesterase activity relative to a placebo [104], an extract of Turkish oregano boosted PON1 paraoxonase and arylesterase activities relative to the control after 3 months [105], and a 2-month red sage extract incremented paraoxonase activity when compared to baseline [106]. An intervention trial with yerba mate tea (1000 mL/day) increased PON1 circulating levels relative to the control [32]. However, another intervention (1000 mL/day for 3 months) did not modify PON1 arylesterase activity [107]. Null changes were reported in three RCTs studying a polyphenol-enriched tomato juice, an oatmeal porridge enriched with sea buckthorn flavonols, and dietary doses of vitamin E (15 mg/day) [108,109,110]. Finally, regarding other HDL properties potentially related to antioxidant/anti-inflammatory potential, an increased consumption of lycopene decreased the levels of HDL-bound acute-phase cytokines, such as serum amyloid A [91].
From a total of 26 studies with antioxidants, some effect on HDL function was reported in 5 out of 11 studies with healthy individuals, and 11 out of 15 in subjects with cardiovascular risk-related pathologies. A considerable part of the effects described in subjects with pathologies employed PON methodologies.
3.5.2. Antioxidant-Rich Dietary Patterns
Five human trials assessed the overall effect on HDL functions of an increase in the intake of dietary antioxidants through healthy dietary patterns (Table 7).

Table 7.
Studies with antioxidant-rich dietary patterns.
Two interventions in a long-term RCT (1 year) with Mediterranean diets enriched with virgin olive oil or mixed nuts were linked to increases in CEC values relative to baseline [19]. The same intervention with a Mediterranean diet enriched with virgin olive oil was also associated with decreased CETP activity relative to baseline and an improvement in an indirect indicator of LCAT function [19]. CETP activity also decreased relative to baseline in another 1-year intervention with an olive-oil-enriched Mediterranean diet [28]. A diet rich in fruits and vegetables (6 portions/day of fruit and vegetables for 8 weeks) was related to an increase in LCAT activity relative to baseline [111]. In contrast, a diet rich in vegetables, berries, and apples presented no changes in LCAT activity at 6 weeks [96].
Regarding the relationship between HDL and oxidative stress in RCTs, HDLs became more resistant to oxidation relative to baseline after the intervention with a Mediterranean diet enriched with virgin olive oil [19]. They also increased their content of carotenoids (α-carotene, β-cryptoxanthin, lutein, and lycopene) after an intervention with a diet rich in fruits and vegetables, relative to a diet lacking in plant-based foods [111]. In addition, HDL capacity to directly decrease LDL oxidation improved after the intervention with a virgin olive-oil-rich Mediterranean diet [19]. Following this same Mediterranean diet and a diet rich in fruits and vegetables also increased PON1 arylesterase activity when compared to control diets in two large RCTs [19,111]. Conversely, a 5-week vegetable-rich diet decreased PON1 activity relative to a low-vegetable diet [112]. A second trial based on a 6-week high intake of vegetables, berries, and apples decreased PON1 activity relative to baseline [96]. Antioxidant-rich diets have additionally been shown to improve the role of HDL in low-grade inflammation. In an RCT, a Mediterranean diet enriched with virgin olive oil decreased the concentrations of HDL-bound cytokines (α1-antitrypsin) relative to control interventions [21]. Finally, in two RCTs, following a Mediterranean diet rich in virgin olive oil has been observed to increase the activity of another antioxidant/antithrombotic enzyme carried by HDLs (HDL-bound phospholipase A2, also known as platelet-activating factor acetylhydrolase), as well as HDL capacity to promote the endothelial release of nitric oxide in vitro [19,21], relative to the control intervention in both cases.
Briefly, while one study with an antioxidant-rich dietary pattern was performed in healthy subjects without benefits to HDL function, four more were conducted in subjects at high cardiovascular risk, with benefits reported in all of them.
3.6. Ethanol and HDL Function
Moderate consumption of alcohol was analyzed in eight studies comparing the intake of wine, beer, gin, and whisky (from 15 g/day to 40 g/day) with non-alcoholic beverages (Table 8).

Table 8.
Studies on ethanol and HDL function.
Ethanol intake increased CEC in healthy populations in studies comparing: red wine (30 g alcohol/day for 2 weeks) with alcohol-free wine [113]; red wine, beer, and gin (40 g alcohol/day for 3 weeks) with an equivalent volume of carbonated water [114]; white wine with grape juice (24 g alcohol/day for 3 weeks) [115]; whisky (40 g alcohol/day for 17 days) with an equivalent volume of water [116]; and beer (30 g/day alcohol –men–, 15 g/day –women– for 4 weeks) with alcohol-free beer [117]. A marginally significant increase was also observed in a trial comparing beer (1 L/day 36 g ethanol/day– for 4 weeks) with an equivalent volume of non-alcoholic beverages [118]. In contrast, alcohol intake did not modify CETP activity in two RCTs comparing red wine with alcohol-free wine (30 g alcohol/day for 2 weeks) [113] and white wine with grape juice (24 g alcohol/day for 3 weeks) [115].
Finally, alcohol intake promoted PON1 paraoxonase activity in an RCT after a 3-week consumption of 40 g alcohol/day (as red wine, beer, or gin) relative to carbonated water [119], as well as beer (40 g alcohol/day –men–, 30 g/day –women– for 3 weeks) relative to alcohol-free beer [120]. These results are consistent with the observed increase in HDL antioxidant capacity relative to baseline in LDLs after consumption of beer during a 4-week period, (30 g alcohol/day for men and 15 g/day for women) [117].
From the eight studies with ethanol intake benefits in CEC, improvements were specially observed in four studies with healthy subjects and in one with overweight/obese participants. Two studies with healthy individuals showed improvements in PON1 activity.
3.7. Physical Activity, Calorie Restriction, and HDL Function
Physical activity, caloric restriction, and their combinations were studied as possible promoters of HDL function in thirteen RCTs (Table 9).

Table 9.
Studies with physical activity, calorie restriction, and HDL function.
Regarding CEC, the effect of a high levels of vigorous-sintensity activity (16 kcal/kg/week at 75% VO2 reserve) during a 6-month period increased radio-labeled CEC compared with two groups with different amounts of moderate-intensity physical activity and a group combining moderate-intensity exercise with a low-fat diet [20]. A 6-month high-level endurance-training intervention (with a caloric goal of 20 kcal/kg/week at 65–85% VO2) increased only the non-ABCA1 cholesterol efflux relative to a control group without exercise [20]. In the previous two studies, the same interventions evaluated with fluorescent-labeled CEC technique did not find any changes in CEC. Moreover, an RCT based on aerobic/resistance training (3 times/week for 24 weeks) also reported no effects [18]. In a similar manner, another intervention with aerobic/resistance training (4 times/week for 12 weeks) did not report changes in CEC [121]. A very low-calorie restricted diet (500 kcal/day for 6 weeks), which was accompanied by an average weight loss of 10 kg, also led to no changes in CEC [122].
The combination of physical activity and a hypocaloric diet was associated with improvements in CEC relative to usual care in a small group of obese adolescents with a diet of 1500–1800 kcal combined with supervised exercise sessions for 10 months (aerobic/resistance training 3 times/week and 2 h/day of lifestyle activities) [23]. A second trial reported improvements in CEC relative to baseline levels after a combination of aerobic training (4 times/week) and a DASH diet (with reductions of 600 kcal/day) for 12 weeks in a small group of metabolic syndrome participants [123]. However, no differences were found in a trial combining calorie-restriction counselling and aerobic exercise (mainly brisk walking 5 times per week) [124].
Regarding CETP, 6 months of aerobic training (4 times/week) did not modify its activity [22]. Nevertheless, a 6-week endurance-training intervention (3–5 times/week) decreased CETP activity relative to an untrained control group [125]. Two weight-loss interventions did not modify CETP, either in an intervention with the National Cholesterol Education Program Step I or in a 4-week intervention [126] with a very low-calorie diet (500 kcal/day for 6 weeks) [8,126]. LCAT activity and plasma concentrations were unaffected by aerobic activity (ranging from 11 weeks to 1 year) in three RCTs performed some years ago [27,127,128].
A study with of aerobic and resistance exercise programs for 12 weeks in obese women decreased serum PON1 activity relative to a control without exercise. However PON1 expression in isolated HDLs remained unaltered [121]. The same study also found no changes in the expression of isolated HDLs of the antioxidant/antithrombotic enzyme phospholipase A2 bound in HDLs [121].
Finally, regarding HDL endothelial properties, an intervention with a healthy diet combined with exercise (1500–1800 kcal/day) and aerobic/resistance training improved the HDL role in the activation of endothelial nitric oxide synthase compared to a usual-care intervention [23]. In contrast, another RCT found an aerobic and resistance exercise program for 12 weeks in obese women did not modify HDL anti-inflammatory capacity in endothelial cells [121].
In summary, there were six studies of healthy subjects with physical activity and/or calorie restriction, and in one of them, a benefit to CETP was described. From eight papers with participants presenting cardiovascular risk-related pathologies, some effect—mainly on CEC—was reported in three of them.
3.8. Other Lifestyle Interventions
Very few studies have evaluated dietary interventions unrelated to fats, antioxidants, ethanol, and physical activity/calorie intake (Table 10).

Table 10.
Other lifestyle interventions.
The effect of a prebiotic and probiotic-enriched pasta (with β-glucans 2.3 g/100 g- and Bacillus coagulans) was evaluated in a 12-week intervention. This study increased ABCG1-mediated CEC relative to a control pasta [129].
Only one RCT has evaluated the effects of the isocaloric exchange between hig- and low-glycemic-index carbohydrates on HDL function. This study found no effects on CEC [130].
Interventions with soy protein reported no changes in CEC after 6 weeks of 25–50 g soy protein and a control [131], no changes in CETP mass and LCAT activity after a 4-week, 20 g/day intake of soy protein [132], and an increase in PON1 activity in a trial of 50 g/soy protein relative to a placebo [133].
One RCT evaluated the association of carbohydrate restriction and HDL functions and found it was related to decreased LCAT activity relative to baseline but no changes in CETP function [134].
A whole dietary intervention with TLC/Step 2 diet for 32 days, a dietary pattern low in fats, and rich in vegetables, carbohydrates and fiber, did not change CETP activity [135].
Two RCTs investigated the effects of a supplement of psyllium fiber (one of them also included plant sterols) and reported a decrease in CETP activity relative to placebo pills but no changes in LCAT function [136,137].
Finally, plant sterols were also studied in two RCTs and were associated with decreases in CETP mass relative to a control after 4 weeks of margarine with phytosterols (1.68 g/day) [138] and relative to baseline values after 4 weeks of 2–3 g/day plant stanol [30]. Soy intervention did not modify LCAT activity [138].
There were 11 studies with interventions other than those previously referred to, five papers (some effect on HDL function in three) with healthy subjects, and six studies (some effect in four) with cardiovascular risk-related pathologies.
4. Discussion
In this systematic review, we have summarized the existing evidence regarding the effect of lifestyle changes on HDL functional traits. Short-term consumption of dietary antioxidants and alcohol was more clearly related to HDL functional improvements in subjects with cardiovascular risk and healthy individuals, respectively, especially regarding CEC and HDL antioxidant properties. Additionally, in subjects at cardiovascular risk, an effect on HDL functions was suggested after the intake of MUFAs and long-chain PUFAs, whilst an antioxidant-rich dietary pattern was able to improve HDL function in both groups, healthy individuals and subjects at high cardiovascular risk.
MUFA and long-chain PUFA intake has been analyzed in a considerable number of studies, with controversial results. Such diverse findings can be partially explained by the high heterogeneity of the study designs, for instance, the different types of fatty acids used in diets/supplements and control arms, and the wide range of doses employed. The clearest improvements in HDL functions were observed when MUFAs and PUFAs were compared to low-fat, SFA, and TFA interventions. However, the comparisons between MUFAs and PUFAs and between different types of PUFAs (linoleic acid, linolenic acid, EPA, and DHA) did not show differences regarding HDL functionalities. MUFA interventions reported increased levels of CEC, CETP, and LCAT only when compared with SFAs and TFAs. Such results are consistent with previous non-randomized studies in humans in which increases in CEC [139], CETP [140,141], and LCAT activity were also reported [142]. All long-chain PUFA studies consistently improved HDL antioxidant capacity through augmented PON1 activity. Antioxidant and anti-inflammatory capacities were also described in non-randomized trials in which doses of EPA-DHA (1.8–2 g/day) increased PON1 mass and arylesterase activity and decreased the expression/secretion of pro-inflammatory proteins, such as VCAM-1, alpha-1-antitrypsin, and complement proteins [143,144]. Long-chain fatty-acid intake also suggested improvements in CEC (when not compared to other unsaturated fatty acid interventions), accompanied by improvements in CETP and LCAT activities. Changes in CEC could be caused by increments in apolipoprotein A-I, the major apolipoprotein in HDL involved in CEC, as observed after the consumption of EPA + DHA [61,144]. On the other hand, a detrimental effect of higher doses of EPA and DHA (>3 g/day) on LCAT and HDL inflammatory indices, with no changes in CEC, has been proposed. Hypotheses describing the potential beneficial effects of MUFAs and PUFAs on HDL function are diverse. A first explanation lies in the capacity of these fatty acids to increase HDL particle fluidity. HDLs, which are more fluid, are thought to have a greater capacity to adapt to the shape of cholesterol transporters in cells and allow for the export of cholesterol excess [139,145,146]. Second, omega-3 PUFAs are known ligands for peroxisome-proliferator-activated receptor α (PPARα). This cell receptor, the activation of which leads to an increased production of apolipoprotein A-I (the HDL main active protein), may improve HDL function beyond an increase in HDL-C levels [147,148]. An increase in apolipoprotein A-I levels could also mediate a greater stability and antioxidant function of PON1 [149] beyond the intrinsic potential capacity of PUFAs to increase hepatic synthesis and its release [150]. Third, omega-3 PUFAs are able to decrease low-grade inflammation. This may lead to a reduction in the circulating levels of cytokines and acute-phase proteins, thus favoring a lower binding of these molecules to HDL particles [151]. Moreover, most of these interventions are potentially rich in dietary antioxidants, which may additionally contribute to the effects observed [152]. Finally, we cannot exclude the fact that the decrease in triglyceride concentrations due to PUFAs may play a role in explaining some of the benefits on HDL cholesterol metabolism. PUFAs can reduce hepatic synthesis of triglycerides and very low-density lipoproteins (VLDL), activating PPAR receptors [153]. Considering the close relationship between both lipids, decreases in triglycerides and VLDL could decrease CETP enzymatic activity and improve HDL cholesterol metabolism [154].
Saturated and trans fats presented opposite effects to MUFAs and PUFAs, with lower CEC and LCAT and increased CETP. Nevertheless, an article that studied different sources of saturated fats (from cheese and butter) described contraposed effects in CEC [33]. These findings could be due to the fact that the toxic effect of long-chain SFAs, such as palmitic acid [155], is not present in shorter chain species [156,157]. A few studies have also suggested a diminished HDL profile (worse PON1 and CETP activities) after TFAs when compared with SFA-rich diets [48,68,72]. Impairment in HDL functions is probably secondary to the increment of total cholesterol and fractions induced by SFAs and TFAs [155,158].
Dietary cholesterol intake promotes increases in CEC, CETP, and LCAT activities. Augmented intake may increment the pool of cholesterol in circulation (in both LDLs and HDLs), which could be related to increased cholesterol levels in the organism, consequently leading to a greater uptake of cholesterol from peripheral cells (CEC) and a greater necessity to metabolize cholesterol by CETP and LCAT in oder to transfer it back to the liver. However, increases in CEC caused by cholesterol intake could not reflect a better atheroprotective capacity of HDL. Cholesterol intake showed increases in large HDL particles and HDL diameter [82,86], which are associated with greater cardiovascular risk [6]. In addition, HDLs may be more oxidized and contain higher levels of acute-phase proteins, such as SAA, suggesting increased levels of oxidative stress and low-grade inflammation.
Under chronic oxidative stress and inflammation, HDL lipoprotein loses its atheroprotective capacity and becomes dysfunctional [11]. Thus, antioxidant-rich dietary patterns are promising interventions for the preservation of HDL atheroprotective capacity. As described in this review, olive oil enriched with phenolic compounds, anthocyanins, carotene extracts, and supplements is clearly associated with improvements in HDL functionalities. The Mediterranean diet, which includes all the previously mentioned beneficial antioxidants, presents a positive effect on a wide battery of functions (CEC, antioxidant, anti-inflammatory, and endothelial protection capacity of HDLs) [21,28,89]. In contrast, the few interventions that only increased dietary vegetable content failed to improve CETP, LCAT, and PON1 activities of HDLs [96,112]. It is possible changes in vegetable quantity without other beneficial compounds (such as olive oil rich in polyphenols, nuts, and other sources of omega-3 fatty acids) are not enough to change HDL functions in a whole dietary pattern. Several intertwined molecular mechanisms have been suggested as hypothetical explanations. First, a number of HDL proteins involved in reverse cholesterol transport (apolipoprotein A-I, LCAT) and antioxidant capacity (PON1) have been described as having their functional capacity decreased if they become oxidized [159,160,161]. Thus, antioxidants may keep these proteins non-oxidized and functional. Second, oxidized lipids are also known to become less fluid. Therefore, antioxidants may enhance HDL lipid fluidity [162]. Third, some phenolic compounds may induce a slight boost of AMP-activated protein kinase (AMPK), a cellular metabolic regulator capable of activating PPARα and the subsequent synthesis of apolipoprotein A-I [152,163]. Fourth, antioxidant-rich HDLs are hypothesized to employ the antioxidant compounds they carry to counteract the oxidation of other lipids. Fifth, regarding HDL anti-inflammatory potential, the decrease in reactive oxygen species due to dietary antioxidants may moderate the activation of the nuclear factor kappa beta (NF-κβ), a pivotal regulator of inflammatory responses [164]. This, in turn, can reduce the concentrations of cytokine/acute-phase proteins that bind to HDLs. Some particular antioxidants, such as flavonoids, have been reported to be able to directly downregulate NF-κβ activation [165]. In addition, several phenolic compounds are also able to promote an AMPK-mediated decrease in the production of low-grade inflammation signals [166,167]. Finally, the capacity of some phenolic antioxidants to decrease hepatic liver synthesis through an AMPK-dependent mechanism could also lead to a decrease in circulating levels of triglycerides [163]. This could be linked to an enhancement in HDL cholesterol metabolism due to the close relationship between HDL-C and triglycerides [154].
Alcohol consumption is clearly associated with higher levels of HDL-C, accompanied by increments of apolipoprotein A-I [168]. In the same manner, moderate ethanol consumption has presented consistent increases in CEC and PON1 activity. Although two RCTs did not demonstrate changes in CETP, some non-randomized trials have reported improvements in CETP and LCAT activities [169,170] and detrimental effects in CETP after alcohol withdrawal [171,172]. The main hypothesis to explain the potentially beneficial effects of alcohol on HDL metabolism is related to the transient increase in acetate, the main ethanol metabolite after its ingestion [173]. Acetate is a short-chain fatty acid known to be capable of decreasing lipolysis [174], which lowers levels of non-esterified fatty acids released into circulation. These fatty acids are physiologically transformed into triglycerides in the liver and subsequently packed in VLDLs. A transient decrease in VLDLs is therefore expected after alcohol intake [173]. VLDLs are the main destination of cholesterol esters collected by HDL particles in CEC. Thus, if transiently low VLDL levels are present, CETP activity is halted, and HDL-C concentrations increase due to greater cholesterol content per HDL particle (larger HDLs) [60,175]. Finally, an increment in CEC directed to these large HDLs, mediated by specific cholesterol transporters, such as ABCG1 and SR-BI, could be expected, and a subsequent esterification of the collected cholesterol by LCAT takes place [173]. In addition, the promotion in PON1 antioxidant function could be partially justified by the high content of dietary antioxidants in some alcoholic beverages [176,177].
The effect of physical activity on HDL function is still unclear. Regarding CEC, only large amounts of vigorous exercise were associated with improvements [20]. Moderate-intensity activities did not show any changes in CEC, CETP, or LCAT activities, suggesting a possible dose-dependent relationship. Further evidence was found in non-randomized trials, which also reported the absence of effect of moderate physical activity [128,178]. In addition, a very low calorific diet changed neither CEC nor CETP activity (Talbot et al., 2018). In fact, non-randomized studies even found negative effects on CEC [179,180]. Nevertheless, interventions combining physical activity with modest calorie restriction suggested increases in CEC and decreases in CETP activity [23,123] in non-randomized trials [181,182]. Physical activity and/or calorie restriction are known to promote the activation of AMPK [183], a mechanism that seems essential to the hypothetical explanation of the molecular effects of these two lifestyle modifications on HDL function [184]. First, as already mentioned, AMPK is capable of stimulating PPARα, a transcription factor that promotes the hepatic synthesis of apolipoprotein A-I and the release of cholesterol transporters in peripheral cells, such as macrophages, leading to increases in HDL-C circulating levels and potential improvements in CEC values [148]. In parallel, the beneficial decrease in CETP activity could be secondary to the reduction in triglyceride plasma levels due to the ability of AMPK to lower the production of triglyceride synthesis enzymes in the liver [185]. Second, AMPK simulation has also been linked to a greater production of several antioxidant enzymes [186,187,188]. This antioxidant protection could be related to a stronger preservation of HDL proteins, such as apolipoprotein A-I and PON1, in a non-oxidized, functional state, boosting CEC and HDL antioxidant capacities [159,161]. Finally, AMPK stimulation may counteract low-grade inflammation, which, in turn, may be related to decreased levels of cytokines and acute-phase proteins bound to HDLs and a reduction in the pro-inflammatory potential of the lipoprotein [166,167].
This systematic review has some limitations to be considered. First, the high percentage of studies classified as unclear risk could conceal the presence of bias, leading to questions of their quality. Second, there is a marked heterogeneity in the laboratory procedures used to evaluate HDL functions. For example, to evaluate CEC, the analyzed studies employed six different types of cell cultures (J774, THP-1, Fu5AH, CHO, RAW264.7, and PBMCs cells) and three different types of labeled cholesterol (2 radio-labeled and 1 fluorescent). Some of these assays could hinder standardization and measurement precision, and it remains to be proven whether they constitute adequate surrogate endpoints. Third, there was heterogeneity in the lifestyle modifications and trial designs. Fourth, most studies did not specify whether the LCAT activity was beta or alpha, and consequently, whether the activity is specifically linked to HDL. Finally, most of the studies evaluated were short-term interventions with modest sample sizes. For all these reasons, results need to be interpreted with caution.
As far as the strengths of this review, we have reviewed many publications, including an exhaustively wide range of studies of both HDL functional abilities and lifestyle interventions.
5. Conclusions
Given that healthy diet and lifestyle are consistently related to decreased cardiovascular disease risk, their link to improved HDL function has been widely investigated in human trials. In brief, beyond an improvement in HDL-C levels, mainly in short-term clinical trials, the consumption of MUFAs, PUFAs (particularly long-chain, omega-3 MUFAs and PUFAs in fish), and dietary antioxidants, such as phenolic compounds (in dietary or near-dietary doses), showed benefits in HDL functionality, mainly in subjects with cardiovascular risk factors. In this regard, antioxidant-rich dietary patterns were able to improve HDL function in both healthy individuals and subjects at high cardiovascular risk. In addition, reverse cholesterol transport with ethanol at moderate quantities, in studies mainly performed with healthy individuals, was able to enhance CEC. Finally, cholesterol dietary interventions with eggs increased circulant cholesterol fractions (total and HDL cholesterol) and, in concordance, increase CEC, CETP, and LCAT activity.
Such findings suggest the capacity of dietary and lifestyle modifications to modulate cardiovascular risk factors. Nevertheless, given the marked heterogeneity in study design and procedures used to assess HDL functions, more homogeneous, large-scale, long-term, randomized, controlled trials are required to confirm these results. Moreover, such trials should be performed over different periods, in varying populations, and with individuals presenting diverse pathologies.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10245897/s1, Table S1: Risk of bias evaluation, Supplementary File S1: List of terms used for article selection.
Author Contributions
Á.H. and M.F. conceptualized the review. A.S., Á.H. and M.F. selected studies and assessed risk of bias. A.S. extracted data from articles selected. A.S., Á.H. and M.F. wrote the manuscript draft. C.L., M.T.S.-F. and O.C. reviewed and edited the text. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Instituto de Salud Carlos III [grant numbers: CB06/03/0028, CD17/00122, IFI16/00012, PI15/00047, and PI18/00020], Fundació La Marató de TV3 [grant number: 201512.31], Agència de Gestió d’Ajuts Universitaris i de Recerca [grant number: 2017 BP 00021, 2017 SGR 222, SLT002/16/00088], and the European Regional Development Fund (ERDF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boekholdt, S.M.; Arsenault, B.J.; Hovingh, G.K.; Mora, S.; Pedersen, T.R.; LaRosa, J.C.; Welch, K.M.A.; Amarenco, P.; DeMicco, D.A.; Tonkin, A.M.; et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients. Circulation 2013, 128, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; Packard, C.J.; et al. Major lipids, apolipoproteins, and risk of vascular disease. J. Am. Med. Assoc. 2009, 302, 1993–2000. [Google Scholar]
- Keene, D.; Price, C.; Shun-Shin, M.J.; Francis, D.P. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: Meta-analysis of randomised controlled trials including 117,411 patients. BMJ 2014, 349, g4379. [Google Scholar] [CrossRef]
- Holmes, M.V.; Asselbergs, F.W.; Palmer, T.M.; Drenos, F.; Lanktree, M.B.; Nelson, C.P.; Dale, C.E.; Padmanabhan, S.; Finan, C.; Swerdlow, D.I.; et al. Mendelian randomization of blood lipids for coronary heart disease. Eur. Heart J. 2015, 36, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef]
- Prats-Uribe, A.; Sayols-Baixeras, S.; Fernández-Sanlés, A.; Subirana, I.; Carreras-Torres, R.; Vilahur, G.; Civeira, F.; Marrugat, J.; Fitó, M.; Hernáez, Á.; et al. High-density lipoprotein characteristics and coronary artery disease: A Mendelian randomization study. Metabolism 2020, 112, 154351. [Google Scholar] [CrossRef]
- Soria-Florido, M.T.; Schröder, H.; Grau, M.; Fitó, M.; Lassale, C. High density lipoprotein functionality and cardiovascular events and mortality: A systematic review and meta-analysis. Atherosclerosis 2020, 302, 36–42. [Google Scholar] [CrossRef]
- Talbot, C.P.J.; Plat, J.; Ritsch, A.; Mensink, R.P. Determinants of cholesterol efflux capacity in humans. Prog. Lipid Res. 2018, 69, 21–32. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Hama, S.Y.; Hough, G.P.; Subbanagounder, G.; Reddy, S.T.; Fogelman, A.M. A Cell-Free Assay for Detecting HDL That is Dysfunctional in Preventing the Formation of or Inactivating Oxidized Phospholipids. J. Lipid Res. 2001, 42, 1308–1317. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef]
- Besler, C.; Lüscher, T.F.; Landmesser, U. Molecular mechanisms of vascular effects of High-density lipoprotein: Alterations in cardiovascular disease. EMBO Mol. Med. 2012, 4, 251–268. [Google Scholar] [CrossRef]
- Birner-Gruenberger, R.; Schittmayer, M.; Holzer, M.; Marsche, G. Understanding high-density lipoprotein function in disease: Recent advances in proteomics unravel the complexity of its composition and biology. Prog. Lipid Res. 2014, 56, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Soria-Florido, M.T.; Castañer, O.; Lassale, C.; Estruch, R.; Salas-Salvadó, J.; Martínez-González, M.Á.; Corella, D.; Ros, E.; Arós, F.; Elosua, R.; et al. Dysfunctional high-density lipoproteins are associated with a greater incidence of acute coronary syndrome in a population at high cardiovascular risk: A nested case-control study. Circulation 2020, 141, 444–453. [Google Scholar] [CrossRef]
- Khera, A.V.; Demler, O.V.; Adelman, S.J.; Collins, H.L.; Glynn, R.J.; Ridker, P.M.; Rader, D.J.; Mora, S. Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events. Circulation 2017, 135, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Sanllorente, A.; Castañer, O.; Lassale, C.; Almanza-Aguilera, E.; Elosua, R.; Vila, J.; Soldado, M.; Blanchart, G.; Muñoz-Aguayo, D.; Subirana, I.; et al. High-density lipoprotein functional traits and coronary artery disease in a general population: A case–cohort study. Eur. J. Prev. Cardiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343. [Google Scholar] [CrossRef]
- Albaghdadi, M.S.; Wang, Z.; Gao, Y.; Mutharasan, R.K.; Wilkins, J. High-density lipoprotein subfractions and cholesterol efflux capacity are not affected by supervised exercise but are associated with baseline interleukin-6 in patients with peripheral artery disease. Front. Cardiovasc. Med. 2017, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, A.; Castañer, O.; Elosua, R.; Pinto, X.; Estruch, R.R.; Salas-Salvado, J.; Corella, D.; Aros, F.; Serra-Majem, L.; Fiol, M.; et al. Mediterranean diet improves high-density lipoprotein function in high-cardiovascular-risk individuals: A randomized controlled trial. Circulation 2017, 135, 633–643. [Google Scholar] [CrossRef]
- Sarzynski, M.A.; Ruiz-Ramie, J.J.; Barber, J.L.; Slentz, C.A.; Apolzan, J.W.; McGarrah, R.W.; Harris, M.N.; Church, T.S.; Borja, M.S.; He, Y.; et al. Effects of increasing exercise intensity and dose on multiple measures of HDL (high-density lipoprotein) function. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 943–952. [Google Scholar] [CrossRef]
- Hernáez, Á.; Castañer, O.; Tresserra-Rimbau, A.; Pintó, X.; Fitó, M.; Casas, R.; Martínez-González, M.Á.; Corella, D.; Salas-Salvadó, J.; Lapetra, J.; et al. Mediterranean diet and atherothrombosis biomarkers: A randomized controlled trial. Mol. Nutr. Food Res. 2020, 64, 2000350. [Google Scholar] [CrossRef] [PubMed]
- Tiainen, S.; Luoto, R.; Ahotupa, M.; Raitanen, J.; Vasankari, T. 6-mo aerobic exercise intervention enhances the lipid peroxide transport function of HDL. Free Radic. Res. 2016, 50, 1279–1285. [Google Scholar] [CrossRef]
- Wesnigk, J.; Bruyndonckx, L.; Hoymans, V.Y.; De Guchtenaere, A.; Fischer, T.; Schuler, G.; Vrints, C.J.; Adams, V. Impact of lifestyle intervention on HDL-induced eNOS activation and cholesterol efflux capacity in obese adolescent. Cardiol. Res. Pract. 2016, 2016, 2820432. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-T.; Fitschen, P.J.; Kistler, B.M.; Jeong, J.H.; Chung, H.R.; Aviram, M.; Phillips, S.A.; Fernhall, B.; Wilund, K.R. Effects of pomegranate extract supplementation on cardiovascular risk factors and physical function in hemodialysis patients. J. Med. Food 2015, 18, 941–949. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, X.; Zhang, Y.; Wang, Y.; Liu, Y.; Sun, R.; Xia, M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014, 99, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the american college of cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T.; Albers, J.J.; Krauss, R.M.; Wood, P.D.S. Associations of lecithin: Cholesterol acyltransferase (LCAT) mass concentrations with exercise, weight loss, and plasma lipoprotein subfraction concentrations in men. Atherosclerosis 1990, 82, 53–58. [Google Scholar] [CrossRef]
- Damasceno, N.R.T.; Sala-Vila, A.; Cofán, M.; Pérez-Heras, A.M.; Fitó, M.; Ruiz-Gutiérrez, V.; Martínez-González, M.Á.; Corella, D.; Arós, F.; Estruch, R.; et al. Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis 2013, 230, 347–353. [Google Scholar] [CrossRef]
- Liu, X.; Garban, J.; Jones, P.J.; Heuvel, J.V.; Lamarche, B.; Jenkins, D.J.; Connelly, P.W.; Couture, P.; Pu, S.; Fleming, J.A.; et al. Diets low in saturated fat with different unsaturated fatty acid profiles similarly increase serum-mediated cholesterol efflux from THP-1 macrophages in a population with or at risk for metabolic syndrome: The canola oil multicenter intervention trial. J. Nutr. 2018, 148, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Ikeda, I.; Ishikawa, T.; Tateno, M.; Sugano, M.; Nakamura, H. Decrease in plasma low-density lipoprotein cholesterol, apolipoprotein B, cholesteryl ester transfer protein, and oxidized low-density lipoprotein by plant stanol ester-containing spread: A randomized, placebo-controlled trial. Nutrition 2003, 19, 369–374. [Google Scholar] [CrossRef]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.; Liu, J.; Mou, H.; Cao, L.; Ling, W. Anthocyanin supplementation improves serum LDL-and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef]
- Balsan, G.; Pellanda, L.C.; Sausen, G.; Galarraga, T.; Zaffari, D.; Pontin, B.; Portal, V.L. Effect of yerba mate and green tea on paraoxonase and leptin levels in patients affected by overweight or obesity and dyslipidemia: A randomized clinical trial. Nutr. J. 2019, 18, 5. [Google Scholar] [CrossRef]
- Brassard, D.; Arsenault, B.J.; Boyer, M.; Bernic, D.; Tessier-Grenier, M.; Talbot, D.; Tremblay, A.; Levy, E.; Asztalos, B.; Jones, P.J.H.; et al. Saturated fats from butter but not from cheese increase HDL-Mediated cholesterol efflux capacity from J774 macrophages in men and women with abdominal obesity. J. Nutr. 2018, 148, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Solà, R.; La Ville, A.E.; Richard, J.L.; Motta, C.; Bargalló, M.T.; Girona, J.; Masana, L.; Jacotot, B. Oleic acid rich diet protects against the oxidative modification of high density lipoprotein. Free Radic. Biol. Med. 1997, 22, 1037–1045. [Google Scholar] [CrossRef]
- Lagrost, L.; Mensink, R.P.; Guyard-Dangremont, V.; Temme, E.H.M.; Desrumaux, C.; Athias, A.; Hornstra, G.; Gambert, P. Variations in serum cholesteryl ester transfer and phospholipid transfer activities in healthy women and men consuming diets enriched in lauric, palmitic or oleic acids. Atherosclerosis 1999, 142, 395–402. [Google Scholar] [CrossRef]
- Vega-López, S.; Ausman, L.M.; Jalbert, S.M.; Erkkilä, A.T.; Lichtenstein, A.H. Palm and partially hydrogenated soybean oils adversely alter lipoprotein profiles compared with soybean and canola oils in moderately hyperlipidemic subjects. Am. J. Clin. Nutr. 2006, 84, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Baudet, M.F.; Jacotot, B. Dietary fats and lecithin-cholesterol acyltransferase activity in healthy humans. Ann. Nutr. Metab. 1988, 32, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Jaeger, W.; Berger, I.; Barleben, H.; Wirth, M.; Richter-Heinrich, E.; Voigt, S.; Gödicke, W. Effects of dietary oleic, linoleic and alpha-linolenic acids on blood pressure, serum lipids, lipoproteins and the formation of eicosanoid precursors in patients with mild essential hypertension. J. Hum. Hypertens. 1990, 4, 227–233. [Google Scholar]
- Andraski, A.B.; Singh, S.A.; Lee, L.H.; Higashi, H.; Smith, N.; Zhang, B.; Aikawa, M.; Sacks, F.M. Effects of replacing dietary monounsaturated fat with carbohydrate on HDL (high-density lipoprotein) protein metabolism and proteome composition in humans. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2411–2430. [Google Scholar] [CrossRef] [PubMed]
- Stirban, A.; Nandrean, S.; Götting, C.; Stratmann, B.; Tschoepe, D. Effects of n-3 polyunsaturated fatty acids (PUFAs) on circulating adiponectin and leptin in subjects with type 2 diabetes mellitus. Horm. Metab. Res. 2014, 46, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Berryman, C.E.; Fleming, J.A.; Kris-Etherton, P.M. Inclusion of almonds in a cholesterol-lowering diet improves plasma HDL subspecies and cholesterol efflux to serum in normal-weight individuals with elevated LDL cholesterol. J. Nutr. 2017, 147, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Holligan, S.D.; West, S.G.; Gebauer, S.K.; Kay, C.D.; Kris-Etherton, P.M. A moderate-fat diet containing pistachios improves emerging markers of cardiometabolic syndrome in healthy adults with elevated LDL levels. Br. J. Nutr. 2014, 112, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Tindall, A.M.; Kris-Etherton, P.M.; Petersen, K.S. Replacing saturated fats with unsaturated fats from walnuts or vegetable oils lowers atherogenic lipoprotein classes without increasing lipoprotein(a). J. Nutr. 2020, 150, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Buonacorso, V.; Nakandakare, E.R.; Nunes, V.S.; Passarelli, M.; Quintão, E.C.R.; Lottenberg, A.M.P. Macrophage cholesterol efflux elicited by human total plasma and by HDL subfractions is not affected by different types of dietary fatty acids. Am. J. Clin. Nutr. 2007, 86, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Kralova Lesna, I.; Suchanek, P.; Kovar, J.; Stavek, P.; Poledne, R. Replacement of dietary saturated FAs by PUFAs in diet and reverse cholesterol transport. J. Lipid Res. 2008, 49, 2414–2418. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Yamanaka-Okumura, H.; Naniwa-Kuroki, Y.; Sakuma, M.; Taketani, Y.; Takeda, E. Flaxseed oil intake reduces serum small dense low-density lipoprotein concentrations in Japanese men: A randomized, double blind, crossover study. Nutr. J. 2015, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.; Mann, J.; Sutherland, W.; Chisholm, A.; Skeaff, M. Effects of coconut oil, butter, and safflower oil on lipids and lipoproteins in persons with moderately elevated cholesterol levels. J. Lipid Res. 1995, 36, 1787–1795. [Google Scholar] [CrossRef]
- Van Tol, A.; Zock, P.L.; van Gent, T.; Scheek, L.M.; Katan, M.B. Dietary trans fatty acids increase serum cholesterylester transfer protein activity in man. Atherosclerosis 1995, 115, 129–134. [Google Scholar] [CrossRef]
- De Souza, R.G.M.; Gomes, A.C.; de Castro, I.A.; Mota, J.F. A baru almond–enriched diet reduces abdominal adiposity and improves high-density lipoprotein concentrations: A randomized, placebo-controlled trial. Nutrition 2018, 55, 154–160. [Google Scholar] [CrossRef]
- Gebauer, S.K.; West, S.G.; Kay, C.D.; Alaupovic, P.; Bagshaw, D.; Kris-Etherton, P.M. Effects of pistachios on cardiovascular disease risk factors and potential mechanisms of action: A dose-response study. Am. J. Clin. Nutr. 2008, 88, 651–659. [Google Scholar] [CrossRef]
- Chung, B.H.; Cho, B.H.S.; Liang, P.; Doran, S.; Osterlund, L.; Oster, R.A.; Darnell, B.; Franklin, F. Contribution of postprandial lipemia to the dietary fat-mediated changes in endogenous lipoprotein-cholesterol concentrations in humans. Am. J. Clin. Nutr. 2004, 80, 1145–1158. [Google Scholar] [CrossRef][Green Version]
- Lottenberg, A.M.P.; Nunes, V.S.; Lottenberg, S.A.; Shimabukuro, A.F.M.; Carrilho, A.J.F.; Malagutti, S.; Nakandakare, E.R.; McPherson, R.; Quintão, E.C.R. Plasma cholesteryl ester synthesis, cholesteryl ester transfer protein concentration and activity in hypercholesterolemic women: Effects of the degree of saturation of dietary fatty acids in the fasting and postprandial states. Atherosclerosis 1996, 126, 265–275. [Google Scholar] [CrossRef]
- Abbey, M.; Clifton, P.; Kestin, M.; Belling, B.; Nestel, P. Effect of fish oil on lipoproteins, lecithin: Cholesterol Acyltransferase, and lipid Transfer Protein Activity in Humans. Arteriosclerosis 1989, 10, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muniz, F.J.; Canales, A.; Nus, M.; Bastida, S.; Guillén, M.; Corella, D.; Olmedilla-Alonso, B.; Granado-Lorencio, F.; Benedí, J. The antioxidant status response to low-fat and walnut paste-enriched meat differs in volunteers at high cardiovascular Risk carrying different PON-1 polymorphisms. J. Am. Coll. Nutr. 2012, 31, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Canales, A.; Sánchez-Muniz, F.J.; Bastida, S.; Librelotto, J.; Nus, M.; Corella, D.; Guillen, M.; Benedi, J. Effect of walnut-enriched meat on the relationship between VCAM, ICAM, and LTB4 levels and PON-1 activity in ApoA4 360 and PON-1 allele carriers at increased cardiovascular risk. Eur. J. Clin. Nutr. 2011, 65, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Canales, A.; Benedí, J.; Nus, M.; Librelotto, J.; Sánchez-Montero, J.M.; Sánchez-Muniz, F.J. Effect of walnut-enriched restructured meat in the antioxidant status of overweight/obese senior subjects with at least one extra chd-risk factor. J. Am. Coll. Nutr. 2007, 26, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Pfeuffer, M.; Fielitz, K.; Laue, C.; Winkler, P.; Rubin, D.; Helwig, U.; Giller, K.; Kammann, J.; Schwedhelm, E.; Böger, R.H.; et al. CLA does not impair endothelial function and decreases body weight as compared with safflower oil in overweight and obese male subjects. J. Am. Coll. Nutr. 2011, 30, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Manninen, S.; Lankinen, M.; Erkkilä, A.; Nguyen, S.D.; Ruuth, M.; de Mello, V.; Öörni, K.; Schwab, U. The effect of intakes of fish and Camelina sativa oil on atherogenic and anti-atherogenic functions of LDL and HDL particles: A randomized controlled trial. Atherosclerosis 2019, 281, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, L.; Villa, B.; Canavesi, M.; Sirtori, C.R.; James, R.W.; Bernini, F.; Franceschini, G. An omega-3 polyunsaturated fatty acid concentrate increases plasma high-density lipoprotein 2 cholesterol and paraoxonase levels in patients with familial combined hyperlipidemia. Metab. Clin. Exp. 2004, 53, 153–158. [Google Scholar] [CrossRef]
- Pownall, H.J.; Brauchi, D.; Kilinç, C.; Osmundsen, K.; Pao, Q.; Payton-Ross, C.; Gotto, A.M.; Ballantyne, C.M. Correlation of serum triglyceride and its reduction by ω-3 fatty acids with lipid transfer activity and the neutral lipid compositions of high- density and low-density lipoproteins. Atherosclerosis 1999, 143, 285–297. [Google Scholar] [CrossRef]
- Lambert, C.; Cubedo, J.; Padró, T.; Sánchez-Hernández, J.; Antonijoan, R.M.; Perez, A.; Badimon, L. Phytosterols and omega 3 supplementation exert novel regulatory effects on metabolic and inflammatory pathways: A proteomic study. Nutrients 2017, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanihaghjo, A.; Kolahi, S.; Seifirad, S.; Rashtchizadeh, N.; Argani, H.; Hajialilo, M.; Khabazi, A.; Alizadeh, S.; Bahreini, E. Effect of fish oil supplements on serum paraoxonase activity in female patients with rheumatoid arthritis: A double-blind randomized controlled trial. Arch. Iran. Med. 2012, 15, 549–552. [Google Scholar] [PubMed]
- Golzari, M.H.; Hosseini, S.; Koohdani, F.; Yaraghi, A.A.S.; Javanbakht, M.H.; Mohammadzadeh-Honarvar, N.; Djalali, M. The effect of eicosapentaenoic acid on the serum levels and enzymatic activity of paraoxonase 1 in the patients with type 2 diabetes mellitus. Acta Med. Iran. 2017, 55, 486–495. [Google Scholar]
- Shidfar, F.; Amani, S.; Vafa, M.; Shekarriz, R.; Hosseini, S.; Shidfar, S.; Eshraghian, M.; Mousavi, S.N. Effects of iron supplementation with and without docosahexaenoic acid on the cardiovascular disease risk based on paraoxonase-1, hs-CRP, and ApoB/ApoA-I ratio in women with iron deficiency anemia. Biol. Trace Elem. Res. 2016, 169, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wurm, R.; Schrutka, L.; Hammer, A.; Moertl, D.; Berger, R.; Pavo, N.; Lang, I.M.; Goliasch, G.; Huelsmann, M.; Distelmaier, K. Polyunsaturated fatty acids supplementation impairs anti-oxidant high-density lipoprotein function in heart failure. Eur. J. Clin. Investig. 2018, 48, e12998. [Google Scholar] [CrossRef]
- Schwab, U.S.; Niskanen, L.K.; Maliranta, H.M.; Savolainen, M.J.; Kesaniemi, Y.A.; Uusitupa, M.I.J. Lauric and palmitic acid-enriched diets have minimal impact on serum lipid and lipoprotein concentrations and glucose metabolism in healthy young women. J. Nutr. 1995, 125, 466–473. [Google Scholar] [PubMed]
- Tholstrup, T.; Ehnholm, C.; Jauhiainen, M.; Petersen, M.; Høy, C.-E.; Lund, P.; Sandström, B. Effects of medium-chain fatty acids and oleic acid on blood lipids, lipoproteins, glucose, insulin, and lipid transfer protein activities. Am. J. Clin. Nutr. 2004, 79, 564–569. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Jauhiainen, M.; McGladdery, S.; Ausman, L.M.; Jalbert, S.M.; Vilella-Bach, M.; Ehnholm, C.; Frohlich, J.; Schaefer, E.J. Impact of hydrogenated fat on high density lipoprotein subfractions and metabolism. J. Lipid Res. 2001, 42, 597–604. [Google Scholar] [CrossRef]
- Matthan, N.R.; Cianflone, K.; Lichtenstein, A.H.; Ausman, L.M.; Jauhiainen, M.; Jones, P.J. Hydrogenated fat consumption affects acylation-stimulating protein levels and cholesterol esterification rates in moderately hypercholesterolemic women. J. Lipid Res. 2001, 42, 1841–1848. [Google Scholar] [CrossRef]
- Vega-López, S.; Matthan, N.R.; Ausman, L.M.; Ai, M.; Otokozawa, S.; Schaefer, E.J.; Lichtenstein, A.H. Substitution of vegetable oil for a partially-hydrogenated fat favorably alters cardiovascular disease risk factors in moderately hypercholesterolemic postmenopausal women. Atherosclerosis 2009, 207, 208–212. [Google Scholar] [CrossRef][Green Version]
- Chardigny, J.M.; Destaillats, F.; Malpuech-Brugère, C.; Moulin, J.; Bauman, D.E.; Lock, A.L.; Barbano, D.M.; Mensink, R.P.; Bezelgues, J.B.; Chaumont, P.; et al. Do trans fatty acids from industrially produced sources and from natural sources have the same effect on cardiovascular disease risk factors in healthy subjects? Results of the trans fatty acids collaboration (TRANSFACT) study. Am. J. Clin. Nutr. 2008, 87, 558–566. [Google Scholar] [CrossRef] [PubMed]
- De Roos, N.M.; Schouten, E.G.; Scheek, L.M.; Van Tol, A.; Katan, M.B. Replacement of dietary saturated fat with trans fat reduces serum paraoxonase activity in healthy men and women. Metabolism 2002, 51, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Molina, A.; Castro, G.; Martín-Escalante, D.; Bravo, D.; López-Miranda, J.; Castro, P.; López-Segura, F.; Fruchart, J.C.; Ordovás, J.M.; Pérez-Jiménez, F. Effects of different dietary cholesterol concentrations on lipoprotein plasma concentrations and on cholesterol efflux from Fu5AH cells. Am. J. Clin. Nutr. 1998, 68, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Sawrey-Kubicek, L.; Zhu, C.; Bardagjy, A.S.; Rhodes, C.H.; Sacchi, R.; Randolph, J.M.; Steinberg, F.M.; Zivkovic, A.M. Whole egg consumption compared with yolk-free egg increases the cholesterol efflux capacity of high-density lipoproteins in overweight, postmenopausal women. Am. J. Clin. Nutr. 2019, 110, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J.; Blesso, C.N.; Lee, J.; Barona, J.; Shah, D.; Thomas, M.J.; Fernandez, M.L. Egg consumption modulates HDL lipid composition and increases the cholesterol-accepting capacity of serum in metabolic syndrome. Lipids 2013, 48, 557–567. [Google Scholar] [CrossRef]
- Herron, K.L.; Vega-Lopez, S.; Conde, K.; Ramjiganesh, T.; Roy, S.; Shachter, N.S.; Fernandez, M.L. Pre-menopausal women, classified as hypo-or hyper-responders, do not alter their LDL/HDL ratio following a high dietary Cholesterol challenge. J. Am. Coll. Nutr. 2002, 21, 250–258. [Google Scholar] [CrossRef]
- Herron, K.L.; Vega-Lopez, S.; Conde, K.; Ramjiganesh, T.; Shachter, N.S.; Fernandez, M.L. Men classified as hypo-or hyperresponders to dietary cholesterol feeding exhibit differences in lipoprotein metabolism. J. Nutr. 2003, 133, 1036–1042. [Google Scholar] [CrossRef]
- Herron, K.L.; Lofgren, I.E.; Sharman, M.; Volek, J.S.; Fernandez, M.L. High intake of cholesterol results in less atherogenic low-density lipoprotein particles in men and women independent of response classification. Metabolism 2004, 53, 823–830. [Google Scholar] [CrossRef]
- Martin, L.J.; Connelly, P.W.; Nancoo, D.; Wood, N.; Zhang, Z.J.; Maguire, G.; Quinet, E.; Tall, A.R.; Marcel, Y.L.; McPherson, R. Cholesteryl ester transfer protein and high density lipoprotein responses to cholesterol feeding in men: Relationship to apolipoprotein E genotype. J. Lipid Res. 1993, 34, 437–446. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Karmally, W.; Siddiqui, M.; Holleran, S.; Tall, A.R.; Rumsey, S.C.; Deckelbaum, R.J.; Blaner, W.S.; Ramakrishnan, R. A dose-response study of the effects of dietary cholesterol on fasting and postprandial lipid and lipoprotein metabolism in healthy young men. Arterioscler. Thromb. Vasc. Biol. 1994, 14, 576–586. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Karmally, W.; Siddiqui, M.; Holleran, S.; Tall, A.R.; Blaner, W.S.; Ramakrishnan, R. Increases in dietary cholesterol are associated with modest increases in both LDL and HDL cholesterol in healthy young women. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 169–178. [Google Scholar] [CrossRef]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volek, J.S.; Fernandez, M.L. Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk-free egg substitute in individuals with metabolic syndrome. Metabolism 2013, 62, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Missimer, A.; Fernandez, M.L.; DiMarco, D.M.; Norris, G.H.; Blesso, C.N.; Murillo, A.G.; Vergara-Jimenez, M.; Lemos, B.S.; Medina-Vera, I.; Malysheva, O.V.; et al. Compared to an oatmeal breakfast, two eggs/day increased plasma carotenoids and choline without increasing trimethyl amine n-oxide concentrations. J. Am. Coll. Nutr. 2018, 37, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.; Clark, R.M.; Greene, C.M.; Contois, J.H.; Fernandez, M.L. Change in plasma lutein after egg consumption is positively associated with plasma cholesterol and lipoprotein size but negatively correlated with body size in postmenopausal women. J. Nutr. 2007, 137, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Vorster, H.H.; Benadé, A.J.; Barnard, H.C.; Locke, M.M.; Silvis, N.; Venter, C.S.; Smuts, C.M.; Engelbrecht, G.P.; Marais, M.P. Egg intake does not change plasma lipoprotein and coagulation profiles. Am. J. Clin. Nutr. 1992, 55, 400–410. [Google Scholar] [CrossRef]
- Mutungi, G.; Waters, D.; Ratliff, J.; Puglisi, M.; Clark, R.M.; Volek, J.S.; Fernandez, M.L. Eggs distinctly modulate plasma carotenoid and lipoprotein subclasses in adult men following a carbohydrate-restricted diet. J. Nutr. Biochem. 2010, 21, 261–267. [Google Scholar] [CrossRef]
- Morgantini, C.; Trifirò, S.; Tricò, D.; Meriwether, D.; Baldi, S.; Mengozzi, A.; Reddy, S.T.; Natali, A. A short-term increase in dietary cholesterol and fat intake affects high-density lipoprotein composition in healthy subjects. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 575–581. [Google Scholar] [CrossRef]
- Hernáez, A.; Fernández-Castillejo, S.; Farràs, M.; Catalán, U.; Subirana, I.; Montes, R.; Solà, R.; Muñoz-Aguayo, D.; Gelabert-Gorgues, A.; Díaz-Gil, O.; et al. Olive oil polyphenols enhance high-density lipoprotein function in humans: A randomized controlled trial. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2115–2119. [Google Scholar] [CrossRef]
- Farràs, M.; Fernández-Castillejo, S.; Rubió, L.; Arranz, S.; Catalán, Ú.; Subirana, I.; Romero, M.P.; Castañer, O.; Pedret, A.; Blanchart, G.; et al. Phenol-enriched olive oils improve HDL antioxidant content in hypercholesterolemic subjects. A randomized, double-blind, cross-over, controlled trial. J. Nutr. Biochem. 2018, 51, 99–104. [Google Scholar] [CrossRef]
- Millar, C.L.; Duclos, Q.; Garcia, C.; Norris, G.H.; Lemos, B.S.; Dimarco, D.M.; Fernandez, M.L.; Blesso, C.N. Effects of freeze-dried grape powder on high-density lipoprotein function in adults with metabolic syndrome: A randomized controlled pilot study. Metab. Syndr. Relat. Disord. 2018, 16, 464–469. [Google Scholar] [CrossRef]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef]
- Zern, T.L.; Wood, R.J.; Greene, C.; West, K.L.; Liu, Y.; Aggarwal, D.; Shachter, N.S.; Fernandez, M.L. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J. Nutr. 2005, 135, 1911–1917. [Google Scholar] [CrossRef]
- Puglisi, M.J.; Mutungi, G.; Brun, P.J.; McGrane, M.M.; Labonte, C.; Volek, J.S.; Fernandez, M.L. Raisins and walking alter appetite hormones and plasma lipids by modifications in lipoprotein metabolism and up-regulation of the low-density lipoprotein receptor. Metabolism 2009, 58, 120–128. [Google Scholar] [CrossRef]
- De Roos, B.; Van Tol, A.; Urgert, R.; Scheek, L.M.; Van Gent, T.; Buytenhek, R.; Princen, H.M.G.; Katan, M.B. Consumption of French-press coffee raises cholesteryl ester transfer protein activity levels before LDL cholesterol in normolipidaemic subjects. J. Intern. Med. 2000, 248, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Farràs, M.; Castañer, O.; Martín-Peláez, S.; Hernáez, A.; Schröder, H.; Subirana, I.; Muñoz-Aguayo, D.; Gaixas, S.; Torre, R.D.L.; Farré, M.; et al. Complementary phenol-enriched olive oil improves HDL characteristics in hypercholesterolemic subjects. A randomized, double-blind, crossover, controlled trial. The VOHF study. Mol. Nutr. Food Res. 2015, 59, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Freese, R.; Alfthan, G.; Jauhiainen, M.; Basu, S.; Erlund, I.; Salminen, I.; Aro, A.; Mutanen, M. High intakes of vegetables, berries, and apples combined with a high intake of linoleic or oleic acid only slightly affect markers of lipid peroxidation and lipoprotein metabolism in healthy subjects. Am. J. Clin. Nutr. 2002, 76, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Van Tol, A.; Urgert, R.; de Jong-Caesar, R.; van Gent, T.; Scheek, L.M.; de Roos, B.; Katan, M.B. The cholesterol-raising diterpenes from coffee beans increase serum lipid transfer protein activity levels in humans. Atherosclerosis 1997, 132, 251–254. [Google Scholar] [CrossRef]
- Baralic, I.; Djordjevic, B.; Dikic, N.; Kotur-Stevuljevic, J.; Spasic, S.; Jelic-Ivanovic, Z.; Radivojevic, N.; Andjelkovic, M.; Pejic, S. Effect of astaxanthin supplementation on paraoxonase 1 activities and oxidative stress status in young soccer players. Phyther. Res. 2013, 27, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Bub, A.; Barth, S.; Watzl, B.; Briviba, K.; Herbert, B.M.; Lührmann, P.M.; Neuhäuser-Berthold, M.; Rechkemmer, G. Paraoxonase 1 Q192R (PON1-192) polymorphism is associated with reduced lipid peroxidation in R-allele-carrier but not in QQ homozygous elderly subjects on a tomato-rich diet. Eur. J. Nutr. 2002, 41, 237–243. [Google Scholar] [CrossRef]
- Bub, A.; Barth, S.W.; Watzl, B.; Briviba, K.; Rechkemmer, G. Paraoxonase 1 Q192R (PON1-192) polymorphism is associated with reduced lipid peroxidation in healthy young men on a low-carotenoid diet supplemented with tomato juice. Br. J. Nutr. 2005, 93, 291. [Google Scholar] [CrossRef]
- Lazavi, F.; Mirmiran, P.; Sohrab, G.; Nikpayam, O.; Angoorani, P.; Hedayati, M. The barberry juice effects on metabolic factors and oxidative stress in patients with type 2 diabetes: A randomized clinical trial. Complement. Ther. Clin. Pract. 2018, 31, 170–174. [Google Scholar] [CrossRef]
- Cherki, M.; Derouiche, A.; Drissi, A.; El Messal, M.; Bamou, Y.; Idrissi-Ouadghiri, A.; Khalil, A.; Adlouni, A. Consumption of argan oil may have an antiatherogenic effect by improving paraoxonase activities and antioxidant status: Intervention study in healthy men. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Castillejo, S.; García-Heredia, A.-I.; Solà, R.; Camps, J.; López de la Hazas, M.-C.; Farràs, M.; Pedret, A.; Catalán, Ú.; Rubió, L.; Motilva, M.-J.; et al. Phenol-enriched olive oils modify paraoxonase-related variables: A randomized, crossover, controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600932. [Google Scholar] [CrossRef]
- Shidfar, F.; Rajab, A.; Rahideh, T.; Khandouzi, N.; Hosseini, S.; Shidfar, S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J. Complement. Integr. Med. 2015, 12, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, B.; Ekbul, A.; Topal, N.; Sarandol, E.; Sag, S.; Baser, K.; Cordan, J.; Gullulu, S.; Tuncel, E.; Baran, I.; et al. Effects of origanum onites on endothelial function and serum biochemical markers in hyperlipidaemic patients. J. Int. Med. Res. 2008, 36, 1326–1334. [Google Scholar] [CrossRef]
- Qian, Q.; Qian, S.; Fan, P.; Huo, D.; Wang, S. Effect of salvia miltiorrhiza hydrophilic extract on antioxidant enzymes in diabetic patients with chronic heart disease: A randomized controlled trial. Phytother. Res. 2012, 26, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Boaventura, B.C.B.; Di Pietro, P.F.; Stefanuto, A.; Klein, G.A.; de Morais, E.C.; de Andrade, F.; Wazlawik, E.; da Silva, E.L. Association of mate tea (Ilex paraguariensis) intake and dietary intervention and effects on oxidative stress biomarkers of dyslipidemic subjects. Nutrition 2012, 28, 657–664. [Google Scholar] [CrossRef]
- Michaličková, D.; Belović, M.; Ilić, N.; Kotur-Stevuljević, J.; Slanař, O.; Šobajić, S. Comparison of polyphenol-enriched tomato juice and standard tomato juice for cardiovascular benefits in subjects with stage 1 hypertension: A randomized controlled study. Plant Foods Hum. Nutr. 2019, 74, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Suomela, J.P.; Ahotupa, M.; Yang, B.; Vasankari, T.; Kallio, H. Absorption of flavonols derived from sea buckthorn (Hippophaë rhamnoides L.) and their effect on emerging risk factors for cardiovascular disease in humans. J. Agric. Food Chem. 2006, 54, 7364–7369. [Google Scholar] [CrossRef] [PubMed]
- Dalgård, C.; Christiansen, L.; Jonung, T.; Mackness, M.I.; De Maat, M.P.M.; Hørder, M. No influence of increased intake of orange and blackcurrant juices and dietary amounts of vitamin E on paraoxonase-1 activity in patients with peripheral arterial disease. Eur. J. Nutr. 2007, 46, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.-A.; Mulligan, C.; McCance, D.; Woodside, J.V.; Patterson, C.; Young, I.S.; McEneny, J. A randomised controlled trial of increasing fruit and vegetable intake and how this influences the carotenoid concentration and activities of PON-1 and LCAT in HDL from subjects with type 2 diabetes. Cardiovasc. Diabetol. 2014, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.; Silaste, M.; Tuominen, A.; Kaikkonen, J.; Salonen, J.T.; Alfthan, G.; Aro, A.; Kesa, Y.A. Nutrient-gene interactions dietary modifications and gene polymorphisms alter serum paraoxonase activity in healthy women 1. Public Health 2002, 132, 3012–3017. [Google Scholar]
- Senault, C.; Betoulle, D.; Luc, G.; Hauw, P.; Rigaud, D.; Fumeron, F. Beneficial effects of a moderate consumption of red wine on cellular cholesterol efflux in young men. Nutr. Metab. Cardiovasc. Dis. 2000, 10, 63–69. [Google Scholar]
- Van der Gaag, M.S.; van Tol, A.; Vermunt, S.H.; Scheek, L.M.; Schaafsma, G.; Hendriks, H.F. Alcohol consumption stimulates early steps in reverse cholesterol transport. J. Lipid Res. 2001, 42, 2077–2083. [Google Scholar] [CrossRef]
- Sierksma, A.; Vermunt, S.H.F.; Lankhuizen, I.M.; van der Gaag, M.S.; Scheek, L.M.; Grobbee, D.E.; van Tol, A.; Hendriks, H.F.J. Effect of moderate alcohol consumption on parameters of reverse cholesterol transport in postmenopausal women. Alcohol. Clin. Exp. Res. 2004, 28, 662–666. [Google Scholar] [CrossRef]
- Beulens, J.W.J.; Sierksma, A.; van Tol, A.; Fournier, N.; van Gent, T.; Paul, J.-L.; Hendriks, H.F.J. Moderate alcohol consumption increases cholesterol efflux mediated by ABCA1. J. Lipid Res. 2004, 45, 1716–1723. [Google Scholar] [CrossRef]
- Padro, T.; Muñoz-García, N.; Vilahur, G.; Chagas, P.; Deyà, A.; Antonijoan, R.M.; Badimon, L. Moderate beer intake and cardiovascular health in overweight individuals. Nutrients 2018, 10, 1237. [Google Scholar] [CrossRef]
- Králová Lesná, I.; Suchánek, P.; Stávek, P.; Poledne, R. May alcohol-induced increase of HDL be considered as atheroprotective? Physiol. Res. 2010, 59, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Van der Gaag, M.S.; van Tol, A.; Scheek, L.M.; James, R.W.; Urgert, R.; Schaafsma, G.; Hendriks, H.F.J. Daily moderate alcohol consumption increases serum paraoxonase activity; a diet-controlled, randomised intervention study in middle-aged men. Atherosclerosis 1999, 147, 405–410. [Google Scholar] [CrossRef]
- Sierksma, A.; Van Der Gaag, M.S.; Van Tol, A.; James, R.W.; Hendriks, H.F.J. Kinetics of HDL cholesterol and paraoxonase activity in moderate alcohol consumers. Alcohol. Clin. Exp. Res. 2002, 26, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Woudberg, N.J.; Mendham, A.E.; Katz, A.A.; Goedecke, J.H.; Lecour, S. Exercise intervention alters HDL subclass distribution and function in obese women. Lipids Health Dis. 2018, 17, 232. [Google Scholar] [CrossRef] [PubMed]
- Talbot, C.P.J.; Plat, J.; Joris, P.J.; Konings, M.; Kusters, Y.H.A.M.; Schalkwijk, C.G.; Ritsch, A.; Mensink, R.P. HDL cholesterol efflux capacity and cholesteryl ester transfer are associated with body mass, but are not changed by diet-induced weight loss: A randomized trial in abdominally obese men. Atherosclerosis 2018, 274, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Mundra, P.A.; Straznicky, N.E.; Nestel, P.J.; Wong, G.; Tan, R.; Huynh, K.; Ng, T.W.; Mellett, N.A.; Weir, J.M.; et al. Weight loss and exercise alter the high-density lipoprotein lipidome and improve high-density lipoprotein functionality in metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Dokras, A.; Playford, M.; Kris-Etherton, P.M.; Kunselman, A.R.; Stetter, C.M.; Williams, N.I.; Gnatuk, C.L.; Estes, S.J.; Sarwer, D.B.; Allison, K.C.; et al. Impact of hormonal contraception and weight loss on high-density lipoprotein cholesterol efflux and lipoprotein particles in women with polycystic ovary syndrome. Clin. Endocrinol. 2017, 86, 739–746. [Google Scholar] [CrossRef]
- Vislocky, L.M.; Pikosky, M.A.; Rubin, K.H.; Vega-López, S.; Gaine, P.C.; Martin, W.F.; Zern, T.L.; Lofgren, I.E.; Fernandez, M.L.; Rodriguez, N.R. Habitual consumption of eggs does not alter the beneficial effects of endurance training on plasma lipids and lipoprotein metabolism in untrained men and women. J. Nutr. Biochem. 2009, 20, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Miida, T.; Yamaguchi, T.; Tsuda, T.; Okada, M. High preβ1-HDL levels in hypercholesterolemia are maintained by probucol but reduced by a low-cholesterol diet. Atherosclerosis 1998, 138, 129–134. [Google Scholar] [CrossRef]
- Thomas, T.R.; Adeniran, S.B.; Iltis, P.W.; Aquiar, C.A.; Albers, J.J. Effects of interval and continuous running on HDL-cholesterol, apoproteins A-1 and B, and LCAT. Can. J. Appl. Sport Sci. 1985, 10, 52–59. [Google Scholar]
- Rönnemaa, T.; Marniemi, J.; Puukka, P.; Kuusi, T. Effects of long-term physical exercise on serum lipids, lipoproteins and lipid metabolizing enzymes in type 2 (non-insulin-dependent) diabetic patients. Diabetes Res. 1988, 7, 79–84. [Google Scholar] [PubMed]
- Favari, E.; Angelino, D.; Cipollari, E.; Adorni, M.P.; Zimetti, F.; Bernini, F.; Ronda, N.; Pellegrini, N. Functional pasta consumption in healthy volunteers modulates ABCG1-mediated cholesterol efflux capacity of HDL. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1768–1776. [Google Scholar] [CrossRef]
- Meng, H.; Matthan, N.R.; Fried, S.K.; Berciano, S.; Walker, M.E.; Galluccio, J.M.; Lichtenstein, A.H. Effect of dietary carbohydrate type on serum cardiometabolic risk indicators and adipose tissue inflammatory markers. J. Clin. Endocrinol. Metab. 2018, 103, 3430–3438. [Google Scholar] [CrossRef]
- Richter, C.K.; Skulas-Ray, A.C.; Fleming, J.A.; Link, C.J.; Mukherjea, R.; Krul, E.S.; Kris-Etherton, P.M. Effects of isoflavone-containing soya protein on ex vivo cholesterol efflux, vascular function and blood markers of CVD risk in adults with moderately elevated blood pressure: A dose-response randomised controlled trial. Br. J. Nutr. 2017, 117, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Abata, S.; Iwamoto, N.; Ogura, M.; Yamashita, T.; Ishikawa, O.; Ohslzu, F.; Nakamura, H. Effects of soy protein on levels of remnant-like particles cholesterol and vitamin E in healthy men. J. Nutr. Sci. Vitaminol. 2001, 47, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Shidfar, F.; Ehramphosh, E.; Heydari, I.; Haghighi, L.; Hosseini, S.; Shidfar, S. Effects of soy bean on serum paraoxonase 1 activity and lipoproteins in hyperlipidemic postmenopausal women. Int. J. Food Sci. Nutr. 2009, 60, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.J.; Volek, J.S.; Liu, Y.; Shachter, N.S.; Contois, J.H.; Fernandez, M.L. Carbohydrate restriction alters lipoprotein metabolism by modifying VLDL, LDL, and HDL subfraction distribution and size in overweight men. J. Nutr. 2006, 136, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Ausman, L.M.; Jalbert, S.M.; Vilella-Bach, M.; Jauhiainen, M.; McGladdery, S.; Erkkilä, A.T.; Ehnholm, C.; Frohlich, J.; Schaefer, E.J. Efficacy of a therapeutic lifestyle change/step 2 diet in moderately hypercholesterolemic middle-aged and elderly female and male subjects. J. Lipid Res. 2002, 43, 264–273. [Google Scholar] [CrossRef]
- Shrestha, S.; Freake, H.C.; McGrane, M.M.; Volek, J.S.; Fernandez, M.L. A combination of psyllium and plant sterols alters lipoprotein metabolism in hypercholesterolemic subjects by modifying the intravascular processing of lipoproteins and increasing LDL uptake. J. Nutr. 2007, 137, 1165–1170. [Google Scholar] [CrossRef]
- Vega-López, S.; Vidal-Quintanar, R.L.; Femandez, M.L. Sex and hormonal status influence plasma lipid responses to psyllium. Am. J. Clin. Nutr. 2001, 74, 435–441. [Google Scholar] [CrossRef]
- Lottenberg, A.M.; Nunes, V.S.; Nakandakare, E.R.; Neves, M.; Bernik, M.; Lagrost, L.; dos Santos, J.E.; Quintao, E. The human cholesteryl ester transfer protein I405V polymorphism is associated with plasma cholesterol concentration and its reduction by dietary phytosterol esters. J. Nutr. 2003, 133, 1800–1805. [Google Scholar] [CrossRef]
- Sola, R.; Motta, C.; Maille, M.; Bargallo, M.T.; Boisnier, C.; Richard, J.L.; Jacotot, B. Dietary monounsaturated fatty acids enhance cholesterol efflux from human fibroblasts. Relation to fluidity, phospholipid fatty acid composition, overall composition, and size of HDL3. Arterioscler. Thromb. J. Vasc. Biol. 1993, 13, 958–966. [Google Scholar] [CrossRef]
- Jansen, S.; Lôpez-Miranda, J.; Castro, P.; López-Segura, F.; Marín, C.; Ordovás, J.M.; Paz, E.; Jiménez-Perepérez, J.; Fuentes, F.; Pérez-Jiménez, F. Low-fat and high-monounsaturated fatty acid diets decrease plasma cholesterol ester transfer protein concentrations in young, healthy, normolipemic men. Am. J. Clin. Nutr. 2000, 72, 36–41. [Google Scholar] [CrossRef][Green Version]
- Abbey, M.; Nestel, P.J. Plasma cholesteryl ester transfer protein activity is increased when trans-elaidic acid is substituted for cis-oleic acid in the diet. Atherosclerosis 1994, 106, 99–107. [Google Scholar] [CrossRef]
- Pieke, B.; von-Eckardstein, A.; Gulbahce, E.; Chirazi, A.; Schulte, H.; Assmann, G.; Wahrburg, U. Treatment of hypertriglyceridemia by two diets rich either in unsaturated fatty acids or in carbohydrates: Effects on lipoprotein subclasses, lipolytic enzymes, lipid transfer proteins, insulin and leptin. Int. J. Obes. 2000, 24, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Ishida, T.; Nagao, M.; Mori, T.; Monguchi, T.; Sasaki, M.; Mori, K.; Kondo, K.; Nakajima, H.; Honjo, T.; et al. Administration of high dose eicosapentaenoic acid enhances anti-inflammatory properties of high-density lipoprotein in Japanese patients with dyslipidemia. Atherosclerosis 2014, 237, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Burillo, E.; Mateo-Gallego, R.; Cenarro, A.; Fiddyment, S.; Bea, A.M.; Jorge, I.; Vázquez, J.; Civeira, F. Beneficial effects of omega-3 fatty acids in the proteome of high-density lipoprotein proteome. Lipids Health Dis. 2012, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Gillotte, K.L.; Lund-Katz, S.; De La Llera-Moya, M.; Parks, J.S.; Rudel, L.L.; Rothblat, G.H.; Phillips, M.C. Dietary modification of high density lipoprotein phospholipid and influence on cellular cholesterol efflux. J. Lipid Res. 1998, 39, 2065–2075. [Google Scholar] [CrossRef]
- Harayama, T.; Shimizu, T. Roles of polyunsaturated fatty acids, from mediators to membranes. J. Lipid Res. 2020, 61, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Bragt, M.C.E.; Popeijus, H.E. Peroxisome proliferator-activated receptors and the metabolic syndrome. Physiol. Behav. 2008, 94, 187–197. [Google Scholar] [CrossRef]
- Duval, C.; Müller, M.; Kersten, S. PPARalpha and dyslipidemia. Biochim. Biophys. Acta 2007, 1771, 961–971. [Google Scholar] [CrossRef]
- Pizzini, A.; Lunger, L.; Demetz, E.; Hilbe, R.; Weiss, G.; Ebenbichler, C.; Tancevski, I. The role of omega-3 fatty acids in reverse cholesterol transport: A review. Nutrients 2017, 9, 1099. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T. Effect of dietary lipids on paraoxonase-1 activity and gene expression. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 88–94. [Google Scholar] [CrossRef]
- Ichimura, A.; Hara, T.; Hirasawa, A. Regulation of energy homeostasis via GPR120. Front. Endocrinol. 2014, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Hernáez, A.; Farràs, M.; Fitó, M. Olive oil phenolic compounds and high-density lipoprotein function. Curr. Opin. Lipidol. 2016, 27, 47–53. [Google Scholar] [CrossRef][Green Version]
- Zuliani, G.; Galvani, M.; Leitersdorf, E.; Volpato, S.; Cavalieri, M.; Fellin, R. The role of polyunsaturated fatty acids (PUFA) in the treatment of dyslipidemias. Curr. Pharm. Des. 2009, 15, 4087–4093. [Google Scholar] [CrossRef]
- Lamarche, B.; Rashid, S.; Lewis, G.F. HDL metabolism in hypertriglyceridemic states: An overview. Clin. Chim. Acta 1999, 286, 145–161. [Google Scholar] [CrossRef]
- Sun, Y.; Neelakantan, N.; Wu, Y.; Lote-Oke, R.; Pan, A.; van Dam, R.M. Palm oil consumption increases LDL cholesterol compared with vegetable oils low in saturated fat in a meta-analysis of clinical trials. J. Nutr. 2015, 145, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Karupaiah, T.; Tan, C.H.; Chinna, K.; Sundram, K. The chain length of dietary saturated fatty acids affects human postprandial lipemia. J. Am. Coll. Nutr. 2011, 30, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, C.M.; Hapugaswatta, H.; Samarawickrama, G.R.; Jayathilaka, N.; Seneviratne, K.N. Effect of chain length and saturation of the fatty acids in dietary triglycerides on lipid metabolism in Wistar rats. J. Food Biochem. 2020, 45, e13664. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, V.; Gulia, N.; Ahlawat, K.S.; Khatkar, B.S. Trans fats-sources, health risks and alternative approach—A review. J. Food Sci. Technol. 2011, 48, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Shao, B. Site-specific oxidation of apolipoprotein A-I impairs cholesterol export by ABCA1, a key cardioprotective function of HDL. Biochim. Biophys. Acta 2012, 1821, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Subbaiah, P.V. Importance of the free sulfhydryl groups of lecithin-cholesterol acyltransferase for its sensitivity to oxidative inactivation. Biochim. Biophys. Acta 2000, 1488, 268–277. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Billecke, S.; Erogul, J.; Sorenson, R.; Bisgaier, C.L.; Newton, R.S.; La Du, B. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic. Biol. Med. 1999, 26, 892–904. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D.; Motta, C.; Khalil, A.O.; Sola, R.; La Ville, A.E.; Delattre, J.; Gardès-Albert, M. Physicochemical changes in human high-density lipoproteins (HDL) oxidized by gamma radiolysis-generated oxyradicals. Effect on their cholesterol effluxing capacity. Biochim. Biophys. Acta 1995, 1255, 23–30. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.J.; Kroemer, G. Caloric restriction mimetics against age-associated disease: Targets, mechanisms, and therapeutic potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef] [PubMed]
- Salt, I.P.; Hardie, D.G. AMP-activated protein kinase. Circ. Res. 2017, 120, 1825–1841. [Google Scholar] [CrossRef] [PubMed]
- Brien, S.E.; Ronksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ 2011, 342, 480. [Google Scholar] [CrossRef] [PubMed]
- Hagiage, M.; Marti, C.; Rigaud, D.; Senault, C.; Fumeron, F.; Apfelbaum, M.; Girard-Globa, A. Effect of a moderate alcohol intake on the lipoproteins of normotriglyceridemic obese subjects compared with normoponderal controls. Metabolism 1992, 41, 856–861. [Google Scholar] [CrossRef]
- Serdyuk, A.P.; Metelskaya, V.A.; Ozerova, I.N.; Kovaltchouk, N.V.; Olferiev, A.M.; Bubnova, M.G.; Perova, N.V.; Jauhiainen, M.; Lasselin, C.; Castro, G. Effects of alcohol on the major steps of reverse cholesterol transport. Biochemistry 2000, 65, 1310–1315. [Google Scholar] [PubMed]
- Savolainen, M.J.; Hannuksela, M.; Seppänen, S.; Kervinen, K.; Kesäniemi, Y.A. Increased high-density lipoprotein cholesterol concentration in alcoholics is related to low cholesteryl ester transfer protein activity. Eur. J. Clin. Investig. 1990, 20, 593–599. [Google Scholar] [CrossRef]
- Välimäki, M.; Kahri, J.; Laitinen, K.; Lahdenperä, S.; Kuusi, T.; Ehnholm, C.; Jauhiainen, M.; Bard, J.M.; Fruchart, J.C.; Taskinen, M.R.; et al. High density lipoprotein subfractions, apolipoprotein A-I containing lipoproteins, lipoprotein (a), and cholesterol ester transfer protein activity in alcoholic women before and after ethanol withdrawal. Eur. J. Clin. Investig. 1993, 23, 406–417. [Google Scholar] [CrossRef]
- Rosales, C.; Gillard, B.K.; Gotto, A.M.; Pownall, H.J. The alcohol–high-density lipoprotein athero-protective axis. Biomolecules 2020, 10, 987. [Google Scholar] [CrossRef]
- Ge, H.; Li, X.; Weiszmann, J.; Wang, P.; Baribault, H.; Chen, J.L.; Tian, H.; Li, Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 2008, 149, 4519–4526. [Google Scholar] [CrossRef]
- Dai, W.S.; Laporte, R.E.; Hom, D.L.; Kuller, L.H.; D’antonio, J.A.; Gutai, J.P.; Wozniczak, M.; Wohlfahrt, B. Alcohol consumption and high density lipoprotein cholesterol concentration among alcoholics. Am. J. Epidemiol. 1985, 122, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine polyphenol content and its influence on wine quality and properties: A review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gomez, A.; Caballero, I.; Blanco, C.A. Phenols and melanoidins as natural antioxidants in beer. Structure, reactivity and antioxidant activity. Biomolecules 2020, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Sang, H.; Yao, S.; Zhang, L.; Li, X.; Yang, N.; Zhao, J.; Zhao, L.; Si, Y.; Zhang, Y.; Lv, X.; et al. Walk-run training improves the anti-inflammation properties of high-density lipoprotein in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2015, 100, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, M.; Tchoua, U.; Gillard, B.K.; Jones, P.H.; Ballantyne, C.M.; Pownall, H.J. Modest diet-induced weight loss reduces macrophage cholesterol efflux to plasma of patients with metabolic syndrome. J. Clin. Lipidol. 2011, 7, 661–670. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aicher, B.O.; Haser, E.K.; Freeman, L.A.; Carnie, A.V.; Stonik, J.A.; Wang, X.; Remaley, A.T.; Kato, G.J.; Cannon, R.O. Diet-induced weight loss in overweight or obese women and changes in high-density lipoprotein levels and function. Obesity 2012, 20, 2057–2062. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.V.; Li, L.; Byun, J.; Guo, Y.; Michailidis, G.; Jaiswal, M.; Chen, Y.E.; Pop-Busui, R.; Pennathur, S. Therapeutic lifestyle changes improve HDL function by inhibiting myeloperoxidase-mediated oxidation in patients with metabolic syndrome. Diabetes Care 2018, 41, 2431–2437. [Google Scholar] [CrossRef]
- Boyer, M.; Mitchell, P.L.; Poirier, P.; Alméras, N.; Tremblay, A.; Bergeron, J.; Després, J.P.; Arsenault, B.J. Impact of a one-year lifestyle modification program on cholesterol efflux capacities in men with abdominal obesity and dyslipidemia. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E460–E468. [Google Scholar] [CrossRef]
- Richter, E.A.; Ruderman, N.B. AMPK and the biochemistry of exercise: Implications for human health and disease. Biochem. J. 2009, 418, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Hernáez, Á.; Soria-Florido, M.T.; Castañer, O.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Alonso-Gómez, Á.; Martínez-González, M.Á.; Schröder, H.; et al. Leisure time physical activity is associated with improved HDL functionality in high cardiovascular risk individuals: A cohort study. Eur. J. Prev. Cardiol. 2021, 28, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.; Rye, K.A. Cholesteryl ester transfer protein: Its role in plasma lipid transport. Clin. Exp. Pharmacol. Physiol. 1994, 21, 663–672. [Google Scholar] [CrossRef]
- Greer, E.L.; Banko, M.R.; Brunet, A. AMP-activated protein kinase and FoxO transcription factors in dietary restriction-induced longevity. Ann. N. Y. Acad. Sci. 2009, 1170, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of Berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).