Abstract
This paper presents a comprehensive literature review surveying the most important polymer materials used for electrospinning processes and applied as membranes for the removal of emerging pollutants. Two types of processes integrate these membrane types: separation processes, where electrospun polymers act as a support for thin film composites (TFC), and adsorption as single or coupled processes (photo-catalysis, advanced oxidation, electrochemical), where a functionalization step is essential for the electrospun polymer to improve its properties. Emerging pollutants (EPs) released in the environment can be efficiently removed from water systems using electrospun membranes. The relevant results regarding removal efficiency, adsorption capacity, and the size and porosity of the membranes and fibers used for different EPs are described in detail.
1. Introduction
The application of electrospun membranes for wastewater treatment has been the subject of numerous review papers, and their application for the removal of emergent pollutants has been studied in recent years in several review papers, with their numbers constantly increasing [1]. The data regarding nanofibers presented in this paper cover developments from 1998 to 2021. Because the significance of these emerging pollutants is increasing, the aim of this literature review is to contribute to developing a possible database covering their decontamination performance. Thus, this paper presents tested membrane types along with their characteristics, as well as concrete results for different classes of emerging pollutants.
Today, among the many environmental risks, the water crisis is one of the most important. Thus, it is imperative that a technological approach be developed to deliver high-quality standards for water and welfare. Appropriate treatment methodologies should limit the presence of pathogens, toxic chemicals, pharmaceuticals, heavy metals, fertilizers, and endocrine disruptors, as emergent pollutants in water [2,3,4,5,6]. For these types of pollutants, classical water treatment methods are insufficient for achieving efficient degradation, and thus researchers today are focused on hybrid technologies in which classical methods are combined with hybrid techniques such as chlorination and UV radiation [7]. The results are not yet technologically and economically effective, and the challenge of producing high-quality water remains an issue.
One of the most significant issues regarding the presence of emergent pollutants in the environment is their persistence and their risk of diffusing directly from the soil to groundwater, making their treatment more complex. Today there are several membrane-based techniques, such as microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), reverse osmosis (RO), and forward osmosis (FO). Any of these techniques can be integrated into hybrid water treatment systems, but there is currently only a small number of such applications on an industrial scale.
There are numerous data on the experimental parameters by which membranes based on electrospun fibers can be obtained, including the initial concentration and type of polymer and solvent, electrospinning speed, temperature, type of collector, and electrospinning method. This work highlights the performance that these types of materials can have in the removal of some emerging pollutants, for which viable solutions are still being sought with respect to decontamination, and also in the integration of solutions found in existing decontamination systems. Thus far, as will be shown in the course of the paper, the basic purpose, at the application level, is to integrate these materials as a support for thin film composites (TFC) that subsequently form membranes used in separation processes. In recent years, these types of materials have been experimentally tested in single adsorption or hybrid processes in which they have been combined with advanced oxidation or photocatalytic techniques.
This review article outlines the performances of electrospun membranes when applied for the removal of emergent pollutants from waters, based on their nanofiber properties and efficiency. This paper also includes a comprehensive discussion of membrane materials as advanced materials with high characteristics associated with filtration processes dedicated to emergent pollutants.
2. Emerging Pollutants
2.1. Classification
Water pollution still represents an urgent issue at the global level, with respect to both quality and quantity [2,8]. Emerging pollutants, according to the Norman Substance Database, are defined as synthetic or natural pollutants that should potentially be included in future regulations due to their ecotoxicity and impact on life and the environment, that have not yet been introduced into routine monitoring plans [9,10]. Emerging pollutants can be classified, according to their physico-chemical properties, into the following categories: organics (such as pharmaceuticals, industrial chemicals, pesticides), inorganics (such as trace metals), and contaminating particles (such as nanoparticles and microplastics) [3,8].
There are two different pollution sources from which emerging pollutants can be released into the environment: through wastewater treatment plants in urban or industrial areas (as point sources), and through atmospheric deposition, crops, and animal production (diffuse sources).
According to Stone, when an emerging pollutant is defined as a contaminant, this may either be as a result of the identification of a new source or pathway into the human body, or as a result of the development of a new detection method or treatment process [11].
According to the EPA, those pollutants recently discovered, often as a result of improvements in analytical detection performance, and which are not necessarily new chemicals, often found in the environment but not monitored until recent years, are defined as “contaminants of emerging concern” [12]. Thus, there are more than 20 classes of compounds under the umbrella of emerging pollutants, among which, we can mention:
- -
- Persistent organic pollutants (POPs) from flame retardants, furniture foam, plastics, etc.;
- -
- Pharmaceuticals and personal care products (PPCPs) from prescribed drugs (antidepressants, blood pressure, etc.) to over-the-counter medications (ibuprofen, acetaminophen, etc.), as well as bactericides (such as triclosan), sunscreens, and synthetic musks;
- -
- Veterinary medicines such as antimicrobials, antibiotics, antifungals, and growth hormones;
- -
- Endocrine-disrupting chemicals (EDCs), including synthetic estrogens and androgens, and naturally occurring estrogens, along with organochlorine pesticides and alkylphenols, well-known to alter normal hormonal functions and steroidal synthesis in aquatic organisms;
- -
- Nanomaterials such as carbon nanotubes or nano-scale particulate titanium dioxide, with little being known about either their environmental fate or effects.
All these products contain compounds that have a high probability of being concentrated in biological species and transferred to the food chain. Their identification has raised an urgent need to establish efficient removal technologies that are technically and economically feasible. Their presence in domestic and industrial streams has demonstrated that current conventional water and sludge treatment plants are not able to provide the required efficiency [13].
These emerging pollutants represent a constant issue due to the high rate of urbanization, consumption trends, and industrial technologies, all of which are focused on elevated standards of life quality [10]. The main categories of emerging pollutants (EPs) that influence aquatic, air and terrestrial environments are presented in Figure 1. Thus, new products appear on the market as a result of society’s requirements. Along with these, the development of new analytical methods for the correct detection of the substances that are part of these products, and the high costs involved, represent an additional challenge [10,14,15]. On this basis, advanced water treatment methods can be identified and integrated into hybrid systems alongside classical methods. Because membrane systems that involve a filtration step are essential, the materials underlying the design of filters and membranes are also of increasing interest.
Figure 1.
Influence of emerging pollutants (EPs) on environments.
There are plentiful data regarding the occurrence, sources, behavior, impacts, and risks of emerging pollutants in water, sediments, soil, and the atmosphere [14,16,17], but with little data from investigations of their toxicity. The most studied emerging pollutants are endocrine disruptors, and the most frequently detected in the environment are pharmaceutical products (CECs), personal care products (PPCPs), and flame retardants. According to the WHO, one of the biggest food safety and health problems is antibiotic resistance, especially where these can be acquired without a prescription, and thus their spread and resistance has become noticeable in the environment [18,19]. Antibiotics can cause some bacteria to become resistant to low concentrations of these classes of substances, so removing them from environments is becoming even more complicated [20].
On the list of emergent pollutants, another category consists of personal care products (PCPs), comprising especially ultraviolet radiation screening compounds or organic UV filters [21]. Their intensive use has caused them to be actively introduced into waters (rivers, lakes, seawater, groundwater, sediments, and biota) through recreational activities. Most of them are detected in wastewater treatment plants, where the actual treatment steps are not adequate to remove them.
2.2. Environmental Impact
All these above-mentioned aspects related to the persistence in the environment and the occurrence in water treatment systems of these types of pollutants have been studied in recent years in order to solve the deficiencies occurring in conventional treatment systems. The literature indicates that membrane technologies, activated sludge technologies, sorption processes, advanced oxidation processes, phytoremediation, and bioremediation are processes suitable for the removal of emerging pollutants. Each of these processes has demonstrated advantages and disadvantages, depending on the category of emerging pollutant being removed from the system, the complex matrix, and the level of concentration. The main advantages and future challenges regarding conventional and non-conventional processes applied for EP removal are presented in Table 1.

Table 1.
Advantages and future challenges for applying conventional and non-conventional processes for EP removal.
The efficiency of their removal from wastewater effluent is related to the risk of their appearance in surface water, sediment, soil, groundwater, and seas [20,22,23]. The challenge arises when concentrations are low and these substances are present in complex matrices (micro- or nanograms per liter) [3], so in addition to analytical methods that must be sufficiently sensitive for proper trace detection, studies on ecotoxicology, risk assessment, and spatial distribution must be performed to establish effective treatment methods for their removal from contaminated aqueous systems [20].
Today’s technologies for water/wastewater treatment are adequate and cost effective in specific applications [24]. Often, wastewater contains a variety of compounds, including metals, microorganisms, organic compounds, as natural or synthetics (pharmaceuticals), and a single technology is unable to meet the required quality standards associated with a circular model of water management [25]. When there are effective methods (such as chlorination for drinking water, reverse osmosis for the desalination of seawater, and activated sludge for organic matter, phosphorus, and nitrogen), in the case of a complex water matrix, treatment plants may employ various techniques in combination to remove heavy metals, solid suspensions, and persistent organic pollutants. Thus, hybrid technologies can provide the efficiency and quality required by water standards [26]. Thus, hybrid systems combining active sludge, as a classical method, with membrane systems based on ultra-, micro-, or nanofiltration offer effluent quality, containing solid suspensions, organics, and nutrients [20]. Several hybrid configurations adapted to emerging pollutants have been tested at pilot level in the EU, and represent a new generation of water technologies, but these are associated with considerable investment costs, delaying broad application [27,28]. In order for these systems to meet the requirements regarding efficiency, cost, and environmental impact, the experimental conditions must coincide with real ones in terms of parameters, whereas to date, the literature includes some notable differences. Additionally, given the performance of membrane systems, these need to be further developed. The types of materials underlying these membranes are important in their manufacture, being responsible for the efficient removal of pollutants. Importantly, in the case of the degradation of these emerging pollutants, the compounds resulting from the degradation can sometimes be more hazardous than the original ones, leading to high ecotoxicity, as water treatment systems are not always efficient. This phenomenon occurs especially in the case of advanced oxidation systems, and such cases have been described in the literature [20,29,30,31,32].
3. Membranes
3.1. Treatment Technologies for Electrospun Functional Membranes
The technologies applied for the preparation of electrospun membranes are designed to introduce new functions to the membrane, such as excellent hydrophobicity and mechanical properties, as well as chemical stability, in order for the membrane to be used in the treatment of emerging pollutants in water [33].
Electrospun functional membranes are the subject of pretreatment and post-treatment treatment technologies, after the preparation step. By means of pretreatment tehcnologies, other functional components can be added directly to spinnable polymer solutions in order to provide membrane functionality. The solvent and functional components have to be compatible, and the dispersion of functional solid components in the working polymer solution should also be considered [34]. Conversely, precipitation and possible clogging of the spinneret could take place, and thus, viscosity, solubility, and dispersion are compulsory parameters to control [35,36].
Post-treatment technologies involve the treatment of the membrane (calcination treatment or surface coating) to obtain a large specific surface area, while hydrophilic functional groups on the surface of the fibers extends the possibilities of performing water treatment using certain hydrophobic polymers that possess good mechanical strength [34,37]. The post-treatment could be a physical modification, but without a total change in fiber properties. Additionally, chemical modification is applied to enhance the internal fiber properties; for example, the functionalization of cellulose nanofibers using sulfhydryl group can be successfully performed by means of the deacetylation of electrospun cellulose acetate nanofiber membrane, followed by temperature treatment (at 80 °C for 22 h) and the esterification of hydroxyl groups with 3,3′-dithiodipropionic acid and the additional reductive cleavage treatment of the disulfide bond [38].
The pretreatment and post-treatment technologies can be combined; for example, an easily recycled photocatalyst for antibiotics degradation can be synthesized using dispersed graphitic carbon nitride (g-C3N4) embedded in polyethylene terephthalate (PET) by electrospinning with subsequent hydrothermal treatment [39].
The role of a membrane is indicated by its selectivity with respect to pollutant separations resulting from their transport between two phases [40,41]. Depending on the pore structures of materials, membranes can be classified as porous or dense (non-porous) [42].
3.2. Dense (Non-Porous) Membranes
Focusing on environmental applications, membrane biofilm reactors are designed within non-porous-based materials [43,44]. Flat-sheet membranes with pore sizes lower than 0.2 nm are considered to be non-porous membranes [43]. Today, composite non-porous membranes from the polyurethane layer between polyethylene micro-porous layers are commercially available, because porous layers usually offer structural support to thin non-porous membranes [44]. One of the most significant features of these membranes is their ability to demonstrate gas transfer resistance only if a thin layer is present. Some applications include the removal of organic compounds from contaminated gas streams, with good results being obtained in benzene removal using latex non-porous membrane [45]. Usually, dense-phase materials (for example, silicone rubber) are used for the design of semipermeable hydrophobic membranes.
For water decontamination, in order to separate impurities, oils, organics and biological species, and other emerging pollutants, purification methods are classified, on the basis of the pore size of the materials used, into nano- and micro-porous methods [46]. Nano-porous membranes act as a dense film with a thickness of only a few hundred nanometers, in which pollutant molecules are separated through a solution–diffusion process directed by pressure, concentration, and potential gradient across the membrane [40,47]. These types of membranes are applied in processes in which reverse osmosis (RO), forward osmosis (FO), nanofiltration (NF), and membrane distillation (MD) are used [48].
NF and RO membranes are applied in desalination water treatments, where high pressure is necessary compared with FO [40,49]. A very crucial aspect when polymeric membranes are used, especially for NF, is the accurate control of their morphology, as well as their chemical, thermal and mechanical integrity, in order to completely remove target pollutants without affecting the permeate flux [50,51]. Under these considerations, thin-film composite (TFC) membranes have been used extensively in NF applications. These TFC membranes are designed on an asymmetric porous support obtained using the phase inversion method [52]. Recently, conventional support systems in TFC membranes have been replaced with electrospun scaffolds that exhibit excellent interactions between the barrier and support layers, improving the separation efficiency [40,51,53,54,55,56,57]. Electrospun structures offer thin layers with good mechanical properties, and could also be a reliable option for FO and, combined with electrospun scaffolds, could lead to high flux due to the interconnected pore structure [33,58,59,60,61,62].
3.3. Porous Membranes
Porous membranes are usually used for micro (MF) and ultrafiltration (UF) applications, where the main characteristic is the pressure acting on the membrane, and only the smallest particles pass through it. In the case of UF, the membrane is able to retain particles greater than 0.001 μm in size [63]. As in the case of dense nano-membranes, the pore size and the distribution are crucial features necessary for mechanical strength.
Sundarrajan and colleagues indicated that conventional UF membranes can be integrated into TFC-type configurations, which incorporate three layers: (i) a micro-fibrous nonwoven support for mechanical strength, (ii) a UF membrane for assuring the permeate flow resistance, coated with (iii) a thin film as a barrier layer for solute exclusion and flux rate monitoring [46].
Another application of micro-porous membranes is in membrane distillation (MD) processes, where the water is maintained on one side, and the vapors cross through the membrane pores. This MD process requires hydrophobic membranes that possess a narrow pore size distribution, good mechanical strength, and high values for liquid entry pressure (LEP) [64,65,66]. The phase inversion technique is one of the most facile techniques used as a preparation method, and possesses the advantage of obtaining a large variety of pore sizes in accordance with polymer type and concentration, as well as precipitation method and temperature [43]. Electrospun membranes are appropriate candidates for MD processes, due to their effectiveness in the control of material characteristics and design [40,64].
4. Electrospinning Technique
4.1. Principles, Characteristics, Parameters
Electrospinning is a technique that has been known since ancient times, and electrospun shapes exist even in nature [67]. Xue et al. mention in their review the history of this technique’s emergence, with the most common examples in nature being the spider’s feathers, which have diameters of between 2 and 5 μm, or the filaments of cocoons built by silkworms [68]. Nature has thus been a source of inspiration for man, with the textile industry’s achievements in wool and cotton yarn being one example. Progress in chemistry, particularly in the polymer industry, has led to the development of synthetic fibers, with nylon representing the greatest advance in this field. Subsequently, numerous synthetic fibers have been developed using wet, dry, melt, or gel methods [69,70].
Today, electrospun nanofibers have applications in areas such as the textile industry [71,72,73,74], medicine [75,76,77,78,79], sensor manufacturing [80,81], cosmetics [82,83,84], and water purification [40]. In short, obtaining fibers using the wet method involves placing an extruded polymer solution from a spinneret into a chemical bath, where it is solidified into fibers as a result of a chemical reaction or dilution. In the dry method, the fibers are formed as a result of the extrusion of the polymer through the air onto a surface, with the solvent of the solution being evaporated into the atmosphere by gentle heating or airflow. In the case of the melt method, the fibers are formed by cooling, with the generation of extruded polymer from the spinneret. To obtain fibers with special properties, mainly high mechanical strength, a polymer solution gel is used, which is able to form fiber by drying in air and then in a liquid bath. Depending on the application, these types of methods can be chosen to obtain submicron-sized, high-strength fibers.
The arrangement of the fibers obtained by electrospinning results in the formation of membranes, the main advantage of which is the small diameter of the fibers, but also the low pore size, which induces a large specific surface area, high porosity, surface roughness, and low weight [34,68]. The advantages of such a membrane also arise from the ease with which it can be functionalized/decorated later with different compounds with advanced properties for use in fields in which these types of membranes are applied. Thus, nanofiber-based composites may emerge that are more efficient than bare nanofibers (e.g., physical, chemical, and catalytic properties can be enhanced).
The principle of the electrospinning technique is really simple. A high-voltage device, a spinneret, and a collector are required for fiber formation [68,85]. Polymer droplets upset the surface tension, and as they exit the spinneret, they form one or more ultrafine jets that are captured on a collector device as thin fibers.
Applications have proven the need to obtain fibers with controlled morphology; as such, the parameters are driven by system, processing, and environmental factors [86]. Fibrous structures display important characteristics useful for many applications, especially when nanoscale diameters are obtained. Thus, fabrication techniques remain a challenge, with a focus on nanofibers. Nowadays, the electrospinning technique is designated as an emerging technique that offers good control and operating conditions for the production of highly porous smooth non-woven structures.
In contrast to traditional methods of manufacturing phase inversion membranes, those obtained by electrospinning show a structure with a relatively uniform pore size distribution, with high interconnectivity between the pores and with a high porosity of about 80% [40,87,88,89]. These arguments support the use of such membranes in separation processes with fiber diameter sizes in the nanometer range.
Nanofibers represent a nexus class of nanomaterials with exceptional properties due to their nanometric scale and high specific surface activity.
Electrospun membranes are effective in water purification processes, including membrane distillation (MD) and pretreatment steps prior to reverse osmosis (RO) or nanofiltration (NF), with the effect of removing divalent metal ions, oils, and other contaminants. Thin film composite (TFC) membranes for reverse osmosis (RO) and nanofiltration (NF) desalination are often fabricated on electrospun polymer supports [46,90].
There are numerous data on the practical applicability of such membranes in air and water filtration [91,92,93], with focus on membrane architecture, new types of materials, synthesis and characterization methods for simple or functionalized membranes, especially in case of membrane distillation (MD) processes [68], and or other water treatment processes [94]. Post-treatment methods have also been intensively studied [95]. Developments in recent years in the production of electrospun membranes have been described in detail, especially for membrane processes involving RO, FO, NF. Additionally, electrospun nanofibers as a support layer in the fabrication of TFCs have demonstrated their usefulness. However, fewer cases have been studied in which these types of membranes combining electrospinning with the TFC production method have been applied for the removal of emerging pollutants. However, the literature provides substantial information on the use of electrospun membranes in adsorption or photocatalysis, as well as other advanced oxidation processes for the retention of emerging pollutants, mostly at laboratory, pilot plant level. There is thus a need to develop studies in the direction of the application of electrospun nanofibrous membranes as a barrier layer for water treatment with the removal of emerging pollutants, with a focus on the consolidation and post-treatment of electrospun membranes.
The parameters influencing the morphology of electrospun fibers can be classified into the following groups: system, process, and environmental [34,68,96]. System parameters are focused on polymer molecular weight and solution concentration, conductivity, dielectric constant, surface tension, viscosity, and solvent type [34]. When the polymer has a high molecular weight, the formed fiber has a large diameter and is straight, without beads.
Additionally, the polymer concentration is one of the essential parameters in the electrospinning process. Low concentrations lead to jet instability and fracture, and the final product will take the form of beads. Multiple attempts are usually required to achieve a specific concentration range to form fibers of uniform diameter. Insulating polymers are difficult to electrospin, so ionic compounds or salts are used to improve the conductivity of the polymer solution [40].
If the concentration range is appropriate, the fibers are formed without defects, while a high concentration prevents the formation of straight fibers. As the electrical conductivity increases in a specific range, the diameter of the fibers decreases, and the fibers do not show pearls, while excessively increasing the conductivity values makes it impossible to form straight fibers of uniform diameter. The decrease in surface tension leads to the formation of smooth fibers, together with an increase in vascularity, the latter leading to an increase in diameter. Additionally, an increased viscosity will lead to bead-like fibers and clogging, and extremely low viscosity will lead to the electrospraying phenomenon. High solubility gives the appearance of fibers with a well-defined morphology, together with appropriate volatility [85,97].
Thus, the rheology of the polymer solution is essential in the formation of fibers, and the molecular weight and concentration of the polymer directly influence the properties of the obtained fibers. It is known that at low concentrations, when the viscosity is low, the phenomenon of “electrospraying” occurs, in which particles are formed instead of fibers [40,98]. For example, a solution of 5% poly (vinylidene fluoride) (PVDF) in a solution of N,N-dimethylformamide (DMF) leads to droplets, with smooth fibers only appearing at concentrations above 10% (wt), with diameters being of about 500 nm, while in the case of the addition of DMF/acetone, the obtained matte fibers are of about 330 nm, acetone acting to decrease the viscosity of the solution without influencing the fiber formation capacity [99].
Solvent volatility has a major impact on the resulting membrane fiber morphology. When a low-volatility solvent is used, evaporation does not occur rapidly, and thus wet fibers are formed. On the other hand, when using highly volatile solvents, the fibers solidify immediately upon exit from the needle, and the polymer jet no longer leads to the formation of fibers.
The solubility of the solvent induces a homogeneous polymer solution suitable for electrospinning [68,100,101]. The most used solvents include acetone, dimethyl sulfoxide (DMSO), chloroform, dimethylformamide (DMF), dichloromethane, tetrahydrofuran (THF), hexafluoroisopropanol (HFIP), and alcohols. Some applications involve the mixture of different solvents to produce an efficient formulation. Water is not an adequate solvent for electrospinning, due to its depletion of electrostatic repulsions, having a high dielectric constant.
The spinning voltage is the most basic processing parameter of electrospinning; it leads to the formation of the Taylor cone when a critical voltage is reached, after which, by increasing the voltage required to obtain perfectly stretched fibers, the formation of a jet responsible for fiber formation takes place [85]. The increased tension leads not only to the stretching of the fibers, but also to their thinning, with the diameter being decreased to the order of nanometers.
The speed at which the spinning solution is injected is extremely important in the formation of fibers of different diameters. Adjusting the pumping speed through the syringe can lead either to small diameters when the electrospinning period is long (when the speed is low) or to large diameters or even droplet formation at high speeds. Additionally, the distance between the syringe through which the polymer is extruded and the manifold is important in terms of solidification and fiber formation time. With a short distance, the solvent will volatilize, causing the fibers to adhere and increase in diameter. In addition, solvents with increased volatility lead to fibers wrapping around the needle, just as low volatility leads to the inability of the jet to be stretched; therefore, the use of mixed solvents to obtain ideal materials for electrospinning is recommended. As the distance increases, the formation of smaller diameters will be possible, reaching as low as nanometers.
The applied voltage influences the shape and morphology of the fibers. An example of this is the electrospinning of the mixed solution of poly (vinyl alcohol) and sodium alginate at values between 28 kV and 35 kV, for which the shape of the fibers is different [20,102].
For example, at 28 kV, broken fibers were formed, while the length of the fibers became continuous at voltages greater than 35 kV. Authors suggest that at high stresses, long, matte fibers with small diameters are typically formed [40], although there is still no exact relationship between voltage and fiber diameter, which must be correlated with the concentration of the solution and the tip–collector distance. However, it has been found that bead formation occurs at very high voltages [102].
Environmental conditions such as humidity and temperature can influence fiber morphology [103]. Thus, low relative humidity can accelerate solvent evaporation, leading to the formation of fibers with small diameters. Temperature acts in two ways on fiber morphology. As the temperature increases, the evaporation of solvent occurs rapidly, leading to difficulty in stretching the jet. When the temperature decreases, the viscosity of the solution decreases, and thus the formation of fibers with small diameter occurs.
Relative humidity (RH) influences the evaporation rate of the solvent, and thus the formation of pores on the surface of the fibers. For example, electrospinning of polystyrene (PS) fibers in tetrahydrofuran (THF) at a low humidity of 25% leads to smooth, pore-free fibers on the surface [104]. At values above 30%, more pores begin to appear on the fibers. It was thus found that the porosity and pore diameter increase with increasing moisture [40].
The effects of humidity and temperature when obtaining cellulose acetate (CA) and poly (vinylpyrrolidone) (PVP) fibers, respectively, have also been studied [105]. For CA, the fiber diameter increased with increasing humidity, while the diameter of PVP fibers decreased due to the absorption of water from the environment, which induced a slower solidification, and therefore a longer jet elongation time, resulting in the formation of fibers with thinner diameters. However, at a high relative humidity of 60%, PVP nanofibers begin to adhere to each other, thus resulting in larger apparent diameters.
In CA, with increasing RH, water absorption leads to faster precipitation, leading to the formation of larger diameters. The two materials follow opposite trends due to different interactions between the polymer/solvent system and water vapor, causing the evaporation of the solvent and the solidification rate to react differently.
In summary, electrospinning represents an advanced fiber preparation technology based on the interaction between a polymeric solution and an electric field, generating fibers as products [34,106]. A schematic depiction of the process is presented in Figure 2.
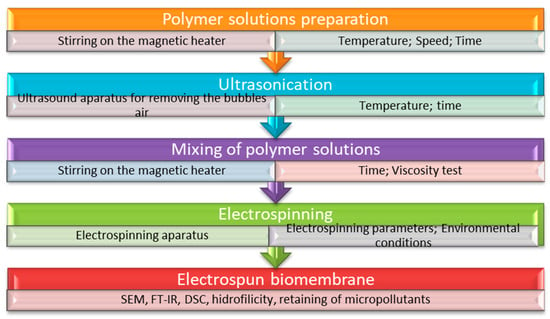
Figure 2.
Electrospinning process steps applied for membrane fabrication.
The electrospinning device works based on three principles that represent the operating steps [107]. (1) Extrusion of polymeric liquid from the syringe through the spinneret. Here, the droplet has a spherical shape at the tip of the spinneret due to the balance between surface tension and gravitational acceleration. (2) The morphology of the droplet changes, with the application of electric field force, from a spherical to a conical shape with increasing electric charge, a phenomenon based on gravitational acceleration and Coulomb force, which must be greater than the surface tension to generate a straight jet forming the cone. (3) According to the principle of Rayleigh instability and the interaction of positive charges on the jet surface in the electric field, the duration during which the jet is straight is short, resulting in deflection. Based on the Coulomb repulsive force and gravity, the jet in the deflection section will solidify rapidly at the same time as the solvent evaporates.
The use of electrospun membranes in the treatment of wastewater contaminated with emerging pollutants such as antibiotics has been studied intensively in recent years. Thus, it is possible to establish, on the basis of the results presented so far in the literature, specific conditions that need to be met when using electrospun membranes [34,108,109,110,111,112,113,114,115].
It is important that the polymer used to obtain the fibers to be used as membranes be environmentally friendly, biodegradable, and without secondary environmental pollution. Additionally, the obtained membranes have to possess selectivity for the target pollutants, which can be developed by functionalization with specific groups. Thus, the polymers used for electrospinning can be adsorbent, and can be used as such; additionally, substances possessing specific adsorbent functional groups can be added to the electrospinning solution, or the obtained membrane can be subjected to post-treatment techniques after the electrospinning process, such as coating [108,109], heat treatment [110,111], and cross-linking [112,113,114,115].
Other essential conditions for obtaining these electrospun membranes include water insolubility combined with hydrophilicity of the polymers, as well as good mechanical properties and chemical stability in the obtained fibers.
4.2. Materials
Because fibers with nanometric scale demonstrate high efficiency in wastewater treatments, we focus on their properties and structures. These types of materials can be obtained using a variety of techniques, as described by Ahmed and colleagues [40] in their review, including self-assembly and electrospinning as emerging techniques [116,117,118], drawing [119,120,121,122], template synthesis [103,123,124,125], and phase separation [126,127,128]. The type of polymer controls the morphology, shape, size, and strength of the nanofibers. Within an appropriate operation control, the obtained nanofibers exhibit porosity, favorable conditions for functionalization with nanoparticles, defect-free fibers, rigidity, and tensile strength. Electrospinning parameters, including polymer solution properties, can be changed and controlled in order to obtain different nanofiber morphologies.
Electrospun nanofibers are excellent candidates for use as membranes in water and wastewater treatment, including dye degradation [129,130,131,132], heavy metal ion adsorption [133,134,135], oily water separation [136,137], and microbial disinfection [130,138,139]. There are many types of materials, and these are briefly presented in Figure 3.
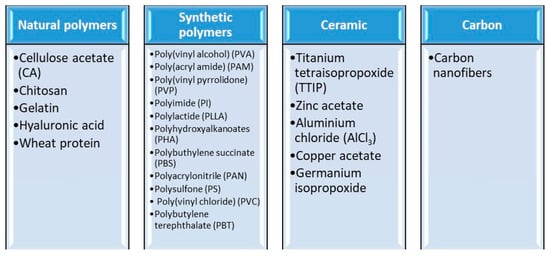
Figure 3.
Main materials applied for the electrospinning process.
Nanofibers exhibit new and advanced properties that can be enhanced by their functionalization. For example, due to their high specific surface area, nanoparticles, nanorods, nanowires, nanotubes, nanosheets, zeolites, and metal–organic frameworks can be immobilized, allowing the high-selectivity removal of water contaminants [140]. The functionalization can be performed through the direct electrospinning of the nanofibers using self-assembly techniques. In addition to this, nanofibers can be packed into a fixed-bed column or use membrane reactors, depending on the requirements of the separation process.
4.2.1. Polymers
Usually, organic polymers are dissolved in appropriate solvents and used directly as solutions, or are melted without degradation. The advantage of using electrospun solutions is the formation of a stretched, elongated, and thinned polymer solution jet, where the solvent is evaporated, and the fibers are collected on a support [68].
Electrospun polymer solutions made from natural polymers (collagen [141,142,143,144], gelatin [145], chitosan [146,147], hyaluronic acid [148], silk fibrion [149], and/or synthetic polymers poly(lactic acid) (PLA) [150], polyurethane (PU) [151,152], poly(ε-caprolactone) (PCL) [152,153,154], poly(lactic-coglycolic acid) (PLGA) [155,156,157], poly(ethylene-co-vinylacetate) (PEVA) [158], and poly(l-lactide-co-ε-caprolactone) (PLLA-CL) [159,160] have been studied intensively. There are more than 100 polymers that can be used either individually or as a mixture; thus, the final product will mainly be a polymer fiber or a ceramic [161].
Synthetic polymers have demonstrated their high capacity for being electrospun into nanofibers, such as polystyrene (PS) and poly(vinyl chloride) (PVC), with good results in environmental protection applications. Additionally, natural biopolymers, such as silk fibroin, fibrinogens, dextran, chitin, chitosan, alginate, collagen, and/or gelatin, can be used for nanofiber preparation in various applications. Additionally, conductive polymers such as polyaniline (PANi) and polypyrrole (PPy) are able to be electrospun into nanofibers as well as poly(vinylidene fluoride) (PVDF) [68].
Among these, synthetic polymers are still used, possessing the advantages of low cost, high mechanical properties, ease of production. Some disadvantages, such as long-term health and environmental impacts due to their toxicity, nonbiodegradability, and disposal, have to be noted. In terms of waste minimization, there is an urgent need to replace them with recyclable and biodegradable green materials [140,162,163].
Additionally, some polymers that are insoluble solvents appropriate for electrospinning, such as polyethylene and polypropylene (PP), are melted and electrospun directly [164].
To be melted, a polymer has to be thermally stable (except for thermoset polymers and proteins) and non-degradable, such as thermoplastic polymers (e.g., PP) and polyesters (e.g., polyurethane, PCL, PLA, and PLGA). In the case of PCL, which has a low melting point, the thermal stability and processability are suitable for the melting electrospinning process. There are some common industrial polymers with adequate melting capacity for electrospinning: nylon-6, polyethylene, poly(methyl methacrylate) (PMMA), and poly (ethylene terephthalate) (PET). Usually, the melting process for a polymer prior to electrospinning depends on its viscosity and electrical conductivity [165].
Additionally, classes of polymers such as polyolefins and polyamides, soluble in specific solvents, are mostly processed into nanofibers using the melting electrospinning process.
CS is widely applied as a biopolymer in wastewater treatment, and its ability to be electrospun makes it a more interesting material, with a high capacity for the adsorption/degradation of different pollutants, including pharmaceutical compounds. Pure chitosan dissolved in acetic acid solvent can be used to prepare nanofibers, and subsequently membranes, for wastewater treatment [1,166]. Usually, the fiber diameter is proportionally linked with the concentration of the solution, and only 2% or less of CS is acceptable in order to obtain homogenous nanofibers with higher molecular weight, as the other polymer-processing conditions and molecular weight, together with deacetylation degree, are crucial for nanofiber processing [167].
The functionality of CS can be enhanced by mixture with other polymers and metals or metal oxides by means of amino groups [168]. For example, PEO, a synthetic polyether, was used to prepare 80:20 wt% CS/PEO solution for the removal of pharmaceuticals such as ibuprofen [169] and CS/PEO/permutit electrospun nanofibers [170]. A commonly used mixture for wastewater treatment is CS/PVA (polyvinyl alcohol), with PVA being known as a synthetic polymer that is soluble in water, and is usually employed as a film. CS/PVA nanofibers at different concentrations and temperatures were prepared using the electrospinning technique, with the tetracycline (TC) being degraded on this nanofiber [171,172,173,174,175,176,177]. Mixture blends with different compounds lead to different classes of adsorbents, such as the immobilization of ZnO into PVA/Alg/CS polymeric composite nanofibers for phenol degradation [178].
β-cyclodextrin (β-CD) is an excellent compound that is useful for the adsorption of large ions from water, and membranes prepared with electrospun β-cyclodextrin (β-CD)/chitosan/PVA nanofibers are capable of simultaneously removing organic and inorganic pollutants, the most notable of which is Bisphenol A (BPA) [179]. An important feature of these membranes is their decreasing porosity with increasing thickness. A number of CS formulations have been studied, including CS/PVA [171], CS/polyethylene oxide (PEO) [169], β-CD/CS/PVA [179], M-ZnO/PVA/Alg/CS [178], and CS-g-PNVCL/ZIF-8 [180], in order to analyze the adsorption efficiencies on pharmaceutical compounds (tetracycline, ibuprofen, Bisphenol A, and phenol).
Good adsorption capacities were obtained, with β-CD/CS/PVA nanofiber being compatible for integration as a membrane in drinking water treatments [179].
Usually, cyclodextrin derivatives induce high viscosity in solution, as it is able to form aggregates via hydrogen bonding, and possesses a viscoelastic solid-like behavior [68].
Cyclodextrin (CD) enhances the removal capacity of PAN nanofibers (91.46% for atrazine) and polyethersulfone (PES) nanofibers (for steroid hormones), and has also been investigated in combination with triglycidyl ether and triphenylmethane triglycidyl ether as crosslinkers (95% for estradiol). It was observed that when the diameter is decreased from 557 nm to 497 nm, in the case of CD-PAN, the specific surface area increases [140]. Additionally, the synergistic effect of hydrophobic interaction and hydrogen bonding influences the removal process in the case of CD-PES [181,182,183].
PVA and CD are used for the removal of emerging pollutants from wastewaters, especially when cyclodextrins (CD) act as macrocyclic hosts, and their high surface area makes them optimal substrates for the adsorption of large molecules. Additionally, PVA decorated with alkali lignin, after thermo-stabilization, offers efficient antibiotics removal [184].
Cellulose is a sustainable polymer that can be applied to obtain nanofibers [185,186,187,188,189], and has been studied intensively in recent years. In addition to the use of ionic liquids to produce cellulose fibers, the electrospinning method is also applied [185,190]. One important aspect is the defects that can appear at the nanoscale level [191]. It has been observed that low-density nanocellulose and the reactive surface of –OH groups allow the grafting of chemical species in order to acquire new functionalities as substrates for wastewater treatment [191].
Some examples of cellulose electrospun nanofibers configured as membrane systems are based on the use of amino-ionic liquids (ILs). For example, polyvinylidene fluoride-cohexafluoropropylene (PVP-HFP) nanofibers modified with cellulose using [Emim]Ac as ionic liquid possess good porosity, pore size, wettability, mechanical and thermal strength for use as nanofibers for oil separation [192]. Other examples are based on ethylcellulose nanofibrous matrix doped with ionic liquid [Emim]BF4 or electrically conductive cellulose nanofibers containing carbon nanotubes; the ionic liquid [Bmim]Cl was used to dissolve cellulose solution, for application in water desalination [185,193,194].
Cellulose acetate/cellulose triacetate (CA/CTA), along with other polymers such as polyamide, polysulphone (Psf), etc., have been used for FO membrane design [195].
There are commercial CTA membranes for application in FO that exhibit a water flux and salt rejection of 96% in comparison with RO [196]. Double-skinned CA membranes reduce fouling and exhibit high salt rejection due to their dense layer structure [197,198,199].
Various parameters influence the morphology and properties of the membrane, such as polymer–solvent concentration, evaporation and annealing time, casting substrate, coagulation time, etc. [200,201]. The functional groups play an essential role in separation efficiency, including acetyl, hydroxyl, propionyl, and butyryl groups [195].
Polysulfone (PSf) is another polymer widely applied as an electrospun substrate in the fabrication of thin-film nanocomposite membranes, especially for FO. It exhibits excellent mechanical, thermal and chemical stabilities, with the disadvantage of fouling due to its hydrophobic properties [195,202,203]. Mixture with modifiers or hydrophilic nanofillers, such as oxides (titanium and silica), can be carried out to boost hydrophilicity [204]. Furthermore, the addition of graphene to Psf leads to pH and chemical resistance due to the sulphonyl group [205].
Polyethersulfone (PES) is also known to be an electrospinnable polymer substrate, especially for FO membranes, possessing good thermal, chemical and mechanical properties, as well as pH resistance [206].
The PES membrane increases the porosity, and improves permeability and tensile strength, and mixture with carbon nanotubes was demonstrated to be efficient for seawater desalination and wastewater treatment [195].
There are various polymers that are included in membrane system configurations, and their electrospinnability properties are used in order to obtain controlled nanostructures. Thus, the polymers act as a substrate both for TFC membranes (used in membrane separation processes) and for the integration of other active compounds (used in adsorption and degradation/advanced oxidation processes).
4.2.2. Composites Using Carrier Polymers as Substrate
Nano or colloidal particles, as well as other small molecules, can be electrospun into fibers with the help of polymer solutions as carriers. The process is sustained by adequate intramolecular interactions when self-assembled structures appear [68].
Composites are prepared by adding sol−gel precursors or other nanoparticles into polymer electrospinnable solutions, where the polymer has a “carrier” function. The electrospinning process is linked to the characteristics of the sol−gel precursor and the carrier polymer, in order to obtain adequate composite nanofibers [68].
An inorganic phase is built up as a continuous network in the polymer matrix, and the formation of inorganic−polymer composite nanofibers takes place. PVP is one of the polymers most widely used as a carrier polymer due to its high solubility in ethanol and water and good affinity for different sol−gel precursors. In addition to this, PEO, poly(vinyl alcohol) (PVA), and poly(acrylic acid) are also used [207,208,209]. The viscosity and electrical conductivity of the solution are important characteristics for obtaining an electrospinnable solution, and the carrier polymer has to expose high Mw [68,210]. Salts such as NaCl and (CH3)4NCl) ensure electrical conductivity of the solution and determine whether thin fibers are obtained. For example, nanofibers from PVP and amorphous TiO2 were produced from a mixture of PVP and titanium tetraisopropoxide (Ti(OiPr)4, as a precursor for TiO2, dissolved in alcohol [207]. The sol−gel precursor reaction (hydrolysis, condensation, and gelation) has to begin when the electrospinning jet reaches the surrounding air [68,207,208]. The sol–gel reactions are influenced by precursor type; thus, rapid hydrolysis results in the clogging of the spinneret, and a quick gelation creates a non-flexible jet and thicker fibers [208]. Precursors such as alkoxides, nitrates, acetates, chlorides, and sulfates are intensively used, and in order to ensure a proper hydrolysis and/or gelation process, additives such as acetic acid, hydrochloric acid, or propionic acid can be added [211,212]. Additionally, composites were prepared by dispersing nanoscale components into polymer solutions through stirring or ultrasonication. Some well-known examples include Ag, Au, TiO2, and SiO2 nanoparticles, carbon nanotubes, clay tablets or metal−organic framework (MOF) compounds [213]. The electrospinning process depends on the morphology, size and concentration of the nanocomponents. It is necessary to ensure a stable dispersion in the polymer solution of the nanoscale components in order to obtain homogeneous nanofibers. Ag nanoparticles can be well dispersed in an aqueous PVA solution or PVP to obtain controlled composite nanofibers, and SiO2 particles dispersed in PVA solution fibers composite fibers with a necklace-like structure [213,214,215].
TiO2 nanofibers are deposited onto a polyvinylidene difluoride (PVDF) membrane as a support. This type of material, as a composite, was tested for the degradation of emerging pollutants. Good results were achieved for BPA degradation using a UV light source, with an efficiency of about 63–85% [140,216]. Additionally, the electrospinning method was applied for the preparation of ZnO–carbon composite nanofibers, using PAN, polystyrene (PS) and polyvinyl pyrrolidone (PVP) as precursors for caffeine (80.4%) and diclofenac (79.5%) removal under photocatalytic degradation [217]. A TiO2 nanofiber layer with a thickness of between 10 and 29 µm was embedded on a stainless-steel filter, with PVDF in between as a binder, was tested, and demonstrated high efficiency for cimetidine (90%) [218]. TiO2 photocatalysts are classified into two arrangements, suspended and immobilized in/on the support, with higher photocatalytic capacity for absorbing more UV light in the case of suspended TiO2 due to the high surface area in contact with pollutants, but with the disadvantage of a low recovery rate from solution. Satisfactory results were obtained for photocatalytic oxidation of BPA (84.53%) using a PVDF/TiO2 nanocomposite membrane with a uniform structure [216]. The composite membrane presents a porous-like structure, and TiO2 was immobilized on a PVDF substrate.
Numerous emerging contaminants were evaluated as antibiotics in terms of their degradation efficiency (over 90%) when PAN nanofibers with TiO2 nanoparticles dispersed in the polymeric matrix were applied as nanofiber composite filters [140,219]. The dispersant of the nanoparticles was phthalic acid, which increased the diameter of the nanofibers and their porosity by means of high viscosity and volatilization rate.
TiO2 nanoparticles can be added to the polymer solution of PS in order to obtain a hydrophilic electrospun membrane. This structure is then coated with nano g-C3N4 using melamine as the precursor, and a core–shell CNCT functional nanofiber membrane is obtained for TC and Escherichia coli degradation [34,220]. Additionally, a high concentration of metal nanoparticles dispersed in polymer solution results in a spinnable mixture. Ag nanoparticles were attached to the surface of PAN fibers, using AgNO3 precursor solution and irradiation of the fibers under a UV lamp to reduce Ag+ to Ag nanoparticles [221].
Colloidal particles can be electrospun when inorganic sols are the result of the hydrolysis and condensation of metal alkoxides, or when metal salts possess viscosity. A silica sol obtained from tetraethyl orthosilicate (TEOS) as a precursor generates silica fibers with sizes between 0.4 and 1 μm or smaller than 400 nm, depending on the working parameters [68,222].
Ag nanoparticles were directly dispersed in ethylene glycol, and after thermal annealing, conductive Ag nanofibers were obtained [68].
The electrospinning method was also applied for hollow and porous Fe-doped PAN nanofibers developed for the removal of BPA. The nanoparticles were uniformly immobilized, and 100% BPA removal was achieved [140,223]. To degrade the emerging pollutants, nanofiber membranes can be used in electrochemical processes. For example, antimony tin oxide doped with ruthenium oxide (ATO-RO) nanoparticles was integrated into PVP nanofibers. The material acts as the anode for electrochemical BPA degradation [224]. Additionally, TC solutions were subjected to degradation using Fe/Co alloy on PVP nanofibers, with efficiencies of up to 100%. Efficiencies over 95% were registered when tubular carbon nanofibers with activated alumina were integrated into a PVC support and used as the anode material for the electrocoagulation process [225,226].
Electrospun composites based on carbon were recently developed for the adsorption of antibiotics. Carbon nanofiber (CNF)–carbon nanotube (CNT) composites, with high specific surface area and mechanical strength due to the nanofibers, exhibited high uptake capacity (>90%) and fast kinetics [140,227].
4.3. Performances of Electrospun Materials Used for Adsorption and Advanced Degradation Processes
The literature already offers solutions, in addition to existing membrane systems, of combined methods for the retention of emerging pollutants using electrospun membrane structures, decorated with nanoparticles with adsorbent or photocatalyst role [140]. However, a classification according to the type of category or emerging pollutant, compared to other types of membrane systems, has not been made. Nanofibers act as adsorbents for emerging pollutants. Through their structure and appearance, they can be designed to incorporate target compounds with advanced properties due to their functional groups, and their high specific surface area, which possesses a high selectivity that subsequently acts on the degradation process of pollutants. Thus, once immobilized on the nanofiber surface, emerging pollutants can be degraded by advanced oxidation, or catalytic or electrochemical degradation processes. Nanofibers can act as a carrier for reactive nanoparticles with high degradation potential, but which, when used alone in the system, may have a tendency to agglomerate, reducing their effect, or which, due to their tendency to disperse in aqueous media, can pass through classical filtration systems into natural systems, their size making them difficult to collect from waters. Thus, their incorporation into nanofibers leads to the development of adsorption systems and the simultaneous degradation of emerging pollutants. Another important aspect is the possibility of regenerating these functionalized nanofibers and using them for multiple cycles.
Additionally, as was mentioned, electrospun fibers can be integrated into TFC as support due to their mechanical properties and flexibility, and the electrospun structures (micro- and/or nano-dimensional in size) are responsible for membrane separation phenomena, in addition to adsorption or photo- and electro-catalytic degradation [40].
In summary, the most significant decontamination processes in which nanofibers have been tested are presented in Table 2, with a focus on the main EP types and categories.

Table 2.
Examples of nanofibers with efficiencies in adsorption and advanced decontamination processes for EP.
4.4. Performances of Electrospun Materials Integrated into Separation Membranes Processes
There are many types of membranes used in the separation (filtration) or adsorption retention of emerging pollutants. Among these, the type of membrane used in these processes is very important, as they must meet certain characteristics regarding pore size, active surface, permeability, material type. Thus, today, a variety of materials based on polymers, ceramics, zeolites, etc., are known to be used in the manufacture of membranes. Ultrafiltration (UF), microfiltration (MF), nanofiltration (NF), reverse osmosis (RO), and forward osmosis (FO) are defined in the literature as processes that are dependent on pore size, morphology, and specific characteristics of the type of water and pollutants [2]. In Table 3, the most significant materials integrated into membrane processes are presented.

Table 3.
Membrane materials used in EP separation processes.
It can be seen that membranes are an effective means of reducing emerging pollutants during both drinking water and wastewater treatment. The degree of removal is directly related to the characteristics of the membrane and, to some extent, to the molecular properties of the contaminant in question [271].
As a rule, microfiltration/ultrafiltration (MF/UF) systems are recommended primarily due to space limitations, and show different efficiencies depending on the class of emerging pollutants. As a rule, for organic compounds, performance is limited unless coupled with RO, but there are promising results in the removal of steroids.
Nanofiltration and reverse osmosis are effective for the removal of emerging pollutants although some compounds could be detected in traces in the permeate. In addition, continuing concerns about possible quality of life impacts of emerging pollutants require viable solutions to be found and tested, and these techniques are proving effective.
5. Conclusions
The implementation of different functional membranes in water treatment is greatly increasing, with a focus on emerging pollutants.
The most used materials applied in membrane design are polymers, both as support for TFC, with application in separation processes, and also as functional materials applied in adsorption and other advanced processes. Electrospinning represents a facile and adequate method for fiber preparation (especially nanosized), where surface characteristics and functionalization enhance material properties. Using electrospun fibers, various emerging pollutants can be degraded, especially pharmaceutical compounds. Some reliable results have been reported on the use of electrospun membranes for separation processes in pilot and/or full-scale applications. An significant number of efficient electrospun nanofibers have been investigated in the literature in systems testing adsorption, advanced photodegradation, and other advanced processes. Good characteristics of the fibers include their reusability and their controlled surface area.
Due to their performances, an integrated holistic strategy could be developed at an industrial level in order to control and preserve the aquatic environment when emerging pollutants threaten the ecosystem’s equilibrium. Setting new standards for the quality of wastewater treatment is dependent on material performances that contribute to water management systems. Research should be focused on the development of hybrid systems for degradation and removal of emerging pollutants from wastewaters.
Author Contributions
Conceptualization, E.M.; methodology, C.I.C.-M.; validation, C.P. and M.R.; investigation, A.M.P. and A.A.Ţ.; writing—original draft preparation, E.M.; writing—review and editing, C.I.C.-M. and M.R.; visualization, A.M.P. and A.A.Ţ.; supervision, M.R.; funding acquisition, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by EU Horizon 2020 (InNoPlastic), GA no. 101000612.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sakib, M.N.; Mallik, A.K.; Rahman, M.M. Update on chitosan-based electrospun nanofibers for wastewater treatment: A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100064. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Nataraj, S.K.; Nadagouda, M.; Reddy, K.R.; Shukla, S.S.; Aminabhavi, T.M. Membrane-based separation of potential emerging pollutants. Sep. Purif. Technol. 2019, 210, 850–866. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Mueller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Nasseri, S.; Ebrahimi, S.; Abtahi, M.; Saeedi, R. Synthesis and characterization of polysulfone/graphene oxide nano composite membranes for removal of bisphenol A from water. J. Environ. Manag. 2018, 205, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Gilca, A.F.; Teodosiu, C.; Fiore, S.; Musteret, C.P. Emerging disinfection byproducts: A review on their occurrence and control in drinking water treatment processes. Chemosphere 2020, 259, 127476. [Google Scholar] [CrossRef]
- Jones, O.A.H.; Voulvoulis, N.; Lester, J.N. Human pharmaceuticals in wastewater treatment processes. Crit. Rev. Environ. Sci. Technol. 2005, 35, 401–427. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; Van der Ploeg, M.; Van de Zee, S.E.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Available online: www.norman-network.net (accessed on 11 November 2021).
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and fate of emerging pollutants in water environment and options for their removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Stone, V.; Donaldson, K. Signs of stress. Nat. Nanotechnol. 2006, 1, 23–24. [Google Scholar] [CrossRef]
- Ankley, G.; Hoff, D.; Mount, D.; Lazorchak, J.; Beaman, J.; Linton, T.; Erickson, R. Aquatic Life Criteria for Contaminants of Emerging Concern; Prepared by the Office of Water and Office of Research and Development Emerging Contaminants Workgroup; US Environmental Protection Agency: Washington, DC, USA, 2008; Part I; pp. 1–46.
- Kaur, H.; Hippargi, G.; Pophali, G.R.; Bansiwal, A.K. Treatment methods for removal of pharmaceuticals and personal care products from domestic wastewater. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 129–150. [Google Scholar]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Chaminda, G.T.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, M.; Yang, W.; Li, H.; Zhong, Y.; Mo, L.; Liang, Y.; Ma, X.; Sun, X. Emerging pollutants in water environment: Occurrence, monitoring, fate, and risk assessment. Water Environ. Res. 2019, 91, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—Degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Emerging contaminants: Here today, there tomorrow! Environ. Nanotechnol. Monit. Manag. 2018, 10, 122–126. [Google Scholar] [CrossRef]
- Richardson, S.D. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2009, 81, 4645–4677. [Google Scholar] [CrossRef]
- Suárez, S.; Carballa, M.; Omil, F.; Lema, J.M. How are pharmaceutical and personal care products (PPCPs) removed from urban wastewaters? Rev. Environ. Sci. Bio/Technol. 2008, 7, 125–138. [Google Scholar] [CrossRef]
- Tondera, K.; Blecken, G.-T.; Tournebize, J.; Viklander, M.; Österlund, H.; Wikström, A.A.; Tanner, C.C. Emerging contaminants: Occurrence, treatment efficiency and accumulation under varying flows. In Ecotechnologies for the Treatment of Variable Stormwater and Wastewater Flows; Springer: Berlin/Heidelberg, Germany, 2018; pp. 93–109. [Google Scholar]
- Radjenović, J.; Petrović, M.; Ventura, F.; Barceló, D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008, 42, 3601–3610. [Google Scholar] [CrossRef]
- Anastasi, A.; Spina, F.; Romagnolo, A.; Tigini, V.; Prigione, V.; Varese, G.C. Integrated fungal biomass and activated sludge treatment for textile wastewaters bioremediation. Bioresour. Technol. 2012, 123, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Tadkaew, N.; Hai, F.I.; McDonald, J.A.; Khan, S.J.; Nghiem, L.D. Removal of trace organics by MBR treatment: The role of molecular properties. Water Res. 2011, 45, 2439–2451. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef] [PubMed]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef] [Green Version]
- Ferrando-Climent, L.; Gonzalez-Olmos, R.; Anfruns, A.; Aymerich, I.; Corominas, L.; Barceló, D.; Rodriguez-Mozaz, S. Elimination study of the chemotherapy drug tamoxifen by different advanced oxidation processes: Transformation products and toxicity assessment. Chemosphere 2017, 168, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Von Gunten, U. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003, 37, 1469–1487. [Google Scholar] [CrossRef]
- Cruz-Morató, C.; Lucas, D.; Llorca, M.; Rodriguez-Mozaz, S.; Gorga, M.; Petrovic, M.; Barceló, D.; Vicent, T.; Sarrà, M.; Marco-Urrea, E. Hospital wastewater treatment by fungal bioreactor: Removal efficiency for pharmaceuticals and endocrine disruptor compounds. Sci. Total Environ. 2014, 493, 365–376. [Google Scholar] [CrossRef]
- Miralles-Cuevas, S.; Oller, I.; Agüera, A.; Llorca, M.; Pérez, J.S.; Malato, S. Combination of nanofiltration and ozonation for the remediation of real municipal wastewater effluents: Acute and chronic toxicity assessment. J. Hazard. Mater. 2017, 323, 442–451. [Google Scholar] [CrossRef]
- Riaz, T.; Ahmad, A.; Saleemi, S.; Adrees, M.; Jamshed, F.; Hai, A.M.; Jamil, T. Synthesis and characterization of polyurethane-cellulose acetate blend membrane for chromium (VI) removal. Carbohydr. Polym. 2016, 153, 582–591. [Google Scholar] [CrossRef]
- Zhao, K.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Electrospun functional nanofiber membrane for antibiotic removal in water. Polymers 2021, 13, 226. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Yu, D.-G.; Zhu, M.-J.; Zhao, M.; Williams, G.R. Tunable zero-order drug delivery systems created by modified triaxial electrospinning. Chem. Eng. J. 2019, 356, 886–894. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, W.; Yu, D.-G.; Wang, G.; Williams, G.R.; Zhang, Z. Tunable drug release from nanofibers coated with blank cellulose acetate layers fabricated using tri-axial electrospinning. Carbohydr. Polym. 2019, 203, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Sagitha, P.; Reshmi, C.; Sundaran, S.P.; Sujith, A. Recent advances in post-modification strategies of polymeric electrospun membranes. Eur. Polym. J. 2018, 105, 227–249. [Google Scholar] [CrossRef]
- An, S.; Jeon, B.; Bae, J.H.; Kim, I.S.; Paeng, K.; Kim, M.; Lee, H. Thiol-based chemistry as versatile routes for the effective functionalization of cellulose nanofibers. Carbohydr. Polym. 2019, 226, 115259. [Google Scholar] [CrossRef]
- Qin, D.; Lu, W.; Wang, X.; Li, N.; Chen, X.; Zhu, Z.; Chen, W. Graphitic carbon nitride from burial to re-emergence on polyethylene terephthalate nanofibers as an easily recycled photocatalyst for degrading antibiotics under solar irradiation. ACS Appl. Mater. Interfaces 2016, 8, 25962–25970. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef] [Green Version]
- Ravanchi, M.T.; Kaghazchi, T.; Kargari, A. Application of membrane separation processes in petrochemical industry: A review. Desalination 2009, 235, 199–244. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L. Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Hai, F.I.; Nghiem, L.D.; Modin, O. Biocatalytic membrane reactors for the removal of recalcitrant and emerging pollutants from wastewater. Handb. Membr. React. 2013, 763–807. [Google Scholar] [CrossRef]
- Fitch, M.; Neeman, J.; England, E. Mass transfer and benzene removal from air using latex rubber tubing and a hollow-fiber membrane module. Appl. Biochem. Biotechnol. 2003, 104, 199–214. [Google Scholar] [CrossRef]
- Sundarrajan, S.; Balamurugan, R.; Kaur, S.; Ramakrishna, S. Potential of engineered electrospun nanofiber membranes for nanofiltration applications. Dry. Technol. 2013, 31, 163–169. [Google Scholar] [CrossRef]
- Vrentas, J.; Vrentas, C. Transport in nonporous membranes. Chem. Eng. Sci. 2002, 57, 4199–4208. [Google Scholar] [CrossRef]
- Geise, G.M.; Lee, H.S.; Miller, D.J.; Freeman, B.D.; McGrath, J.E.; Paul, D.R. Water purification by membranes: The role of polymer science. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1685–1718. [Google Scholar] [CrossRef]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of membrane support layer hydrophobicity on water flux in osmotically driven membrane processes. J. Membr. Sci. 2008, 318, 458–466. [Google Scholar] [CrossRef]
- Kaur, S.; Sundarrajan, S.; Rana, D.; Matsuura, T.; Ramakrishna, S. Influence of electrospun fiber size on the separation efficiency of thin film nanofiltration composite membrane. J. Membr. Sci. 2012, 392, 101–111. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hoek, E.M. Impacts of support membrane structure and chemistry on polyamide–polysulfone interfacial composite membranes. J. Membr. Sci. 2009, 336, 140–148. [Google Scholar] [CrossRef]
- Yoon, K.; Kim, K.; Wang, X.; Fang, D.; Hsiao, B.S.; Chu, B. High flux ultrafiltration membranes based on electrospun nanofibrous PAN scaffolds and chitosan coating. Polymer 2006, 47, 2434–2441. [Google Scholar] [CrossRef]
- Kaur, S.; Sundarrajan, S.; Gopal, R.; Ramakrishna, S. Formation and characterization of polyamide composite electrospun nanofibrous membranes for salt separation. J. Appl. Polym. Sci. 2012, 124, E205–E215. [Google Scholar] [CrossRef]
- Tang, Z.; Wei, J.; Yung, L.; Ji, B.; Ma, H.; Qiu, C.; Yoon, K.; Wan, F.; Fang, D.; Hsiao, B.S. UV-cured poly (vinyl alcohol) ultrafiltration nanofibrous membrane based on electrospun nanofiber scaffolds. J. Membr. Sci. 2009, 328, 1–5. [Google Scholar] [CrossRef]
- Wang, X.; Fang, D.; Yoon, K.; Hsiao, B.S.; Chu, B. High performance ultrafiltration composite membranes based on poly (vinyl alcohol) hydrogel coating on crosslinked nanofibrous poly (vinyl alcohol) scaffold. J. Membr. Sci. 2006, 278, 261–268. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Fabrication and characterization of cellulose nanofiber based thin-film nanofibrous composite membranes. J. Membr. Sci. 2014, 454, 272–282. [Google Scholar] [CrossRef]
- Hoover, L.A.; Schiffman, J.D.; Elimelech, M. Nanofibers in thin-film composite membrane support layers: Enabling expanded application of forward and pressure retarded osmosis. Desalination 2013, 308, 73–81. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z.; Sun, D.D. Nano gives the answer: Breaking the bottleneck of internal concentration polarization with a nanofiber composite forward osmosis membrane for a high water production rate. Adv. Mater. 2011, 23, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Qiu, C.; Liao, Y.; Chou, S.; Wang, R. Preparation of polyamide thin film composite forward osmosis membranes using electrospun polyvinylidene fluoride (PVDF) nanofibers as substrates. Sep. Purif. Technol. 2013, 118, 727–736. [Google Scholar] [CrossRef]
- Bui, N.-N.; Lind, M.L.; Hoek, E.M.; McCutcheon, J.R. Electrospun nanofiber supported thin film composite membranes for engineered osmosis. J. Membr. Sci. 2011, 385, 10–19. [Google Scholar] [CrossRef]
- Bui, N.-N.; McCutcheon, J.R. Hydrophilic nanofibers as new supports for thin film composite membranes for engineered osmosis. Environ. Sci. Technol. 2013, 47, 1761–1769. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Tijing, L.D.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Recent progress of membrane distillation using electrospun nanofibrous membrane. J. Membr. Sci. 2014, 453, 435–462. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Shirazi, M.M.A.; Bastani, D.; Kargari, A.; Tabatabaei, M. Characterization of polymeric membranes for membrane distillation using atomic force microscopy. Desalination Water Treat. 2013, 51, 6003–6008. [Google Scholar] [CrossRef]
- Demir, M.M.; Yilgor, I.; Yilgor, E.; Erman, B. Electrospinning of polyurethane fibers. Polymer 2002, 43, 3303–3309. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- GuptaV, B.; Kothari, V. Manufactured Fiber Technology; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Luo, C.; Stoyanov, S.D.; Stride, E.; Pelan, E.; Edirisinghe, M. Electrospinning versus fibre production methods: From specifics to technological convergence. Chem. Soc. Rev. 2012, 41, 4708–4735. [Google Scholar] [CrossRef]
- Lee, S.; Kay Obendorf, S. Developing protective textile materials as barriers to liquid penetration using melt-electrospinning. J. Appl. Polym. Sci. 2006, 102, 3430–3437. [Google Scholar] [CrossRef]
- Han, X.J.; Huang, Z.M.; He, C.L.; Liu, L.; Wu, Q.S. Coaxial electrospinning of PC (shell)/PU (core) composite nanofibers for textile application. Polym. Compos. 2006, 27, 381–387. [Google Scholar] [CrossRef]
- Ma, M.; Mao, Y.; Gupta, M.; Gleason, K.K.; Rutledge, G.C. Superhydrophobic fabrics produced by electrospinning and chemical vapor deposition. Macromolecules 2005, 38, 9742–9748. [Google Scholar] [CrossRef]
- Virovska, D.; Paneva, D.; Manolova, N.; Rashkov, I.; Karashanova, D. Electrospinning/electrospraying vs. electrospinning: A comparative study on the design of poly (l-lactide)/zinc oxide non-woven textile. Appl. Surf. Sci. 2014, 311, 842–850. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.J.; Park, W.H. Electrospinning of polysaccharides for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1020–1032. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.-H.; Yu, D.-G.; Williams, G.R. Tunable biphasic drug release from ethyl cellulose nanofibers fabricated using a modified coaxial electrospinning process. Nanoscale Res. Lett. 2014, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Russo, G.; Lamberti, G. Electrospinning of drug-loaded polymer systems: Preparation and drug release. J. Appl. Polym. Sci. 2011, 122, 3551–3556. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.-H.; Wu, D.-Q.; Chu, C.-C. Dual functions of polyvinyl alcohol (PVA): Fabricating particles and electrospinning nanofibers applied in controlled drug release. J. Nanoparticle Res. 2013, 15, 1–14. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Y.; Yang, M. Polyaniline nanofiber humidity sensor prepared by electrospinning. Sens. Actuators B Chem. 2012, 161, 967–972. [Google Scholar] [CrossRef]
- Landau, O.; Rothschild, A.; Zussman, E. Processing-microstructure-properties correlation of ultrasensitive gas sensors produced by electrospinning. Chem. Mater. 2009, 21, 9–11. [Google Scholar] [CrossRef]
- Zanin, M.H.A.; Cerize, N.N.; de Oliveira, A.M. Production of nanofibers by electrospinning technology: Overview and application in cosmetics. In Nanocosmetics and Nanomedicines; Springer: Berlin/Heidelberg, Germany, 2011; pp. 311–332. [Google Scholar]
- Camerlo, A.; Vebert-Nardin, C.; Rossi, R.M.; Popa, A.-M. Fragrance encapsulation in polymeric matrices by emulsion electrospinning. Eur. Polym. J. 2013, 49, 3806–3813. [Google Scholar] [CrossRef]
- Fang, J.; Niu, H.; Lin, T.; Wang, X. Applications of electrospun nanofibers. Chin. Sci. Bull. 2008, 53, 2265–2286. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Li, F.; Wang, Y.; Zhang, Q.; Ma, W.; Huang, C. Electrospun nanofiber membranes for wastewater treatment applications. Sep. Purif. Technol. 2020, 250, 117116. [Google Scholar] [CrossRef]
- Hou, L.; Wang, N.; Wu, J.; Cui, Z.; Jiang, L.; Zhao, Y. Bioinspired superwettability electrospun micro/nanofibers and their applications. Adv. Funct. Mater. 2018, 28, 1801114. [Google Scholar] [CrossRef]
- Cloete, T.E. Nanotechnology in Water Treatment Applications; Caister Academic Press: Norfolk, UK, 2010. [Google Scholar]
- Zhu, X.; Cui, W.; Li, X.; Jin, Y. Electrospun fibrous mats with high porosity as potential scaffolds for skin tissue engineering. Biomacromolecules 2008, 9, 1795–1801. [Google Scholar] [CrossRef]
- Xiong, J.; Huo, P.; Ko, F.K. Fabrication of ultrafine fibrous polytetrafluoroethylene porous membranes by electrospinning. J. Mater. Res. 2009, 24, 2755–2761. [Google Scholar] [CrossRef]
- Subramanian, S.; Seeram, R. New directions in nanofiltration applications—are nanofibers the right materials as membranes in desalination? Desalination 2013, 308, 198–208. [Google Scholar] [CrossRef]
- Balamurugan, R.; Sundarrajan, S.; Ramakrishna, S. Recent trends in nanofibrous membranes and their suitability for air and water filtrations. Membranes 2011, 1, 232–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barhate, R.S.; Ramakrishna, S. Nanofibrous filtering media: Filtration problems and solutions from tiny materials. J. Membr. Sci. 2007, 296, 1–8. [Google Scholar] [CrossRef]
- Yoon, K.; Hsiao, B.S.; Chu, B. Functional nanofibers for environmental applications. J. Mater. Chem. 2008, 18, 5326–5334. [Google Scholar] [CrossRef]
- Feng, C.; Khulbe, K.; Matsuura, T.; Tabe, S.; Ismail, A.F. Preparation and characterization of electro-spun nanofiber membranes and their possible applications in water treatment. Sep. Purif. Technol. 2013, 102, 118–135. [Google Scholar] [CrossRef]
- Kaur, S.; Sundarrajan, S.; Rana, D.; Sridhar, R.; Gopal, R.; Matsuura, T.; Ramakrishna, S. The characterization of electrospun nanofibrous liquid filtration membranes. J. Mater. Sci. 2014, 49, 6143–6159. [Google Scholar] [CrossRef]
- Kumar, P.S.; Sundaramurthy, J.; Sundarrajan, S.; Babu, V.J.; Singh, G.; Allakhverdiev, S.I.; Ramakrishna, S. Hierarchical electrospun nanofibers for energy harvesting, production and environmental remediation. Energy Environ. Sci. 2014, 7, 3192–3222. [Google Scholar] [CrossRef]
- Wang, M.; Hai, T.; Feng, Z.; Yu, D.-G.; Yang, Y.; Annie Bligh, S. The relationships between the working fluids, process characteristics and products from the modified coaxial electrospinning of zein. Polymers 2019, 11, 1287. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, C. Effects of working parameters on electrospinning. In One-Dimensional Nanostructures; Springer: Berlin/Heidelberg, Germany, 2013; pp. 15–28. [Google Scholar]
- Costa, L.M.M.; Bretas, R.E.S.; Gregorio, R. Effect of solution concentration on the electrospray/electrospinning transition and on the crystalline phase of PVDF. Mater. Sci. Appl. 2010, 1, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.; Nangrejo, M.; Edirisinghe, M. A novel method of selecting solvents for polymer electrospinning. Polymer 2010, 51, 1654–1662. [Google Scholar] [CrossRef]
- Luo, C.; Stride, E.; Edirisinghe, M. Mapping the influence of solubility and dielectric constant on electrospinning polycaprolactone solutions. Macromolecules 2012, 45, 4669–4680. [Google Scholar] [CrossRef]
- Şener, A.G.; Altay, A.S.; Altay, F. Effect of voltage on morphology of electrospun nanofibers. In Proceedings of the 2011 7th International Conference on Electrical and Electronics Engineering (ELECO), Bursa, Turkey, 1–4 December 2011; pp. I-324–I-328. [Google Scholar]
- Feng, L.; Li, S.; Zhai, J.; Song, Y.; Jiang, L.; Zhu, D. Template based synthesis of aligned polyacrylonitrile nanofibers using a novel extrusion method. Synth. Met. 2003, 135–136, 817–818. [Google Scholar] [CrossRef]
- Casper, C.L.; Stephens, J.S.; Tassi, N.G.; Chase, D.B.; Rabolt, J.F. Controlling surface morphology of electrospun polystyrene fibers: Effect of humidity and molecular weight in the electrospinning process. Macromolecules 2004, 37, 573–578. [Google Scholar] [CrossRef]
- De Vrieze, S.; Van Camp, T.; Nelvig, A.; Hagström, B.; Westbroek, P.; De Clerck, K. The effect of temperature and humidity on electrospinning. J. Mater. Sci. 2009, 44, 1357–1362. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; R Williams, G. Energy-saving electrospinning with a concentric teflon-core rod spinneret to create medicated nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Wang, M.; Yu, D.-G.; Li, X.; Williams, G.R. The development and bio-applications of multifluid electrospinning. Mater. Highlights 2020, 1, 1–13. [Google Scholar] [CrossRef]
- Kupka, V.; Dvořáková, E.; Manakhov, A.; Michlíček, M.; Petruš, J.; Vojtová, L.; Zajíčková, L. Well-blended PCL/PEO electrospun nanofibers with functional properties enhanced by plasma processing. Polymers 2020, 12, 1403. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, J.; Lu, J.; Huang, J.; Zhang, F.; Hu, Y.; Liu, C.; An, R.; Miao, H.; Chen, Y. Preparation and properties of plant-oil-based epoxy acrylate-like resins for UV-curable coatings. Polymers 2020, 12, 2165. [Google Scholar] [CrossRef]
- Liu, N.; Qu, R.; Chen, Y.; Cao, Y.; Zhang, W.; Lin, X.; Wei, Y.; Feng, L.; Jiang, L. In situ dual-functional water purification with simultaneous oil removal and visible light catalysis. Nanoscale 2016, 8, 18558–18564. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, C.; He, A.; Yang, S.J.; Wu, G.P.; Darling, S.B.; Xu, Z.K. Photocatalytic nanofiltration membranes with self-cleaning property for wastewater treatment. Adv. Funct. Mater. 2017, 27, 1700251. [Google Scholar] [CrossRef]
- Sikhwivhilu, K.; Moutloali, R.M. Functionalized PVDF membrane-immobilized Fe/Ni bimetallic nanoparticles for catalytic degradation of methyl orange dye: A comparative study. Mater. Today Proc. 2015, 2, 4070–4080. [Google Scholar] [CrossRef]
- Rojas-Lema, S.; Terol, J.; Fages, E.; Balart, R.; Quiles-Carrillo, L.; Prieto, C.; Torres-Giner, S. Microencapsulation of copper (II) sulfate in ionically cross-linked chitosan by spray drying for the development of irreversible moisture indicators in paper packaging. Polymers 2020, 12, 2039. [Google Scholar] [CrossRef]
- Zhao, S.; Ba, C.; Yao, Y.; Zheng, W.; Economy, J.; Wang, P. Removal of antibiotics using polyethylenimine cross-linked nanofiltration membranes: Relating membrane performance to surface charge characteristics. Chem. Eng. J. 2018, 335, 101–109. [Google Scholar] [CrossRef]
- Satilmis, B.; Uyar, T. Amine modified electrospun PIM-1 ultrafine fibers for an efficient removal of methyl orange from an aqueous system. Appl. Surf. Sci. 2018, 453, 220–229. [Google Scholar] [CrossRef]
- Ramakrishna, S. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005. [Google Scholar]
- Smith, L.; Ma, P. Nano-fibrous scaffolds for tissue engineering. Colloids Surf. B Biointerfaces 2004, 39, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Xing, X.; Wang, Y.; Li, B. Nanofiber drawing and nanodevice assembly in poly (trimethylene terephthalate). Opt. Express 2008, 16, 10815–10822. [Google Scholar] [CrossRef]
- Suzuki, A.; Mikuni, T.; Hasegawa, T. Nylon 66 nanofibers prepared by CO2 laser supersonic drawing. J. Appl. Polym. Sci. 2014, 131, 6. [Google Scholar] [CrossRef]
- Suzuki, A.; Arino, K. Polypropylene nanofiber sheets prepared by CO2 laser supersonic multi-drawing. Eur. Polym. J. 2012, 48, 1169–1176. [Google Scholar] [CrossRef]
- Nain, A.S.; Amon, C.; Sitti, M. Proximal probes based nanorobotic drawing of polymer micro/nanofibers. IEEE Trans. Nanotechnol. 2006, 5, 499–510. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Wang, W.; Li, G.; Ma, X.; Li, X.; Zhang, Z.; Qian, Y. Template-assisted synthesis of porous molybdenum dioxide nanofibers and nanospheres by redox etching method. J. Cryst. Growth 2006, 290, 96–102. [Google Scholar] [CrossRef]
- Tao, S.L.; Desai, T.A. Aligned arrays of biodegradable poly (ϵ-caprolactone) nanowires and nanofibers by template synthesis. Nano Lett. 2007, 7, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Che, G.; Lakshmi, B.; Martin, C.; Fisher, E.; Ruoff, R.S. Chemical vapor deposition based synthesis of carbon nanotubes and nanofibers using a template method. Chem. Mater. 1998, 10, 260–267. [Google Scholar] [CrossRef]
- Ma, P.X.; Zhang, R. Synthetic nano-scale fibrous extracellular matrix. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1999, 46, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Ichimori, T.; Mizuma, K.; Uchida, T.; Yamazaki, S.; Kimura, K. Morphological diversity and nanofiber networks of poly (p-oxybenzoyl) generated by phase separation during copolymerization. J. Appl. Polym. Sci. 2013, 128, 1282–1290. [Google Scholar] [CrossRef]
- Shao, J.; Chen, C.; Wang, Y.; Chen, X.; Du, C. Early stage evolution of structure and nanoscale property of nanofibers in thermally induced phase separation process. React. Funct. Polym. 2012, 72, 765–772. [Google Scholar] [CrossRef]
- Nasir, A.M.; Awang, N.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yajid, M.A.M. Recent progress on fabrication and application of electrospun nanofibrous photocatalytic membranes for wastewater treatment: A review. J. Water Process Eng. 2021, 40, 101878. [Google Scholar] [CrossRef]
- Wang, L.; Ali, J.; Zhang, C.; Mailhot, G.; Pan, G. Simultaneously enhanced photocatalytic and antibacterial activities of TiO2/Ag composite nanofibers for wastewater purification. J. Environ. Chem. Eng. 2020, 8, 102104. [Google Scholar] [CrossRef]
- Ni, Y.; Yan, K.; Xu, F.; Zhong, W.; Zhao, Q.; Liu, K.; Yan, K.; Wang, D. Synergistic effect on TiO2 doped poly (vinyl alcohol-co-ethylene) nanofibrous film for filtration and photocatalytic degradation of methylene blue. Compos. Commun. 2019, 12, 112–116. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Song, J.; Si, Y.; Zhuang, X.; Yu, J.; Ding, B. In situ synthesis of flexible hierarchical TiO2 nanofibrous membranes with enhanced photocatalytic activity. J. Mater. Chem. A 2015, 3, 22136–22144. [Google Scholar] [CrossRef]
- Habiba, U.; Afifi, A.M.; Salleh, A.; Ang, B.C. Chitosan/(polyvinyl alcohol)/zeolite electrospun composite nanofibrous membrane for adsorption of Cr6+, Fe3+ and Ni2+. J. Hazard. Mater. 2017, 322, 182–194. [Google Scholar] [CrossRef]
- Zahakifar, F.; Keshtkar, A.R.; Talebi, M. Performance evaluation of sodium alginate/polyvinyl alcohol/polyethylene oxide/ZSM5 zeolite hybrid adsorbent for ion uptake from aqueous solutions: A case study of thorium (IV). J. Radioanal. Nucl. Chem. 2021, 327, 65–72. [Google Scholar] [CrossRef]
- Shariful, M.I.; Sharif, S.B.; Lee, J.J.L.; Habiba, U.; Ang, B.C.; Amalina, M.A. Adsorption of divalent heavy metal ion by mesoporous-high surface area chitosan/poly (ethylene oxide) nanofibrous membrane. Carbohydr. Polym. 2017, 157, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Loh, C.-H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Ozbey-Unal, B.; Gezmis-Yavuz, E.; Eryildiz, B.; Koseoglu-Imer, D.Y.; Keskinler, B.; Koyuncu, I. Boron removal from geothermal water by nanofiber-based membrane distillation membranes with significantly improved surface hydrophobicity. J. Environ. Chem. Eng. 2020, 8, 104113. [Google Scholar] [CrossRef]
- Ghafoor, S.; Inayat, A.; Aftab, F.; Duran, H.; Kirchhoff, K.; Waseem, S.; Arshad, S.N. TiO2 nanofibers embedded with g-C3N4 nanosheets and decorated with Ag nanoparticles as Z-scheme photocatalysts for environmental remediation. J. Environ. Chem. Eng. 2019, 7, 103452. [Google Scholar] [CrossRef]
- Kim, J.H.; Joshi, M.K.; Lee, J.; Park, C.H.; Kim, C.S. Polydopamine-assisted immobilization of hierarchical zinc oxide nanostructures on electrospun nanofibrous membrane for photocatalysis and antimicrobial activity. J. Colloid Interface Sci. 2018, 513, 566–574. [Google Scholar] [CrossRef]
- Agrawal, S.; Ranjan, R.; Lal, B.; Rahman, A.; Singh, S.P.; Selvaratnam, T.; Nawaz, T. Synthesis and water treatment applications of nanofibers by electrospinning. Processes 2021, 9, 1779. [Google Scholar] [CrossRef]
- Hwang, J.; Harrington, D.; Klok, H.-A.; Stupp, S. Cell-Synthetic Surface Interactions: Self-Assembling Biomaterials; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef]
- Gersbach, C.A.; Byers, B.A.; Pavlath, G.K.; Guldberg, R.E.; García, A.J. Runx2/Cbfa1-genetically engineered skeletal myoblasts mineralize collagen scaffolds in vitro. Biotechnol. Bioeng. 2004, 88, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Shields, K.J.; Beckman, M.J.; Bowlin, G.L.; Wayne, J.S. Mechanical properties and cellular proliferation of electrospun collagen type II. Tissue Eng. 2004, 10, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, H.; Lim, C.T.; Ramakrishna, S.; Huang, Z.M. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72, 156–165. [Google Scholar] [CrossRef]
- Bhattarai, N.; Edmondson, D.; Veiseh, O.; Matsen, F.A.; Zhang, M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials 2005, 26, 6176–6184. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.-H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Um, I.C.; Fang, D.; Hsiao, B.S.; Okamoto, A.; Chu, B. Electro-spinning and electro-blowing of hyaluronic acid. Biomacromolecules 2004, 5, 1428–1436. [Google Scholar] [CrossRef]
- Jin, H.-J.; Fridrikh, S.V.; Rutledge, G.C.; Kaplan, D.L. Electrospinning Bombyx mori silk with poly (ethylene oxide). Biomacromolecules 2002, 3, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly (L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef]
- Verreck, G.; Chun, I.; Rosenblatt, J.; Peeters, J.; Van Dijck, A.; Mensch, J.; Noppe, M.; Brewster, M.E. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. J. Control. Release 2003, 92, 349–360. [Google Scholar] [CrossRef]
- Riboldi, S.A.; Sampaolesi, M.; Neuenschwander, P.; Cossu, G.; Mantero, S. Electrospun degradable polyesterurethane membranes: Potential scaffolds for skeletal muscle tissue engineering. Biomaterials 2005, 26, 4606–4615. [Google Scholar] [CrossRef] [Green Version]
- Li, W.J.; Danielson, K.G.; Alexander, P.G.; Tuan, R.S. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly (ϵ-caprolactone) scaffolds. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2003, 67, 1105–1114. [Google Scholar]
- Li, M.; Mondrinos, M.J.; Gandhi, M.R.; Ko, F.K.; Weiss, A.S.; Lelkes, P.I. Electrospun protein fibers as matrices for tissue engineering. Biomaterials 2005, 26, 5999–6008. [Google Scholar] [CrossRef] [PubMed]
- Luu, Y.; Kim, K.; Hsiao, B.; Chu, B.; Hadjiargyrou, M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA–PEG block copolymers. J. Control. Release 2003, 89, 341–353. [Google Scholar] [CrossRef]
- Kim, K.; Luu, Y.K.; Chang, C.; Fang, D.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Incorporation and controlled release of a hydrophilic antibiotic using poly (lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J. Control. Release 2004, 98, 47–56. [Google Scholar] [CrossRef]
- Uematsu, K.; Hattori, K.; Ishimoto, Y.; Yamauchi, J.; Habata, T.; Takakura, Y.; Ohgushi, H.; Fukuchi, T.; Sato, M. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials 2005, 26, 4273–4279. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of tetracycline hydrochloride from electrospun poly (ethylene-co-vinylacetate), poly (lactic acid), and a blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Mo, X.; Weber, H.J. Electrospinning P (LLA-CL) nanofiber: A tubular scaffold fabrication with circumferential alignment. In Macromolecular Symposia; Wiley-VCH Verlag: Weinheim, Germany, 2004; pp. 413–416. [Google Scholar]
- Mo, X.; Xu, C.; Kotaki, M.; Ramakrishna, S. Electrospun P (LLA-CL) nanofiber: A biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials 2004, 25, 1883–1890. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Maggi, F.; Petrelli, R.; Nicoletti, M. Commentary: Making green pesticides greener? The potential of plant products for nanosynthesis and pest control. J. Clust. Sci. 2017, 28, 3–10. [Google Scholar] [CrossRef]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Torre, L.; Jiménez, A.; Kenny, J. Effects of modified cellulose nanocrystals on the barrier and migration properties of PLA nano-biocomposites. Carbohydr. Polym. 2012, 90, 948–956. [Google Scholar] [CrossRef]
- Ortiz, J.; Chabot, B. Electrospun nanofibers for the removal of heavy metals from aqueous solutions. In Mitacs Globalink Intership Report; Monterrey Institute of Technology and Higher Education: Monterrey, Mexico, 2016. [Google Scholar]
- Li, S.; Yue, X.; Jing, Y.; Bai, S.; Dai, Z. Fabrication of zonal thiol-functionalized silica nanofibers for removal of heavy metal ions from wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2011, 380, 229–233. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Li, Y.; Yang, C. Removal of Cr (VI) with a spiral wound chitosan nanofiber membrane module via dead-end filtration. J. Membr. Sci. 2017, 544, 333–341. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer–Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Park, D.; Lee, Y.-C. Chitosan combined with ZnO, TiO2 and Ag nanoparticles for antimicrobial wound healing applications: A mini review of the research trends. Polymers 2017, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Paradis-Tanguay, L.; Camiré, A.; Renaud, M.; Chabot, B.; Lajeunesse, A. Sorption capacities of chitosan/polyethylene oxide (PEO) electrospun nanofibers used to remove ibuprofen in water. J. Polym. Eng. 2019, 39, 207–215. [Google Scholar] [CrossRef]
- Wang, P.; Wang, L.; Dong, S.; Zhang, G.; Shi, X.; Xiang, C.; Li, L. Adsorption of hexavalent chromium by novel chitosan/poly (ethylene oxide)/permutit electrospun nanofibers. New J. Chem. 2018, 42, 17740–17749. [Google Scholar] [CrossRef]
- Abdolmaleki, A.Y.; Zilouei, H.; Khorasani, S.N.; Zargoosh, K. Adsorption of tetracycline from water using glutaraldehyde-crosslinked electrospun nanofibers of chitosan/poly (vinyl alcohol). Water Sci. Technol. 2018, 77, 1324–1335. [Google Scholar] [CrossRef]
- Esmaeili, A.; Beni, A.A. Optimization and design of a continuous biosorption process using brown algae and chitosan/PVA nano-fiber membrane for removal of nickel by a new biosorbent. Int. J. Environ. Sci. Technol. 2018, 15, 765–778. [Google Scholar] [CrossRef]
- Karim, M.R.; Aijaz, M.O.; Alharth, N.H.; Alharbi, H.F.; Al-Mubaddel, F.S.; Awual, M.R. Composite nanofibers membranes of poly (vinyl alcohol)/chitosan for selective lead (II) and cadmium (II) ions removal from wastewater. Ecotoxicol. Environ. Saf. 2019, 169, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Mokhtari-Shourijeh, Z. Preparation of PVA-chitosan blend nanofiber and its dye removal ability from colored wastewater. Fibers Polym. 2015, 16, 1861–1869. [Google Scholar] [CrossRef]
- Mei, Y.; Runjun, S.; Yan, F.; Honghong, W.; Hao, D.; Chengkun, L. Preparation, characterization and kinetics study of chitosan/PVA electrospun nanofiber membranes for the adsorption of dye from water. J. Polym. Eng. 2019, 39, 459–471. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, N.; Tiwari, S.; Tiwari, S.K.; Dhakate, S.R. Cerium functionalized PVA–chitosan composite nanofibers for effective remediation of ultra-low concentrations of Hg (II) in water. RSC Adv. 2015, 5, 16622–16630. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.-X.; Zheng, G.-F.; Li, W.-W.; Zhong, L.-B.; Zheng, Y.-M. Electrospun chitosan nanofiber membrane for adsorption of Cu (II) from aqueous solution: Fabrication, characterization and performance. J. Nanosci. Nanotechnol. 2018, 18, 5624–5635. [Google Scholar] [CrossRef] [PubMed]
- Elkady, M.; Salama, E.; Amer, W.A.; Ebeid, E.-Z.M.; Ayad, M.M.; Shokry, H. Novel eco-friendly electrospun nanomagnetic zinc oxide hybridized PVA/alginate/chitosan nanofibers for enhanced phenol decontamination. Environ. Sci. Pollut. Res. 2020, 27, 43077–43092. [Google Scholar] [CrossRef]
- Fan, J.-P.; Luo, J.-J.; Zhang, X.-H.; Zhen, B.; Dong, C.-Y.; Li, Y.-C.; Shen, J.; Cheng, Y.-T.; Chen, H.-P. A novel electrospun β-CD/CS/PVA nanofiber membrane for simultaneous and rapid removal of organic micropollutants and heavy metal ions from water. Chem. Eng. J. 2019, 378, 122232. [Google Scholar] [CrossRef]
- Bahmani, E.; Koushkbaghi, S.; Darabi, M.; ZabihiSahebi, A.; Askari, A.; Irani, M. Fabrication of novel chitosan-g-PNVCL/ZIF-8 composite nanofibers for adsorption of Cr (VI), As (V) and phenol in a single and ternary systems. Carbohydr. Polym. 2019, 224, 115148. [Google Scholar] [CrossRef] [PubMed]
- Chabalala, M.B.; Al-Abri, M.Z.; Mamba, B.B.; Nxumalo, E.N. Mechanistic aspects for the enhanced adsorption of bromophenol blue and atrazine over cyclodextrin modified polyacrylonitrile nanofiber membranes. Chem. Eng. Res. Des. 2021, 169, 19–32. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, J.; Liu, K.; Jiang, Y.; Yang, G.; Liu, Y.; Lin, C.; Ye, X.; Shi, Y.; Liu, M. Rapid elimination of trace bisphenol pollutants with porous β-cyclodextrin modified cellulose nanofibrous membrane in water: Adsorption behavior and mechanism. J. Hazard. Mater. 2021, 403, 123666. [Google Scholar] [CrossRef]
- Khalil, A.M.; Schäfer, A.I. Cross-linked β-cyclodextrin nanofiber composite membrane for steroid hormone micropollutant removal from water. J. Membr. Sci. 2021, 618, 118228. [Google Scholar] [CrossRef]
- Camiré, A.; Espinasse, J.; Chabot, B.; Lajeunesse, A. Development of electrospun lignin nanofibers for the adsorption of pharmaceutical contaminants in wastewater. Environ. Sci. Pollut. Res. 2020, 27, 3560–3573. [Google Scholar] [CrossRef]
- Da Silva, B.A.; de Sousa Cunha, R.; Valerio, A.; Junior, A.D.N.; Hotza, D.; González, S.Y.G. Electrospinning of cellulose using ionic liquids: An overview on processing and applications. Eur. Polym. J. 2021, 147, 110283. [Google Scholar] [CrossRef]
- Jonas, R.; Farah, L.F. Production and application of microbial cellulose. Polym. Degrad. Stab. 1998, 59, 101–106. [Google Scholar] [CrossRef]
- Rodrigues Filho, G.; Monteiro, D.S.; da Silva Meireles, C.; de Assunção, R.M.N.; Cerqueira, D.A.; Barud, H.S.; Ribeiro, S.J.; Messadeq, Y. Synthesis and characterization of cellulose acetate produced from recycled newspaper. Carbohydr. Polym. 2008, 73, 74–82. [Google Scholar] [CrossRef]
- Reddy, K.O.; Zhang, J.; Zhang, J.; Rajulu, A.V. Preparation and properties of self-reinforced cellulose composite films from Agave microfibrils using an ionic liquid. Carbohydr. Polym. 2014, 114, 537–545. [Google Scholar] [CrossRef]
- Na, B.; Zhang, P.; Lv, R.; Tian, R.; Ju, Y.; Liu, Q. Effect of ionic liquids on the morphology and mesophase formation of electrospun polylactide nanofibers. Polymer 2015, 65, 55–62. [Google Scholar] [CrossRef]
- Yarin, A.L.; Koombhongse, S.; Reneker, D.H. Taylor cone and jetting from liquid droplets in electrospinning of nanofibers. J. Appl. Phys. 2001, 90, 4836–4846. [Google Scholar] [CrossRef] [Green Version]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hilal, N.; Hashaikeh, R. Underwater superoleophobic cellulose/electrospun PVDF–HFP membranes for efficient oil/water separation. Desalination 2014, 344, 48–54. [Google Scholar] [CrossRef]
- Kacmaz, S.; Ertekin, K.; Gocmenturk, M.; Suslu, A.; Ergun, Y.; Celik, E. Selective sensing of Fe3+ at pico-molar level with ethyl cellulose based electrospun nanofibers. React. Funct. Polym. 2013, 73, 674–682. [Google Scholar] [CrossRef]
- Gouda, M.; Abu-Abdeen, M. Highly conductive cellulosic nanofibers for efficient water desalination. Fibers Polym. 2017, 18, 2111–2117. [Google Scholar] [CrossRef]
- Jain, H.; Garg, M.C. Fabrication of polymeric nanocomposite forward osmosis membranes for water desalination—A review. Environ. Technol. Innov. 2021, 23, 101561. [Google Scholar] [CrossRef]
- Lee, C.; Chae, S.H.; Yang, E.; Kim, S.; Kim, J.H.; Kim, I.S. A comprehensive review of the feasibility of pressure retarded osmosis: Recent technological advances and industrial efforts towards commercialization. Desalination 2020, 491, 114501. [Google Scholar] [CrossRef]
- Duong, P.H.; Nunes, S.P.; Chung, T.-S. Dual-skinned polyamide/poly (vinylidene fluoride)/cellulose acetate membranes with embedded woven. J. Membr. Sci. 2016, 520, 840–849. [Google Scholar] [CrossRef]
- Ong, R.C.; Chung, T.-S.; de Wit, J.S.; Helmer, B.J. Novel cellulose ester substrates for high performance flat-sheet thin-film composite (TFC) forward osmosis (FO) membranes. J. Membr. Sci. 2015, 473, 63–71. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, K.Y.; Chung, T.-S.; Chen, H.; Jean, Y.; Amy, G. Well-constructed cellulose acetate membranes for forward osmosis: Minimized internal concentration polarization with an ultra-thin selective layer. J. Membr. Sci. 2010, 360, 522–535. [Google Scholar] [CrossRef]
- Sairam, M.; Sereewatthanawut, E.; Li, K.; Bismarck, A.; Livingston, A. Method for the preparation of cellulose acetate flat sheet composite membranes for forward osmosis—Desalination using MgSO4 draw solution. Desalination 2011, 273, 299–307. [Google Scholar] [CrossRef]
- Wu, Q.-Y.; Xing, X.-Y.; Yu, Y.; Gu, L.; Xu, Z.-K. Novel thin film composite membranes supported by cellulose triacetate porous substrates for high-performance forward osmosis. Polymer 2018, 153, 150–160. [Google Scholar] [CrossRef]
- Li, M.-N.; Chen, X.-J.; Wan, Z.-H.; Wang, S.-G.; Sun, X.-F. Forward osmosis membranes for high-efficiency desalination with Nano-MoS2 composite substrates. Chemosphere 2021, 278, 130341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, J.; Ren, Z.; Shi, W.; Zhang, Z.; Xu, Y.; Gao, S.; Cui, F. High performance thin-film composite (TFC) forward osmosis (FO) membrane fabricated on novel hydrophilic disulfonated poly (arylene ether sulfone) multiblock copolymer/polysulfone substrate. J. Membr. Sci. 2016, 520, 529–539. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Esteves, C.M.; Figueiredo, J.L.; Silva, A.M. Thin-film composite forward osmosis membranes based on polysulfone supports blended with nanostructured carbon materials. J. Membr. Sci. 2016, 520, 326–336. [Google Scholar] [CrossRef]
- Camacho, L.M.; Pinion, T.A.; Olatunji, S.O. Behavior of mixed-matrix graphene oxide–polysulfone membranes in the process of direct contact membrane distillation. Sep. Purif. Technol. 2020, 240, 116645. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, R.; Ge, Q.; Wang, H.; Xu, T. Preparation of polyethersulfone/carbon nanotube substrate for high-performance forward osmosis membrane. Desalination 2013, 330, 70–78. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Fabrication of titania nanofibers by electrospinning. Nano Lett. 2003, 3, 555–560. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, W.; Formo, E.; Sun, Y.; Xia, Y. Ceramic nanofibers fabricated by electrospinning and their applications in catalysis, environmental science, and energy technology. Polym. Adv. Technol. 2011, 22, 326–338. [Google Scholar] [CrossRef]
- Malwal, D.; Gopinath, P. Fabrication and characterization of poly (ethylene oxide) templated nickel oxide nanofibers for dye degradation. Environ. Sci. Nano 2015, 2, 78–85. [Google Scholar] [CrossRef]
- Saquing, C.D.; Tang, C.; Monian, B.; Bonino, C.A.; Manasco, J.L.; Alsberg, E.; Khan, S.A. Alginate–polyethylene oxide blend nanofibers and the role of the carrier polymer in electrospinning. Ind. Eng. Chem. Res. 2013, 52, 8692–8704. [Google Scholar] [CrossRef]
- Viswanathamurthi, P.; Bhattarai, N.; Kim, H.; Khil, M.; Lee, D.; Suh, E.-K. GeO2 fibers: Preparation, morphology and photoluminescence property. J. Chem. Phys. 2004, 121, 441–445. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, S.G.; Im, S.S.; Kim, S.H.; Joo, Y.L. Silica nanofibers from electrospinning/sol-gel process. J. Mater. Sci. Lett. 2003, 22, 891–893. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Yu, S.-H. Nanoparticles meet electrospinning: Recent advances and future prospects. Chem. Soc. Rev. 2014, 43, 4423–4448. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, D.; Kang, D.; Jiang, X. Fabrication of necklace-like structures via electrospinning. Langmuir 2010, 26, 1186–1190. [Google Scholar] [CrossRef]
- Zhang, C.L.; Lv, K.P.; Hu, N.Y.; Yu, L.; Ren, X.F.; Liu, S.L.; Yu, S.H. Macroscopic-scale alignment of ultralong Ag nanowires in polymer nanofiber mat and their hierarchical structures by magnetic-field-assisted electrospinning. Small 2012, 8, 2936–2940. [Google Scholar] [CrossRef]
- Nor, N.; Jaafar, J.; Ismail, A.; Mohamed, M.A.; Rahman, M.; Othman, M.; Lau, W.; Yusof, N. Preparation and performance of PVDF-based nanocomposite membrane consisting of TiO2 nanofibers for organic pollutant decomposition in wastewater under UV irradiation. Desalination 2016, 391, 89–97. [Google Scholar] [CrossRef]
- Gadisa, B.T.; Kassahun, S.K.; Appiah-Ntiamoah, R.; Kim, H. Tuning the charge carrier density and exciton pair separation in electrospun 1D ZnO-C composite nanofibers and its effect on photodegradation of emerging contaminants. J. Colloid Interface Sci. 2020, 570, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Ramasundaram, S.; Yoo, H.N.; Song, K.G.; Lee, J.; Choi, K.J.; Hong, S.W. Titanium dioxide nanofibers integrated stainless steel filter for photocatalytic degradation of pharmaceutical compounds. J. Hazard. Mater. 2013, 258, 124–132. [Google Scholar] [CrossRef]
- Greenstein, K.E.; Nagorzanski, M.R.; Kelsay, B.; Verdugo, E.M.; Myung, N.V.; Parkin, G.F.; Cwiertny, D.M. Carbon–titanium dioxide (C/TiO2) nanofiber composites for chemical oxidation of emerging organic contaminants in reactive filtration applications. Environ. Sci. Nano 2021, 8, 711–722. [Google Scholar] [CrossRef]
- Song, J.; Wu, X.; Zhang, M.; Liu, C.; Yu, J.; Sun, G.; Si, Y.; Ding, B. Highly flexible, core-shell heterostructured, and visible-light-driven titania-based nanofibrous membranes for antibiotic removal and E. coil inactivation. Chem. Eng. J. 2020, 379, 122269. [Google Scholar] [CrossRef]
- Yu, D.-G.; Zhou, J.; Chatterton, N.P.; Li, Y.; Huang, J.; Wang, X. Polyacrylonitrile nanofibers coated with silver nanoparticles using a modified coaxial electrospinning process. Int. J. Nanomed. 2012, 7, 5725. [Google Scholar] [CrossRef] [Green Version]
- Geltmeyer, J.; De Roo, J.; Van den Broeck, F.; Martins, J.C.; De Buysser, K.; De Clerck, K. The influence of tetraethoxysilane sol preparation on the electrospinning of silica nanofibers. J. Sol-Gel Sci. Technol. 2016, 77, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Li, M.; Zhang, M.; Wang, C.; Luo, R.; Yan, X.; Zhang, H.; Qi, J.; Sun, X.; Li, J. Metal organic framework derived one-dimensional porous Fe/N-doped carbon nanofibers with enhanced catalytic performance. J. Hazard. Mater. 2021, 416, 126101. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-C.; Oh, S.-I.; Kang, W.; Yoo, H.-Y.; Lee, J.; Kim, D.-W. Superior anodic oxidation in tailored Sb-doped SnO2/RuO2 composite nanofibers for electrochemical water treatment. J. Catal. 2019, 374, 118–126. [Google Scholar] [CrossRef]
- Xie, W.; Shi, Y.; Wang, Y.; Zheng, Y.; Liu, H.; Hu, Q.; Wei, S.; Gu, H.; Guo, Z. Electrospun iron/cobalt alloy nanoparticles on carbon nanofibers towards exhaustive electrocatalytic degradation of tetracycline in wastewater. Chem. Eng. J. 2021, 405, 126585. [Google Scholar] [CrossRef]
- Yang, G.C.; Yen, C.-H. The use of different materials to form the intermediate layers of tubular carbon nanofibers/carbon/alumina composite membranes for removing pharmaceuticals from aqueous solutions. J. Membr. Sci. 2013, 425, 121–130. [Google Scholar] [CrossRef]
- Peter, K.T.; Vargo, J.D.; Rupasinghe, T.P.; De Jesus, A.; Tivanski, A.V.; Sander, E.A.; Myung, N.V.; Cwiertny, D.M. Synthesis, optimization, and performance demonstration of electrospun carbon nanofiber–carbon nanotube composite sorbents for point-of-use water treatment. ACS Appl. Mater. Interfaces 2016, 8, 11431–11440. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Niu, J.; Zhang, X.; Liu, J.; Cao, G.; Kong, X. Sorption of triclosan on electrospun fibrous membranes: Effects of pH and dissolved organic matter. Emerg. Contam. 2015, 1, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Sun, M.; Liu, Y.; Liu, H.; Qu, J.; Li, J. Ionic liquid assisted electrospun cellulose acetate fibers for aqueous removal of triclosan. Langmuir 2015, 31, 1820–1827. [Google Scholar] [CrossRef]
- Zhao, R.; Shi, X.; Ma, T.; Rong, H.; Wang, Z.; Cui, F.; Zhu, G.; Wang, C. Constructing mesoporous adsorption channels and MOF–polymer interfaces in electrospun composite fibers for effective removal of emerging organic contaminants. ACS Appl. Mater. Interfaces 2020, 13, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Camiré, A.; Chabot, B.; Lajeunesse, A. Sorption capacities of a lignin-based electrospun nanofibrous material for pharmaceutical residues remediation in water. In Sorption in 2020s; IntechOpen: London, UK, 2019; Volume 25. [Google Scholar]
- Li, A.; Zhou, M.; Luo, P.; Shang, J.; Wang, P.; Lyu, L. Deposition of MOFs on polydopamine-modified electrospun polyvinyl alcohol/silica nanofibers mats for chloramphenicol adsorption in water. Nano 2020, 15, 2050046. [Google Scholar] [CrossRef]
- Zhang, Y.; Ou, H.; Liu, H.; Ke, Y.; Zhang, W.; Liao, G.; Wang, D. Polyimide-based carbon nanofibers: A versatile adsorbent for highly efficient removals of chlorophenols, dyes and antibiotics. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 92–101. [Google Scholar] [CrossRef]
- Chao, S.; Li, X.; Li, Y.; Wang, Y.; Wang, C. Preparation of polydopamine-modified zeolitic imidazolate framework-8 functionalized electrospun fibers for efficient removal of tetracycline. J. Colloid Interface Sci. 2019, 552, 506–516. [Google Scholar] [CrossRef]
- Das, S.; Barui, A.; Adak, A. Montmorillonite impregnated electrospun cellulose acetate nanofiber sorptive membrane for ciprofloxacin removal from wastewater. J. Water Process Eng. 2020, 37, 101497. [Google Scholar] [CrossRef]
- Park, J.-A.; Nam, A.; Kim, J.-H.; Yun, S.-T.; Choi, J.-W.; Lee, S.-H. Blend-electrospun graphene oxide/Poly (vinylidene fluoride) nanofibrous membranes with high flux, tetracycline removal and anti-fouling properties. Chemosphere 2018, 207, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; You, Q.; Wang, Q.; Liao, G.; Wang, D. Highly efficient removal of antibiotics and dyes from water by the modified carbon nanofibers composites with abundant mesoporous structure. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 392–401. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Fan, X.; Quan, X.; Tan, F.; Zhang, Y.; Gao, J. Adsorption of ciprofloxacin, bisphenol and 2-chlorophenol on electrospun carbon nanofibers: In comparison with powder activated carbon. J. Colloid Interface Sci. 2015, 447, 120–127. [Google Scholar] [CrossRef]
- Kebede, T.; Seroto, M.; Chokwe, R.; Dube, S.; Nindi, M. Adsorption of antiretroviral (ARVs) and related drugs from environmental wastewaters using nanofibers. J. Environ. Chem. Eng. 2020, 8, 104049. [Google Scholar] [CrossRef]
- Khalil, A.M.; Hashem, T.; Gopalakrishnan, A.; Schäfer, A.I. Cyclodextrin composite nanofiber membrane: Impact of the crosslinker type on steroid hormone micropollutant removal from water. ACS Appl. Polym. Mater. 2021, 3, 2646–2656. [Google Scholar] [CrossRef]
- Bobirică, C.; Bobirică, L.; Râpă, M.; Matei, E.; Predescu, A.M.; Orbeci, C. Photocatalytic degradation of ampicillin using PLA/TiO2 hybrid nanofibers coated on different types of fiberglass. Water 2020, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-P.; Yang, L.-M.; Chen, J.P.; Zheng, Y.-M. Electrospun spongy zero-valent iron as excellent electro-Fenton catalyst for enhanced sulfathiazole removal by a combination of adsorption and electro-catalytic oxidation. J. Hazard. Mater. 2019, 371, 576–585. [Google Scholar] [CrossRef]
- Kizildag, N.; Geltmeyer, J.; Ucar, N.; De Buysser, K.; De Clerck, K. Coaxial nanofibers containing TiO2 in the shell for water treatment applications. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Hong Kong, China, 12–14 December 2017; p. 102007. [Google Scholar]
- Gadisa, B.T.; Appiah-Ntiamoah, R.; Kim, H. In-situ derived hierarchical ZnO/Zn-C nanofiber with high photocatalytic activity and recyclability under solar light. Appl. Surf. Sci. 2019, 491, 350–359. [Google Scholar] [CrossRef]
- Qin, D.; Lu, W.; Zhu, Z.; Li, N.; Xu, T.; Wang, G.; Chen, W. Free channel formation around graphitic carbon nitride embedded in porous polyethylene terephthalate nanofibers with excellent reusability for eliminating antibiotics under solar irradiation. Ind. Eng. Chem. Res. 2017, 56, 11151–11160. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Organic fouling of forward osmosis membranes: Fouling reversibility and cleaning without chemical reagents. J. Membr. Sci. 2010, 348, 337–345. [Google Scholar] [CrossRef]
- Owen, R.; Jobling, S. The hidden costs of flexible fertility. Nature 2012, 485, 441. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Gómez-Serrano, V.; Álvarez, P.; Alvim-Ferraz, M.; Dias, J. Activated carbon modifications to enhance its water treatment applications. An overview. J. Hazard. Mater. 2011, 187, 1–23. [Google Scholar] [CrossRef]
- Kümmerer, K. Pharmaceuticals in the environment. Annu. Rev. Environ. Resour. 2010, 35, 57–75. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Gerrity, D.; Snyder, S. Wastewater and drinking water treatment technologies. In Human Pharmaceuticals in the Environment; Springer: Berlin/Heidelberg, Germany, 2012; pp. 225–255. [Google Scholar]
- Packer, J.L.; Werner, J.J.; Latch, D.E.; McNeill, K.; Arnold, W.A. Photochemical fate of pharmaceuticals in the environment: Naproxen, diclofenac, clofibric acid, and ibuprofen. Aquat. Sci. 2003, 65, 342–351. [Google Scholar] [CrossRef]
- Wert, E.C.; Rosario-Ortiz, F.L.; Drury, D.D.; Snyder, S.A. Formation of oxidation byproducts from ozonation of wastewater. Water Res. 2007, 41, 1481–1490. [Google Scholar] [CrossRef]
- Langlais, B.; Reckhow, D.A.; Brink, D.R. Ozone in water treatment. Appl. Eng. 1991, 558-592, 558–592. [Google Scholar]
- Benner, J.; Salhi, E.; Ternes, T.; von Gunten, U. Ozonation of reverse osmosis concentrate: Kinetics and efficiency of beta blocker oxidation. Water Res. 2008, 42, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.M.; GÖbel, A.; Joss, A.; Hermann, N.; LÖffler, D.; McArdell, C.S.; Ried, A.; Siegrist, H.; Ternes, T.A.; von Gunten, U. Oxidation of pharmaceuticals during ozonation of municipal wastewater effluents: A pilot study. Environ. Sci. Technol. 2005, 39, 4290–4299. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Huang, H.; Cho, H.-H. Carbon nanotube composite membranes for microfiltration of pharmaceuticals and personal care products: Capabilities and potential mechanisms. J. Membr. Sci. 2015, 479, 165–174. [Google Scholar] [CrossRef]
- Chon, K.; Cho, J.; Shon, H.K. A pilot-scale hybrid municipal wastewater reclamation system using combined coagulation and disk filtration, ultrafiltration, and reverse osmosis: Removal of nutrients and micropollutants, and characterization of membrane foulants. Bioresour. Technol. 2013, 141, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Benítez, F.J.; Acero, J.L.; Leal, A.I.; González, M. The use of ultrafiltration and nanofiltration membranes for the purification of cork processing wastewater. J. Hazard. Mater. 2009, 162, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Javier Benitez, F.; Acero, J.L.; Real, F.J.; Roldán, G.; Rodriguez, E. Ultrafiltration and nanofiltration membranes applied to the removal of the pharmaceuticals amoxicillin, naproxen, metoprolol and phenacetin from water. J. Chem. Technol. Biotechnol. 2011, 86, 858–866. [Google Scholar] [CrossRef]
- Ruíz, F.N.; Arévalo, A.A.; Álvarez, J.C.D.; Cisneros, B.J. Operating conditions and membrane selection for the removal of conventional and emerging pollutants from spring water using nanofiltration technology: The Tula Valley case. Desalination Water Treat. 2012, 42, 117–124. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I.; Elimelech, M. Nanofiltration of hormone mimicking trace organic contaminants. Sep. Sci. Technol. 2005, 40, 2633–2649. [Google Scholar] [CrossRef]
- López-Muñoz, M.J.; Sotto, A.; Arsuaga, J.M. Nanofiltration removal of pharmaceutically active compounds. Desalination Water Treat. 2012, 42, 138–143. [Google Scholar] [CrossRef]
- Semião, A.J.; Schäfer, A.I. Removal of adsorbing estrogenic micropollutants by nanofiltration membranes. Part A—Experimental evidence. J. Membr. Sci. 2013, 431, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Khouni, I.; Marrot, B.; Moulin, P.; Amar, R.B. Decolourization of the reconstituted textile effluent by different process treatments: Enzymatic catalysis, coagulation/flocculation and nanofiltration processes. Desalination 2011, 268, 27–37. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, P.-K.; Lee, C.-H.; Kwon, H.-H. Surface modification of nanofiltration membranes to improve the removal of organic micro-pollutants (EDCs and PhACs) in drinking water treatment: Graft polymerization and cross-linking followed by functional group substitution. J. Membr. Sci. 2008, 321, 190–198. [Google Scholar] [CrossRef]
- Sun, S.P.; Hatton, T.A.; Chung, T.-S. Hyperbranched polyethyleneimine induced cross-linking of polyamide−imide nanofiltration hollow fiber membranes for effective removal of ciprofloxacin. Environ. Sci. Technol. 2011, 45, 4003–4009. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, L.D.; Schäfer, A.I.; Elimelech, M. Role of electrostatic interactions in the retention of pharmaceutically active contaminants by a loose nanofiltration membrane. J. Membr. Sci. 2006, 286, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Zhang, H.; Feng, Y.; Yang, F.; Zhang, J. Removal of trace antibiotics from wastewater: A systematic study of nanofiltration combined with ozone-based advanced oxidation processes. Chem. Eng. J. 2014, 240, 211–220. [Google Scholar] [CrossRef]
- Snyder, S.; Wert, E.; Yoon, Y.; Westerhoff, P. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals during water treatment processes. Desalination 2007, 202, 156–181. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).