Abstract
Nanofiltration application for the separation of Mg2+-Li+ from salt-lake brines was attempted in the present work. Four different nanofiltration membranes identified in the manuscript as DL, DK, NF-270, and NF-90 were used to treat salt brine with a magnesium to lithium ratio (MLR) of 61, additionally contaminated by the other ions such as Na+, K+, Ca2+, etc. The effect of the dilution factor, operating pressure, circulation rate, and feed pH were assessed to identify the optimal operating conditions for each membrane based on the retention efficiency of each ion. The results showed an insignificant effect of Ca2+ on the retention performance of Mg2+-Li+. Na+ and K+ had a smaller hydration radius and larger diffusion coefficient, which competed with Li+ and altered the separation of Mg2+-Li+. Under the optimal conditions (dilution factor: 40; operating pressure: 1.2 MPa; circulation flow rate: 500 L/h; pH: 7), the retention efficiency of lithium was as low as 5.17%, separation factor (SF) was as low as 0.074, and the MLR in the permeate reduced to 0.088.
1. Introduction
Lithium is a key raw material for energy storage batteries. The proportion of motor vehicles operated with rechargeable batteries is increasing year on year, which drives the demand for lithium and widespread attention on lithium metal extraction [1,2]. The prime source of lithium is ore, which continues to decline due to huge exploitation [3,4]. The exploitation of prime lithium ore resources such as spodumene and lepidolite has been widely documented [5]. Because the ore is a non-sustainable source and due to the drastic decline in the lithium resource reserves, the industry and scientific society efforts are persistent for alternative resources [6]. These persistent effects have led to the development of newer resources, of which salt lakes stand out in recent years [7]. Lithium resources in salt lakes account for nearly 70% of the basic reserves in 2021 [8,9]. China has the third largest lithium reserves in the world, of which 71% is accounted for in the salt lakes. Hence, the lithium resources from salt lakes are expected to be the main source in the near future catering to the increasing local and global demand [10].
The extraction of lithium from salt lakes is marred by two issues. Firstly, the Mg2+/Li+ ratio of Chinese salt lake brine (MLR = 1577) is too high compared to lithium-rich salt lakes elsewhere (MLR = 1.37) [11,12]. The efficient enrichment of lithium resources is hampered by the similar properties of magnesium and lithium [8,13]. Secondly, due to the presence of a large number of other ions (such as Na+, K+, Ca2+, Ba2+, etc.), the separation difficulty is added by the ionic valence as well as high concentration (0.0004-0.112 M). [14]. Therefore, it is necessary to understand the effects of other ions on host ions, so as to develop efficient separation processes.
At present, a variety of methods have been reported for extracting lithium from salt lakes, which include precipitation, adsorption, extraction, and membrane methods [15]. The precipitation method precipitates contaminant ions and enriches lithium by evaporation. It is a simple process being widely adopted in industry, but applicable only for brine with low MLR, and the high cost of the precipitation renders it unfeasible [16,17]. In comparison, the adsorption can handle high MLR brine, but is limited by the low adsorption capacity of the adsorbents and the need of adopting the cyclic adsorption–desorption process [18]. The extraction process can separate lithium using an appropriate solvent, but limited by the difficulties of phase separation and the solvent losses [19]. As an emerging alternative, nanofiltration is a pressure-driven membrane separation process, which offers the advantages of high separation efficiency, being less energy-intensive, being environmentally benign, and easy operation at large scales [20,21]. Based on the Don-nan repulsion and pore screening effect, a high interception efficiency for polyvalent ions and good permeability can be achieved for the effective separation of Mg2+-Li+ [22,23]. Thus, nanofiltration has the potential to serve to be a better separation process to affect the lithium extraction from salt lakes.
Recently, commercial polyamide nanofiltration membranes are widely use for the separation of Mg2+-Li+ from salt lakes, commonly known as NF, DK, and DL [24]. Ji et al. [25] had confirmed that high MLR is not conducive for separation using the DK-2540 membrane, as a significant competition of Na+, K+, and Li+ while Ca2+ is not significant. Sun et al. [26] had compared the separation performance of DL-2540 and DK-2540 membranes and reported similar performance of both membranes. The results showed high separation factor at low MLR, and the separation factor is insignificant while the MLR is higher than 20. Pramanik et al. [27] had compared the Mg2+-Li+ separation performance of NF-90 and NF-270 and reported that NF-90 reduces MLR from 10 to 0.19 (Li+ extraction 23%), while NF-270 from 10 to 2.1 (Li+ extraction 44%).
The present work attempted to assess the Mg2+-Li+ separation performance of four nanofiltration membranes, namely DL, DK, NF-270, and NF-90, due to the understanding of the hydration radius of each ion and the basic properties of the NF membrane (MWCO, MgSO4 retention rate, etc.). The effects of different operating parameters, namely, the dilution, operating pressure (transmembrane pressure), circulation flow rate, and pH, were systematically investigated on the four objective functions, namely, ion retention efficiency, membrane flux, SF, and MLR. The effect of impurity (Na+, K+, Ca2+, etc.) on the retention efficiency of Mg2+-Li+ was also analyzed by the Donnan repulsion effect, pore size sieving effect, and dilution effect.
2. Experimental
2.1. Experimental Materials and Device
The ion composition of the simulated brine resembled the brine of Yiliping Salt Lake, which had a MLR of 61, and the specific ion composition was shown in Table 1. The reagents used in the preparation of the simulated brine were all analytical grade (purity ≥ 99.98%), including lithium chloride (LiCl·H2O), magnesium chloride (MgCl2), magnesium sulfate (MgSO4), potassium chloride (KCl), sodium chloride (NaCl) and calcium chloride (CaCl2). All the aforementioned chemicals were obtained from Sinopharm Chemical Reagent Co., Ltd., (Shanghai, China).

Table 1.
Comparison of ion concentrations of the Yiliping Salt Lake with the simulated solution/wt%.
The triple high-pressure flat membrane test equipment was used for nanofiltration separation experiments. The nanofiltration separation process schematic was shown in Figure 1. The feed solution was pumped to the module by a high-pressure pump, where a small part of them passed through the membrane to the permeate side and most of them returned to the feedstock barrel (retentate). Once the conductivity of the permeate was stable, it would be collected and the membrane flux would be calculated. During the experiment, the temperature of the feedstock barrel was kept constant with a cooling arrangement. The inlet and outlet pressure were controlled by the pressure regulating valve while the feed flow rate was through the control valve.
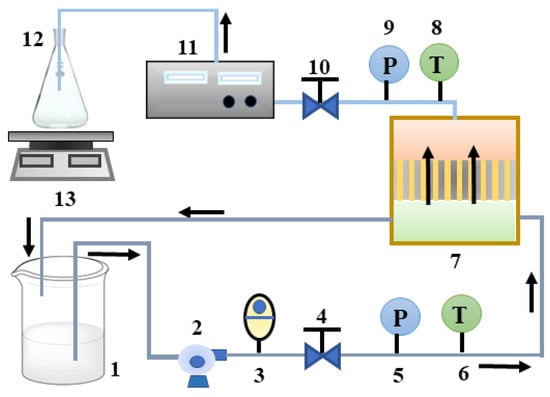
Figure 1.
Experimental flow chart. (1. raw material drum; 2. pressure pump; 3. control valve; 4, 10. pressure valve; 5, 9. pressure gauge 6, 8. thermometer 7. membrane block 11. conductivity meter 12. permeate collection bottle 13. electronic scale).
2.2. Experimental Membrane
The commercial nanofiltration membranes used in the following experiments were supplied by Dow, and their basic properties and operating range were shown in Table 2 [28].

Table 2.
Application scope of commercial nanofiltration membrane with a negative charge.
Four nanofiltration membranes (DL, DK, NF 270, NF 90) with Mg2+-Li+ separation properties were initially screened through the understanding of the hydration radius of each ion and the basic properties of the NF membrane (molecular weight cutoff (MWCO), MgSO4 retention rate, etc.). The effects of different operating parameters, namely, the dilution ratio, solution pH, operating pressure, and circulating flow on the separation of Mg2+-Li+, were assessed for all four membranes chosen in the study. The experiments were conducted within the operating range of commercial nanofiltration membranes (temperature = 25 ± 0.1 °C; pressure = 0–2.5 MPa; feed liquid pH = 3–11).
2.3. Analysis and Characterization Methods
Inductively Coupled Plasma Emission Spectrometer (ICP-OES, 7500CS, Agilent Technologies, Wilmington, DE, USA) was used to measure ion concentrations (such as Li+, Na+, K+, Mg2+, Ca2+) [27]. The pH meter (PHS-3E, Thunderstorm, Shanghai, China) was used to measure the pH of the raw brine. The field emission Scanning Electron Microscope (SEM, Hitachi SU8010, Japan) was used to characterize the surface and side morphology of the nanofiltration membrane. The surface roughness of the nanofiltration membrane was obtained by Atomic Force Microscopy (AFM, Shimadzu SPM-9500 J3, Kyoto, Japan). The contact angle of the membranes was obtained by a contact angle measuring instrument (LSA60, LAUDA Scientific GmbH, Germany, Europe). The solid surface Zeta potential analyzer (SurPASS, Anton Paar, Austria) was used to study the charge properties of the membrane surface.)
2.4. Data Analysis
2.4.1. Membrane Permeate Flux
Membrane flux is the volume of permeate passing through a unit membrane area per unit time [2], as shown in Equation (1):
where: is the membrane flux (L·m−2·h−1); V is the permeate volume (L); A is the membrane area (m2); and t is the time required to collect permeate per unit volume (h).
The permeation volume flux refers to the volume of dialysate passing through the unit effective membrane area per unit time at a given pressure [29], such as Equation (2):
where is the permeability coefficient of the NF membrane (only related to temperature and membrane structure parameters); is the pressure difference on both sides of the membrane (bar); and is the osmotic pressure (bar). Studies have shown that the greater permeation flux, the better the membrane permeability. And = 0 when the solution to be tested is pure water according to the Dissolution-Diffusion Model, pure water permeation flux is show in Equation (3).
The relationship between pure water permeation flux and operating pressure of various nanofiltration membranes was obtained. Some test sites deviated due to the instability of the nanofiltration membrane and the nanofiltration equipment under high pressure conditions while the membrane maintained good stability in the low-pressure range, but the error was within the acceptable range (<5%), which had little impact on the experimental results, as shown in Figure 2. The corresponding permeability coefficient of the membrane () was estimated by fitting the data and the performance was in the increasing order of NF270 (: 174.76) > DK (148.56) > DL (145.63) > NF90 (26.73). refers to the influence of pressure on membrane flux; the larger the is, the more significant effect of pressure on membrane flux is.
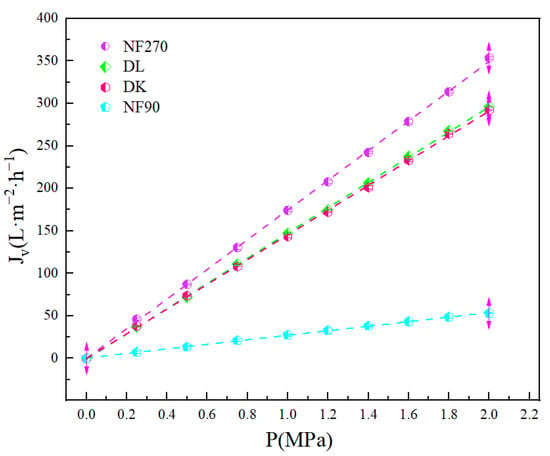
Figure 2.
Response of membrane pure water flux with operating pressure.
2.4.2. Salt Retention
Salt rejection efficiency () refers to the ratio of the concentration of the solute in the permeate to the concentration of the solute in the feed, as shown in Equation (4) [30].
where: and are the permeate and feed concentrations, respectively (unit: g/L). Studies have shown that the higher the salt rejection, the better the separation performance of the nanofiltration membrane.
2.4.3. Separation Factor of Magnesium and Lithium (SF)
The separation factor refers to the ratio of permeate to feed liquid MLR, as shown in Equation (5).
where and are the concentrations of Mg2+ and Li+ (g/L) in the permeate. and are the concentrations of Mg2+ and Li+ (g/L) in raw brine. The separation factor of 1 indicates no separation, while lower than 1 shows preferential permeation of Li+ and the lower the separation factor is, the better the separation performance is.
3. Results and Discussion
3.1. Effect of Dilution Factor on the Separation Performance of Mg2+-Li+
The high salt contents of salt lake brine facilitates the easy formation of ion pairs, ion groups, and molecules contributing to the steric hindrance effect [31]. Ions attach to the membrane surface and block the membrane pores during the separation process, thereby reducing membrane flux and increasing membrane fouling [32]. Therefore, the directly use of nanofiltration technology is not conducive to separation, so it is necessary to dilute the raw material brine so as to reduce the membrane pollution. The hydrophilicity and hydrophobicity of the membrane are important parameters that affect the membrane flux and antifouling performance [33]. Due to the good water absorption of the DL film and the rapid disappearance of droplets, the contact angle could not be accurately measured during the detection process, so the dynamic contact angle fitting curve was used to observe the contact angle. The dynamic contact angle reflects the wetting process of a droplet contacting a membrane surface, including diffusion and osmosis. The contact angles of four commercial nanofiltration membranes were estimated and presented in Figure 3: DL (62.5°) > NF270 (60.7°) > DK (51.7°) > NF90 (47.1°). A contact angle of less than 90° indicates the membrane to be hydrophilic, having better surface wettability, reducing surface adhesion and fouling tendency contributing to increases ions permeability [34]. As the dilution of the feed solution was expected to reduce the adhesion of ions on the membrane surface, the effect of dilution ratios covering a range of 25 to 50 on the separation performance of Mg2+-Li+ was assessed with the indoor temperature of 25 ± 0.1 °C. Each experimental data point had been validated by at least three sets of parallel experiments.
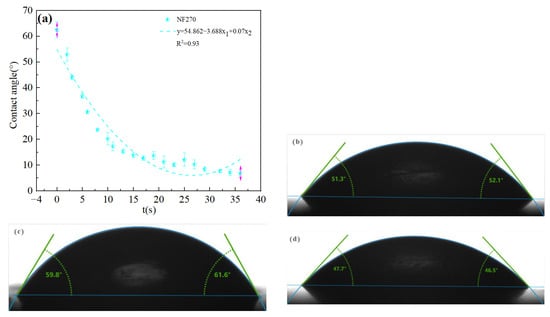
Figure 3.
(a) Dynamic contact angle of DL film and contact angle diagram of (b) DK, (c) NF270, (d) NF90.
It could be seen from Figure 4 that the order of interception effect of nanofiltration membranes on ions was: R(Ca2+) > R(Mg2+) > R(Na+) ≈ R(K+) > R(Li+), except for the NF90 membrane. It was well known from the Donnan repulsion effect that negatively charged membranes were more attractive to divalent cations (Mg2+, Ca2+) than monovalent cations (Na+, K+, Li+), and facilitated the preferential movement of divalent cations to the membrane surface. It could be seen from Table 3 that divalent cations could not pass through the membrane pores smoothly due to the large hydration radius, while monovalent cations could pass through the membrane pores smoothly. The interception of metal cations was related to the degree of enrichment on the membrane surface and was proportional to the ion concentration. Since the concentration of Ca2+ was much lower than that of Mg2+, the competitive permeability of Ca2+ was nearly zero. Comparatively, as the Mg2+ concentration was high, its permeability was around 10% as a small part of ions still could enter the membrane pores through ‘dehydration’. Since, Na+ and K+ had smaller hydration radii and larger diffusion coefficients compared to Li+, their permeation flux was higher than Li+ so that Li+ transmission was limited. However, due to the large concentration of Na+ and K+, there were still a large number of ions that did not pass through, so the interception rate was still above Li+. But in the Figure 4d, we could find that because the pore radius was closed to the hydration radius of Li+ for NF 90 membrane, a significant aperture screening effect was led, and the transmittance of Li+ was limited. Although there was still some Li+ that could penetrate through the membrane pores by removing of the hydrated layer, a large amount of Li+ were still retained in the raw material liquid, which made the interception rate of NF 90 membrane to Li+ higher than that of Na+ and K+, which was not conducive to separation.
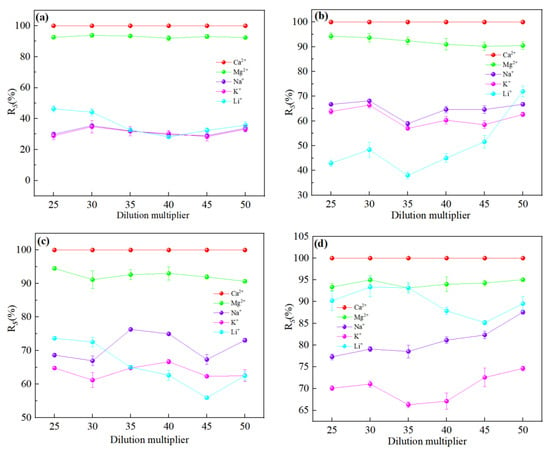
Figure 4.
Retention efficiency (%) of each ion at different dilution multiplier: (a) DL; (b) DK; (c) NF270; (d) NF90. The experimental conditions of the four types of nanofiltration membranes were: operating pressure = 0.5 MPa, circulation flow rate = 600 L/h, and feed pH = 7.

Table 3.
Ionic properties.
Figure 5 showed that the dilution factor had little effect on the permeate flux of the membrane. Although the NF90 membrane had the smallest contact angle and the strongest hydrophilicity, due to the smaller membrane pore size, the solute flux was lower than the water flux, and resulted in the largest. However, the separation factor showed a significant reduction in the increase in the dilution factor, which was conducive to separation. The separation factors of all membranes were different, but less than 1, indicated its effectiveness on the separation of Mg2+-Li+ according to Figure 6.
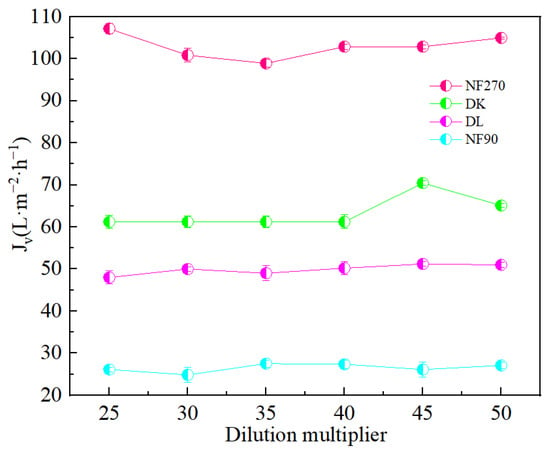
Figure 5.
Effect of different dilution multipliers on the membrane flux (Jv).
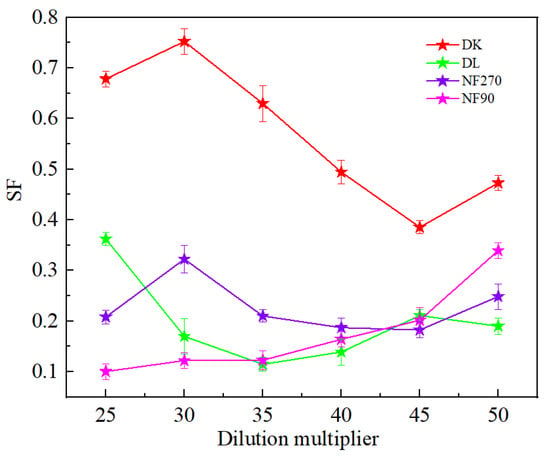
Figure 6.
Effect of different dilution multipliers on the SF.
3.2. Effect of Operating Pressure on the Separation Performance of Mg2+-Li+
The operating pressure is known to strongly influence the membrane flux as it is the driving force for separation and energy consumption. The increase in the transmembrane pressure increases both solvent and solute fluxes. However, the solvent flux increases much more than the solute flux resulting in a more diluted permeate, and a higher solute rejection. Experiments were carried out at low pressure (0–2.5 MPa) to attenuate the effect of the dilution effect as well as the lower power consumption. Each experimental data point had been validated by at least three sets of parallel experiments.
The membrane flux is closely related to the total separation resistance, which is the function of the parameters as shown in Equations (6) and (7), according to Darcy’s law.
where: µ is the dynamic viscosity of the raw material solution (Pa · s); Rt is the total separation resistance (m−1); is the membrane surface resistance; is the concentration polarization resistance; is the adsorption resistance; and is the fouling resistance. With the increase in pressure, the kinetic force of ions across the membrane increases, which weakens the Donnan repulsion effect. At the same time, due to the cross-flow filtration method, the residence time of ions on the membrane surface is shortened, which weakens the concentration polarization effect. The reduction in the enrichment of ions on the membrane surface reduces the fouling resistance and the resistance of ions adsorbed on the membrane surface, all of which contribute to the reduction in separation resistance and increase in permeate flux. Figure 7 showed an increase in the permeate flux with an increase in operating pressure and an increase in the order: NF270 > DK > DL > NF90.
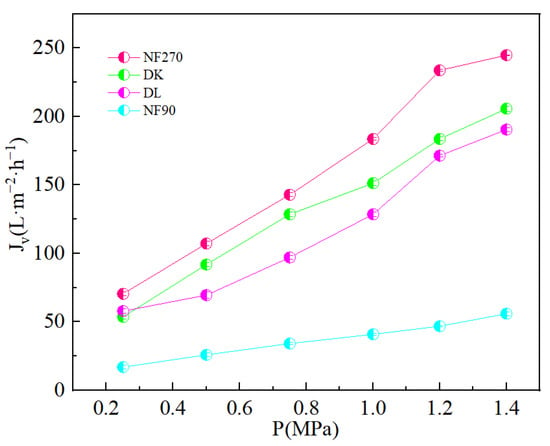
Figure 7.
Effect of different transmembrane pressure on the membrane flux (Jv).
Figure 8 showed the relationship between the percentage of ion rejection and operating pressure. The ions in the dialysate were concentrated, which is because the water flux and membrane flux of each ion were not very different under the low pressure, which resulted in a low interception rate of each ion. The driving force of water molecules and the ions through the membrane increased with the increasing pressure, but because the increase in water flux was significantly higher than the flux of each ion, the concentration of each ion in the dialysate was diluted and the interception rate increased [35]. The dilution effect was unfavorable for ion separation, so the separation process was not good to use large pressure. The Li+ interception rate decreased with the increase in pressure as shown in Figure 8d. Li+ was unable to obtain enough power to pass through the membrane hole under the low pressure, which led to a high interception rate. With the increasing pressure, Li+ obtained sufficient energy to remove the hydration layer and through the membrane, which led to a decrease in the interception rate. Figure 9 showed the SF decreased with an increase in the operating pressure, but the larger pressure could cause significant dilution effect, which was not conducive to ion interception. Therefore, the best operating pressure should be selected according to the interception performance of different nanofiltration membranes.
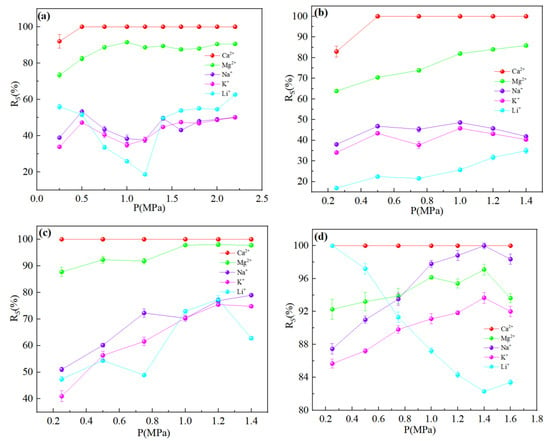
Figure 8.
Retention efficiency (%) of each ion at different transmembrane pressure: (a) DL; (b) DK; (c) NF270; (d) NF90. The experimental conditions: DL (dilution multiplier = 40, circulation flow rate = 600 L/h, and feed pH = 7); DK (dilution multiplier = 35, circulation flow rate = 600 L/h, and feed pH = 7); NF270 (dilution multiplier = 40, circulation flow rate = 600 L/h, and feed pH = 7); NF90 (dilution multiplier = 45, circulation flow rate = 600 L/h, and feed pH = 7).
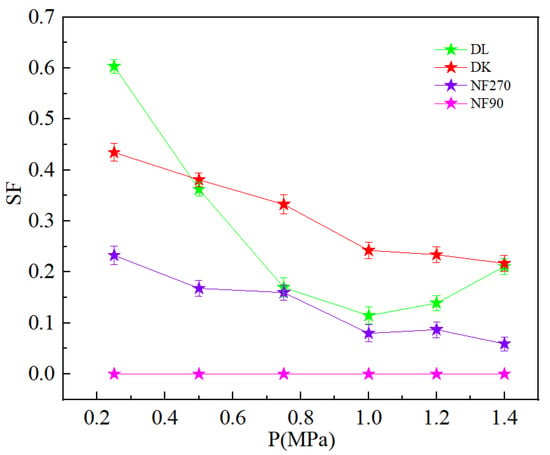
Figure 9.
Effect of different pressure on the SF.
3.3. Effect of Circulating Flow on the Separation Performance of Mg2+-Li+
The circulating flow rate of the feed liquid is known to affect the concentration polarization on the membrane surface, thereby it will affect the separation of Mg2+-Li+. In order to weakened the concentration polarization effect due to high ion concentration, the circulation flow rate of the nanofiltration process was maintained lower than 600 L/h. Each experimental data point had been validated by at least three sets of parallel experiments.
The flow state of the feed liquid on the surface of the membrane was related to the roughness of the membrane surface [36]. Figure 10 showed the AFM images of the membranes, evidenced the variation in the surface roughness. The surface roughness was found to decrease in the order: NF90 (Ra = 42.2 nm) > DK (Ra = 1.31 nm) > DL (Ra = 1.0 nm) > NF270 (Ra = 0.52 nm). The membrane surface had a typical ‘ridge-valley’ structure, and the peak density was found in descending order of NF270 > DL > DK > NF90. The ‘nanoscale’ structure on the surface could accelerate the microscopic mixing of the feed liquid. The hydrodynamic condition was improved by the enhance of the interface contact area, and the permeate flux was increased as a result, as shown in Figure 11.
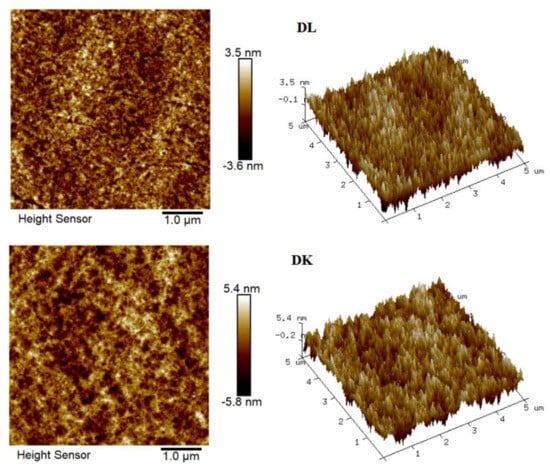
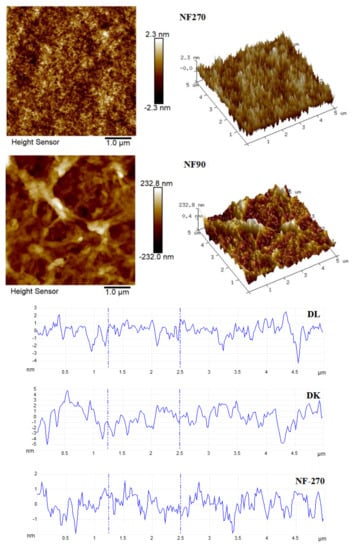
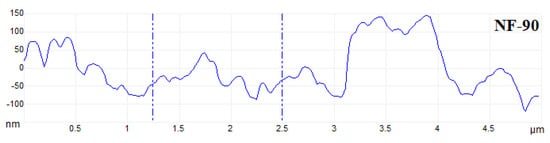
Figure 10.
AFM of membranes.
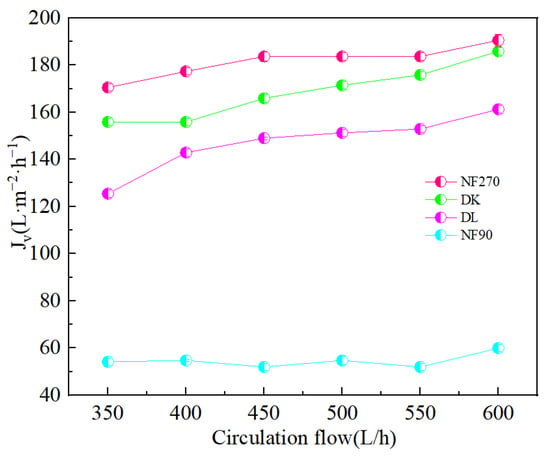
Figure 11.
Effect of different circulation flow on the Membrane flux (Jv).
Figure 12a,b showed the ion rejection efficiency of DL and DK membranes were stable with an increase in circulation rate. This could be attributed to the decrease in the aggregation of ions on the membrane surface, contributed to a decrease in membrane fouling and concentration polarization effects. The reduction of membrane fouling would be contributed to an increase in ion flux, while weakening of the concentration polarization effect would reduce the driving force of ions, as a result of contradicted effects the rejection efficiency remains stable. It could be seen from Figure 12c,d that the Li+ rejection efficiency was opposite to that of Na+ and K+, which could be attributed to the reduction in the residence time of ions on the membrane surface, contributed to a reduction in the ion concentration, thereby reduced the competitive permeability of Na+ and K+. The rejection efficiency decreased as Li+ was more easily permeable through the membrane. The decrease in SF indicated better Mg2+-Li+ separation, as shown in Figure 13.
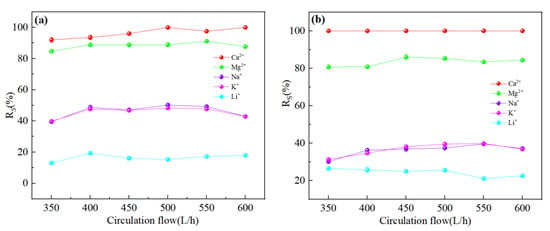
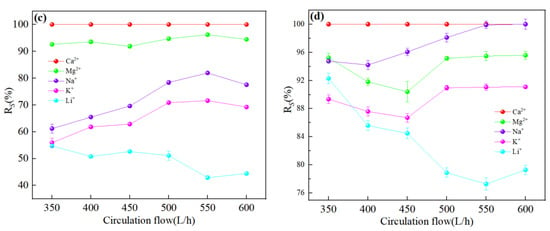
Figure 12.
Retention efficiency (%) of each ion at different circulation flow: (a) DL; (b) DK; (c) NF270; (d) NF90. The experimental conditions: DL (dilution multiplier = 40, operating pressure = 1.2 MPa, and feed pH = 7); DK (dilution multiplier = 35, operating pressure = 1.0 MPa, and feed pH = 7); NF270 (dilution multiplier = 40, operating pressure = 0.75 MPa, and feed pH = 7); NF90 (dilution multiplier = 45, operating pressure = 1.4 MPa, and feed pH = 7).
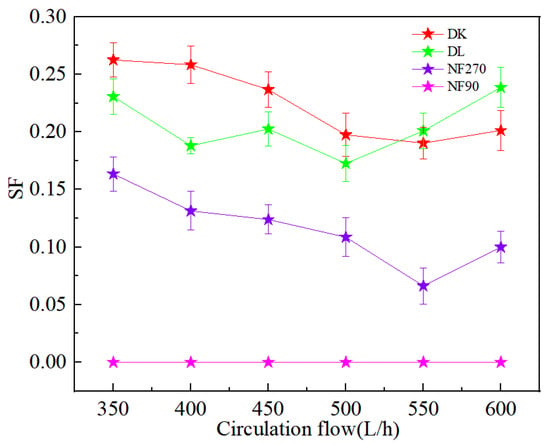
Figure 13.
Effect of different circulation flow on the SF.
3.4. Effect of Brine pH on the Separation Performance of Mg2+-Li+
The pH of the feed solution will affect the membrane surface charge, thereby affecting the Donnan repulsion and the dielectric repulsion effect [37]. The pH of the feed solution was altered using NaOH and HCl (1 mol/L) and varied in the range from 3 to 11. Each experimental data point had been validated by at least three sets of parallel experiments.
The isoelectric point of the membrane (Zeta potential = 0) was shown in Figure 14. The decreasing zeta potential was of the order: DK (3.8) > NF270 (3.53) > DL (3.48) > NF90 (3.31). When the pH was lower than the isoelectric point, the surface of the membrane was positively charged, while it was negatively charged at a pH higher than the isoelectric point [38]. The characteristics of membrane surface charge were related to the dissociation of membrane surface groups and the adsorption of anions and cations (H+, OH−) in solution [39]. Since the nanofiltration membrane had a polyamide functional layer, the residual carboxyl functional group on the surface of the membrane dissociated when in contact with an aqueous solution and became negatively charged. However, the CH+ in the feed solution was relatively high at pH 3, and the accumulation of H+ would cause charge reversal. The zeta potential of the membrane surface decreased with the increase in pH. This was because the composition of salt-lake brine was complex, and there were a large number of anions (Cl−, SO42−) in addition to metal cations. The dehydration ability of anions was stronger than that of cations, so they were more likely to lose binding. When the water reached the surface of the membrane, a large number of anions were adsorbed on the surface of the membrane, thereby increased the negative charge on the surface of the membrane.
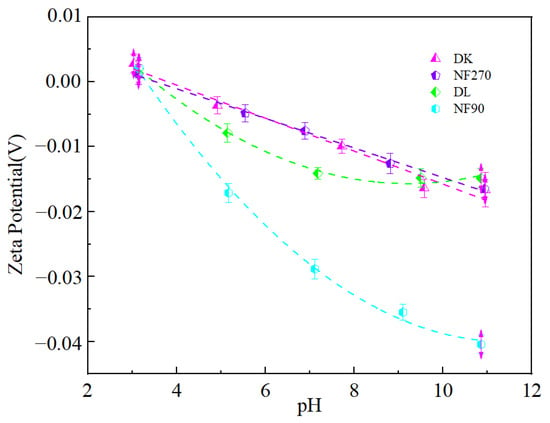
Figure 14.
Zeta potential-pH relation chart.
It could be seen from Figure 15 that pH had little effect on the flux of NF270 and NF90 membranes, while DL and DK membranes showed different trends. As shown in Figure 16, divalent cations were less affected by the pH, and monovalent cations showed a ‘concave’ shape change trend with the pH. When the pH was lower than the isoelectric point, the solution contained excess H+. Since H+ has a smaller hydration radius and a larger diffusion coefficient, it was easier to permeate the membrane and limited the penetration of other monovalent cations, resulted in a higher rejection. When the pH of the feed solution was higher than the isoelectric point, as the pH increased, the CH+ in the feed solution gradually decreased while the COH- gradually increased, which led to the negative charge density on the surface of the membrane increasing and needing to attract cations to maintain electrical neutrality. Based on the Donnan effect, the stronger the negative charge on the membrane surface, the greater the attractive effect on the multivalent cations than on the monovalent cations is, which was not conducive to separation, so the pH of the feed solution could be kept at 7–9 to obtain smaller SF, as shown in Figure 17.
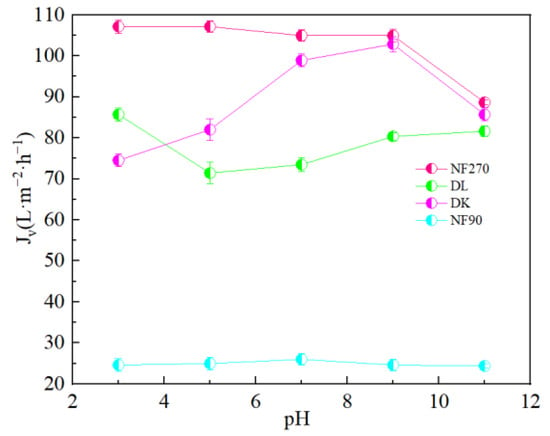
Figure 15.
Effect of different pH on the membrane flux (Jv).
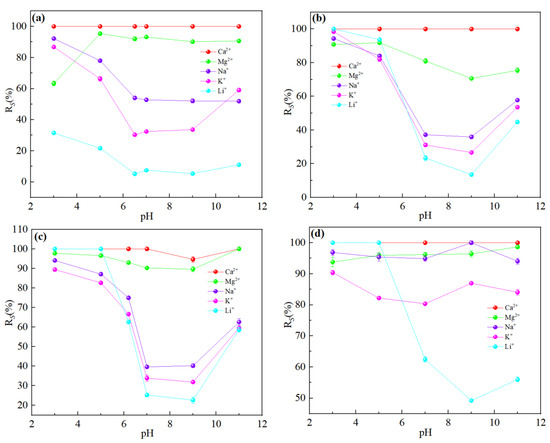
Figure 16.
Retention efficiency (%) of each ion at different pH: (a) DL; (b) DK; (c) NF270; (d) NF90. The experimental conditions: DL (dilution multiplier = 40, operating pressure = 1.2 MPa, and circulation flow rate = 500 L/h); DK (dilution multiplier = 35, operating pressure = 1.0 MPa, and circulation flow rate = 550 L/h); NF270 (dilution multiplier = 40, operating pressure = 0.75 MPa, and circulation flow rate = 550 L/h); NF90 (dilution multiplier = 45, operating pressure = 1.4 MPa, and circulation flow rate = 600 L/h).
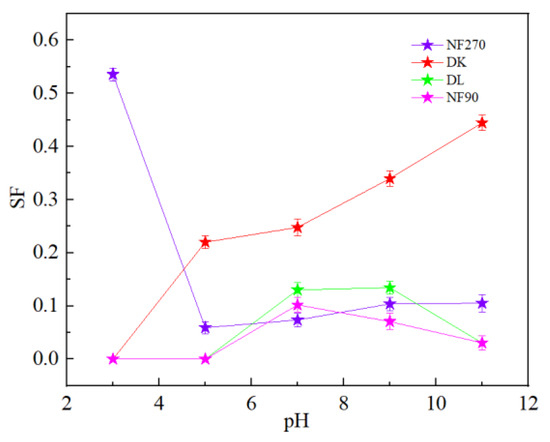
Figure 17.
Effect of different pH on the SF.
To sum up, due to the influence of the hydration radius of ions and the interaction between ions caused by the complex composition of brine, the separation process would be interfered by the impurity ions. This resulted a significant increase at the rejection of Li+, so the separation effect was slightly different from that of the two-component solution of Mg2+-Li+ [28]. The best operating conditions of each membrane were obtained through the above experiments as shown in Table 4. The SFs of the four commercial nanofiltration membranes were all less than 1, so the nanofiltration process achieved the enrichment of Li+, and the smaller the SF and dialysate MLR, the better the separation of Mg2+-Li+. Therefore, the DL membrane was more suitable for the separation of Mg2+-Li+ in salt-lake brine.

Table 4.
Optimum operating conditions and SF, MLR of commercial nanofiltration membranes.
4. Conclusions
Nanofiltration was successfully applied for the separation of Mg2+-Li+ and four commercial nanofiltration membranes were utilized to separate salt-lake brine containing various ions such as Li+, Na+, K+, Mg2+, Ca2+, etc. The results indicated that, among the four different nanofiltration membranes, the performance of the DL membrane was the best one under the optimal operating conditions of a dilution factor of 40; operating pressure of 1.2 MPa; circulation flow rate of 500 L/h, and feed pH of 7. The SF was as low as 0.074, and the MLR (a ratio of molar concentrations) was reduced from 17.406 to 0.088, offering the best separation of Mg2+-Li+, which laid a foundation for further scale-up and commercial adoption of the process.
The salt contents of salt-lake brine are complex and the concentration is large, so the direct use of nanofiltration membrane separation technology is easy to cause membrane pollution, thus the separation efficiency is weakened and the cost increased. Therefore, the optimal concentration of each impurity ion in the salt-lake brine can be studied, and the pretreatment technology can be adopted to remove some impurity ions, so as to reduce the membrane pollution problem of nanofiltration technology and increase the separation effect.
Author Contributions
Conceptualization, T.L. and Y.L.; methodology, T.L.; validation, T.L. and Y.L.; formal analysis, T.L.; investigation, T.L.; resources, T.L.; data curation, T.L.; writing—original draft preparation, T.L.; writing—review and editing, T.L., S.L., X.J., N.Z. and G.Z.; visualization, T.L. and C.S.; supervision, Shaohua Yin; project administration, S.Y. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by National Key research and development Program (Grant No. 2021YFC2901300).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmadi, H.; Zakertabrizi, M.; Hosseini, E.; Cha-Umpong, W.; Abdollahzadeh, M.; Korayem, A.H.; Chen, V.; Shon, H.K.; Asadnia, M.; Razmjou, A. Heterogeneous asymmetric passable cavities within graphene oxide nanochannels for highly efficient lithium sieving. Desalination 2022, 538, 115888. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Usman, M.; Hussain, I.; Ahmad, F.; Guo, S.; Zhang, L. Lithium extraction from high magnesium salt lake brine with an integrated membrane technology. Sep. Purif. Technol. 2022, 302, 122163. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, W.; Wang, Q.; Wu, Z.; Wang, Z. Designed strategies of nanofiltration technology for Mg2+/Li+ separation from salt-lake brine: A comprehensive review. Desalination 2023, 546, 116205. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Ji, Z.-Y.; Chen, Q.-B.; Liu, J.; Zhao, Y.-Y.; Li, F.; Liu, Z.-Y.; Yuan, J.-S. Prefractionation of LiCl from concentrated seawater/salt lake brines by electrodialysis with monovalent selective ion exchange membranes. J. Clean. Prod. 2018, 193, 338–350. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Li, T.; Ren, X.-K.; Wang, J.; Wang, Z.; Zhao, S. Nanofiltration membrane with crown ether as exclusive Li+ transport channels achieving efficient extraction of lithium from salt lake brine. Chem. Eng. J. 2022, 438, 135658. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Ji, Y.; Cui, Z.; Yan, F.; Younas, M.; Li, J.; He, B. Efficiently rejecting and concentrating Li+ by nanofiltration membrane under a reversed electric field. Desalination 2022, 535, 115825. [Google Scholar] [CrossRef]
- Nie, X.-Y.; Sun, S.-Y.; Sun, Z.; Song, X.; Yu, J.-G. Ion-fractionation of lithium ions from magnesium ions by electrodialysis using monovalent selective ion-exchange membranes. Desalination 2017, 403, 128–135. [Google Scholar] [CrossRef]
- Peng, H.; Zhao, Q. A nano-heterogeneous membrane for efficient separation of lithium from high magnesium/lithium ratio brine. Adv. Funct. Mater. 2021, 31, 2009430. [Google Scholar] [CrossRef]
- Shi, D.; Cui, B.; Li, L.; Peng, X.; Zhang, L.; Zhang, Y. Lithium extraction from low-grade salt lake brine with ultrahigh Mg/Li ratio using TBP–kerosene–FeCl3 system. Sep. Purif. Technol. 2019, 211, 303–309. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; He, B.; Shi, W.; Ji, Y.; Cui, Z.; Yan, F.; Mohammad, Y.; Li, J. Ultrahigh-efficient separation of Mg2+/Li+ using an in-situ reconstructed positively charged nanofiltration membrane under an electric field. J. Membr. Sci. 2022, 641, 119880. [Google Scholar] [CrossRef]
- Guo, C.; Li, N.; Qian, X.; Shi, J.; Jing, M.; Teng, K.; Xu, Z. Ultra-thin double Janus nanofiltration membrane for separation of Li+ and Mg2+:“Drag” effect from carboxyl-containing negative interlayer. Sep. Purif. Technol. 2020, 230, 115567. [Google Scholar] [CrossRef]
- Xu, S.; Song, J.; Bi, Q.; Chen, Q.; Zhang, W.-M.; Qian, Z.; Zhang, L.; Xu, S.; Tang, N.; He, T. Extraction of lithium from Chinese salt-lake brines by membranes: Design and practice. J. Membr. Sci. 2021, 635, 119441. [Google Scholar] [CrossRef]
- He, L.; Xu, W.; Song, Y.; Liu, X.; Zhao, Z. Selective removal of magnesium from a lithium-concentrated anolyte by magnesium ammonium phosphate precipitation. Sep. Purif. Technol. 2017, 187, 214–220. [Google Scholar] [CrossRef]
- Li, H.-F.; Li, L.-J.; Peng, X.-W.; Ji, L.-M.; Li, W. Extraction kinetics of lithium from salt lake brine by N, N-bis (2-ethylhexyl) acetamide using Lewis Cell. Hydrometallurgy 2018, 178, 84–87. [Google Scholar] [CrossRef]
- Khalil, A.; Mohammed, S.; Hashaikeh, R.; Hilal, N. Lithium recovery from brine: Recent developments and challenges. Desalination 2022, 528, 115611. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Z.; Ghahreman, A. Novel approaches for lithium extraction from salt-lake brines: A review. Hydrometallurgy 2019, 187, 81–100. [Google Scholar] [CrossRef]
- Li, X.; Mo, Y.; Qing, W.; Shao, S.; Tang, C.Y.; Li, J. Membrane-based technologies for lithium recovery from water lithium resources: A review. J. Membr. Sci. 2019, 591, 117317. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Wang, Y.; Yun, R.; Xiang, X. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine. Sep. Purif. Technol. 2021, 256, 117807. [Google Scholar] [CrossRef]
- Huang, T.; Song, J.; He, H.; Zhang, Y.-B.; Li, X.-M.; He, T. Impact of SPEEK on PEEK membranes: Demixing, morphology and performance enhancement in lithium membrane extraction. J. Membr. Sci. 2020, 615, 118448. [Google Scholar] [CrossRef]
- Xu, P.; Qian, X.; Guo, C.; Xu, Z.; Zhao, L.; Mai, W.; Li, J.; Tian, X.; Duo, Y. Nanofiltration technology used for separation of magnesium and lithium from salt lake brine: A survey. Mater. Rep. 2019, 33, 410–417. [Google Scholar]
- Su, H.; Zhu, Z.; Wang, L.; Qi, T. Advances and prospects of extracting and recovering lithium from salt lake brines. Mater. Rep. 2019, 33, 2119–2126. [Google Scholar]
- Gu, T.; Zhang, R.; Zhang, S.; Shi, B.; Zhao, J.; Wang, Z.; Long, M.; Wang, G.; Qiu, T.; Jiang, Z. Quaternary ammonium engineered polyamide membrane with high positive charge density for efficient Li+/Mg2+ separation. J. Membr. Sci. 2022, 659, 120802. [Google Scholar] [CrossRef]
- Rahighi, R.; Hosseini-Hosseinabad, S.M.; Zeraati, A.S.; Suwaileh, W.; Norouzi, A.; Panahi, M.; Gholipour, S.; Karaman, C.; Akhavan, O.; Khollari, M.A.R. Two-dimensional materials in enhancement of membrane-based lithium recovery from metallic-ions-rich wastewaters: A review. Desalination 2022, 543, 116096. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Asif, M.B.; Roychand, R.; Shu, L.; Jegatheesan, V.; Bhuiyan, M.; Hai, F.I. Lithium recovery from salt-lake brine: Impact of competing cations, pretreatment and preconcentration. Chemosphere 2020, 260, 127623. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Zhang, Z.; Zhang, S.; Sun, S.; Li, P.; Yu, J. Separation properties of magnesium and lithium from brine with high Mg 2+/Li+ ratio by DK nanofiltration membrane. Membr. Sci Technol. 2014, 34, 79–85. [Google Scholar]
- Sun, S.-Y.; Cai, L.-J.; Nie, X.-Y.; Song, X.; Yu, J.-G. Separation of magnesium and lithium from brine using a Desal nanofiltration membrane. J. Water Process Eng. 2015, 7, 210–217. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Asif, M.B.; Kentish, S.; Nghiem, L.D.; Hai, F.I. Lithium enrichment from a simulated salt lake brine using an integrated nanofiltration-membrane distillation process. J. Environ. Chem. Eng. 2019, 7, 103395. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Wang, H.; Wang, M. The application of nanofiltration membrane for recovering lithium from salt lake brine. Desalination. 2019, 468, 114081. [Google Scholar] [CrossRef]
- Lu, D.; Ma, T.; Lin, S.; Zhou, Z.; Li, G.; An, Q.; Yao, Z.; Sun, Q.; Sun, Z.; Zhang, L. Constructing a selective blocked-nanolayer on nanofiltration membrane via surface-charge inversion for promoting Li+ permselectivity over Mg2+. J. Membr. Sci. 2021, 635, 119504. [Google Scholar] [CrossRef]
- He, R.; Xu, S.; Wang, R.; Bai, B.; Lin, S.; He, T. Polyelectrolyte-based nanofiltration membranes with exceptional performance in Mg2+/Li+ separation in a wide range of solution conditions. J. Membr. Sci. 2022, 663, 121027. [Google Scholar] [CrossRef]
- Wen, X.; Ma, P.; Zhu, C.; He, Q.; Deng, X. Preliminary study on recovering lithium chloride from lithium-containing waters by nanofiltration. Sep. Purif. Technol. 2006, 49, 230–236. [Google Scholar] [CrossRef]
- Patel, T.M.; Nath, K. Alleviation of flux decline in cross flow nanofiltration of two-component dye and salt mixture by low frequency ultrasonic irradiation. Desalination 2013, 317, 132–141. [Google Scholar] [CrossRef]
- Song, J.; Li, X.-M.; Zhang, Y.; Yin, Y.; Zhao, B.; Li, C.; Kong, D.; He, T. Hydrophilic nanoporous ion-exchange membranes as a stabilizing barrier for liquid–liquid membrane extraction of lithium ions. J. Membr. Sci. 2014, 471, 372–380. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.; Ang, W.; Chung, Y.; Oatley-Radcliffe, D.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Xu, F.; Dai, L.; Wu, Y.; Xu, Z. Li+/Mg2+ separation by membrane separation: The role of the compensatory effect. J. Membr. Sci. 2021, 636, 119542. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, N.; Shi, J.; Xia, Y.; Zhu, B.; Shao, R.; Min, C.; Xu, Z.; Deng, H. Extra-thin composite nanofiltration membranes tuned by γ-cyclodextrins containing amphipathic cavities for efficient separation of magnesium/lithium ions. Sep. Purif. Technol. 2022, 286, 120419. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Sun, W.; Hu, Y.; Tang, H. Membrane technologies for Li+/Mg2+ separation from salt-lake brines and seawater: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 7–23. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Yu, Q.; Sun, H.; Chen, K.; Ye, H.; Tang, S.; Zhang, H.; Li, P.; Niu, Q.J. Advanced Mg2+/Li+ separation nanofiltration membranes by introducing hydroxypropyltrimethyl ammonium chloride chitosan as a co-monomer. Appl. Surf. Sci. 2023, 616, 156434. [Google Scholar] [CrossRef]
- Sutijan, S.; Darma, S.A.; Hananto, C.M.; Sujoto, V.S.H.; Anggara, F.; Jenie, S.N.A.; Astuti, W.; Mufakhir, F.R.; Virdian, S.; Utama, A.P. Lithium Separation from Geothermal Brine to Develop Critical Energy Resources Using High-Pressure Nanofiltration Technology: Characterization and Optimization. Membranes 2023, 13, 86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).