Abstract
The present pilot study examines subjective reported symptoms of attention-deficit/hyperactivity (AD/H) in adults with Fabry disease (FD) in comparison with existing normative control data. Existing data from 69 adults with FD via the Achenbach System of Empirically Based Assessment Adult Self-Report questionnaire were analyzed. The results demonstrated a higher prevalence of AD/H symptoms in adults with FD than in the general United States population, with a roughly equal endorsement of Inattention/Attention Deficit symptoms (AD), Hyperactivity-Impulsivity (H-I) symptoms, and Combined Inattention/hyperactivity-impulsivity (C) symptoms. No gender differences were observed. While all subjects endorsing H-I symptoms fell into the symptomatic range on the AD/H scale, only two-thirds of subjects endorsing AD did so. This suggests that attention difficulties with FD are not solely explained by ADHD. Adults with FD who endorsed the AD, H-I, and C symptoms were also more likely to report mean adaptive functioning difficulties. These findings support the growing literature regarding attention difficulties in adults with FD, as well as suggesting a previously unrecognized risk of AD/H symptoms. Future research involving the objective assessment of ADHD in adults with FD is recommended. When serving adults with FD clinically, healthcare professionals should address multiple areas of care, including physical, psychological, and cognitive arenas.
1. Introduction
Fabry disease (FD) is an X-linked lysosomal storage disorder (LSD) caused by mutations in the GLA gene, leading to a deficiency of α-galactosidase A (α-gal A; EC 3.2.1.22) and resulting in the storage of globotriaosylceramide (GL3) and related lipids in the lysosome. Its incidence has historically been estimated at 1:40,000 male live births; however recent data suggests as high as 1:3000 [1], with a range of 1250–117,000 worldwide [2]. The symptoms and complications include acroparesthesia, fatigue, anhidrosis, angiokeratomas, gastrointestinal symptoms, kidney failure, cardiovascular problems, and stroke [3,4,5,6,7]. The standard of care treatment is enzyme replacement therapy (ERT) or chaperone therapy (in individuals with amenable GLA mutations).
Historically, research has focused on somatic manifestations of FD, with less attention paid to neuropsychological manifestations. However, recent research suggests difficulties with cognitive functioning, particularly in the realm of attention and concentration, with implications for central nervous system (CNS) functioning in patients with FD.
The initial neuropsychological screening studies of patients with FD reported contradictory results due to varying testing methods and small sample sizes. One initial study found patients with FD performed marginally better on tasks of attention than normal controls and slightly worse on tasks measuring language skills, with unimpaired performances in other cognitive domains [8], while another found patients with FD performed mildly worse on tasks of attention than normal controls [9]. Although the patients initially appeared to perform worse on executive functioning tasks, this difference disappeared once corrected for the effects of depression and remained absent in a subset of patients eight years later [10]. The subsequent early research found that patients with FD performed worse on some tests of attention (especially those involving information processing speed) [11,12,13], as well as some measures of executive functioning [11,13].
The first study to examine neurocognitive functioning in FD using comprehensive and well-validated neuropsychological measures rather than screening tools found that males with FD demonstrated a slower information processing speed and reduced performance on measures of executive functioning compared to both females with FD and 15 age-matched normal controls [14]. However, several confounds were present. None of the females with FD had experienced a stroke or transient ischemic attacks compared to 33% of the males. Males with FD were also more likely to report symptoms of anxiety and depression, which is known to have delirious effects on cognition, including attention, memory, and executive functioning [15]. Taken together with a low sample size and correlational analyses suggesting a link between the cognition and clinical measures of disease severity, these confounds compromised the generalizability.
A more recent study found 29.3% of Danish patients with FD to have cognitive difficulties, with attention, psychomotor speed, and executive functioning once again being the most frequently impaired [16]. Neither depression, disease severity, nor gender predicted objective cognitive impairment; however, depression was associated with the subjective perception of cognition. The subjective perception of cognition was lower than the actual cognitive performance among subjects.
In comparison, subjective perceptions of cognitive impairment among Dutch subjects with FD were found to be much greater (64%) than the objective evidence of impairment (16%) [17]. Objective impairment was found primarily in males, especially those with classical FD. Follow-up testing one year later, however, demonstrated a worsening objective cognitive impairment in only 5.3% of subjects and was found more often among women (three women and one man) [18]. Subjective impairment was prevalent in both genders and correlated with depression [16,17].
Given the increasing evidence of the role of FD in aspects of attention, anecdotal patient reports regarding the use of medication for Attention Deficit Hyperactivity Disorder (ADHD) should perhaps not come as a surprise. Previously referred to as Attention Deficit Disorder (ADD) in the Diagnostic and Statistical Manual of Mental Disorders 3rd Edition (DSM III) [19], one of the core symptoms is a deficiency in attention. The updated label of ADHD in DSM IV and DSM-5 is an umbrella term for a wide range of symptoms and consists of three main types: Inattentive/Attention Deficit (AD), Hyperactive-Impulsive (H-I), and Combination (C) types [20,21]. While attention-deficit/hyperactivity (AD/H) symptoms in patients with FD have been shown to be associated with poorer adaptive functioning (AF) [22], no further exploration of AD/H symptoms in FD has been done. A pilot study specifically documenting and exploring patient reports of attention deficits will be beneficial as a prequel to more in-depth studies of attention deficits in patients with FD.
The present pilot study examines the self-reported symptoms of attention deficits/hyperactivity in adults with FD in comparison with the existing normative control data, as well as potential differences in the frequency between symptoms of attention deficits and symptoms of hyperactivity. In addition, we explored the possible association between attention-deficit/hyperactivity symptoms and poorer adaptive functioning in patients with FD.
2. Materials and Methods
Data was derived from a subset of data in existence at the Emory Lysosomal Storage Disease Center. Specifically, data concerning Attention Deficit/Hyperactivity, Attention, Inattention, Hyperactivity-impulsivity, Somatic Symptoms, Depression, Anxiety, and Mean Adaptive Functioning were utilized from the Achenbach System of Empirically Based Assessment (ASEBA) Adult Self-Report (ASR) questionnaires completed by patients with FD between January 2005 and July 2013. Approval from the Institutional Review Board was granted through Emory University (IRB00068700).
The ASEBA ASR is a reliable, validated measure of social-adaptive and psychological functioning in adults aged 18–59 and the OASR for ages 60–90+ [23]. Norms represent the mix of ethnicities, socioeconomic status, urban–rural–suburban residency, and geography within the US. Raw scores are converted to T-scores to permit comparisons with the general population. Scale scores are normed by gender and age and categorized as normal (<93rd percentile), borderline-clinical (93rd–97th percentiles), or clinical (>97th percentile). The ASEBA is used with a wide variety of medical conditions, including cystic fibrosis, Fabry, Morquio, Turner, Williams, Angelman, and Prader-Willi syndromes [22,23,24,25].
Data Analysis
ASEBA ASR raw data was entered into assessment data manager (ADM, version 9.0) ASEBA scoring software (https://adm-assessment-data-manager.software.informer.com/9.0/, accessed on 1 June 2021), which produces detailed profiles on multiple aspects of psychological functioning. For this study, data from the DSM-Oriented Scale for AD/H, as well as the Attention Problem Syndrome scale, Depression scale, Somatic Complaints scale, and Mean Adaptive Functioning scale, were utilized. Subjects with T-scores in the borderline-clinical and clinical ranges were considered to have symptoms for the purposes of this study.
All data analysis was done using SAS 9.4 (SAS Institute, Cary, NC, USA). Demographic participant characteristics were summarized using frequencies and proportions. Chi-square and Fisher’s exact tests were used to assess the associations between mean adaptive functioning and demographic variables of interest. Similarly, chi-square tests were used to assess the association between depressive symptoms and gender, AD/H symptoms, H-I symptoms, and AD symptoms. The prevalence of AD/H in our study sample was compared to the most recent estimated prevalence of AD/H among the US adult population [26] using Fisher’s exact test. All statistical tests were assessed using an alpha = 0.05.
3. Results
Existing data from 69 adults with FD who completed the ASEBA ASR questionnaire was examined. The demographic information is presented in Table 1. The ages ranged from 18 to 61 years.

Table 1.
Demographic characteristics of the subjects.
Of the 69 subjects who completed the ASEBA ASR, twenty (29%) endorsed symptoms within the borderline-clinical-to-clinical range on the AD/H problems scale (Figure 1). This represents a significantly higher prevalence of AD/H symptoms in our population of adults with FD than in the general population (p < 0.001), using the most recently estimated prevalence (4.4%) of adult ADHD in the United States [26].
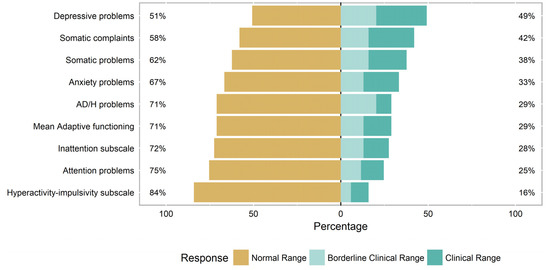
Figure 1.
Prevalence of ASEBA symptoms in adults with Fabry disease.
Among the twenty subjects endorsing symptoms within the borderline-clinical-to-clinical range on the AD/H scale, the source of those scores was almost equally balanced between the symptomatic endorsement of AD items, H-I items, and combined AD/H items, with a final three subjects whose endorsement of items was evenly split such that they fell within the normal ranges on the individual subscales while still falling within the symptomatic range on the overall combined AD/H scale (Table 2). All subjects endorsing the H-I symptoms within borderline-clinical-to-clinical range also scored in the borderline-clinical-to-clinical range on the AD/H scale; however, only 12/19 (63.2%) subjects endorsing AD symptoms also scored in the borderline-clinical-to-clinical range on the AD/H scale.

Table 2.
Subscale breakdown among adults with FD endorsing AD/H symptoms (n = 20).
Almost half of the adults with FD (49%) were also noted to self-report depressive symptoms in the borderline-clinical-to-clinical range on the ASEBA ASR, with no significant differences between the male and female subjects (p = 0.537). A third of the adults with FD (33%) self-reported symptoms of anxiety, with no significant differences between the male and female subjects (p = 0.0870). Almost a third of adults with FD (29%) self-reported difficulties in adaptive functioning, with no significant differences between the male and female subjects (p = 0.060). Over a third of adults with FD (38%) self-reported somatic symptoms in the borderline-clinical-to-clinical range, with no significant differences between the male and female subjects (p = 0.2468).
Adults who scored in the borderline-clinical-to-clinical range on the AD/H scale, AD subscale, and H-I subscale were significantly more likely to self-report both depressive symptoms and somatic problems (Table 3). Adults scoring in the borderline-clinical-to-clinical range on the AD/H scale and H-I scale were significantly more likely to self-report anxiety symptoms as well (Table 3). There were no differences between males and females in any of these categories.

Table 3.
Association between the comorbid symptoms and symptoms of AD/H, AD, and H-I in adults with FD when using the ASEBA Adult Self-Report.
There were no significant demographic differences between those with and without AF deficits; however, the adults with FD who self-reported AD problems, AD/H symptoms, depressive symptoms, and anxiety were also significantly more likely to report AF difficulties (Table 4).

Table 4.
Association between psychological symptoms and adaptive functioning in adults with FD when using the ASEBA Adult Self-Report.
4. Discussion
The present study is a pilot exploration of self-reported attention deficit symptoms in adults with Fabry disease. The results demonstrate a higher prevalence of AD/H symptoms in adults with FD than in the general United States population, with a roughly equal numbers of adults with FD endorsing AD symptoms, H-I symptoms, and Combined symptoms. While ADHD is more common in men than women in the general population [26], and some studies have found greater evidence of cognitive impairment in men with FD than women [14,17], our study found no gender differences in the rate of AD/H, H-I, or AD symptoms amongst adults with FD.
While all subjects endorsing H-I symptoms fell within the symptomatic range on the AD/H scale, only two-thirds of subjects endorsing AD symptoms did so. The remaining third endorsing AD without AD/H symptoms suggests that attention difficulties within FD are not solely linked to AD/H and lends credence to prior research outlining cognitive difficulties in attention in FD [9,11,13,16]. However, the reverse is also true; the endorsement of an equally high rate of H-I symptoms among our FD population suggests a previously unrecognized prevalence of such symptoms among adults with FD separate from attention deficits.
Of note, almost half of adults with FD in the present study endorsed symptoms of depression (49%), with no significant differences between men and women. This replicates the previously reported high rates of depression among adults with FD, with prevalence estimates ranging from 15% to 62% [9,13,25,27,28,29]. The present study likewise supported research demonstrating that depression in FD does not follow gender norms, with males reporting equal or greater rates than females [14,27]. While the most common factor associated with depression in FD is chronic pain [13,27,30], economic status, relationship status, specific coping styles, and somatic symptoms such as anhidrosis and acroparaesthesia have also been associated with depression and a lower QOL [27,30,31].
While depression can have deleterious effects on attention [15], its interaction with hyperactivity-impulsivity goes in the opposite direction; it is more likely to be a consequence of ADHD than a cause. Thus, while adults with FD who reported symptoms of AD/H, AD and H-I were more likely to also report symptoms of depression, this is consistent with previous research demonstrating that people with ADHD are at risk for depression and anxiety as a result of living with ADHD [26,32,33,34].
Finally, the present study found adults with FD-endorsing AD symptoms, AD/H symptoms, depressive symptoms, and anxiety were also significantly more likely to endorse adaptive functioning (AF) difficulties. An indication of the effectiveness with which individuals cope with the demands of everyday tasks and responsibilities as parents, students, employees, etc., AF is measured via evaluations such as the ASEBA focused on individuals’ relationships, jobs, education, substance use, psychological issues, and coping skills. These findings corroborate earlier research in which FD patients had a higher rate of mean AF deficits compared to population norms, with poorer AF associated with greater rates of AD/H, depression, and anxiety [22].
All of the above findings make clear the need to pay attention to the psychological symptoms associated with FD, including the possibility of symptoms of AD/H, and expand our standard of care to include mental health treatments, if necessary. Of note, of the 20 people who self-reported AD/H symptoms in our sample, four had been prescribed medication typically used for ADHD at some point in their life, though only one had undergone a clinical diagnosis for their symptoms. As symptoms of ADHD are more heterogeneous and subtle in adults than children [35,36], with only 25% of adults with ADHD receiving treatment [26], it is possible that ADHD symptoms in adults with FD are being overlooked amidst the urgency of the other symptoms of FD.
The limitations of this study include the use of self-reported symptoms at a single point in time; however, adults with ADHD have been found to be quite reliable in identifying their own symptoms via self-reported measures [35], and an earlier study found that adults with FD were, if anything, more likely to underreport than overreport neurocognitive complaints [16]. Another limitation is the comparison between self-reported symptoms (FD population) and diagnosis (US population). To our knowledge, there is no nationally representative database of self-reported symptoms of AD/H, as compared to the frequency of diagnosis. Previous research has likewise used self-reported ADHD symptoms rather than diagnoses and presented evidence for the use of such as an effective tool [37]. Finally, this study included data primarily from Caucasian adults with FD and may not be generalizable to adults with FD of other ethnicities.
The implications of this study include the need for greater attention to cognitive and psychological health in people with FD, particularly in the areas of attention, AD/H-like symptoms, depression, anxiety, and adaptive functioning. Genetic counselors and other healthcare providers should address such issues in their annual clinic appointments and make referrals as needed to maximize overall treatment for patients with FD.
The recommendations for future research include a more objective assessment of AD/H symptoms in patients with FD, as well as further in-depth neurocognitive studying of attention/concentration in FD. Such research should utilize objective neuropsychological tests with the existing normative data with the general population.
5. Conclusions
In conclusion, the present study suggests that adults with FD are at a higher risk than the general population for attention deficits, as well as symptoms of ADHD, with equal rates among men and women. When serving adults with FD clinically, genetic counselors and other healthcare professionals should address multiple areas of care, including the physical, psychological, and cognitive issues that may accompany the disease.
Author Contributions
Conceptualization, N.A; methodology, N.A., A.C. and D.L.; software, E.W.H.; formal analysis, E.W.H.; writing—original draft preparation, N.A.; writing review and editing, N.A., A.C., E.W.H. and D.L.; and funding acquisition, N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Pfizer Inc., grant number WI194299.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Emory University (IRB00068700).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data availability Statement
The data for this study are available by contacting the corresponding author.
Acknowledgments
The authors wish to thank all patients with Fabry who participate so generously in this research regarding Fabry disease and their lived experiences.
Conflicts of Interest
Nadia Ali, Ph.D. received research support from Sanofi Genzyme, Shire Takeda, BioMarin, Amicus, and Pfizer, as well as lecturers’ honoraria from Sanofi Genzyme, BioMarin, Amicus, and Vitaflo. These activities were monitored and in compliance with the conflicts of interest policies at Emory University. Amanda Caceres, M.MSc, CGC received research support from Genzyme and Pfizer. Eric W Hall, Ph.D. has no conflicts of interest to report. Dawn Laney, M.S., CGC consults for Genzyme, Amicus, and Shire and is a study coordinator in clinical trials sponsored by Genzyme, Amicus, and Protalix. She is a co-founder of ThinkGenetic, Inc. She also received research funding from Alexion, Amicus, Genzyme, Pfizer, Retrophin, Shire, and Synageva. These activities are monitored and are in compliance with the conflicts of interest policies at Emory University. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hopkins, P.V.; Campbell, C.; Klug, T.; Rogers, S.; Raburn-Miller, J.; Kiesling, J. Lysosomal Storage Disorder Screening Implementation: Findings from the First Six Months of Full Population Pilot Testing in Missouri. J. Pediatr. 2015, 166, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Laney, D.A.; Peck, D.S.; Atherton, A.M.; Manwaring, L.; Christensen, K.M.; Shankar, S.P.; Grange, D.K.; Wilcox, W.R.; Hopkin, R.J. Fabry disease in infancy and early childhood: A systematic literature review. Genet. Med. 2015, 17, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Desnick, R.J.; Brady, R.; Barranger, J.; Collins, A.J.; Germain, D.P.; Goldman, M.; Grabowski, G.; Packman, S.; Wilcox, W.R. Fabry Disease, an Under-Recognized Multisystemic Disorder: Expert Recommendations for Diagnosis, Management, and Enzyme Replacement Therapy. Ann. Intern. Med. 2003, 138, 338–346. [Google Scholar] [CrossRef]
- MacDermot, K.D.; Holmes, A.; Miners, A.H. Anderson-Fabry disease: Clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J. Med Genet. 2001, 38, 769–775. [Google Scholar] [CrossRef]
- D’Arco, F.; Hanagandi, P.; Ganau, M.; Krishnan, P.; Taranath, A. Neuroimaging Findings in Lysosomal Disorders. Top. Magn. Reson. Imaging 2018, 27, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Germain, D.P.; Desnick, R.J.; Politei, J.; Mauer, M.; Burlina, A.; Eng, C.; Hopkin, R.J.; Laney, D.; Linhart, A.; et al. Fabry disease revisited: Management and treatment recommendations for adult patients. Mol. Genet. Metab. 2018, 123, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Banerjee, A.; Gandhi, A.B.; Kaleem, I.; Alexander, J.; Hisbulla, M.; Kannichamy, V.; Subas, S.V.; Hamid, P. Stroke and Fabry Disease: A Review of Literature. Cureus 2020, 12, 312083. [Google Scholar]
- Low, M.; Nicholls, K.; Tubridy, N.; Hand, P.; Velakoulis, D.; Kiers, L.; Mitchell, P.; Becker, G. Neurology of Fabry disease. Intern. Med. J. 2007, 37, 436–447. [Google Scholar] [CrossRef]
- Schermuly, I.; Müller, M.J.; Müller, K.-M.; Albrecht, J.; Keller, I.; Yakushev, I.; Beck, M.; Fellgiebel, A. Neuropsychiatric symptoms and brain structural alterations in Fabry disease. Eur. J. Neurol. 2011, 18, 347–353. [Google Scholar] [CrossRef]
- Lelieveld, I.M.; Böttcher, A.; Hennermann, J.B.; Beck, M.; Fellgiebel, A. Eight-Year Follow-Up of Neuropsychiatric Symptoms and Brain Structural Changes in Fabry Disease. PLoS ONE 2015, 10, e0137603. [Google Scholar] [CrossRef]
- Segal, P.; Kohn, Y.; Pollak, Y.; Altarescu, G.; Galili-Weisstub, E.; Raas-Rothschild, A. Psychiatric and cognitive profile in Anderson-Fabry patients: A preliminary study. J. Inherit. Metab. Dis. 2010, 33, 429–436. [Google Scholar] [CrossRef]
- Elstein, D.; Doniger, G.M.; Altarescu, G. Cognitive testing in Fabry disease: Pilot using a brief computerized assessment tool. Isr. Med Assoc. J. IMAJ 2012, 14, 624–628. [Google Scholar] [PubMed]
- Bolsover, F.E.; Murphy, E.; Cipolotti, L.; Werring, D.J.; Lachmann, R.H. Cognitive dysfunction and depression in Fabry disease: A systemic review. J. Inherit. Metab. Dis. 2014, 37, 177–187. [Google Scholar] [CrossRef]
- Sigmundsdottir, L.; Tchan, M.C.; Knopman, A.A.; Menzies, G.C.; Batchelor, J.; Sillence, D.O. Cognitive and Psychological Functioning in Fabry Disease. Arch. Clin. Neuropsychol. 2014, 29, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Rock, P.L.; Roiser, J.P.; Riedel, W.J.; Blackwell, A.D. Cognitive impairment in depression: A systematic review and meta-anlysis. Psychol. Med. 2014, 44, 2029–2040. [Google Scholar] [CrossRef] [PubMed]
- Loeb, J.; Feldt-Rasmussen, U.; Madsen, C.V.; Vogel, A. Cognitive Impairments and Subjective Cognitive Complaints in Fabry Disease: A Nationwide Study and Review of the Literature. J. Inherit. Metab. Dis. Rep. 2018, 41, 73–80. [Google Scholar]
- Körver, S.; Geurtsen, G.J.; Hollak, C.E.; van Schaik, I.N.; Longo, M.G.; Lima, M.R.; Langeveld, M. Predictors of objective cognitive complaints in patients with Fabry disease. Sci. Rep. 2019, 9, 188. [Google Scholar] [CrossRef]
- Körver, S.; Geurtsen, G.J.; Hollak, C.E.; van Schaik, I.N.; Longo, M.G.; Lima, M.R.; Langeveld, M. Cognitive functioning and depressive symptoms in Fabry disease: A follow-up study. J. Inherit. Metab. Dis. 2020, 43, 1070–1081. [Google Scholar] [CrossRef]
- Diagnostic and Statistical Manual of Mental Disorders, 3rd ed.; American Psychiatric Association: Washington, DC, USA, 1980.
- Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994.
- Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013.
- Laney, D.A.; Gruskin, D.J.; Fernhoff, P.M.; Cubells, J.F.; Ousley, O.Y.; Hipp, H.; Mehta, A.J. Social-adaptive and psychological functioning of patients affected by Fabry disease. J. Inherit. Metab. Dis. 2010, 33 (Suppl. 3), S73–S81. [Google Scholar] [CrossRef]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA Adult Forms and Profiles; University of Vermont, Research Center for Children, Youth, and Families: Burlington, VT, USA, 2003. [Google Scholar]
- Ali, N.; Cagle, S. Psychological health in adults with Morquio syndrome. J. Inherit. Metab. Dis. Rep. 2015, 20, 87–93. [Google Scholar] [CrossRef][Green Version]
- Ali, N.; Gillespie, S.; Laney, D.A. Treatment of depression in adults with Fabry disease. J. Inherit. Metab. Dis. Rep. 2017, 38, 13–21. [Google Scholar]
- Kessler, R.C.; Adler, L.; Barkley, R.; Biederman, J.; Conners, C.K.; Demler, O.; Faraone, S.V.; Greenhill, L.L.; Howes, M.J.; Secnik, K.; et al. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. Am. J. Psychiatry 2006, 163, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.L.; Lee, P.J.; Hughes, D.; Deegan, P.; Waldek, S.; Lachmann, R. Depression in adults with Fabry disease: A common and under-diagnosed problem. J. Inherit. Metab. Dis. 2007, 30, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Laney, D.A.; Bennett, R.L.; Clarke, V.; Fox, A.; Hopkin, R.J.; Johnson, J.; O’Rourke, E.; Sims, K.; Walter, G. Fabry Disease Practice Guidelines: Recommendations of the National Society of Genetic Counselors. J. Genet. Couns. 2013, 22, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Löhle, M.; Hughes, D.; Milligan, A.; Richfield, L.; Reichmann, H.; Mehta, A.; Schapira, A. Clinical prodromes of neurodegeneration in Anderson-Fabry disease. Neurology 2015, 84, 1454–1464. [Google Scholar] [CrossRef]
- Körver, S.; Geurtsen, G.J.; Hollak, C.E.M.; Van Schaik, I.N.; Longo, M.G.F.; Lima, M.R.; Vedolin, L.; Dijkgraaf, M.G.W.; Langeveld, M. Depressive symptoms in Fabry disease: The importance of coping, subjective health perception and pain. Orphanet J. Rare Dis. 2020, 15, 28. [Google Scholar] [CrossRef]
- Gold, K.; Pastores, G.; Botteman, M.; Yeh, J.; Sweeney, S.; Aliski, W.; Pashos, C. Quality of life of patients with Fabry disease. Qual. Life Res. 2002, 11, 317–327. [Google Scholar] [CrossRef]
- Chronis-Tuscano, A.; Molina, B.S.; Pelham, W.E.; Applegate, B.; Dahlke, A.; Overmyer, M.; Lahey, B.B. Very early predictors of adolescent depression and suicide attempts in children with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 2010, 67, 1044–1051. [Google Scholar] [CrossRef]
- Furczyk, K.; Thome, J. Adult ADHD and suicide. Atten. Def. Hyp. Disord. 2014, 6, 153–158. [Google Scholar] [CrossRef]
- Biederman, J.; Ball, S.W.; Monuteaux, M.C.; Mick, E.; Spencer, T.J.; McCREARY, M.; Cote, M.; Faraone, S. New Insights into the Comorbidity Between ADHD and Major Depression in Adolescent and Young Adult Females. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 426–434. [Google Scholar] [CrossRef]
- De Quiros, G.B.; Kinsbourne, M. Adult AHDH: Analysis of self-ratings on a behavior questionnaire. Ann. N. Y. Acad. Sci. 2001, 931, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.H.; Wolf, L.E.; Wasserstein, J. Adults with ADHD. An overview. Ann. N. Y. Acad. Sci. 2001, 931, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cho, M.J.; Chang, S.M.; Jeon, H.J.; Cho, S.-J.; Kim, B.-S.; Bae, J.N.; Wang, H.-R.; Ahn, J.H.; Hong, J.P. Prevalence, correlates, and comorbidities of adult ADHD symptoms in Korea: Results of the Korean epidemiologic catchment area study. Psychiatry Res. 2011, 186, 378–383. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).