X-ray Micro-Computed Tomography: A Powerful Device to Analyze the 3D Microstructure of Anode-Electrolyte in BaZr0.8Y0.2O3 Protonic Ceramic Electrochemical Cells and the Reduction Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of the Samples
2.2.1. Microstructure and Composition
2.2.2. X-ray Tomography Analysis
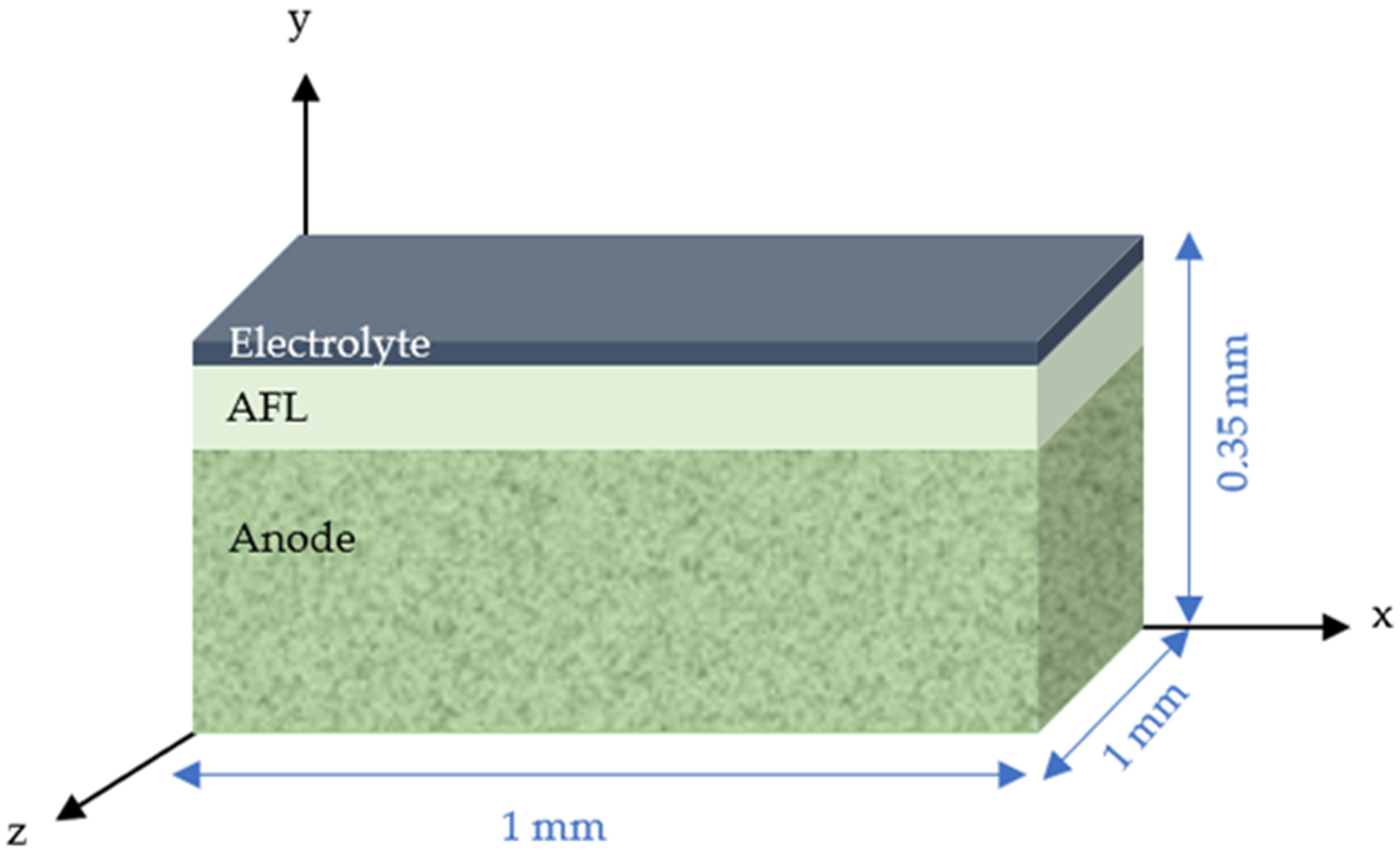
2.3. Volume Reconstruction and Microstructure Characterization
3. Results and Discussion
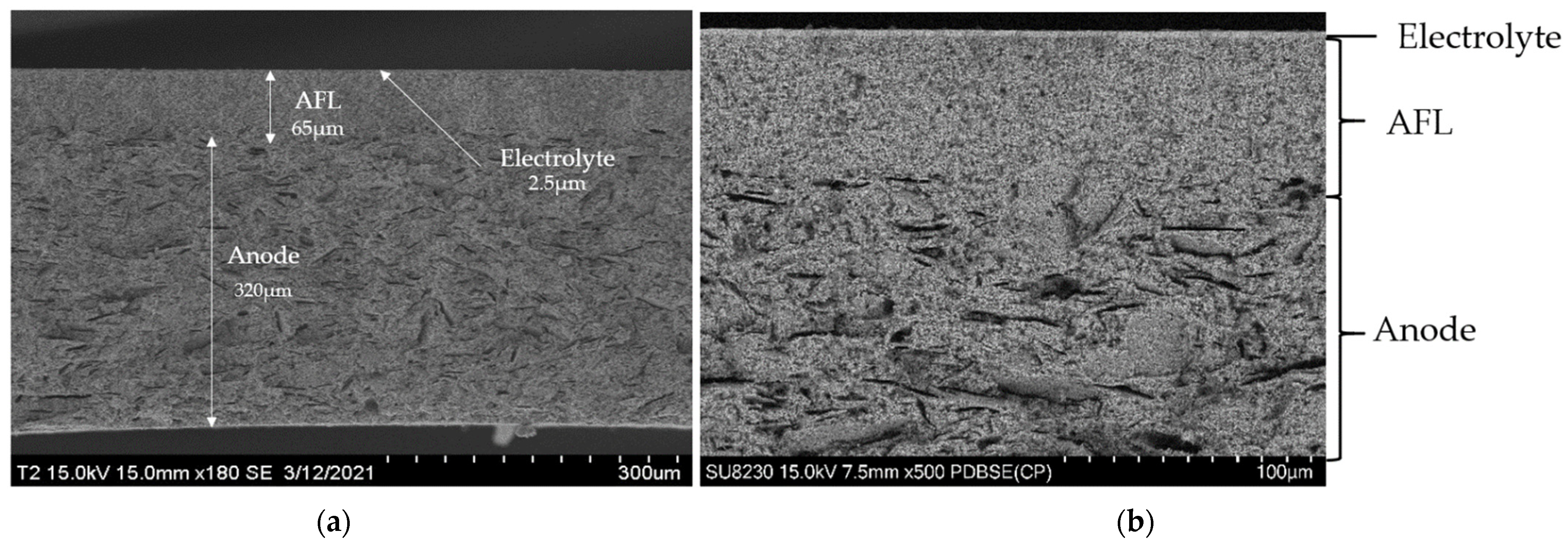
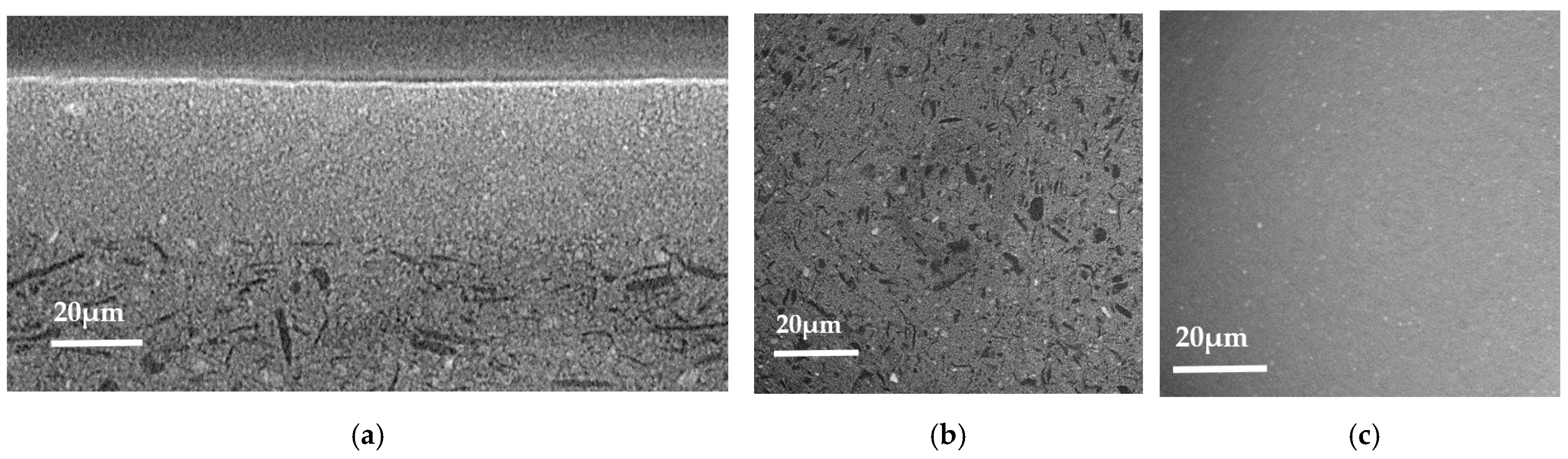
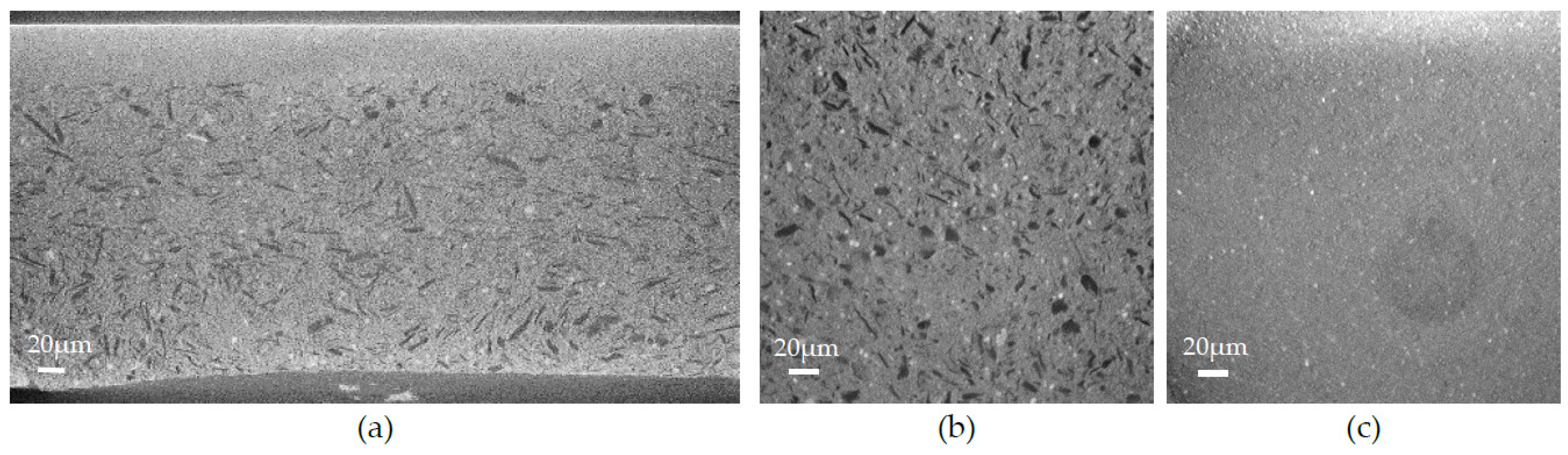
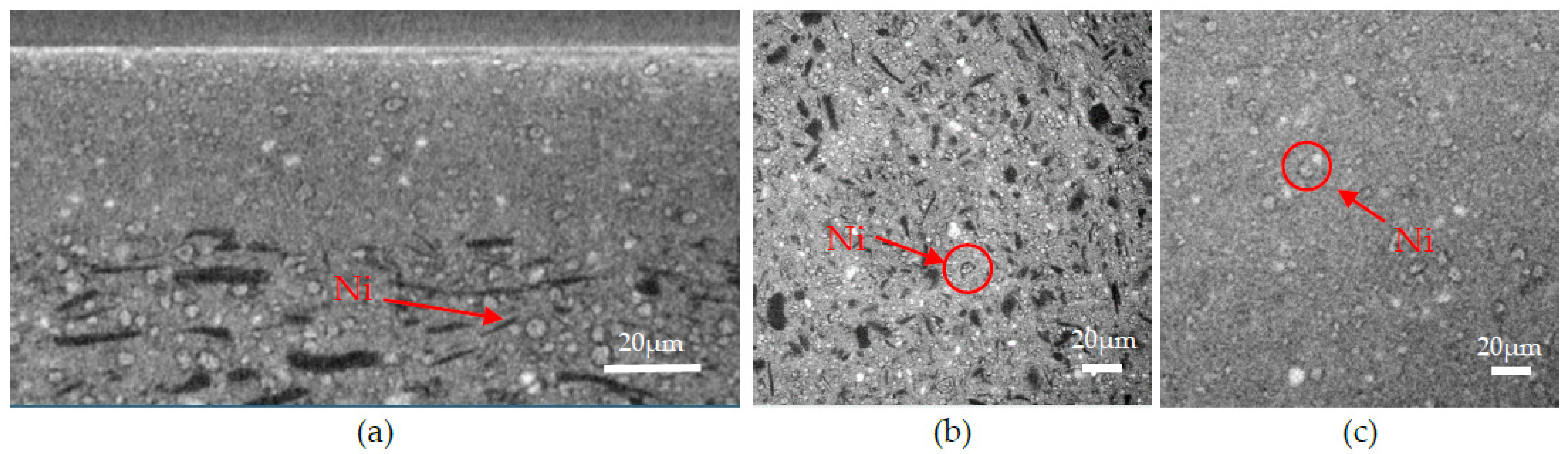
3.1. Scanning Electron Microscopy and Elemental Content Analysis
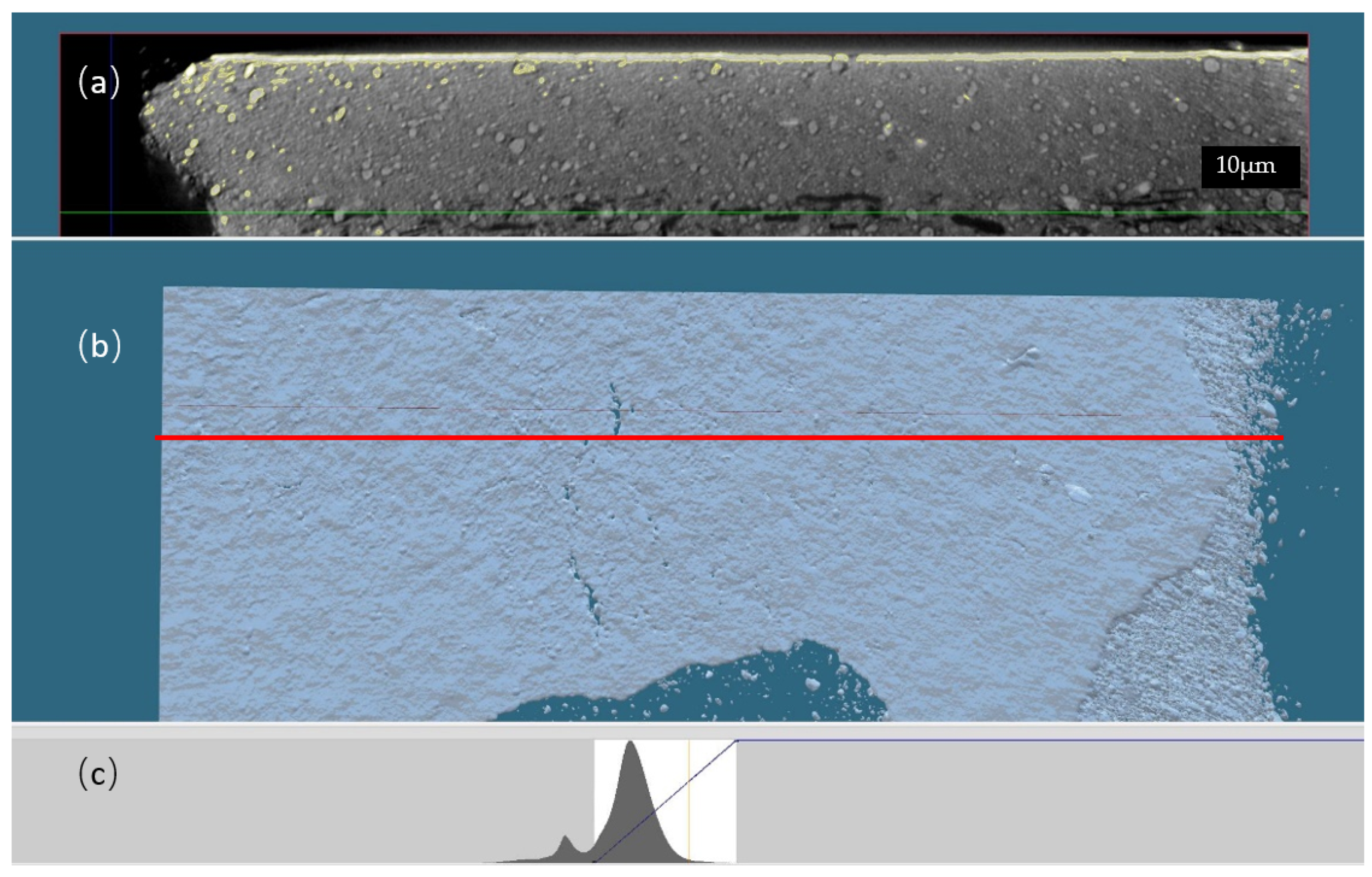
3.2. X-ray Microtomography Characterization
4. Conclusions
- (i) The right microstructure of the three layers composing the half-cell: a dense electrolyte, a dense anode functional layer (AFL), and a porous anode.
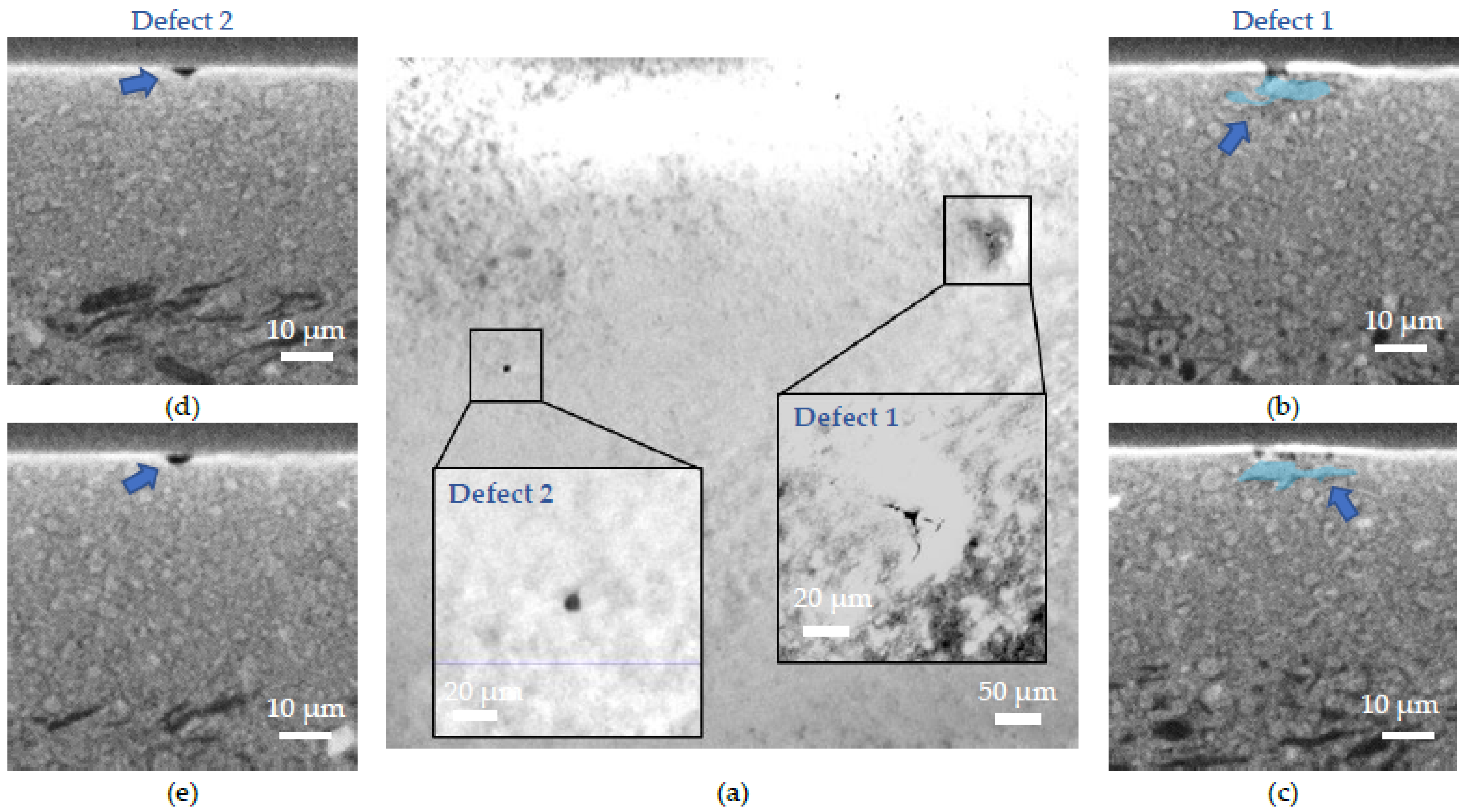
- (ii) A defect similar to a lack of electrolyte matter with no underlying cause, meaning that it comes from the electrolyte deposition by DC magnetron sputtering. This defect, caused by a growth anomaly, is removed during the annealing treatment leading to the formation of a hole.
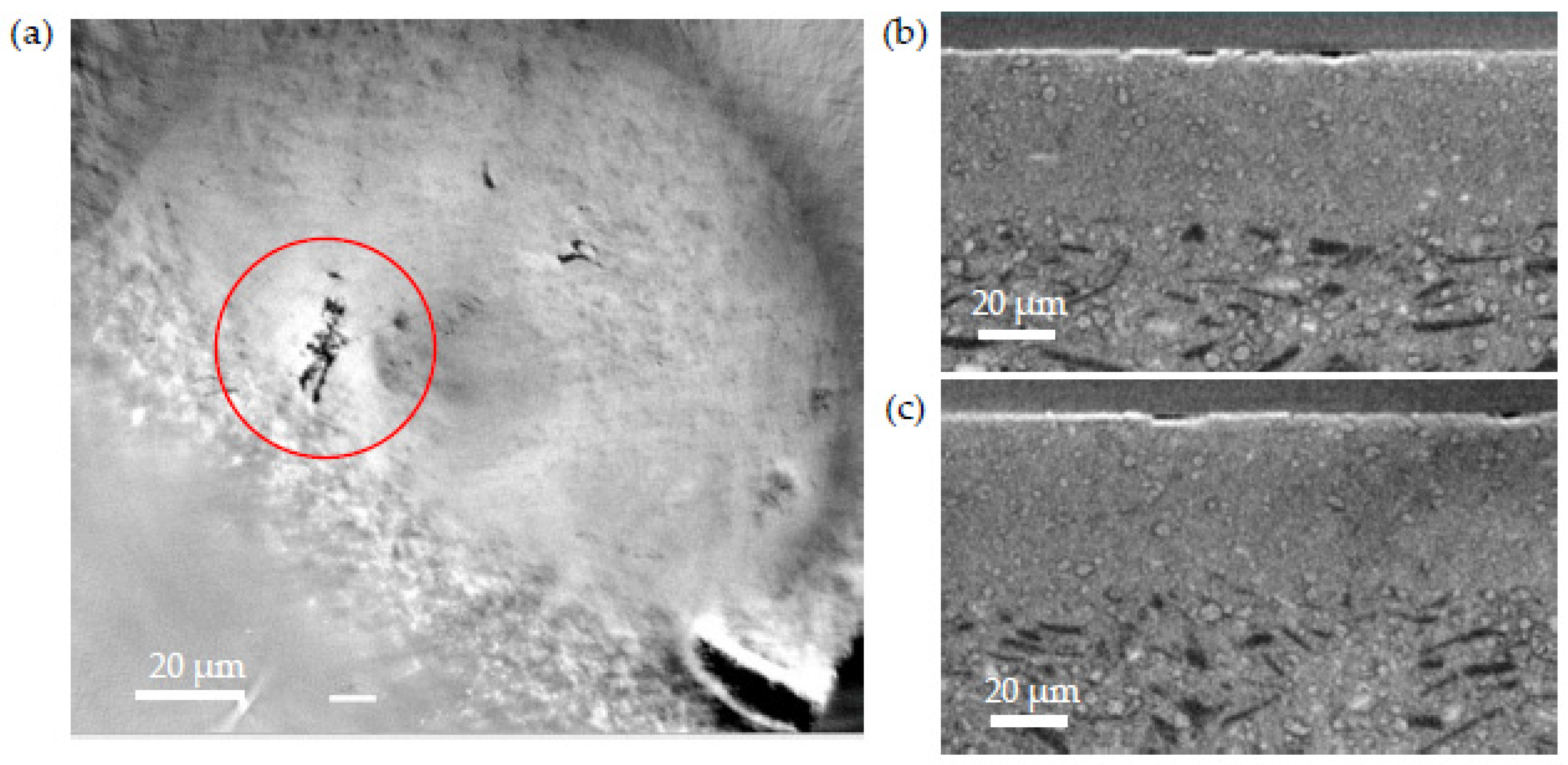
- (iii) The second type of defect is due to the presence of an unusually coarse NiO grain in the AFL. Because of the difference in thermal expansion coefficient (TEC) between NiO and BZY20, the stress caused during the expansion and shrinkage of the cell during the electrolyte deposition lead to crack formation in the electrolyte.
- (iv) Finally, the last type of defect is caused by the NiO reduction. The matter withdrawal, enhanced by the presence of unusually large Ni grains, leads to localized volume reduction, causing mechanical stress in the electrolyte.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, Y.; Gao, J.; Huang, H.; Zou, M.; Duffy, J.; Tong, J.; Brinkman, K.S. A high-performance reversible protonic ceramic electrochemical cell based on a novel Sm-doped BaCe0.7Zr0.1Y0.2O3−δ electrolyte. J. Power Sources 2019, 439, 227093. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.H. Progress in proton-conducting oxides as electrolytes for low-temperature solid oxide fuel cells: From materials to devices. Energy Sci. Eng. 2021, 9, 984–1011. [Google Scholar] [CrossRef]
- Dailly, J.; Taillades, G.; Ancelin, M.; Pers, P.; Marrony, M. High performing BaCe0.8Zr0.1Y0.1O3−δ−Sm0.5Sr0.5CoO3−δ based protonic ceramic fuel cell. J. Power Sources 2017, 361, 221–226. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Jamain, S.N.B.; Zaini, J.H.; Azad, A.K. A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew. Sustain. Energy Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton-Conducting Oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Ryu, K.H.; Haile, S.M. Chemical stability and proton conductivity of doped BaCeO3–BaZrO3 solid solutions. Solid State Ion. 1999, 125, 355–367. [Google Scholar] [CrossRef]
- Wang, S.; Shen, J.; Zhu, Z.; Wang, Z.; Cao, Y.; Guan, X.; Wang, Y.; Wei, Z.; Chen, M. Further optimization of barium cerate properties via co-doping strategy for potential application as proton-conducting solid oxide fuel cell electrolyte. J. Power Sources 2018, 387, 24–32. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kawasaki, Y.; Ito, N.; Enoki, M.; Ishihara, T. Relation Between Electrical Conductivity and Chemical Stability of BaCeO3-Based Proton Conductors with Different Trivalent Dopants. Electrochem. Solid State Lett. 2007, 10, B77. [Google Scholar] [CrossRef]
- Münch, W. Proton diffusion in perovskites: Comparison between BaCeO3, BaZrO3, SrTiO3, and CaTiO3 using quantum molecular dynamics. Solid State Ion. 2000, 136–137, 183–189. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Liu, T.; Chen, F.; Xia, C. Improving the chemical stability of BaCe0.8Sm0.2O3−δ electrolyte by Cl doping for proton-conducting solid oxide fuel cell. Electrochem. Commun. 2013, 28, 87–90. [Google Scholar] [CrossRef]
- Lacz, A. Effect of microstructure on chemical stability and electrical properties of BaCe0.9Y0.1O3−δ. Ionics 2016, 22, 1405–1414. [Google Scholar] [CrossRef][Green Version]
- Tao, S.; Irvine, J.T.S. Conductivity studies of dense yttrium-doped BaZrO3 sintered at 1325 °C. J. Solid State Chem. 2007, 180, 3493–3503. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kang, Y.-M.; Byun, M.-S.; Hwang, K.-T. Study on the chemical stability of Y-doped BaCeO3−δ and BaZrO3−δ films deposited by aerosol deposition. Thin Solid Films 2011, 520, 1015–1021. [Google Scholar] [CrossRef]
- Sun, Z.; Fabbri, E.; Bi, L.; Traversa, E. Electrochemical Properties and Intermediate-Temperature Fuel Cell Performance of Dense Yttrium-Doped Barium Zirconate with Calcium Addition. J. Am. Ceram. Soc. 2012, 95, 627–635. [Google Scholar] [CrossRef]
- Dai, H.; Kou, H.; Wang, H.; Bi, L. Electrochemical performance of protonic ceramic fuel cells with stable BaZrO3-based electrolyte: A mini-review. Electrochem. Commun. 2018, 96, 11–15. [Google Scholar] [CrossRef]
- Yun, D.; Kim, J.; Kim, S.-J.; Lee, J.-H.; Kim, J.-N.; Yoon, H.; Yu, J.; Kwak, M.; Yoon, H.; Cho, Y.; et al. Structural and Electrochemical Properties of Dense Yttria-Doped Barium Zirconate Prepared by Solid-State Reactive Sintering. Energies 2018, 11, 3083. [Google Scholar] [CrossRef]
- Fabbri, E.; Bi, L.; Pergolesi, D.; Traversa, E. Towards the Next Generation of Solid Oxide Fuel Cells Operating Below 600 °C with Chemically Stable Proton-Conducting Electrolytes. Adv. Mater. 2012, 24, 195–208. [Google Scholar] [CrossRef]
- Kim, D.; Lee, D.; Joo, J.H. Effect of Y-doping on the phase relation and electrical properties of Fe-doped BaZrO3. J. Eur. Ceram. Soc. 2018, 38, 535–542. [Google Scholar] [CrossRef]
- Han, D.; Uda, T. The best composition of an Y-doped BaZrO3 electrolyte: Selection criteria from transport properties, microstructure, and phase behavior. J. Mater. Chem. A 2018, 6, 18571–18582. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hernandez-Sanchez, R.; Haile, S.M. High Total Proton Conductivity in Large-Grained Yttrium-Doped Barium Zirconate. Chem. Mater. 2009, 21, 2755–2762. [Google Scholar] [CrossRef]
- Fabbri, E.; Pergolesi, D.; Traversa, E. Materials challenges toward proton-conducting oxide fuel cells: A critical review. Chem. Soc. Rev. 2010, 39, 4355. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; He, J.; Chen, F.; Brinkman, K.S. Spark-plasma-sintered barium zirconate based proton conductors for solid oxide fuel cell and hydrogen separation applications. Int. J. Hydrog. Energy 2015, 40, 5707–5714. [Google Scholar] [CrossRef]
- Bi, L.; Traversa, E. Synthesis strategies for improving the performance of doped-BaZrO3 materials in solid oxide fuel cell applications. J. Mater. Res. 2014, 29, 1–15. [Google Scholar] [CrossRef]
- Loureiro, F.J.A.; Nasani, N.; Reddy, G.S.; Munirathnam, N.R.; Fagg, D.P. A review on sintering technology of proton conducting BaCeO3-BaZrO3 perovskite oxide materials for Protonic Ceramic Fuel Cells. J. Power Sources 2019, 438, 226991. [Google Scholar] [CrossRef]
- Bae, K.; Jang, D.Y.; Choi, H.J.; Kim, D.; Hong, J.; Kim, B.-K.; Lee, J.-H.; Son, J.-W.; Shim, J.H. Demonstrating the potential of yttrium-doped barium zirconate electrolyte for high-performance fuel cells. Nat. Commun. 2017, 8, 14553. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.A.P.; Briois, P.; Billard, A. Influence of the annealing conditions on the structure of BaCe1−xYxO3−α coatings elaborated by DC magnetron sputtering at room temperature. Mater. Chem. Phys. 2009, 117, 178–182. [Google Scholar] [CrossRef]
- Pergolesi, D.; Fabbri, E.; D’Epifanio, A.; Di Bartolomeo, E.; Tebano, A.; Sanna, S.; Licoccia, S.; Balestrino, G.; Traversa, E. High proton conduction in grain-boundary-free yttrium-doped barium zirconate films grown by pulsed laser deposition. Nat. Mater. 2010, 9, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Wang, F.; Wang, D.; Jin, Z. Alternative preparation methods of thin films for solid oxide fuel cells: Review. Mater. Technol. 2020, 35, 212–227. [Google Scholar] [CrossRef]
- Zakaria, Z.; Awang Mat, Z.; Abu Hassan, S.H.; Boon Kar, Y. A review of solid oxide fuel cell component fabrication methods toward lowering temperature. Int. J. Energy Res. 2020, 44, 594–611. [Google Scholar] [CrossRef]
- Faes, A.; Nakajo, A.; Hessler-Wyser, A.; Dubois, D.; Brisse, A.; Modena, S.; Van herle, J. RedOx study of anode-supported solid oxide fuel cell. J. Power Sources 2009, 193, 55–64. [Google Scholar] [CrossRef]
- Liu, X.; Jin, F.; Sun, N.; Li, J.; Shen, Y.; Wang, F.; Li, J. Nd3+-deficiency double perovskite Nd1−BaCo2O5+ and performance optimization as cathode materials for intermediate-temperature solid oxide fuel cells. Ceram. Int. 2021, 47, 33886–33896. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, Z.; Mori, T.; Jiang, S.P. Development of nickel based cermet anode materials in solid oxide fuel cells—Now and future. Mater. Rep. Energy 2021, 1, 100003. [Google Scholar] [CrossRef]
- Onishi, T.; Han, D.; Noda, Y.; Hatada, N.; Majima, M.; Uda, T. Evaluation of performance and durability of Ni-BZY cermet electrodes with BZY electrolyte. Solid State Ion. 2018, 317, 127–135. [Google Scholar] [CrossRef]
- Geng, C.; Yu, X.; Wang, P.; Cheng, J.; Hong, T. The rapid one-step fabrication of bilayer anode for protonic ceramic fuel cells by phase inversion tape casting. J. Eur. Ceram. Soc. 2020, 40, 3104–3110. [Google Scholar] [CrossRef]
- Sivasankaran, V.; Combemale, L.; Pera, M.C.; Caboche, G. Initial Preparation and Characterization of Single Step Fabricated Intermediate Temperature Solid Oxide Fuel Cells (IT-SOFC). Fuel Cells 2014, 14, 533–536. [Google Scholar] [CrossRef]
- François, M.; Carpanese, M.P.; Heintz, O.; Lescure, V.; Clematis, D.; Combemale, L.; Demoisson, F.; Caboche, G. Chemical Degradation of the La0.6Sr0.4Co0.2Fe0.8O3−δ/Ce0.8Sm0.2O2−δ Interface during Sintering and Cell Operation. Energies 2021, 14, 3674. [Google Scholar] [CrossRef]
- Siad, A.; Besnard, A.; Nouveau, C.; Jacquet, P. Critical angles in DC magnetron glad thin films. Vacuum 2016, 131, 305–311. [Google Scholar] [CrossRef]
- Tait, R.N.; Smy, T.; Brett, M.J. Modelling and characterization of columnar growth in evaporated films. Thin Solid Films 1993, 226, 196–201. [Google Scholar] [CrossRef]
- Challali, F.; Mendil, D.; Touam, T.; Chauveau, T.; Bockelée, V.; Sanchez, A.G.; Chelouche, A.; Besland, M.-P. Effect of RF sputtering power and vacuum annealing on the properties of AZO thin films prepared from ceramic target in confocal configuration. Mater. Sci. Semicond. Process. 2020, 118, 105217. [Google Scholar] [CrossRef]
- Zoppi, G.; Beattie, N.S.; Major, J.D.; Miles, R.W.; Forbes, I. Electrical, morphological and structural properties of RF magnetron sputtered Mo thin films for application in thin film photovoltaic solar cells. J. Mater. Sci. 2011, 46, 4913–4921. [Google Scholar] [CrossRef]
- Ahn, S.; Koo, H.; Bae, S.-H.; Chang, I.; Cha, S.; Yoo, Y.-S.; Park, C. The Grain Growth Behavior of NiO in Thermally-Stable Mesoporous Gadolinium-Doped Ceria Network for Intermediate-Temperature Solid Oxide Fuel Cell Anode Materials. J. Nanosci. Nanotechnol. 2014, 14, 8117–8121. [Google Scholar] [CrossRef] [PubMed]
- Wolfi, J.S.; Cavin, O.B. The Effective Thermal Expansion Of Nickel And Nickel Oxide During High-Temperature Oxidation. Adv. X-ray Anal. 1993, 37, 449–456. [Google Scholar] [CrossRef]
- Løken, A.; Ricote, S.; Wachowski, S. Thermal and Chemical Expansion in Proton Ceramic Electrolytes and Compatible Electrodes. Crystals 2018, 8, 365. [Google Scholar] [CrossRef]



| Target | Power (W) | Tension (V) | Intensity (A) | Time Off (µs) | Frequency (kHz) |
|---|---|---|---|---|---|
| Ba | 85 | 161 | 0.56 | 2 | 150 |
| Zr0.8Y0.2 | 80 | 2 [30] | 0.33 | 4 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lescure, V.; Gelin, M.; François, M.; Arab Pour Yazdi, M.; Briois, P.; Demoisson, F.; Combemale, L.; Valton, S.; Caboche, G. X-ray Micro-Computed Tomography: A Powerful Device to Analyze the 3D Microstructure of Anode-Electrolyte in BaZr0.8Y0.2O3 Protonic Ceramic Electrochemical Cells and the Reduction Behavior. Membranes 2022, 12, 68. https://doi.org/10.3390/membranes12010068
Lescure V, Gelin M, François M, Arab Pour Yazdi M, Briois P, Demoisson F, Combemale L, Valton S, Caboche G. X-ray Micro-Computed Tomography: A Powerful Device to Analyze the 3D Microstructure of Anode-Electrolyte in BaZr0.8Y0.2O3 Protonic Ceramic Electrochemical Cells and the Reduction Behavior. Membranes. 2022; 12(1):68. https://doi.org/10.3390/membranes12010068
Chicago/Turabian StyleLescure, Victoire, Morgane Gelin, Mélanie François, Mohammad Arab Pour Yazdi, Pascal Briois, Frédéric Demoisson, Lionel Combemale, Solène Valton, and Gilles Caboche. 2022. "X-ray Micro-Computed Tomography: A Powerful Device to Analyze the 3D Microstructure of Anode-Electrolyte in BaZr0.8Y0.2O3 Protonic Ceramic Electrochemical Cells and the Reduction Behavior" Membranes 12, no. 1: 68. https://doi.org/10.3390/membranes12010068
APA StyleLescure, V., Gelin, M., François, M., Arab Pour Yazdi, M., Briois, P., Demoisson, F., Combemale, L., Valton, S., & Caboche, G. (2022). X-ray Micro-Computed Tomography: A Powerful Device to Analyze the 3D Microstructure of Anode-Electrolyte in BaZr0.8Y0.2O3 Protonic Ceramic Electrochemical Cells and the Reduction Behavior. Membranes, 12(1), 68. https://doi.org/10.3390/membranes12010068







