Exploring Consensus on Preventive Measures and Identification of Patients at Risk of Age-Related Macular Degeneration Using the Delphi Process
Abstract
:1. Introduction
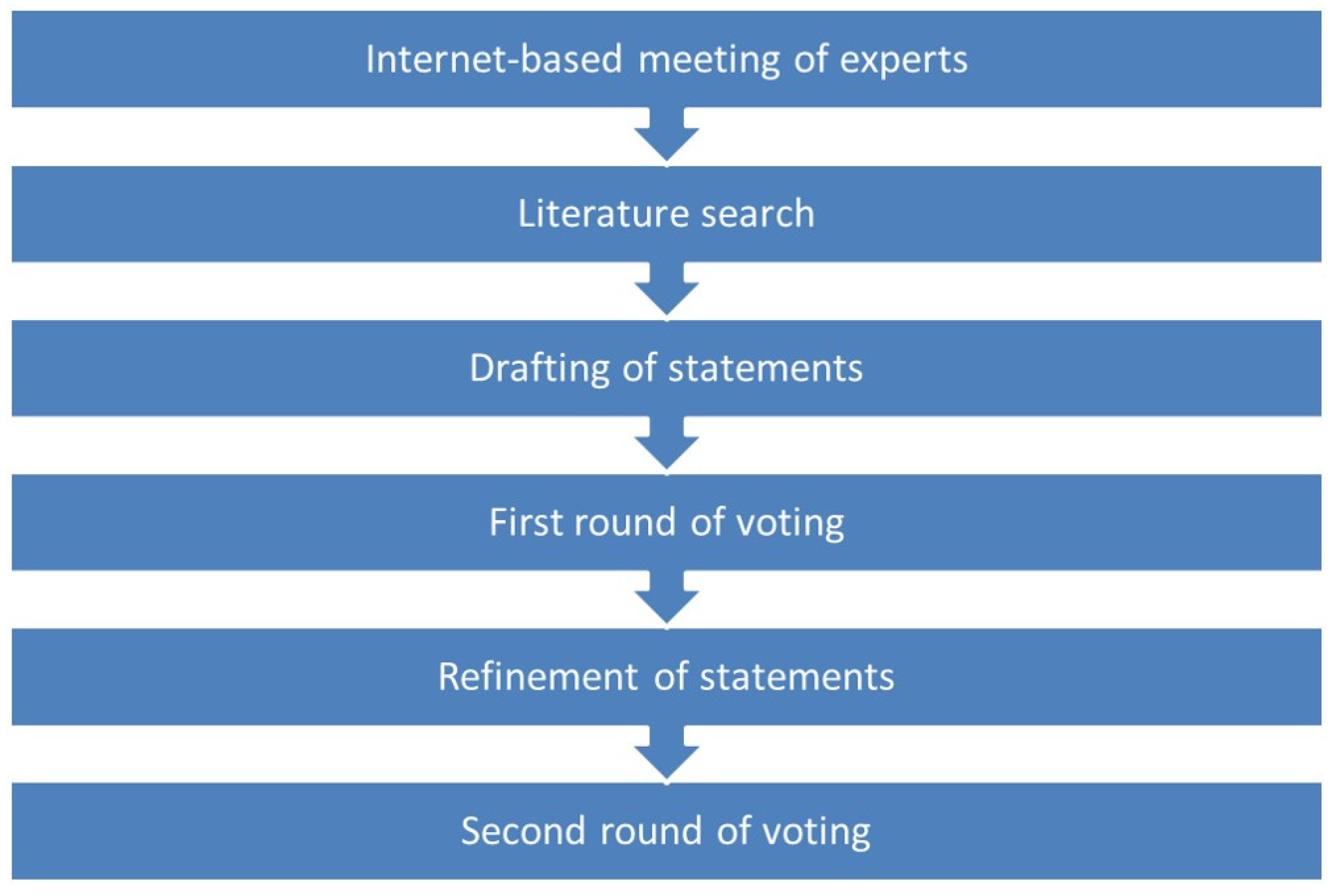
2. Materials and Methods
2.1. Delphi Process
2.2. Analysis of Voting and Determination of Agreement
2.3. Ethics Approval
3. Results
4. Discussion
4.1. General Recommendations
4.2. Use of Evaluation Tools
4.3. General Lifestyle Advice
4.4. AREDS-Based Supplementation
4.5. Moderate and High-Risk Subjects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–349. [Google Scholar] [CrossRef] [Green Version]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group; Maguire, M.G.; Martin, D.F.; Ying, G.S.; Jaffe, G.J.; Daniel, E.; Grunwald, J.E.; Toth, C.A.; Ferris, F.L., 3rd; Fine, S.L. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: The comparison of age-related macular degeneration treatments trials. Ophthalmology 2016, 123, 1751–1761. [Google Scholar] [CrossRef] [Green Version]
- Gillies, M.C.; Campain, A.; Barthelmes, D.; Simpson, J.M.; Arnold, J.J.; Guymer, R.H.; McAllister, I.L.; Essex, R.W.; Morlet, N.; Hunyor, A.P.; et al. Long-term outcomes of treatment of neovascular age-related macular degeneration: Data from an observational study. Ophthalmology 2015, 122, 1837–1845. [Google Scholar] [CrossRef]
- Rofagha, S.; Bhisitkul, R.B.; Boyer, D.S.; Sadda, S.R.; Zhang, K.; SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 2013, 120, 2292–2299. [Google Scholar] [CrossRef]
- Loewenstein, A.; Richard & Hinda Rosenthal Foundation. The significance of early detection of age-related macular degeneration: Richard & hinda rosenthal foundation lecture, the macula society 29th annual meeting. Retina 2007, 27, 873–878. [Google Scholar] [CrossRef] [Green Version]
- Chakravarthy, U.; Wong, T.Y.; Fletcher, A.; Piault, E.; Evans, C.; Zlateva, G.; Buggage, R.; Pleil, A.; Mitchell, P. Clinical risk factors for age-related macular degeneration: A systematic review and meta-analysis. BMC Ophthalmol. 2010, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M. Macular degeneration epidemiology: Nature-nurture, lifestyle factors, genetic risk, and gene-environment interactions—The weisenfeld award lecture. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6513–6528. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Agrón, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D.L.; AREDS and AREDS2 Research Groups. Dietary nutrient intake and progression to late age-related macular degeneration in the age-related eye disease studies 1 and 2. Ophthalmology 2021, 128, 425–442. [Google Scholar] [CrossRef]
- Ho, L.; van Leeuwen, R.; Witteman, J.C.; van Duijn, C.M.; Uitterlinden, A.G.; Hofman, A.; de Jong, P.T.; Vingerling, J.R.; Klaver, C.C. Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and omega-3 fatty acids: The Rotterdam study. Arch. Ophthalmol. 2011, 129, 758–766. [Google Scholar] [CrossRef] [Green Version]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T. Lutein: More than just a filter for blue light. Prog. Retin Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef]
- Merle, B.M.; Silver, R.E.; Rosner, B.; Seddon, J.M. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: A prospective cohort study. Am. J. Clin. Nutr. 2015, 102, 1196–1206. [Google Scholar] [CrossRef]
- Merle, B.M.J.; Colijn, J.M.; Cougnard-Gregoire, A.; De Koning-Backus, A.P.M.; Delyfer, M.N.; Kiefte-de Jong, J.C.; Meester-Smoor, M.; Feart, C.; Verzijden, T.; Samieri, C.; et al. Mediterranean diet and incidence of advanced age-related macular degeneration: The EYE-RISK consortium. Ophthalmology 2019, 126, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, R.; Rosner, B.; Seddon, J.M. Dietary omega-3 fatty acids, other fat intake, genetic susceptibility, and progression to incident geographic atrophy. Ophthalmology 2013, 120, 1020–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seddon, J.M.; Ajani, U.A.; Sperduto, R.D.; Hiller, R.; Blair, N.; Burton, T.C.; Farber, M.D.; Gragoudas, E.S.; Haller, J.; Miller, D.T.; et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA 1994, 272, 1413–1420. [Google Scholar] [CrossRef]
- Souied, E.H.; Aslam, T.; Garcia-Layana, A.; Holz, F.G.; Leys, A.; Silva, R.; Delcourt, C. Omega-3 fatty acids and age-related macular degeneration. Ophthalmic Res. 2015, 55, 62–69. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The age-related eye disease study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Xie, B.; Shen, J.; Yoshida, T.; Yokoi, K.; Hackett, S.F.; Campochiaro, P.A. Oxidative stress promotes ocular neovascularization. J. Cell Physiol. 2009, 219, 544–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorusupudi, A.; Nelson, K.; Bernstein, P.S. The age-related eye disease 2 study: Micronutrients in the treatment of macular degeneration. Adv. Nutr. 2017, 8, 40–53. [Google Scholar] [CrossRef]
- Souied, E.H.; Delcourt, C.; Querques, G.; Bassols, A.; Merle, B.; Zourdani, A.; Smith, T.; Benlian, P.; Nutritional AMD Treatment 2 Study Group. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: The nutritional AMD treatment 2 STUDY. Ophthalmology 2013, 120, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Aslam, T.; Delcourt, C.; Holz, F.; Garcia-Layana, A.; Leys, A.; Silva, R.M.; Souied, E. European survey on the opinion and use of micronutrition in age-related macular degeneration: 10 Years on from the Age-Related Eye Disease Study. Clin. Ophthalmol. 2014, 8, 2045–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.R.; Lawrenson, J.G. A review of the evidence for dietary interventions in preventing or slowing the progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2014, 34, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Lawrenson, J.G.; Evans, J.R. Advice about diet and smoking for people with or at risk of age-related macular degeneration: A cross-sectional survey of eye care professionals in the UK. BMC Public Health 2013, 13, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.Y.; Butt, T.; Chew, E.; Agron, E.; Clemons, T.E.; Egan, C.A.; Lee, C.S.; Tufail, A.; UK EMR AMD Research Group. Cost-effectiveness of age-related macular degeneration study supplements in the UK: Combined trial and real-world outcomes data. Br. J. Ophthalmol. 2018, 102, 465–472. [Google Scholar] [CrossRef]
- Seddon, J.M.; Cote, J.; Davis, N.; Rosner, B. Progression of age-related macular degeneration: Association with body mass index, waist circumference, and waist-hip ratio. Arch. Ophthalmol. 2003, 121, 785–792. [Google Scholar] [CrossRef] [Green Version]
- Seddon, J.M.; Reynolds, R.; Maller, J.; Fagerness, J.A.; Daly, M.J.; Rosner, B. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2044–2053. [Google Scholar] [CrossRef]
- Seddon, J.M.; Reynolds, R.; Yu, Y.; Daly, M.J.; Rosner, B. Risk models for progression to advanced age-related macular degeneration using demographic, environmental, genetic, and ocular factors. Ophthalmology 2011, 118, 2203–2211. [Google Scholar] [CrossRef] [Green Version]
- Seddon, J.M.; Reynolds, R.; Yu, Y.; Rosner, B. Validation of a prediction algorithm for progression to advanced macular degeneration subtypes. JAMA Ophthalmol. 2013, 131, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Buitendijk, G.H.S.; Rochtchina, E.; Myers, C.; van Duijn, C.M.; Lee, K.E.; Klein, B.E.K.; Meuer, S.M.; de Jong, P.; Holliday, E.G.; Tan, A.G.; et al. Prediction of age-related macular degeneration in the general population: The three continent AMD consortium. Ophthalmology 2013, 120, 2644–2655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, C.J.; Mitchell, P.; Klein, R.; Klein, B.E.; Chang, M.L.; Gensler, G.; Taylor, A. A risk score for the prediction of advanced age-related macular degeneration: Development and validation in 2 prospective cohorts. Ophthalmology 2014, 121, 1421–1427. [Google Scholar] [CrossRef] [Green Version]
- Seddon, J.M.; Silver, R.E.; Kwong, M.; Rosner, B. Risk prediction for progression of macular degeneration: 10 Common and rare genetic variants, demographic, environmental, and macular covariates. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2192–2202. [Google Scholar] [CrossRef]
- Seddon, J.M.; Rosner, B. Validated prediction models for macular degeneration progression and predictors of visual acuity loss identify high-risk individuals. Am. J. Ophthalmol. 2019, 198, 223–261. [Google Scholar] [CrossRef]
- Ajana, S.; Cougnard-Gregoire, A.; Colijn, J.M.; Merle, B.M.J.; Verzijden, T.; de Jong, P.; Hofman, A.; Vingerling, J.R.; Hejblum, B.P.; Korobelnik, J.F.; et al. Predicting progression to advanced age-related macular degeneration from clinical, genetic, and lifestyle factors using machine learning. Ophthalmology 2021, 128, 587–597. [Google Scholar] [CrossRef]
- Delcourt, C.; Souied, E.; Sanchez, A.; Bandello, F.; Group, S.S. Development and validation of a risk score for age-related macular degeneration: The STARS questionnaire. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6399–6407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minnella, A.M.; Piccardi, M.; Placidi, G.; Garcia-Layana, A.; Delcourt, C.; Valentini, P.; Falsini, B. Macular function in early and intermediate age-related macular degeneration: Correlation with the simplified thea risk assessment scale (STARS). Transl. Vis. Sci. Technol. 2020, 9, 28. [Google Scholar] [CrossRef]
- Engelman, D.; Fuller, L.C.; Steer, A.C.; International Alliance for the Control of Scabies Delphi Panel. Consensus criteria for the diagnosis of scabies: A Delphi study of international experts. PLoS Negl. Trop. Dis. 2018, 12, e0006549. [Google Scholar] [CrossRef]
- Dalkey, N.; Helmer, O. An experimental application of the Delphi method to the use of experts. Manag. Sci. 1963, 9, 458–467. [Google Scholar] [CrossRef]
- Dalkey, N. The Delphi Method: An Experimental Study of Group Opinion; Rand Corp: Santa Monica, CA, USA, 1969. [Google Scholar]
- Bennett, C.; Vakil, N.; Bergman, J.; Harrison, R.; Odze, R.; Vieth, M.; Sanders, S.; Gay, L.; Pech, O.; Longcroft-Wheaton, G.; et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012, 143, 336–346. [Google Scholar] [CrossRef] [Green Version]
- Meshkat, B.C.S.; Gethin, G.; Ryan, K.; Wiley, M.; Brick, A.; Clarke, E.; Mulligan, E. Using an e-Delphi technique in achieving consensus across disciplines for developing best practice in day surgery in Ireland. J. Hosp. Adm. 2014, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.K.; Black, N.A.; Lamping, D.L.; McKee, C.M.; Sanderson, C.F.; Askham, J.; Marteau, T. Consensus development methods, and their use in clinical guideline development. Health Technol. Assess 1998, 2, 1–88. [Google Scholar] [CrossRef] [Green Version]
- Powell, C. The Delphi technique: Myths and realities. J. Adv. Nurs. 2003, 41, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vakil, N.; Van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R.; Global Consensus, G. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920. [Google Scholar] [CrossRef] [PubMed]
- Fitch, K.; Bernstein, S.J.; Aguilar, D.M.; Burnand, B.; LaCalle, J.R.; Lazaro, P.; van het Loo, M.; McDonnell, J.; Vader, J.; Kahan, J.P. The RAND/UCLA Appropriateness Method User’s Manual; RAND: Santa Monica, CA, USA, 2001. [Google Scholar]
- Androudi, S.; Dastiridou, A.; Pharmakakis, N.; Stefaniotou, M.; Kalogeropoulos, C.; Symeonidis, C.; Charonis, A.; Tsilimbaris, M. Guidelines for the management of wet age-related macular degeneration: Recommendations from a panel of greek experts. Adv. Ther. 2016, 33, 715–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Mones, J.; Richard, G.; Bandello, F.; et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 2014, 98, 1144–1167. [Google Scholar] [CrossRef]
- Pinazo-Duran, M.D.; Gomez-Ulla, F.; Arias, L.; Araiz, J.; Casaroli-Marano, R.; Gallego-Pinazo, R.; Garcia-Medina, J.J.; Lopez-Galvez, M.I.; Manzanas, L.; Salas, A.; et al. Do nutritional supplements have a role in age macular degeneration prevention? J. Ophthalmol. 2014, 2014, 901686. [Google Scholar] [CrossRef] [Green Version]
- Battaglia Parodi, M.; Mollo, M.R.; Romano, F. Micronutrients and benefits of supplementation for reducing the risk of progression of age-related macular degeneration—An update. Eur. Ophthal. Rev. 2018, 12, 39–44. [Google Scholar] [CrossRef]
- Merle, B.M.J.; Rosner, B.; Seddon, J.M. Genetic susceptibility, diet quality, and two-step progression in drusen size. Investig. Ophthalmol. Vis. Sci. 2020, 61, 17. [Google Scholar] [CrossRef]
- Raimundo, M.; Mira, F.; Cachulo, M.D.L.; Barreto, P.; Ribeiro, L.; Farinha, C.; Lains, I.; Nunes, S.; Alves, D.; Figueira, J.; et al. Adherence to a Mediterranean diet, lifestyle and age-related macular degeneration: The Coimbra Eye Study—Report 3. Acta Ophthalmol. 2018, 96, e926–e932. [Google Scholar] [CrossRef] [Green Version]
- McGuinness, M.B.; Le, J.; Mitchell, P.; Gopinath, B.; Cerin, E.; Saksens, N.T.M.; Schick, T.; Hoyng, C.B.; Guymer, R.H.; Finger, R.P. Physical activity and age-related macular degeneration: A systematic literature review and meta-analysis. Am. J. Ophthalmol. 2017, 180, 29–38. [Google Scholar] [CrossRef]
- De Koning-Backus, A.P.M.; Buitendijk, G.H.S.; Kiefte-de Jong, J.C.; Colijn, J.M.; Hofman, A.; Vingerling, J.R.; Haverkort, E.B.; Franco, O.H.; Klaver, C.C.W. Intake of vegetables, fruit, and fish is beneficial for age-related macular degeneration. Am. J. Ophthalmol. 2019, 198, 70–79. [Google Scholar] [CrossRef]
- Chong, E.W.; Kreis, A.J.; Wong, T.Y.; Simpson, J.A.; Guymer, R.H. Dietary omega-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: A systematic review and meta-analysis. Arch. Ophthalmol. 2008, 126, 826–833. [Google Scholar] [CrossRef]
- Seddon, J.M.; Willett, W.C.; Speizer, F.E.; Hankinson, S.E. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA 1996, 276, 1141–1146. [Google Scholar] [CrossRef]
- Velilla, S.; Garcia-Medina, J.J.; Garcia-Layana, A.; Dolz-Marco, R.; Pons-Vazquez, S.; Pinazo-Duran, M.D.; Gomez-Ulla, F.; Arevalo, J.F.; Diaz-Llopis, M.; Gallego-Pinazo, R. Smoking and age-related macular degeneration: Review and update. J Ophthalmol. 2013, 2013, 895147. [Google Scholar] [CrossRef]
- Group, A.R.; Chew, E.Y.; Clemons, T.; SanGiovanni, J.P.; Danis, R.; Domalpally, A.; McBee, W.; Sperduto, R.; Ferris, F.L. The age-related eye disease study 2 (AREDS2): Study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012, 119, 2282–2289. [Google Scholar] [CrossRef] [Green Version]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch. Ophthalmol. 2001, 119, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Richer, S.; Stiles, W.; Statkute, L.; Pulido, J.; Frankowski, J.; Rudy, D.; Pei, K.; Tsipursky, M.; Nyland, J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: The Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004, 75, 216–230. [Google Scholar] [CrossRef]
- Lawrenson, J.G.; Evans, J.R.; Downie, L.E. A critical appraisal of national and international clinical practice guidelines reporting nutritional recommendations for age-related macular degeneration: Are recommendations evidence-based? Nutrients 2019, 11, 823. [Google Scholar] [CrossRef] [Green Version]
- Age-Related Macular Degeneration. Available online: https://www.nice.org.uk/guidance/ng82/chapter/recommendations (accessed on 22 April 2021).
- American Academy of Ophthalmology. Preferred Practice Pattern® Guidelines. Available online: https://www.aao.org/about-preferred-practice-patterns (accessed on 22 April 2021).
- Barrios, M.; Villarroya, A.; Borrego, Á.; Ollé, C. Response rates and data quality in web and mail surveys administered to Ph.D. holders. Soc. Sci. Comput. Rev. 2011, 29, 208–220. [Google Scholar] [CrossRef]
| General Recommendations | IPR | IPRAS | Median | Consensus | |
|---|---|---|---|---|---|
| 1 | Intravitreal injections are the first-choice treatment for patients with wet AMD to stop progression of the disease | 0 | 15.0 | 9.0 | Positive agreement |
| 2 | Patients at high risk of AMD should receive nutritional supplements to help reduce the risk of progression at an early phase after diagnosis of AMD | 2.2 | 10.9 | 9.0 | Positive agreement |
| Evaluation tools | |||||
| 3 | Early detection with simple tools is desirable to detect patients at risk of AMD and treat promptly if needed | 0 | 15.0 | 9.0 | Positive agreement |
| 4 | The STARS® questionnaire is a valid tool to assess risk of AMD in the general population | 1 | 9.4 | 8.0 | Positive agreement |
| 5 | Stratification according to risk of AMD is useful in order to plan lifestyle interventions, give dietary advice and plan follow-up using STARS® and the AREDS category score | 1 | 13.1 | 8.0 | Positive agreement |
| 6 | Ophthalmologists should use the STARS® and AREDS classifications in daily practice to evaluate the risk of AMD and to define the best prevention strategy and follow-up for patients | 2 | 11.3 | 8.0 | Positive agreement |
| General lifestyle advice | |||||
| 7 | All subjects at risk of AMD should be advised to stop smoking, adopt a Mediterranean diet, and carry out regular physical activity | 0 | 15.0 | 9.0 | Positive agreement |
| 8 | Increased intake of vegetables, fruit and fish should be actively encouraged in the aging population as <4% of individuals ≥ 55 years of age achieve adequate intake of these food groups | 1 | 13.1 | 9.0 | Positive agreement |
| 9 | If patients are unable or unwilling to follow a Mediterranean diet, nutritional supplements should be recommended in subjects at high risk of AMD | 2 | 11.3 | 9.0 | Positive agreement |
| AREDS-based supplementation | |||||
| 10 | An AREDS-based formulation significantly reduces the risk of developing advanced AMD in the long-term | 2 | 11.3 | 8.0 | Positive agreement |
| 11 | An AREDS-based formulation decreases the overall risk of moderate vision loss in the long term | 2 | 11.3 | 8.0 | Positive agreement |
| 12A | An AREDS-based formulation has no significant benefit on the progression of dry AMD or development of geographic atrophy in the long term | 4.5 | 0.9 | 5.0 | No agreement |
| 12B | An AREDS-based formulation may have benefit on the progression of dry AMD or development of geographic atrophy in the long term | 3 | 5.6 | 7.0 | Positive agreement |
| 13 | The best-validated supplementation therapy for patients suffering from AMD with geographic atrophy without central involvement of the fovea is an AREDS-based formulation | 3.5 | 4.7 | 7.0 | Positive agreement |
| 14 | Initiating supplementation with an AREDS-based formulation in patients at high risk of AMD is more cost effective than no use of supplements and should be advocated | 2 | 11.3 | 8.0 | Positive agreement |
| Moderate Risk Subjects (STARS® 10–19) | IPR | IPRAS | Median | Consensus | |
|---|---|---|---|---|---|
| 15 | Moderate risk subjects according to STARS® (STARS® score 10–19) and with AREDS category 2 and 55–70 years of age should be asked to carry out self-monitoring (e.g., with Amsler grid) | 2.5 | 10.3 | 7.5 | Positive agreement |
| 16 | Moderate risk subjects according to STARS® (STARS® score 10–19) and with AREDS category 2 and 55–70 years should have follow-up every 2 to 3 years | 3 | 3.8 | 6.5 | Uncertain relevance |
| 17 | Moderate risk subjects according to STARS® (STARS® score 10–19) and with AREDS category 2 and age > 70 years should be asked to carry out self-monitoring (e.g., with Amsler grid). | 2 | 11.3 | 8.0 | Positive agreement |
| 18 | Moderate risk subjects according to STARS® (STARS® score 10–19) and with AREDS category 2 and age > 70 years should be recommended specific nutritional supplements for prevention of AMD | 2.5 | 10.3 | 8.0 | Positive agreement |
| 19 | Moderate risk subjects according to STARS® (STARS® score 10–19) with AREDS category 2 and age > 70 years should have annual follow-up | 2 | 11.3 | 9.0 | Positive agreement |
| High risk subjects (STARS® ≥ 20) | |||||
| 20 | High risk subjects according to STARS® (STARS® ≥ 20), with AREDS category 1 and 55–70 years of age should be asked to carry out self-monitoring (e.g., with Amsler grid) | 2.5 | 10.3 | 8.0 | Positive agreement |
| 21 | High risk subjects according to STARS® (STARS® ≥ 20), with AREDS category 1 and age > 70 years should be asked to carry out self-monitoring | 3 | 9.4 | 8.0 | Positive agreement |
| 22 | High risk subjects according to STARS® (STARS® ≥ 20), with AREDS category 1 and age > 70 years should be recommended specific nutritional supplements for prevention of AMD | 3 | 9.4 | 8.0 | Positive agreement |
| 23 | High risk subjects according to STARS® (STARS® ≥ 20), with AREDS category 2, aged 55 years or more, should be asked to carry out self-monitoring (e.g., with Amsler grid) | 1.5 | 12.2 | 8.0 | Positive agreement |
| 24 | High risk subjects according to STARS® (STARS® ≥ 20), with AREDS category 2, aged 55 years or more, should be recommended specific nutritional supplements for prevention of AMD | 2 | 11.3 | 8.0 | Positive agreement |
| 25 | High risk subjects according to STARS® (STARS® ≥ 20) with AREDS category 2, independently of age, should have follow up every 6 months | 4.5 | 4.7 | 7.0 | Positive agreement |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Layana, A.; Garhöfer, G.; Aslam, T.M.; Silva, R.; Delcourt, C.; Klaver, C.C.W.; Seddon, J.M.; Minnella, A.M. Exploring Consensus on Preventive Measures and Identification of Patients at Risk of Age-Related Macular Degeneration Using the Delphi Process. J. Clin. Med. 2021, 10, 5432. https://doi.org/10.3390/jcm10225432
García-Layana A, Garhöfer G, Aslam TM, Silva R, Delcourt C, Klaver CCW, Seddon JM, Minnella AM. Exploring Consensus on Preventive Measures and Identification of Patients at Risk of Age-Related Macular Degeneration Using the Delphi Process. Journal of Clinical Medicine. 2021; 10(22):5432. https://doi.org/10.3390/jcm10225432
Chicago/Turabian StyleGarcía-Layana, Alfredo, Gerhard Garhöfer, Tariq M. Aslam, Rufino Silva, Cécile Delcourt, Caroline C. W. Klaver, Johanna M. Seddon, and Angelo M. Minnella. 2021. "Exploring Consensus on Preventive Measures and Identification of Patients at Risk of Age-Related Macular Degeneration Using the Delphi Process" Journal of Clinical Medicine 10, no. 22: 5432. https://doi.org/10.3390/jcm10225432
APA StyleGarcía-Layana, A., Garhöfer, G., Aslam, T. M., Silva, R., Delcourt, C., Klaver, C. C. W., Seddon, J. M., & Minnella, A. M. (2021). Exploring Consensus on Preventive Measures and Identification of Patients at Risk of Age-Related Macular Degeneration Using the Delphi Process. Journal of Clinical Medicine, 10(22), 5432. https://doi.org/10.3390/jcm10225432






