Amnesia after Midazolam and Ketamine Sedation in Children: A Secondary Analysis of a Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Aspects
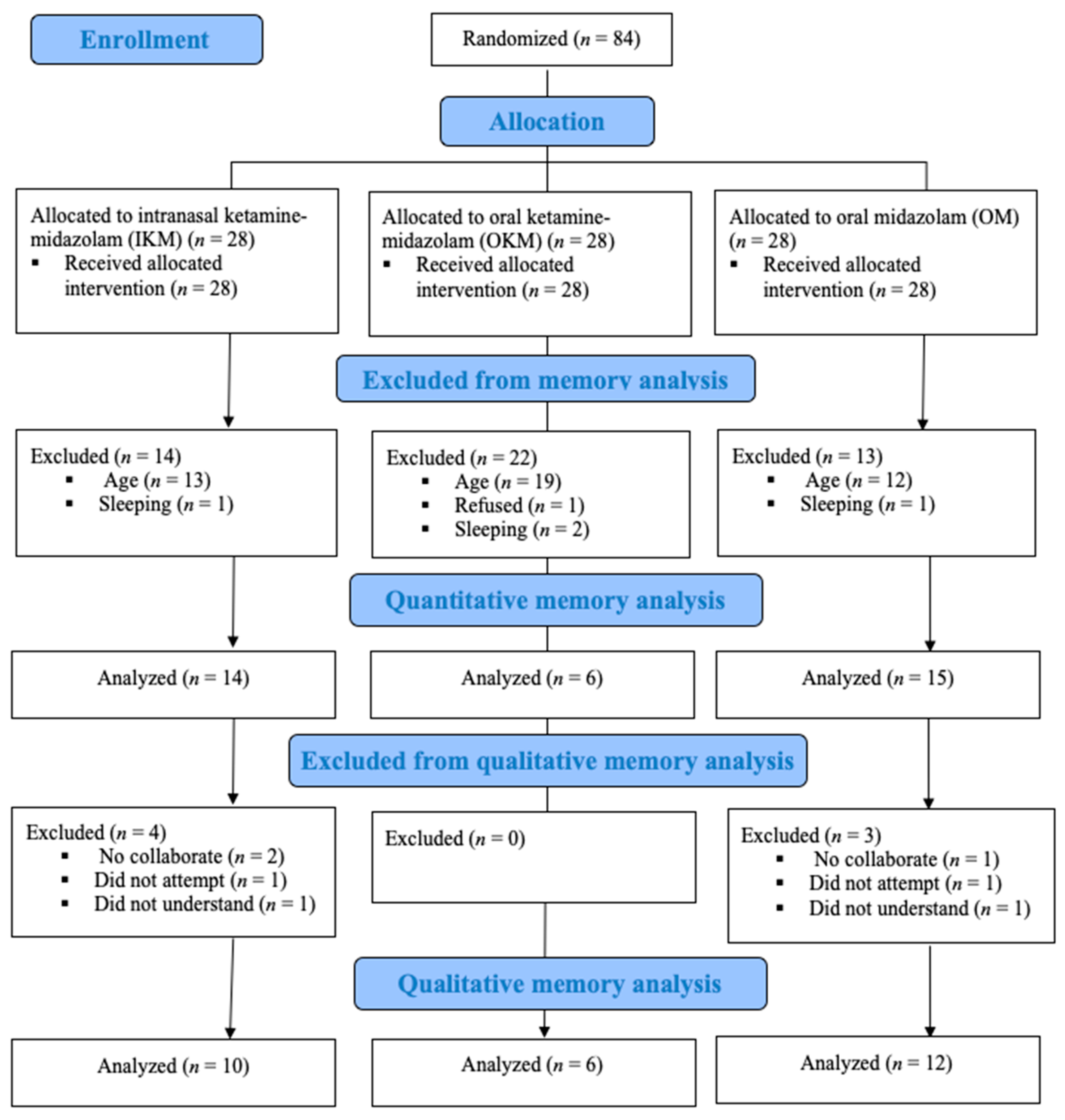
2.2. Participants
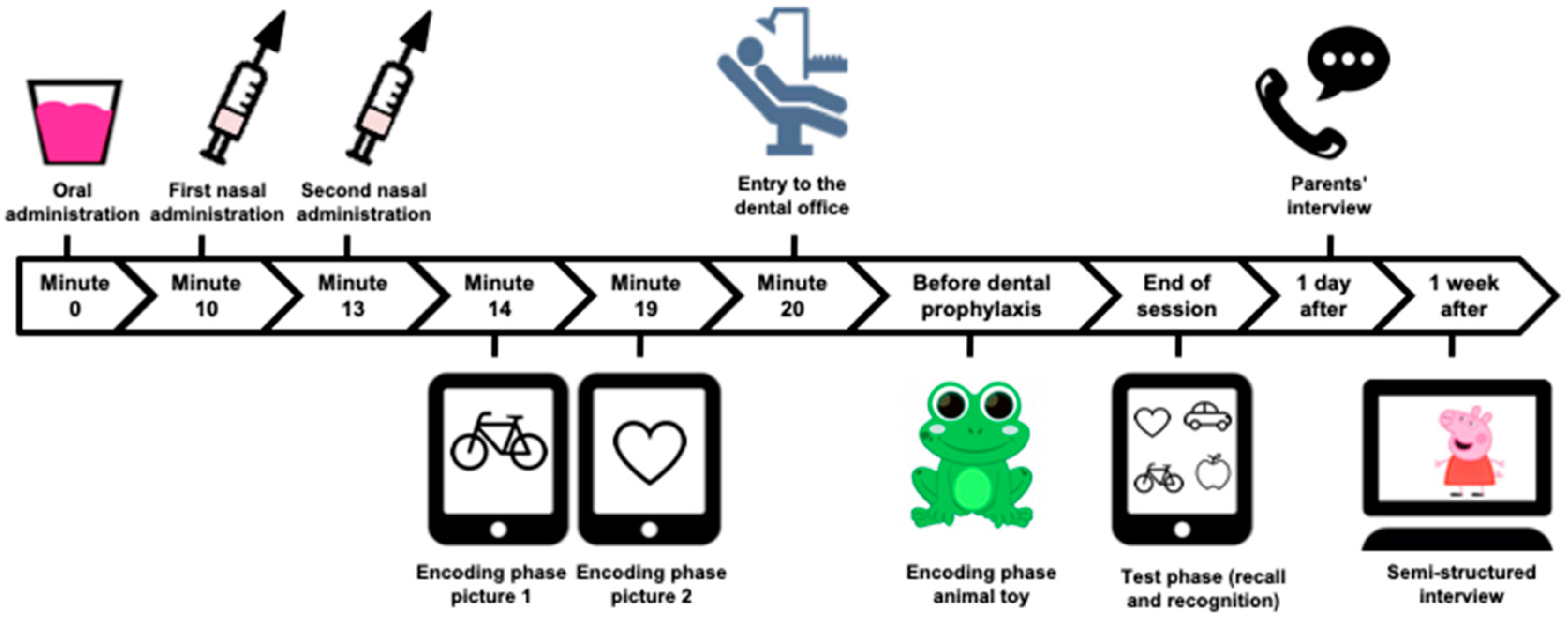
2.3. Proceedings of the Randomized Clinical Trial Phase
2.4. Procedures to Assess Amnesia
2.5. Procedures to Assess Children’s Behavior and Level of Sedation
2.6. Data Analysis
2.7. Quantitative Approach
- (1)
- Lack recall of each picture and the animal toy.
- (2)
- Lack of recognition of pictures, including both the hit rate (correct recognition of target pictures) and false alarm rate (incorrect identification of distractor item as a target picture). A difference between the hit and false alarm rate equal to one was considered recognition.
- (3)
- Lack of recognition of animal toy: measured in a similar way of recognition of pictures.
- (4)
- Amnesia of dental procedure: an absence of recall or recognition of animal toy because it was the stimuli shown during the appointment.
- (5)
- Primary caregivers’ response to the question: “Do you think your son/daughter remembers the performed interventions?”
- (6)
- Amnesia of oral and intranasal administration: taken from interviews.
2.8. Qualitative Approach
3. Results
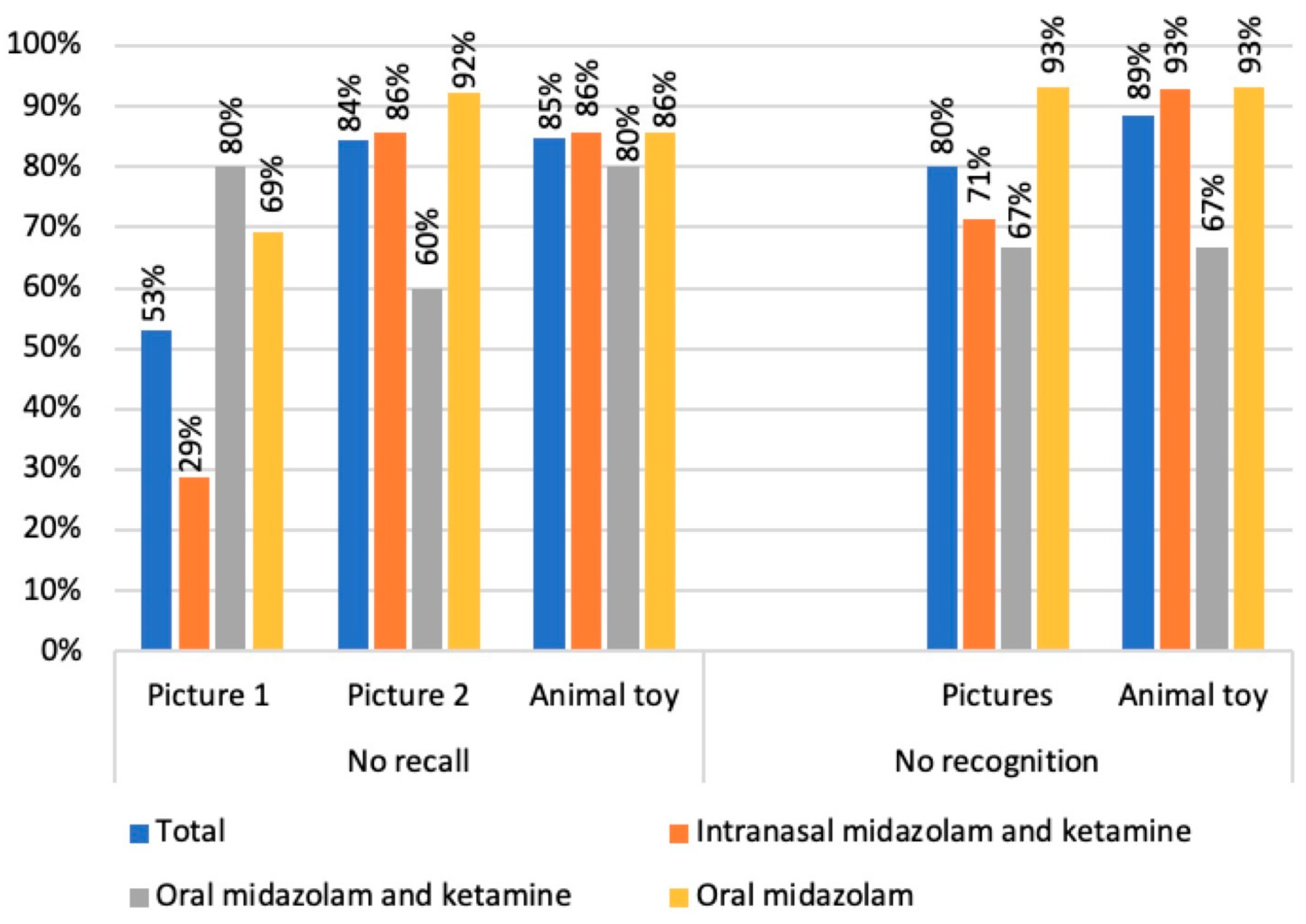
3.1. Quantitative Analysis
3.2. Qualitative Approach
3.3. Category: The Child Clearly Remembered
3.4. Category: The Child Did Not Remember
3.5. Category: The Child Had Uncertain Remembrance
3.6. Category: The Child Had Abilities to Remember
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Academy of Pediatric Dentistry. Behavior guidance for the pediatric dental patient. Pediatr. Dent. 2018, 40, 254–267. [Google Scholar]
- Ghoneim, M.M. Drugs and human memory (part 1): Clinical, theoretical, and methodologic issues. J. Am. Soc. Anesthesiol. 2004, 100, 987–1002. [Google Scholar] [CrossRef]
- Hackmann, T.; Haworth, R.A.; Hong, P.; Gillespie, J.; Chorney, J. Accuracy of children’s perioperative memories. AORN J. 2017, 105, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Ward, D.S.; Carlson, D.; Cravero, J.; Dexter, F.; Lightdale, J.R.; Mason, K.P.; Miner, J.; Vargo, J.J.; Berkenbosch, J.W.; et al. Evaluating patient-centered outcomes in clinical trials of procedural sedation, part 1 efficacy: Sedation consortium on endpoints and procedures for treatment, education, and research recommendations. Anesth. Analg. 2017, 124, 821–830. [Google Scholar] [CrossRef]
- Isik, B.; Baygin, O.; Bodur, H. Premedication with melatonin vs midazolam in anxious children. Paediatr. Anaesth. 2008, 18, 635–641. [Google Scholar] [CrossRef]
- Ghadami Yazdi, A.; Ayatollahi, V.; Hashemi, A.; Behdad, S.; Ghadami Yazdi, E. Effect of two different concentrations of propofol and ketamine combinations (ketofol) in pediatric patients under lumbar puncture or bone marrow aspiration. Iran. J. Ped. Hematol. Oncol. 2014, 3, 187–192. [Google Scholar]
- Godambe, S.A.; Elliot, V.; Matheny, D.; Pershad, J. Comparison of propofol/fentanyl versus ketamine/midazolam for brief orthopedic procedural sedation in a pediatric emergency department. Pediatrics 2003, 112, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Lee-Jayaram, J.J.; Green, A.; Siembieda, J.; Gracely, E.J.; Mull, C.C.; Quintana, E.; Adirim, T. Ketamine/midazolam versus etomidate/fentanyl: Procedural sedation for pediatric orthopedic reductions. Pediatr. Emerg. Care 2010, 26, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Viana, K.A.; Daher, A.; Maia, L.C.; Costa, P.S.; Martins, C.C.; Paiva, S.M.; Costa, L.R. What is the level of evidence for the amnestic effects of sedatives in pediatric patients? A systematic review and meta-analyses. PLoS ONE 2017, 12, e0180248. [Google Scholar] [CrossRef] [Green Version]
- Ghoneim, M.M.; Hinrichs, J.V. Drugs, memory, and sedation: Specificity of effects. Anesthesiology 1997, 87, 734–736. [Google Scholar] [CrossRef]
- Mason, K.P.; Kelhoffer, E.R.; Prescilla, R.; Mehta, M.; Root, J.C.; Young, V.J.; Robinson, F.; Veselis, R.A. Feasibility of measuring memory response to increasing dexmedetomidine sedation in children. Br. J. Anaesth. 2017, 118, 254–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veselis, R.; Kelhoffer, E.; Mehta, M.; Root, J.C.; Robinson, F.; Mason, K.P. Propofol sedation in children: Sleep trumps amnesia. Sleep Med. 2016, 27–28, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuper, A.; Reeves, S.; Levinson, W. An introduction to reading and appraising qualitative research. BMJ 2008, 337, a288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kortesluoma, R.L.; Hentinen, M.; Nikkonen, M. Conducting a qualitative child interview: Methodological considerations. J. Adv. Nurs. 2003, 42, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Sado-Filho, J.; Viana, K.A.; Corrêa-Faria, P.; Costa, L.R.; Costa, P.S. Randomized clinical trial on the efficacy of intranasal or oral ketamine-midazolam combinations compared to oral midazolam for outpatient pediatric sedation. PLoS ONE 2019, 14, e0213074. [Google Scholar] [CrossRef]
- Leech, N.L.; Onwuegbuzie, A.J. Guidelines for conducting and reporting mixed research in the field of counseling and beyond. J. Couns. Dev. 2010, 88, 61–69. [Google Scholar] [CrossRef]
- Creswell, J.W.; Plano Clark, V.L. Designing and Conducting mixed Methods Research, 2nd ed.; SAGE Publications; SAGE: London, UK, 2011. [Google Scholar]
- Bahetwar, S.K.; Pandey, R.K.; Saksena, A.K.; Chandra, G. A comparative evaluation of intranasal midazolam, ketamine and their combination for sedation of young uncooperative pediatric dental patients: A triple blind randomized crossover trial. J. Clin. Pediatr. Dent. 2011, 35, 415–420. [Google Scholar] [CrossRef]
- Warner, D.L.; Cabaret, J.; Velling, D. Ketamine plus midazolam, a most effective paediatric oral premedicant. Paediatr Anaesth. 1995, 5, 293–295. [Google Scholar] [CrossRef]
- Moreira, T.A.; Costa, P.S.; Costa, L.R.; Jesus-França, C.M.; Antunes, D.E.; Gomes, H.S.; Neto, O.A. Combined oral midazolam-ketamine better than midazolam alone for sedation of young children: A randomized controlled trial. Int. J. Paediatr. Dent. 2013, 23, 207–215. [Google Scholar] [CrossRef]
- Reed, M.D.; Rodarte, A.; Blumer, J.L.; Khoo, K.C.; Akbari, B.; Pou, S.; Kearns, G.L.; Pediatric Pharmacology Research Unit Network. The single-dose pharmacokinetics of midazolam and its primary metabolite in pediatric patients after oral and intravenous administration. J. Clin. Pharmacol. 2001, 41, 1359–1369. [Google Scholar] [CrossRef]
- Schrier, L.; Zuiker, R.; Merkus, F.W.; Klaassen, E.S.; Guan, Z.; Tuk, B.; van Gerven, J.M.A.; van der Geest, R.; Jan Groeneveld, G. Pharmacokinetics and pharmacodynamics of a new highly concentrated intranasal midazolam formulation for conscious sedation. Br. J. Clin. Pharmacol. 2017, 83, 721–731. [Google Scholar] [CrossRef] [Green Version]
- Coté, C.J.; Wilson, S.; American Academy of Pediatrics; American Academy of Pediatric Dentistry. Guidelines for monitoring and management of pediatric patients before, during, and after sedation for diagnostic and therapeutic procedures: Update 2016. Pediatrics 2016, 38, 77–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, H.S.; Miranda, A.R.; Viana, K.A.; Batista, A.C.; Costa, P.S.; Daher, A.; Machado, G.C.M.; Sado-Filho, J.; Vieira, L.A.C.; Corrêa-Faria, P.; et al. Intranasal sedation using ketamine and midazolam for pediatric dental treatment (NASO): Study protocol for a randomized controlled trial. Trials 2017, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Society of Anesthesiologists. ASA Physical Status Classification System. 2019. Available online: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system (accessed on 10 April 2021).
- Mallampati, S.R.; Gatt, S.P.; Gugino, L.D.; Desai, S.P.; Waraksa, B.; Freiberger, D.; Liu, P.L. A clinical sign to predict difficult tracheal intubation: A prospective study. Can. Anaesth. Soc. J. 1985, 32, 429–434. [Google Scholar] [CrossRef] [Green Version]
- Frankl, S.; Shiere, F.; Fogels, H. Should the parent remain with the child in the dental operatory? J. Dent. Child 1962, 29, 150–163. [Google Scholar]
- Côté, L.; Turgeon, J. Appraising qualitative research articles in medicine and medical education. Med. Teach. 2005, 27, 71–75. [Google Scholar] [CrossRef]
- Singh, C.; Pandey, R.K.; Saksena, A.K.; Chandra, G. A comparative evaluation of analgo-sedative effects of oral dexmedetomidine and ketamine: A triple-blind, randomized study. Paediatr. Anaesth. 2014, 24, 1252–1259. [Google Scholar] [CrossRef]
- The Joint Commission. Speak up: Anesthesia and Sedation. Available online: https://www.jointcommission.org/assets/1/6/Speak_Up_Anesthesia_infographic_final.pdf (accessed on 10 April 2021).
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, H.F.; Shannon, S.E. Three approaches to qualitative content analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef]
- Pringle, B.; Dahlquist, L.M.; Eskenazi, A. Memory in pediatric patients undergoing conscious sedation for aversive medical procedures. Health Psychol. 2003, 22, 263–269. [Google Scholar] [CrossRef] [PubMed]
- van Houtem, C.M.H.H.; van Wijk, A.J.; de Jongh, A. Presence, content, and characteristics of memories of individuals with dental phobia. Appl. Cogn. Psychol. 2015, 29, 515–523. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists. Continuum of Depth of Sedation Definition of General Anesthesia and Levels of Sedation/Analgesia. 2019. Available online: https://www.asahq.org/standards-and-guidelines/continuum-of-depth-of-sedation-definition-of-general-anesthesia-and-levels-of-sedationanalgesia (accessed on 10 April 2021).
- Pandey, R.K.; Bahetwar, S.K.; Saksena, A.K.; Chandra, G. A comparative evaluation of drops versus atomized administration of intranasal ketamine for the procedural sedation of young uncooperative pediatric dental patients: A prospective crossover trial. J. Clin. Pediatr. Dent. 2011, 36, 79–84. [Google Scholar] [CrossRef]
- Sunbul, N.; Delvi, M.B.; Zahrani, T.A.; Salama, F. Buccal versus intranasal midazolam sedation for pediatric dental patients. Pediatr. Dent. 2014, 36, 483–488. [Google Scholar] [PubMed]
- Wang, J.; Sun, P.; Liang, P. Neuropsychopharmacological effects of midazolam on the human brain. Brain Inform. 2020, 7, 15. [Google Scholar] [CrossRef]
- Vogt, K.M.; Ibinson, J.W.; Smith, C.T.; Citro, A.L.; Norton, C.M.; Karim, H.T.; Popov, V.; Mahajan, A.; Aizenstein, H.J.; Reder, L.M.; et al. Midazolam and Ketamine Produce Distinct Neural Changes in Memory, Pain, and Fear Networksduring Pain. Anesthesiology 2021, 135, 69–82. [Google Scholar] [CrossRef]
- Shields, G.S.; McCullough, A.M.; Ritchey, M.; Ranganath, C.; Yonelinas, A.P. Stress and the medial temporal lobe at rest: Functional connectivity is associated with both memory and cortisol. Psychoneuroendocrinology 2019, 106, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Pavlova, M.; Graham, S.A.; Jordan, A.; Chorney, J.; Vinall, J.; Rasic, N.; Brookes, J.; Hoy, M.; Yunker, W.K.; Noel, M. Socialization of Pain Memories: Parent-ChildReminiscing About Past Painful and Sad Events. J. Pediatr. Psychol. 2019, 44, 679–691. [Google Scholar] [CrossRef]
- Patel, Z.S.; Jensen, S.E.; Lai, J.S. Considerations for conducting qualitative research with pediatric patients for the purpose of PRO development. Qual. Life Res. 2016, 25, 2193–2199. [Google Scholar] [CrossRef]
- Lopez, U.; Habre, W.; Laurençon, M.; Haller, G.; Van der Linden, M.; Iselin-Chaves, I.A. Intra-operative awareness in children: The value of an interview adapted to their cognitive abilities. Anaesthesia 2007, 62, 778–789. [Google Scholar] [CrossRef] [Green Version]
- Upton, P.; Lawford, J.; Eiser, C. Parent-child agreement across child health-related quality of life instruments: A review of the literature. Qual. Life Res. 2008, 17, 895–913. [Google Scholar] [CrossRef] [PubMed]

| Variables | Amnesia of the Dental Procedure (Absence of Toy Recognition or Recall) (n = 35) | Amnesia According to Primary Caregivers’ Report (n = 34) | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Sedation level | ||||
| Minimal | 7 (24.1%) | 4 (66.7%) | 5 (27.8%) | 6 (37.5%) |
| Moderate | 22 (75.9%) | 2 (33.3%) | 13 (72.2%) | 10 (62.5%) |
| Sedative groups | ||||
| Intranasal midazolam and ketamine | 12 (41.4%) | 2 (33.3%) | 8 (44.4%) | 5 (31.3%) |
| Oral midazolam and ketamine | 4 (13.8%) | 2 (33.3%) | 1 (5.6%) | 5 (31.3%) |
| Oral midazolam | 13 (44.8%) | 2 (33.3%) | 9 (50.0%) | 6 (37.5%) |
| Children’s behavior during sedation | ||||
| Negative | 15 (51.7%) | 2 (33.3%) | 10 (55.6%) | 6 (37.5%) |
| Positive | 14 (48.3%) | 4 (66.7%) | 8 (44.4%) | 10 (62.5%) |
| Total | 29 (82.9%) | 6 (17.1%) | 18 (52.9%) | 16 (47.1%) |
| Variables | Groups | ||
| Intranasal Ketamine/Midazolam (n = 14) | Oral Ketamine/Midazolam (n = 6) | Oral Midazolam (n = 15) | |
| Sex, n (%) | |||
| Female | 7 (50.0%) | 3 (50.0%) | 6 (40.0%) |
| Male | 7 (50.0%) | 3 (50.0%) | 9 (60.0%) |
| Age in months, mean (SD) | 54.6 (12.8) | 57.3 (11.2) | 48.6 (11.0) |
| Behavior during the dental session, n (%) | |||
| Positive | 6 (42.9%) | 5 (83.3%) | 7 (46.7%) |
| Negative | 8 (57.1%) | 1 (16.7%) | 8 (53.3.%) |
| Level of sedation | |||
| Minimal | 3 (21.4%) | 2 (33.3%) | 6 (40.0%) |
| Moderate | 11 (78.6%) | 4 (66.7%) | 9 (60.0%) |
| Theme | Categories | Codes–Children… |
|---|---|---|
| Some children can remember in detail procedures performed during dental sedation | Clearly remembered | recalled the sedative administration |
| recalled the animal toy | ||
| recalled specific dental procedures or the whole session | ||
| talked about dental treatment/memory assessment at home | ||
| reported sensations during the procedure (pain, dizziness) | ||
| Did not remember | forgot the animal toy | |
| did not remember specific procedures or the whole session | ||
| Uncertain remembrance | gave some clues of remembering the treatment during the interview, but they were inconsistent | |
| recalled episodes that could have happened during the clinical examination or in the recovery (e.g., slept at the dentist, taken photo with the dentist, sang the same song that dentist sang) | ||
| Abilities to remember (according to primary caregivers) | are clever | |
| have their own opinion | ||
| are agitated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, K.A.; Moterane, M.M.; Green, S.M.; Mason, K.P.; Costa, L.R. Amnesia after Midazolam and Ketamine Sedation in Children: A Secondary Analysis of a Randomized Controlled Trial. J. Clin. Med. 2021, 10, 5430. https://doi.org/10.3390/jcm10225430
Viana KA, Moterane MM, Green SM, Mason KP, Costa LR. Amnesia after Midazolam and Ketamine Sedation in Children: A Secondary Analysis of a Randomized Controlled Trial. Journal of Clinical Medicine. 2021; 10(22):5430. https://doi.org/10.3390/jcm10225430
Chicago/Turabian StyleViana, Karolline A., Mônica M. Moterane, Steven M. Green, Keira P. Mason, and Luciane R. Costa. 2021. "Amnesia after Midazolam and Ketamine Sedation in Children: A Secondary Analysis of a Randomized Controlled Trial" Journal of Clinical Medicine 10, no. 22: 5430. https://doi.org/10.3390/jcm10225430
APA StyleViana, K. A., Moterane, M. M., Green, S. M., Mason, K. P., & Costa, L. R. (2021). Amnesia after Midazolam and Ketamine Sedation in Children: A Secondary Analysis of a Randomized Controlled Trial. Journal of Clinical Medicine, 10(22), 5430. https://doi.org/10.3390/jcm10225430






