Single-Shot Vaccines against Bovine Respiratory Syncytial Virus (BRSV): Comparative Evaluation of Long-Term Protection after Immunization in the Presence of BRSV-Specific Maternal Antibodies
Abstract
1. Introduction
2. Materials and Methods
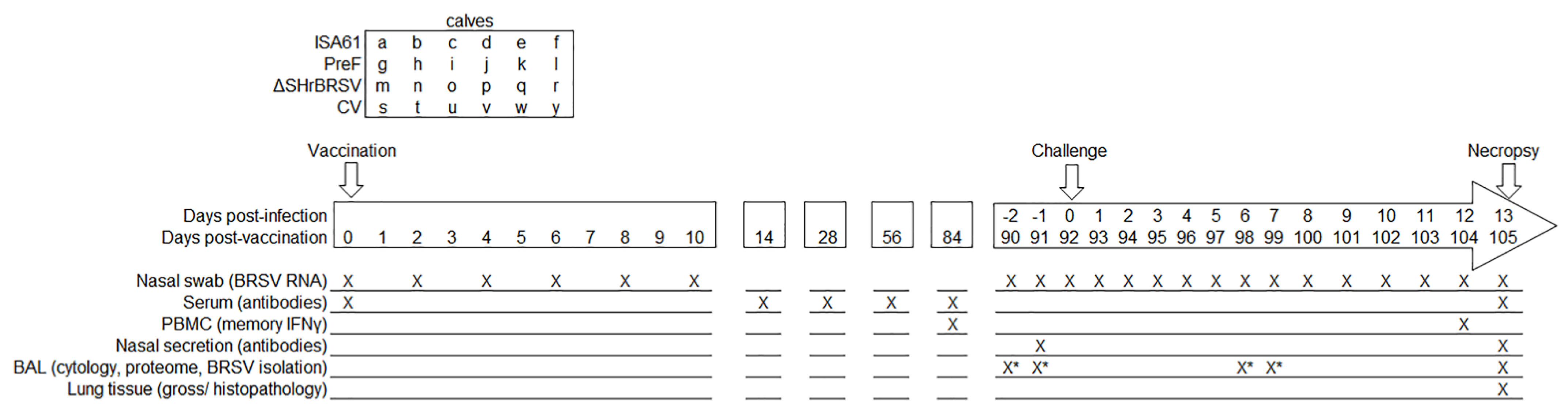
2.1. Calves and Vaccination Protocol
2.2. Experimental Design, Challenge, Clinical Records, and Post-Mortem Analyses
2.3. Sample Collection, Preparation and Storage
2.4. Detection of BRSV RNA and Live BRSV
2.5. Serology
2.6. T-cell Responses
2.7. Histological Analysis of Lung Tissue
2.8. Label-Free Quantitative Mass Spectrometry-Based Proteomics
2.9. Statistical Analysis
2.10. Ethics Statement
3. Results
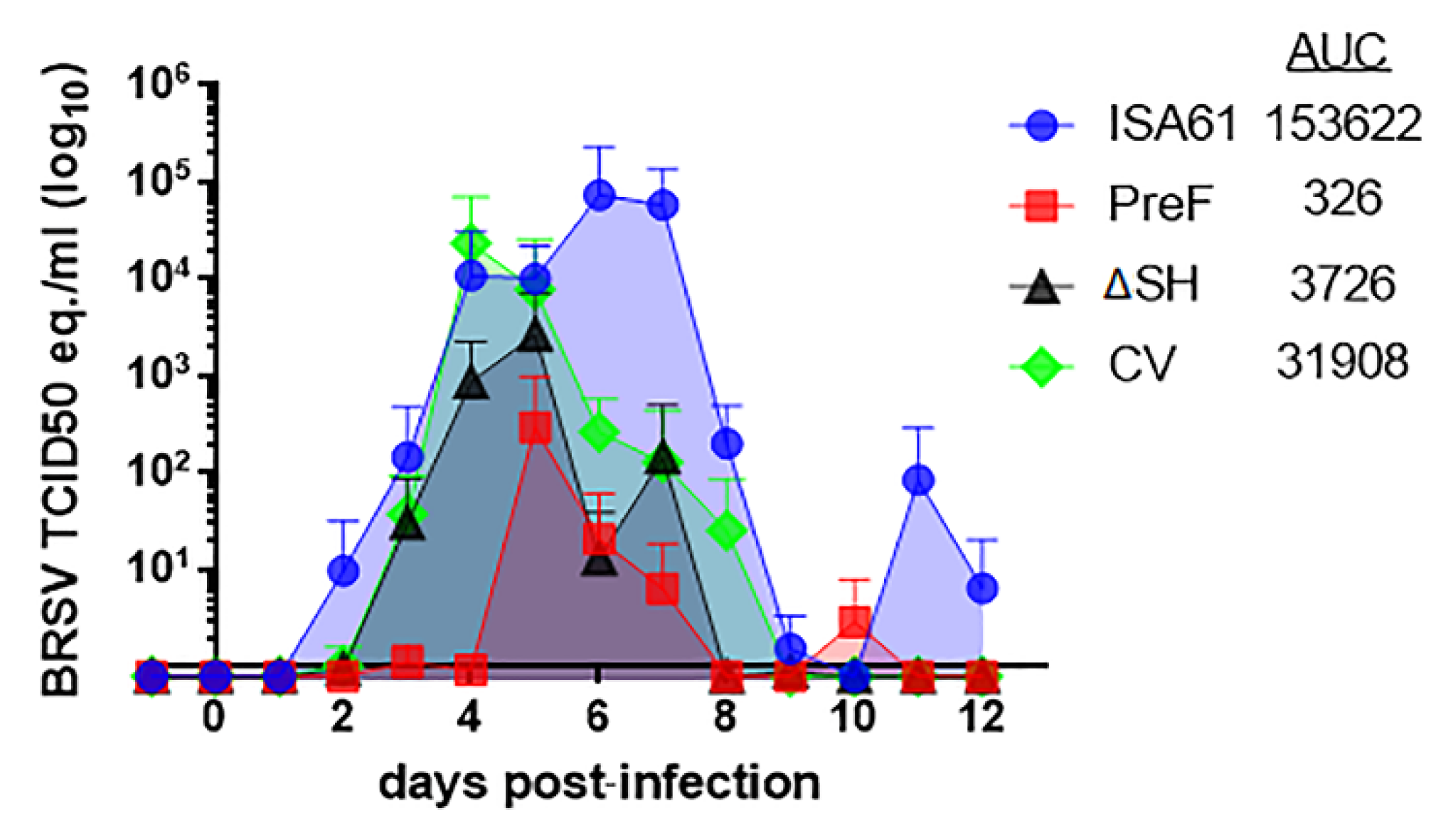
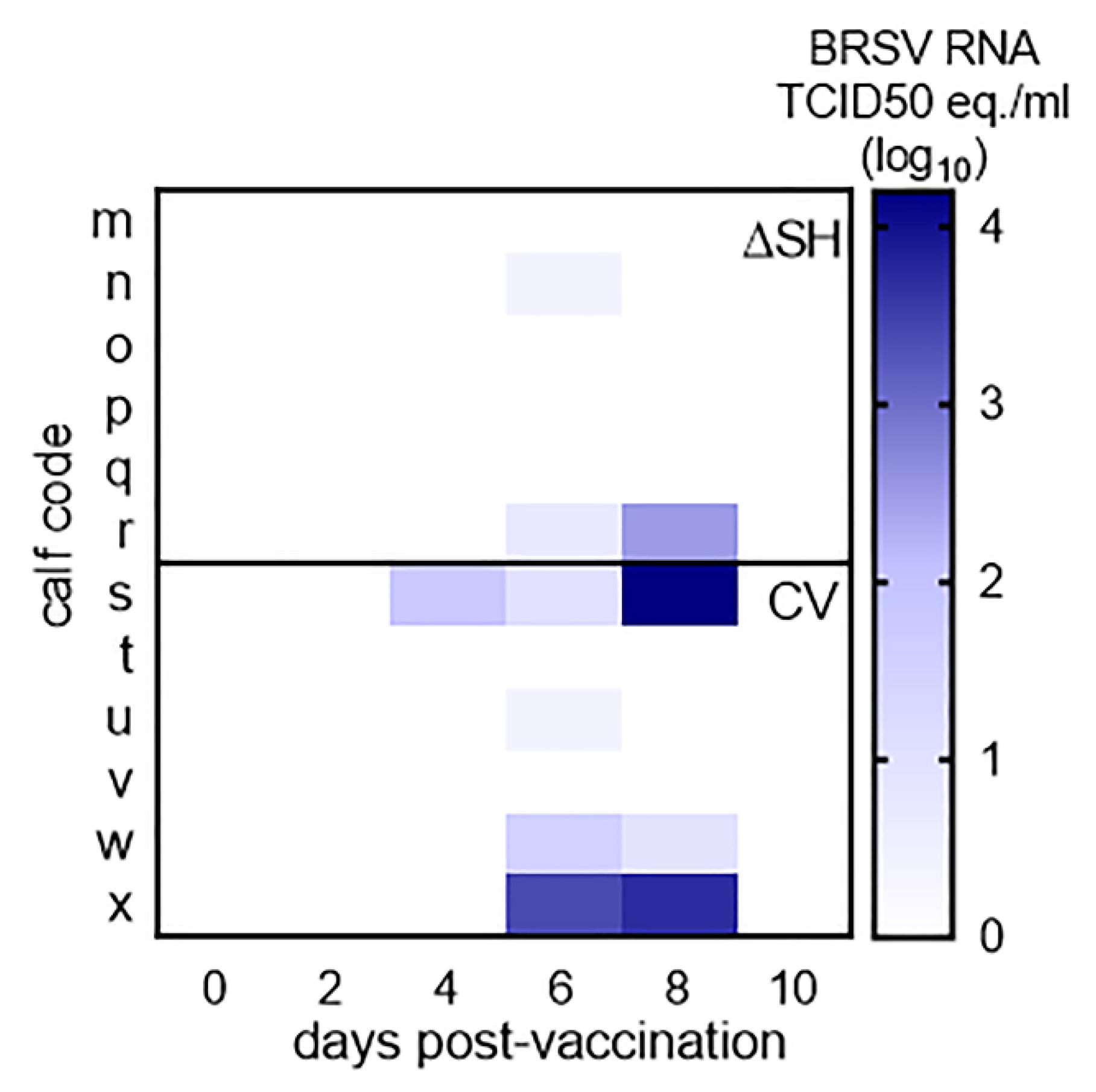
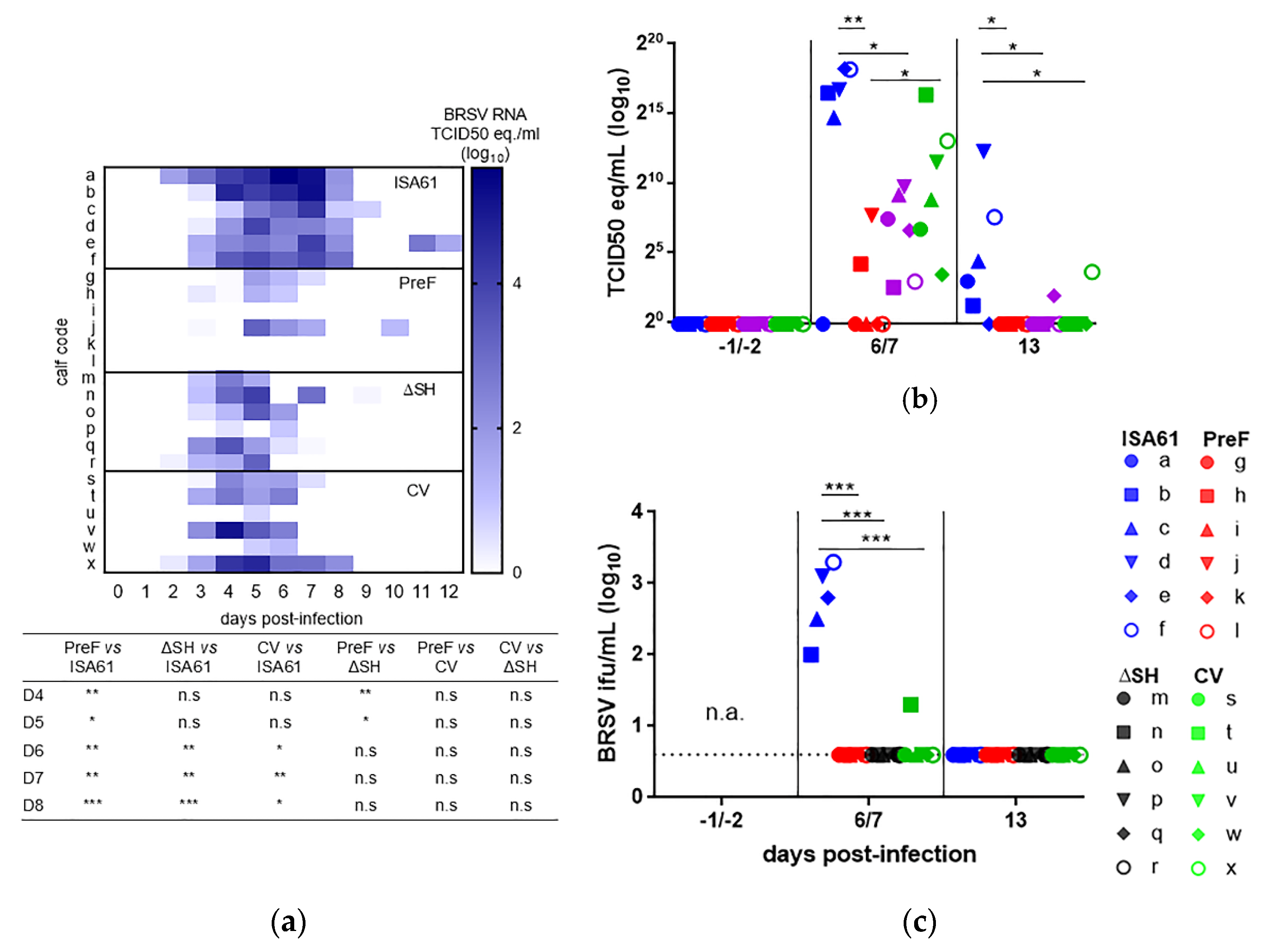
3.1. Virus Replication in Calves Following Intranasal Vaccination with the Live-Attenuated Vaccines
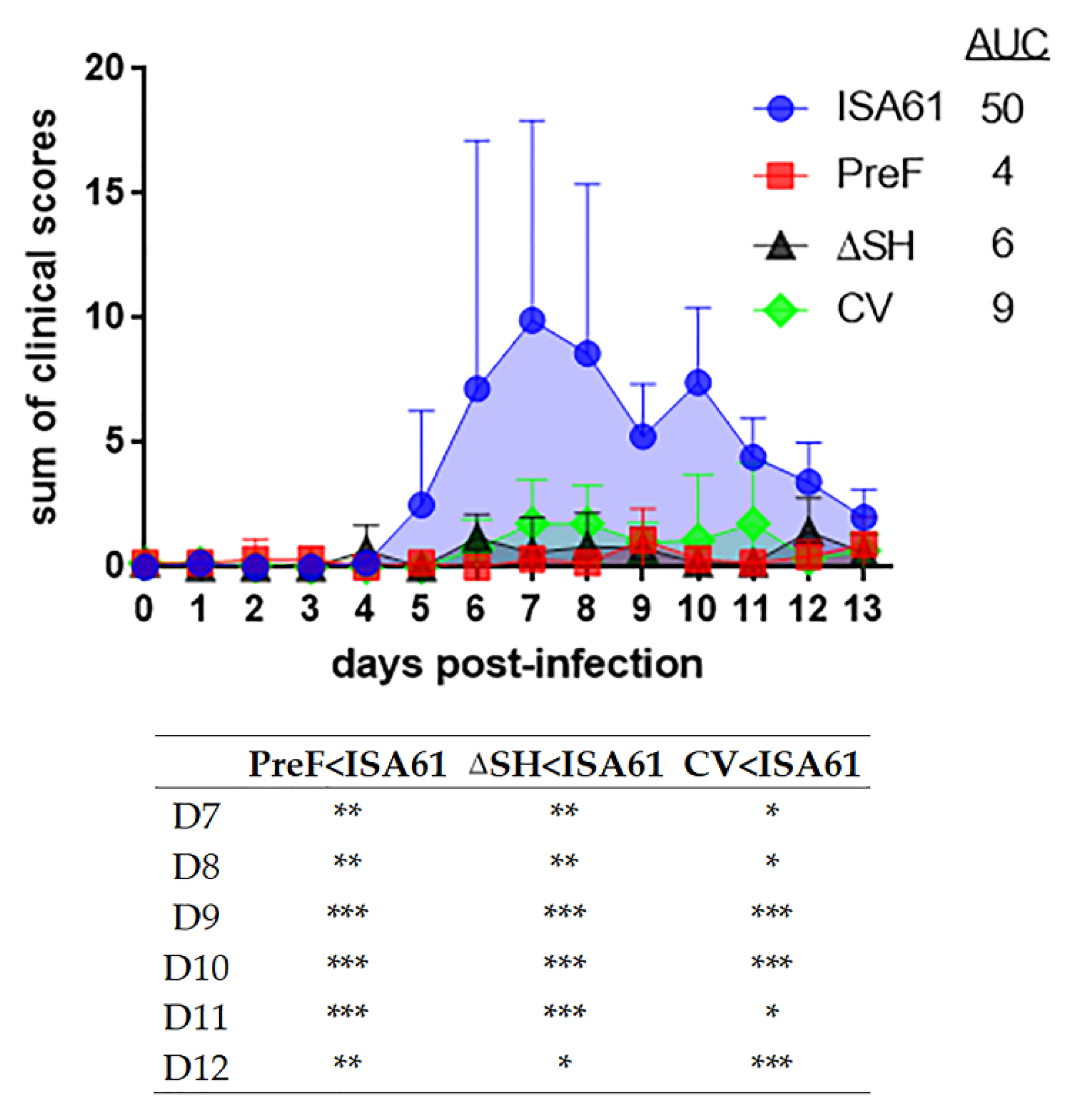
3.2. Mild Clinical Reactions Were Observed after Intranasal and Intramuscular Vaccination
3.3. PreF, ΔSHrBRSV and CV Induced Clinical Protection against BRSV Challenge, Three Months after A Single Immunization
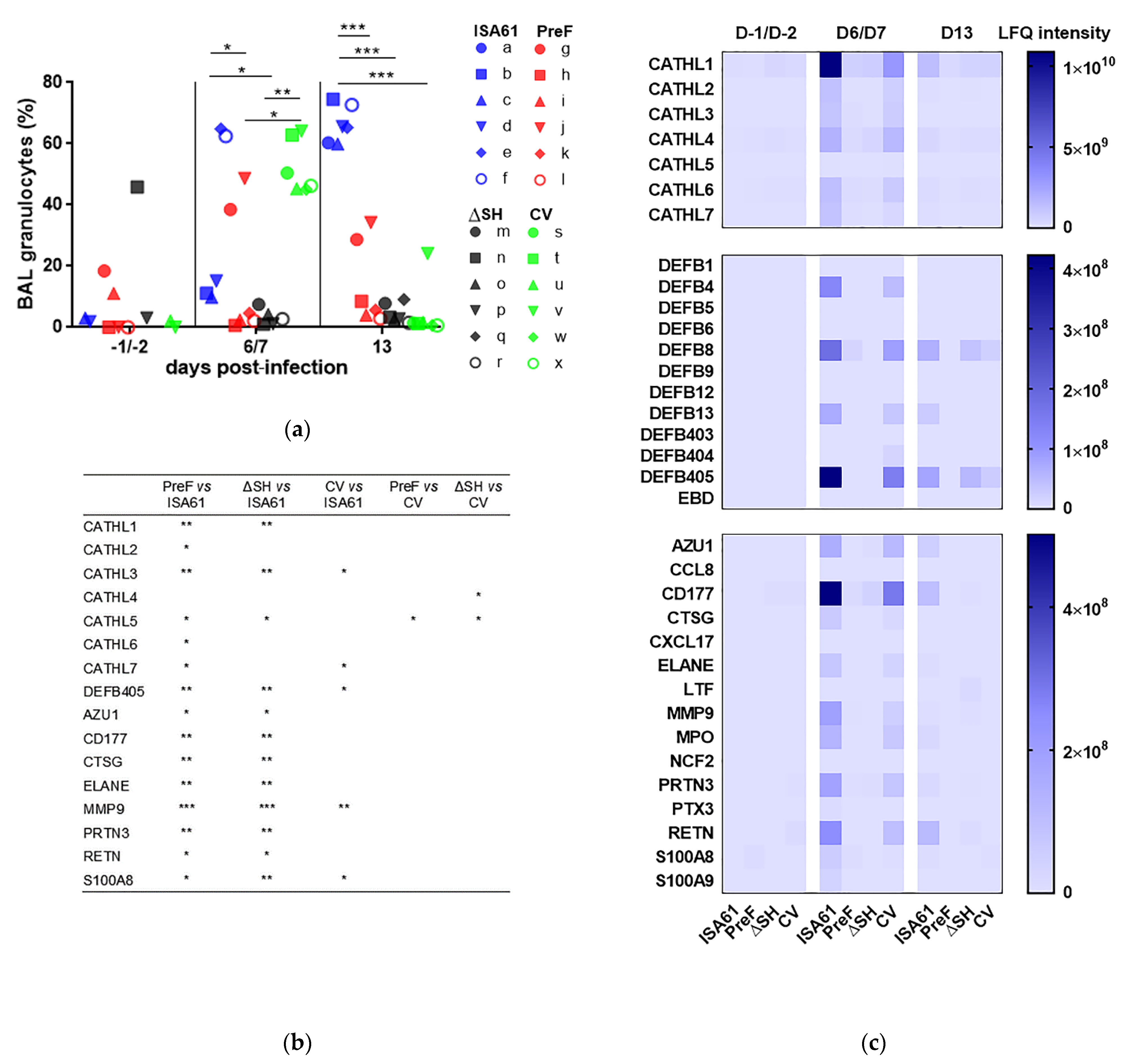
3.4. PreF, ΔSHrBRSV, and CV Induced Protection against Neutrophilic Inflammation Detected in BAL Following BRSV Challenge
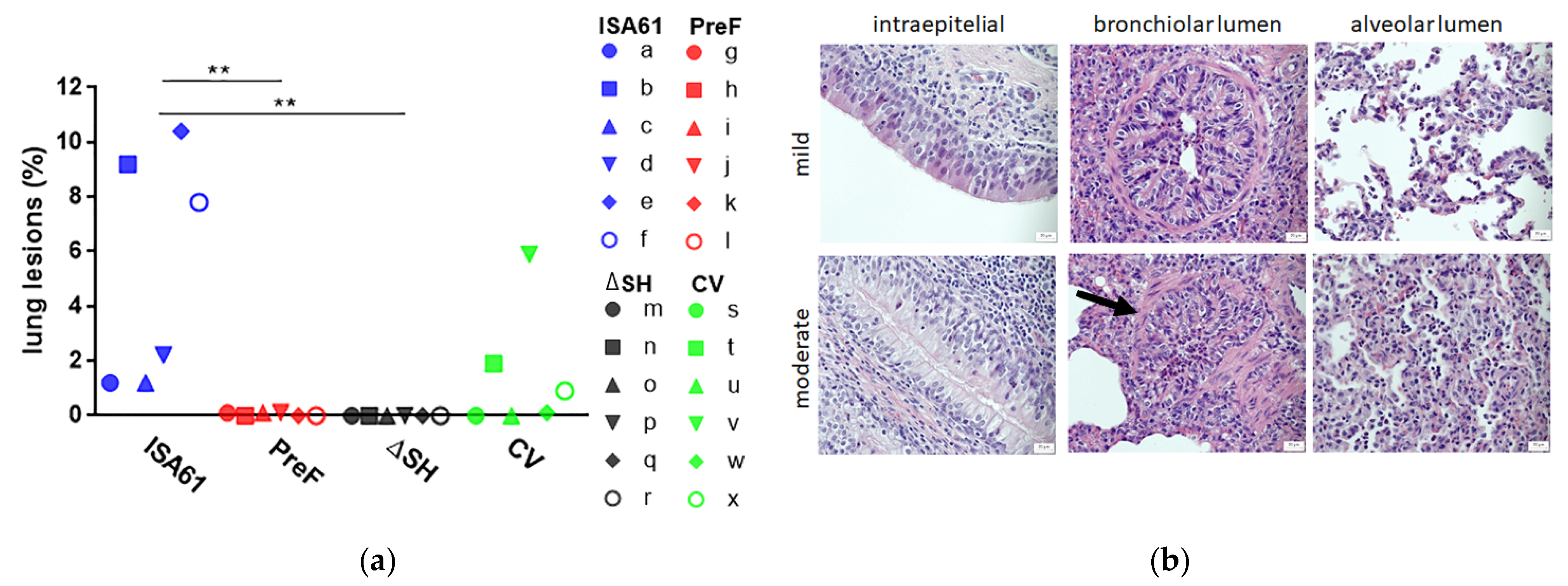
3.5. PreF and ΔSHrBRSV Induced Protection against Macroscopic and Microscopic Lung Lesions Following BRSV Challenge
3.6. PreF, ΔSHrBRSV, and the CV Induced Virological Protection in Decreasing Order
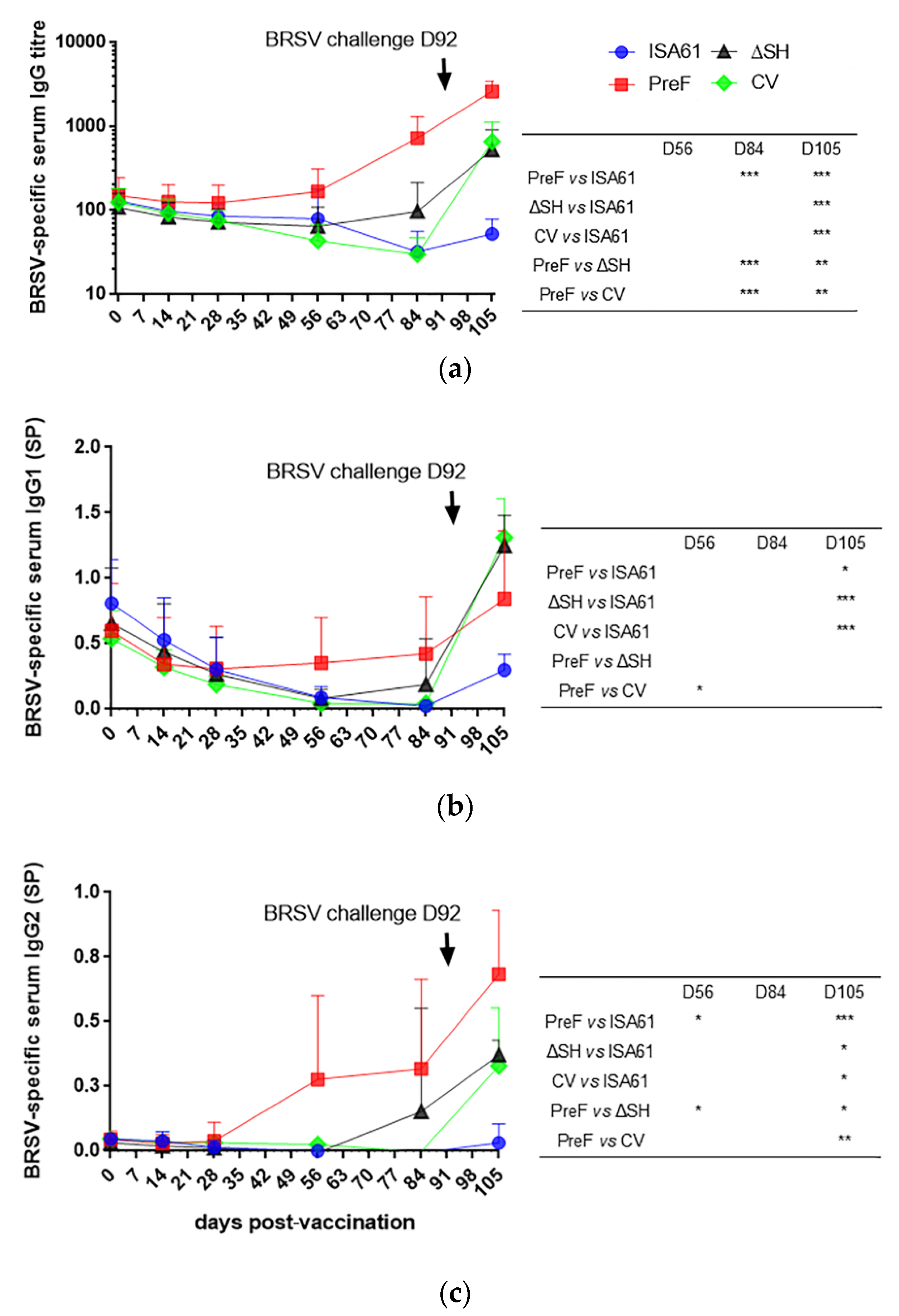
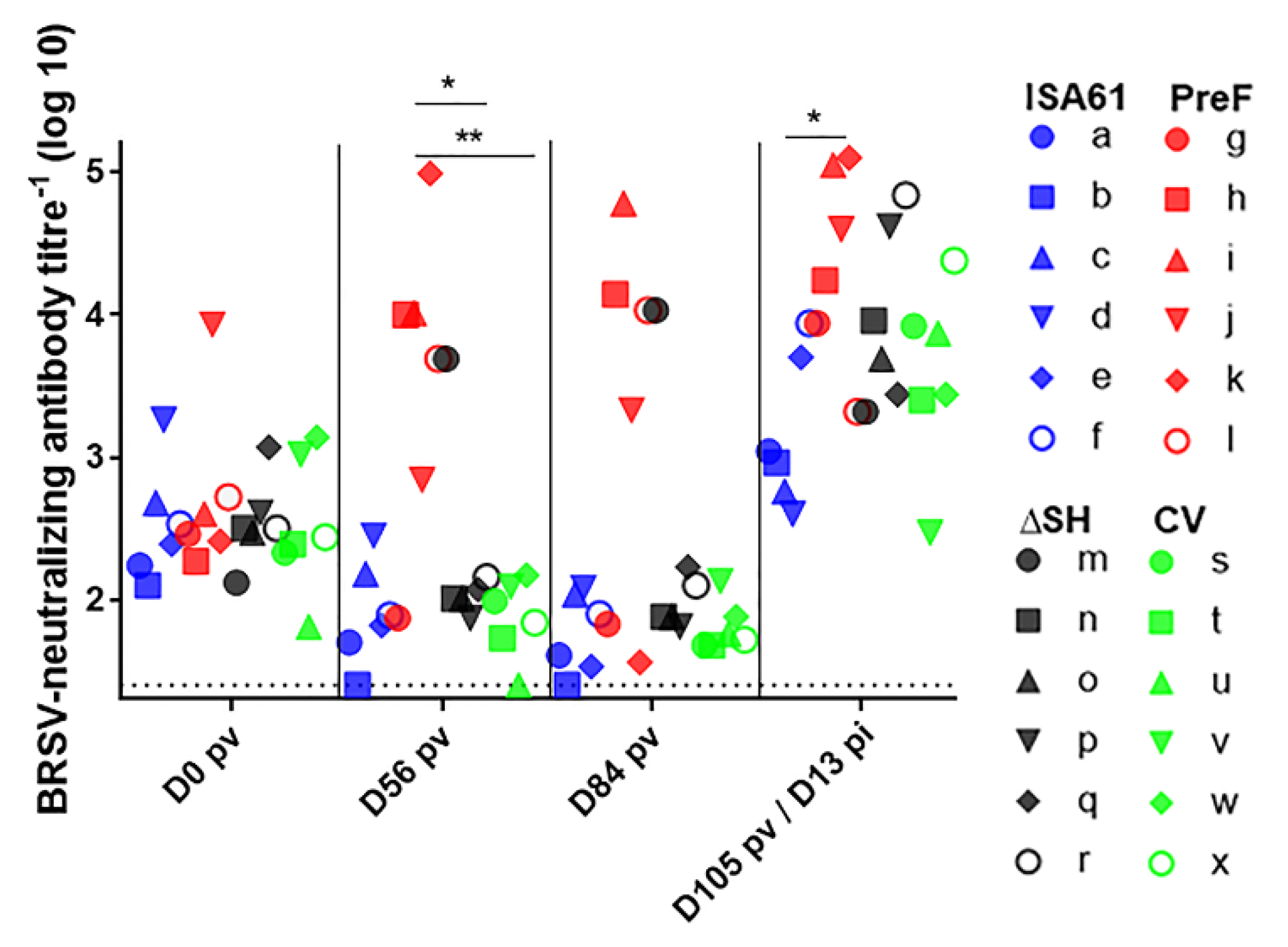
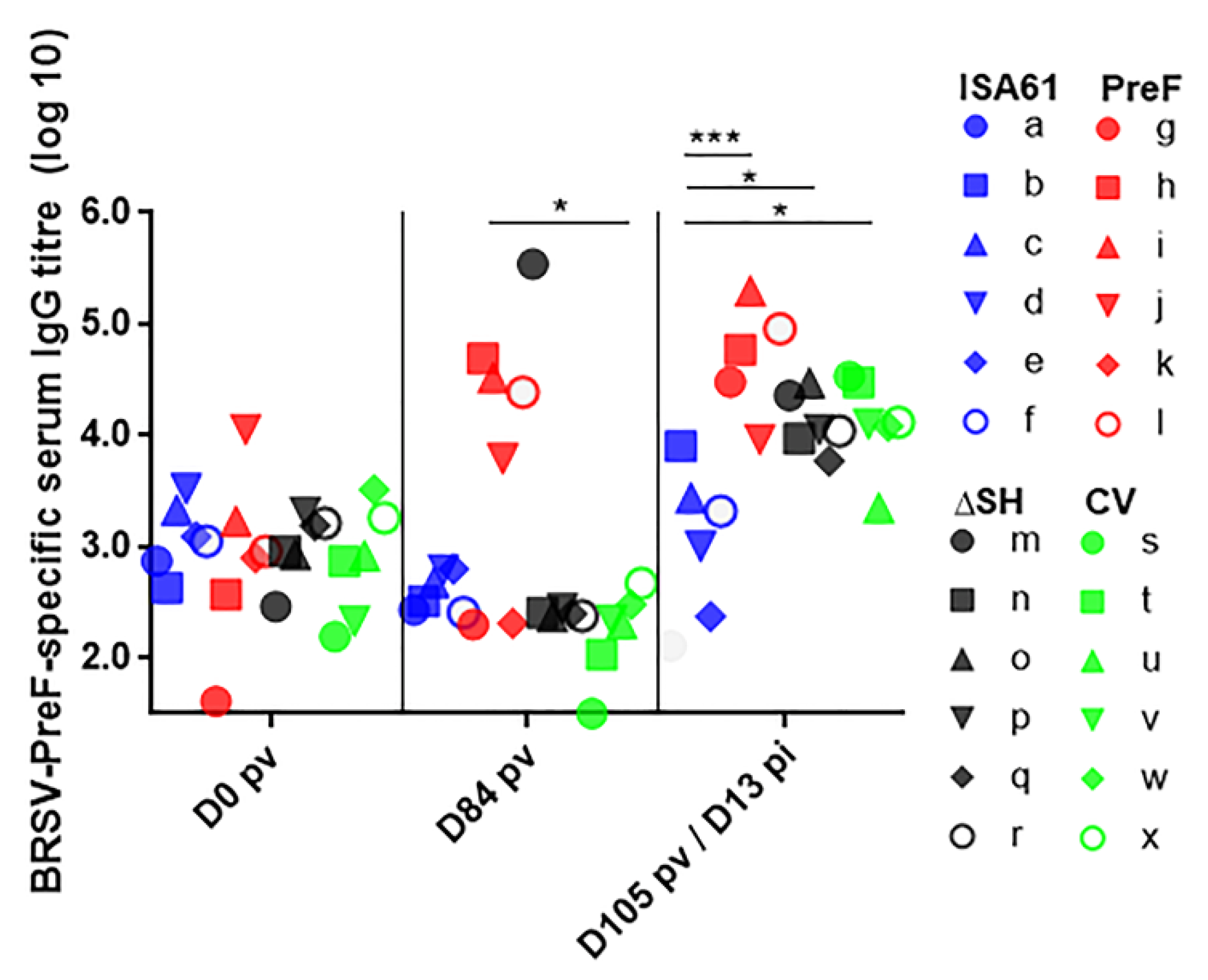
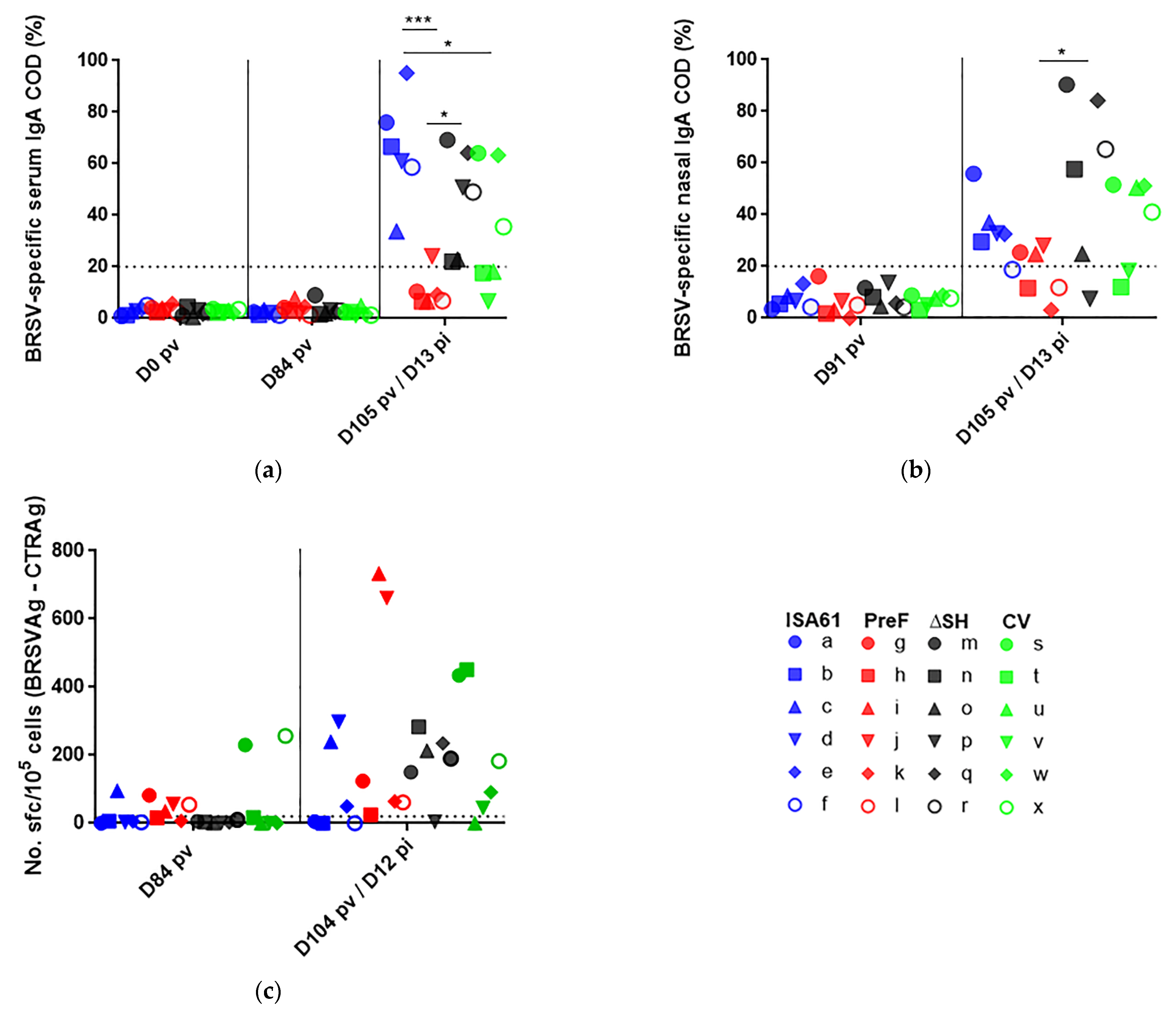
3.7. In Contrast to ΔSHrBRSV and the CV, PreF Induced BRSV-Specific Humoral Responses Pre-Challenge in Most Animals and Primed for Neutralising Antibodies Post-Challenge
3.8. None of the Vaccines Primed for IgA or Circulating BRSV-Specific IFNγ Expressing T Cells
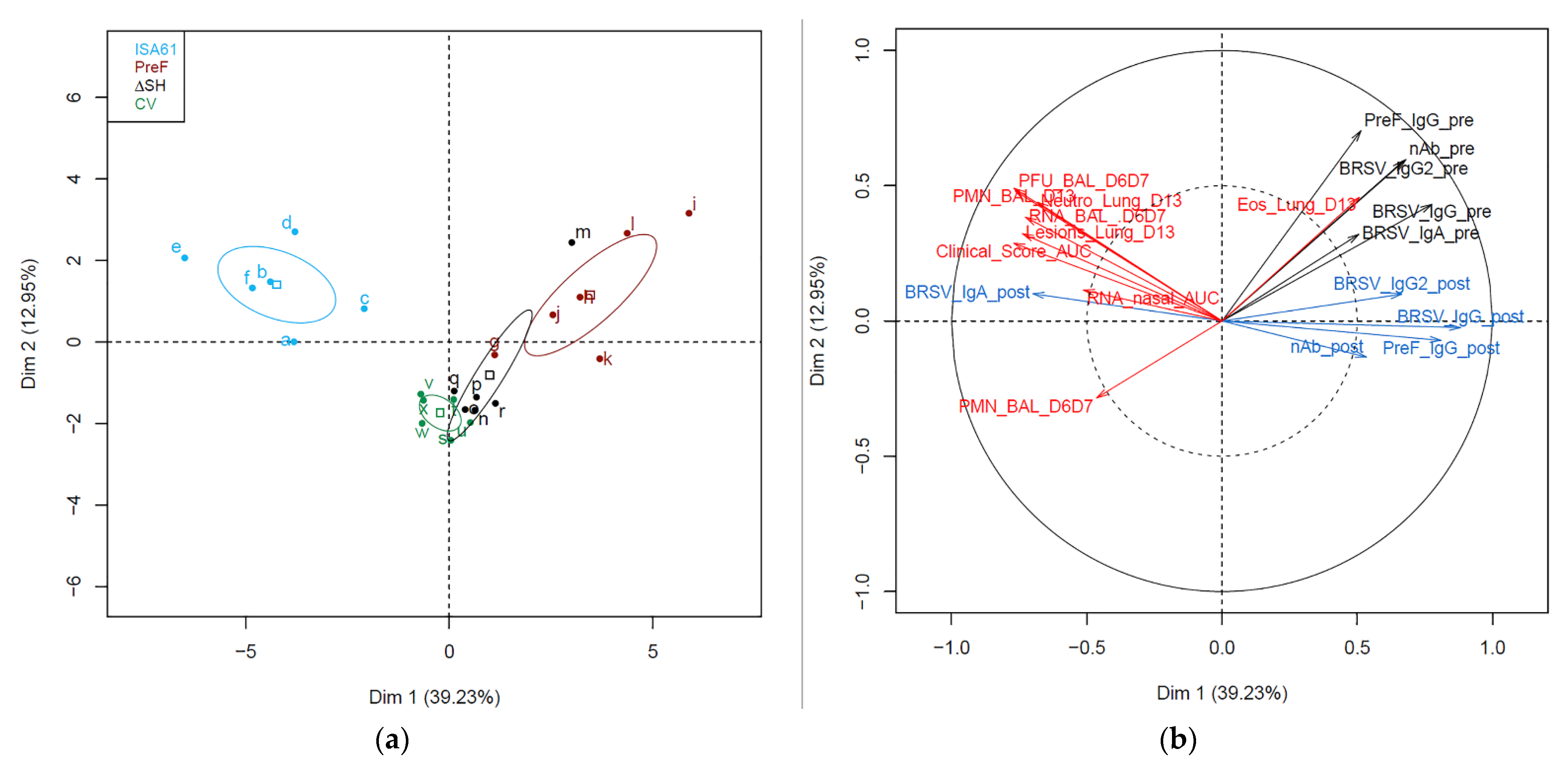
3.9. Immunological Markers before and after Challenge Correlated with Protection against Challenge
3.10. Several Proteins in BAL Obtained before Challenge Correlated with Protection against Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Valarcher, J.F.; Taylor, G. Bovine respiratory syncytial virus infection. Vet. Res. 2007, 38, 153–180. [Google Scholar] [CrossRef]
- ICTV Online. Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report/negative-sense-rna-viruses/w/pneumoviridae/738/genus-orthopneumovirus (accessed on 24 February 2021).
- Klem, T.B.; Kjaestad, H.P.; Kummen, E.; Holen, H.; Stokstad, M. Bovine respiratory syncytial virus outbreak reduced bulls’ weight gain and feed conversion for eight months in a Norwegian beef herd. Acta Vet. Scand. 2016, 58, 8. [Google Scholar] [CrossRef]
- Beaudeau, F.; Ohlson, A.; Emanuelson, U. Associations between bovine coronavirus and bovine respiratory syncytial virus infections and animal performance in Swedish dairy herds. J. Dairy Sci. 2010, 93, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff, J.; Van der Ban, M.; van Nieuwstadt, A.P. Bovine respiratory syncytial virus infections in young dairy cattle: Clinical and haematological findings. Vet. Rec. 1984, 114, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Kimman, T.G.; Westenbrink, F.; Straver, P.J. Priming for local and systemic antibody memory responses to bovine respiratory syncytial virus: Effect of amount of virus, virus replication, route of administration and maternal antibodies. Vet. Immunol. Immunopathol. 1989, 22, 145–160. [Google Scholar] [CrossRef]
- Ellis, J.A.; Gow, S.P.; Goji, N. Response to experimentally induced infection with bovine respiratory syncytial virus following intranasal vaccination of seropositive and seronegative calves. J. Am. Vet. Med. Assoc. 2010, 236, 991–999. [Google Scholar] [CrossRef]
- Ellis, J.A.; Gow, S.P.; Mahan, S.; Leyh, R. Duration of immunity to experimental infection with bovine respiratory syncytial virus following intranasal vaccination of young passively immune calves. J. Am. Vet. Med. Assoc. 2013, 243, 1602–1608. [Google Scholar] [CrossRef]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Guzman, E.; Taylor, G. Immunology of bovine respiratory syncytial virus in calves. Mol. Immunol. 2015, 66, 48–56. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.; Silacci, C.; Thom, M.; Boyington, J.C.; Druz, A.; Joyce, M.G.; Guzman, E.; Kong, W.P.; Lai, Y.T.; et al. Protection of calves by a prefusion-stabilized bovine RSV F vaccine. NPJ Vaccines 2017, 2, 7. [Google Scholar] [CrossRef]
- Riffault, S.; Hagglund, S.; Guzman, E.; Naslund, K.; Jouneau, L.; Dubuquoy, C.; Pietralunga, V.; Laubreton, D.; Boulesteix, O.; Gauthier, D.; et al. A Single Shot Pre-fusion-Stabilized Bovine RSV F Vaccine is Safe and Effective in Newborn Calves with Maternally Derived Antibodies. Vaccines 2020, 8, 231. [Google Scholar] [CrossRef]
- Taylor, G.; Wyld, S.; Valarcher, J.F.; Guzman, E.; Thom, M.; Widdison, S.; Buchholz, U.J. Recombinant bovine respiratory syncytial virus with deletion of the SH gene induces increased apoptosis and pro-inflammatory cytokines in vitro, and is attenuated and induces protective immunity in calves. J. Gen. Virol. 2014, 95, 1244–1254. [Google Scholar] [CrossRef]
- Blodorn, K.; Hagglund, S.; Fix, J.; Dubuquoy, C.; Makabi-Panzu, B.; Thom, M.; Karlsson, P.; Roque, J.L.; Karlstam, E.; Pringle, J.; et al. Vaccine safety and efficacy evaluation of a recombinant bovine respiratory syncytial virus (BRSV) with deletion of the SH gene and subunit vaccines based on recombinant human RSV proteins: N-nanorings, P and M2-1, in calves with maternal antibodies. PLoS ONE 2014, 9, e100392. [Google Scholar] [CrossRef] [PubMed]
- Blodorn, K.; Hagglund, S.; Gavier-Widen, D.; Eleouet, J.F.; Riffault, S.; Pringle, J.; Taylor, G.; Valarcher, J.F. A bovine respiratory syncytial virus model with high clinical expression in calves with specific passive immunity. BMC Vet. Res. 2015, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hagglund, S.; Hu, K.; Vargmar, K.; Pore, L.; Olofson, A.S.; Blodorn, K.; Anderson, J.; Ahooghalandari, P.; Pringle, J.; Taylor, G.; et al. Bovine respiratory syncytial virus ISCOMs-Immunity, protection and safety in young conventional calves. Vaccine 2011, 29, 8719–8730. [Google Scholar] [CrossRef]
- Hagglund, S.; Hu, K.F.; Larsen, L.E.; Hakhverdyan, M.; Valarcher, J.F.; Taylor, G.; Morein, B.; Belak, S.; Alenius, S. Bovine respiratory syncytial virus ISCOMs--protection in the presence of maternal antibodies. Vaccine 2004, 23, 646–655. [Google Scholar] [CrossRef]
- Hagglund, S.; Blodorn, K.; Naslund, K.; Vargmar, K.; Lind, S.B.; Mi, J.; Arainga, M.; Riffault, S.; Taylor, G.; Pringle, J.; et al. Proteome analysis of bronchoalveolar lavage from calves infected with bovine respiratory syncytial virus-Insights in pathogenesis and perspectives for new treatments. PLoS ONE 2017, 12, e0186594. [Google Scholar] [CrossRef]
- Taylor, G.; Bruce, C.; Barbet, A.F.; Wyld, S.G.; Thomas, L.H. DNA vaccination against respiratory syncytial virus in young calves. Vaccine 2005, 23, 1242–1250. [Google Scholar] [CrossRef]
- Hakhverdyan, M.; Hagglund, S.; Larsen, L.E.; Belak, S. Evaluation of a single-tube fluorogenic RT-PCR assay for detection of bovine respiratory syncytial virus in clinical samples. J. Virol. Meth. 2005, 123, 195–202. [Google Scholar] [CrossRef]
- Taylor, G.; Stott, E.J.; Furze, J.; Ford, J.; Sopp, P. Protective epitopes on the fusion protein of respiratory syncytial virus recognized by murine and bovine monoclonal antibodies. J. Gen. Virol. 1992, 73, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.; Stott, E.J.; Bew, M.; Fernie, B.F.; Cote, P.J.; Collins, A.P.; Hughes, M.; Jebbett, J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology 1984, 52, 137–142. [Google Scholar]
- Herve, P.L.; Deloizy, C.; Descamps, D.; Rameix-Welti, M.A.; Fix, J.; McLellan, J.S.; Eleouet, J.F.; Riffault, S. RSV N-nanorings fused to palivizumab-targeted neutralizing epitope as a nanoparticle RSV vaccine. Nanomedicine 2017, 13, 411–420. [Google Scholar] [CrossRef]
- Goris, K.; Uhlenbruck, S.; Schwegmann-Wessels, C.; Kohl, W.; Niedorf, F.; Stern, M.; Hewicker-Trautwein, M.; Bals, R.; Taylor, G.; Braun, A.; et al. Differential sensitivity of differentiated epithelial cells to respiratory viruses reveals different viral strategies of host infection. J. Virol. 2009, 83, 1962–1968. [Google Scholar] [CrossRef]
- Luna, L. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd ed.; McGrawHill: New York, NY, USA, 1968. [Google Scholar]
- Hornaeus, K.; Guillemant, J.; Mi, J.; Hernroth, B.; Bergquist, J.; Lind, S.B. Mass spectrometry data from a quantitative analysis of protein expression in gills of immuno-challenged blue mussels (Mytilus edulis). Data Brief. 2016, 8, 470–473. [Google Scholar] [CrossRef]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: https://www.r-project.org (accessed on 1 December 2020).
- Steff, A.M.; Monroe, J.; Friedrich, K.; Chandramouli, S.; Nguyen, T.L.; Tian, S.; Vandepaer, S.; Toussaint, J.F.; Carfi, A. Pre-fusion RSV F strongly boosts pre-fusion specific neutralizing responses in cattle pre-exposed to bovine RSV. Nat. Commun. 2017, 8, 1085. [Google Scholar] [CrossRef]
- Timsit, E.; Le Drean, E.; Maingourd, C.; Belloc, C.; Guatteo, R.; Bareille, N.; Seegers, H.; Douart, A.; Sellal, E.; Assie, S. Detection by real-time RT-PCR of a bovine respiratory syncytial virus vaccine in calves vaccinated intranasally. Vet. Rec. 2009, 165, 230–233. [Google Scholar] [PubMed]
- Antonis, A.F.; Schrijver, R.S.; Daus, F.; Steverink, P.J.; Stockhofe, N.; Hensen, E.J.; Langedijk, J.P.; van der Most, R.G. Vaccine-induced immunopathology during bovine respiratory syncytial virus infection: Exploring the parameters of pathogenesis. J. Virol. 2003, 77, 12067–12073. [Google Scholar] [CrossRef]
- Ellis, J.A. How efficacious are vaccines against bovine respiratory syncytial virus in cattle? Vet. Microbiol. 2017, 206, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Gershwin, L.J.; Schelegle, E.S.; Gunther, R.A.; Anderson, M.L.; Woolums, A.R.; Larochelle, D.R.; Boyle, G.A.; Friebertshauser, K.E.; Singer, R.S. A bovine model of vaccine enhanced respiratory syncytial virus pathophysiology. Vaccine 1998, 16, 1225–1236. [Google Scholar] [CrossRef]
- Schreiber, P.; Matheise, J.P.; Dessy, F.; Heimann, M.; Letesson, J.J.; Coppe, P.; Collard, A. High mortality rate associated with bovine respiratory syncytial virus (BRSV) infection in Belgian white blue calves previously vaccinated with an inactivated BRSV vaccine. J. Vet. Med. B Inf. Dis. Vet. Pub. Health 2000, 47, 535–550. [Google Scholar] [CrossRef]
- Schneider-Ohrum, K.; Cayatte, C.; Bennett, A.S.; Rajani, G.M.; McTamney, P.; Nacel, K.; Hostetler, L.; Cheng, L.; Ren, K.; O’Day, T.; et al. Immunization with Low Doses of Recombinant Postfusion or Prefusion Respiratory Syncytial Virus F Primes for Vaccine-Enhanced Disease in the Cotton Rat Model Independently of the Presence of a Th1-Biasing (GLA-SE) or Th2-Biasing (Alum) Adjuvant. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Schneider-Ohrum, K.; Snell Bennett, A.; Rajani, G.M.; Hostetler, L.; Maynard, S.K.; Lazzaro, M.; Cheng, L.I.; O’Day, T.; Cayatte, C. CD4(+) T Cells Drive Lung Disease Enhancement Induced by Immunization with Suboptimal Doses of Respiratory Syncytial Virus Fusion Protein in the Mouse Model. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Castilow, E.M.; Legge, K.L.; Varga, S.M. Cutting edge: Eosinophils do not contribute to respiratory syncytial virus vaccine-enhanced disease. J. Immunol. 2008, 181, 6692–6696. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.C.; Ames, T.R.; Markham, R.J. Seroepizootiologic study of bovine respiratory syncytial virus in a dairy herd. Am. J. Vet. Res. 1986, 47, 240–245. [Google Scholar] [PubMed]
- Kimman, T.G.; Westenbrink, F.; Schreuder, B.E.; Straver, P.J. Local and systemic antibody response to bovine respiratory syncytial virus infection and reinfection in calves with and without maternal antibodies. J. Clin. Microbiol. 1987, 25, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Belknap, E.B.; Baker, J.C.; Patterson, J.S.; Walker, R.D.; Haines, D.M.; Clark, E.G. The role of passive immunity in bovine respiratory syncytial virus-infected calves. J. Inf. Dis. 1991, 163, 470–476. [Google Scholar] [CrossRef]
- Lehmkuhl, H.D.; Gough, P.M.; Reed, D.E. Characterization and identification of a bovine respiratory syncytial virus isolated from young calves. Am. J. Vet. Res. 1979, 40, 124–126. [Google Scholar] [PubMed]
- Windeyer, M.C.; Gamsjager, L. Vaccinating Calves in the Face of Maternal Antibodies: Challenges and Opportunities. Vet. Clin. Food Anim. Pract. 2019, 35, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Niewiesk, S. Maternal antibodies: Clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front. Immunol. 2014, 5, 446. [Google Scholar] [CrossRef]
- Harmeyer, S.S.; Murray, J.; Imrie, C.; Wiseman, A.; Salt, J.S. Efficacy of a live bovine respiratory syncytial virus vaccine in seropositive calves. Vet. Rec. 2006, 159, 456–457. [Google Scholar] [CrossRef]
- Kolb, E.A.; Buterbaugh, R.E.; Rinehart, C.L.; Ensley, D.; Perry, G.A.; Abdelsalam, K.W.; Chase, C.C.L. Protection against bovine respiratory syncytial virus in calves vaccinated with adjuvanted modified live vaccine administered in the face of maternal antibody. Vaccine 2020, 38, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Vangeel, I.; Antonis, A.F.; Fluess, M.; Riegler, L.; Peters, A.R.; Harmeyer, S.S. Efficacy of a modified live intranasal bovine respiratory syncytial virus vaccine in 3-week-old calves experimentally challenged with BRSV. Vet. J. 2007, 174, 627–635. [Google Scholar] [CrossRef]
- Ellis, J.; Gow, S.; West, K.; Waldner, C.; Rhodes, C.; Mutwiri, G.; Rosenberg, H. Response of calves to challenge exposure with virulent bovine respiratory syncytial virus following intranasal administration of vaccines formulated for parenteral administration. J. Am. Vet. Med. Assoc. 2007, 230, 233–243. [Google Scholar] [CrossRef]
- Polewicz, M.; Gracia, A.; Garlapati, S.; van Kessel, J.; Strom, S.; Halperin, S.A.; Hancock, R.E.; Potter, A.A.; Babiuk, L.A.; Gerdts, V. Novel vaccine formulations against pertussis offer earlier onset of immunity and provide protection in the presence of maternal antibodies. Vaccine 2013, 31, 3148–3155. [Google Scholar] [CrossRef]
- Goodwin, E.; Gilman, M.S.A.; Wrapp, D.; Chen, M.; Ngwuta, J.O.; Moin, S.M.; Bai, P.; Sivasubramanian, A.; Connor, R.I.; Wright, P.F.; et al. Infants Infected with Respiratory Syncytial Virus Generate Potent Neutralizing Antibodies that Lack Somatic Hypermutation. Immunity 2018, 48, 339–349 e335. [Google Scholar] [CrossRef]
- Zhang, L.; Boeren, S.; van Hooijdonk, A.C.; Vervoort, J.M.; Hettinga, K.A. A proteomic perspective on the changes in milk proteins due to high somatic cell count. J. Dairy. Sci. 2015, 98, 5339–5351. [Google Scholar] [CrossRef]
- Rizvi, S.M.; Del Cid, N.; Lybarger, L.; Raghavan, M. Distinct functions for the glycans of tapasin and heavy chains in the assembly of MHC class I molecules. J. Immunol. 2011, 186, 2309–2320. [Google Scholar] [CrossRef]
- Boyle, L.H.; Hermann, C.; Boname, J.M.; Porter, K.M.; Patel, P.A.; Burr, M.L.; Duncan, L.M.; Harbour, M.E.; Rhodes, D.A.; Skjodt, K.; et al. Tapasin-related protein TAPBPR is an additional component of the MHC class I presentation pathway. Proc. Natl. Acad. Sci. USA 2013, 110, 3465–3470. [Google Scholar] [CrossRef] [PubMed]
- Bingle, L.; Cross, S.S.; High, A.S.; Wallace, W.A.; Rassl, D.; Yuan, G.; Hellstrom, I.; Campos, M.A.; Bingle, C.D. WFDC2 (HE4): A potential role in the innate immunity of the oral cavity and respiratory tract and the development of adenocarcinomas of the lung. Respir. Res. 2006, 7, 61. [Google Scholar] [CrossRef]
- Chhikara, N.; Saraswat, M.; Tomar, A.K.; Dey, S.; Singh, S.; Yadav, S. Human epididymis protein-4 (HE-4): A novel cross-class protease inhibitor. PLoS ONE 2012, 7, e47672. [Google Scholar] [CrossRef] [PubMed]
- Rader, B.A. Alkaline Phosphatase, an Unconventional Immune Protein. Front. Immunol. 2017, 8, 897. [Google Scholar] [CrossRef] [PubMed]
- Bessueille, L.; Briolay, A.; Como, J.; Mebarek, S.; Mansouri, C.; Gleizes, M.; El Jamal, A.; Buchet, R.; Dumontet, C.; Matera, E.L.; et al. Tissue-nonspecific alkaline phosphatase is an anti-inflammatory nucleotidase. Bone 2020, 133, 115262. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.; Gibson, P.G.; Powell, H.; Simpson, J.L.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; et al. A sputum 6-gene signature predicts future exacerbations of poorly controlled asthma. J. Allergy Clin. Immunol. 2019, 144, 51–60 e11. [Google Scholar] [CrossRef]
- McGill, J.L.; Rusk, R.A.; Guerra-Maupome, M.; Briggs, R.E.; Sacco, R.E. Bovine Gamma Delta T Cells Contribute to Exacerbated IL-17 Production in Response to Co-Infection with Bovine RSV and Mannheimia haemolytica. PLoS ONE 2016, 11, e0151083. [Google Scholar] [CrossRef]
| Clinical Signs | ISA61 | PreF | ΔSH | CV |
|---|---|---|---|---|
| Rectal temperature mean of peak, °C (SD) | 40.0 (±0.7) | 39.4 (±0.1) | 39.5 (±0.2) | 39.3 (±0.2) * |
| Respiratory rate, mean of peak, breath/min (SD) | 60 (±20.8) | 38 (±3.2) * | 40 (±3.3) * | 43 (±3.6) |
| Group | id | Neutrophil Score a | Eosinophil | ||
|---|---|---|---|---|---|
| Intra- Epithelial | Lumen Bronchi Bronchiole | Lumen Alveoli | Number b | ||
| ISA61 | a | (+) | - | (+) | 2 |
| b | ++ | ++ | ++ | 11 | |
| c | + | (+) | (+) | 40 | |
| d | ++ | ++ | + | 24 | |
| e | + | ++ | ++ | 21 | |
| f | - | + | + | 10 | |
| PreF | g | ++ | + | - | 2 |
| h | + | - | - | 8 | |
| i | - | - | - | 114 | |
| j | - | - | - | 17 | |
| k | - | - | - | 105 | |
| l | - | - | - | 174 | |
| ΔSH | m | - | (+) | - | 3 |
| n | - | - | - | 21 | |
| o | - | - | - | 14 | |
| p | - | - | - | 13 | |
| q | - | - | - | 22 | |
| r | - | - | - | 7 | |
| CV | s | - | - | - | 5 |
| t | - | - | - | 6 | |
| u | n.a. | n.a. | n.a. | n.a. | |
| v | - | - | - | 2 | |
| w | - | - | - | 0 | |
| x | + | + | (+) | 0 | |
| PreF | ΔSHrBRSV | CV | ||||||
|---|---|---|---|---|---|---|---|---|
| Id a | Disease Parameter | Corr b.c | Id a | Disease Parameter | Corr b.c | Id a | Disease Parameter | Corr b.c |
| Ig | Clincal score AUC | −0.85 | ALPL | BAL PMN D13 | −0.78 | ORM1 | nasal BRSV RNA AUC | −0.78 |
| A5D7Q2 d | BAL BRSV live D13 | −0.82 | BAL BRSV live D13 | −0.78 | GSN | BAL PMN D6/7 | −0.75 | |
| BAL BRSV RNA D6/7 | −0.8 | BAL PMN D6/7 | −0.78 | PGLYRP1 | Eos lung D13 | −0.78 | ||
| Lung lesions D13 | −0.8 | BAL BRSV RNA D13 | −0.78 | SCGB1A1 | PMN lung D13 | −0.94 | ||
| nasal BRSV RNA AUC | −0.78 | BAL BRSV RNA D6/7 | −0.77 | BAL PMN D13 | −0.77 | |||
| ALPL | BAL BRSV RNA D13 | −0.82 | ARHGDIB | BAL BRSV RNA D13 | −0.77 | |||
| ANPEP | BAL BRSV live D13 | −0.78 | CD44 | PMN lung D13 | −0.75 | |||
| G5E5H2 e | nasal BRSV RNA AUC | −0.8 | GPI | BAL PMN D6/7 | −0.82 | |||
| Clinical score AUC | −0.77 | BAL BRSV RNA D6/7 | −0.79 | |||||
| LYS | BAL BRSV live D13 | −0.78 | Clinical score AUC | −0.76 | ||||
| BAL BRSV RNA D6/7 | −0.77 | IGL | BAL PMN D6/7 | −0.77 | ||||
| AOX2 | EOS LUNG D13 | −0.76 | LDHB | BAL PMN D6/7 | −0.75 | |||
| S100A12 | BAL BRSV RNA D6/7 | −0.75 | PGLS | BAL PMN D6/7 | −0.77 | |||
| S100A8 | PMN lung D13 | −0.83 | PIGR | Eos lung D13 | −0.75 | |||
| SCGB2A2 | BAL BRSV RNA D6/7 | −0.75 | WFDC2 | BAL BRSV RNA D13 | −0.89 | |||
| nasal BRSV RNA AUC | −0.74 | |||||||
| WFDC2 | BAL BRSV RNA D13 | −0.85 | ||||||
| nasal BRSV RNA AUC | −0.77 | |||||||
| BAL BRSV live D13 | −0.76 | |||||||
| PMN lung D13 | −0.75 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valarcher, J.F.; Hägglund, S.; Näslund, K.; Jouneau, L.; Malmström, E.; Boulesteix, O.; Pinard, A.; Leguéré, D.; Deslis, A.; Gauthier, D.; et al. Single-Shot Vaccines against Bovine Respiratory Syncytial Virus (BRSV): Comparative Evaluation of Long-Term Protection after Immunization in the Presence of BRSV-Specific Maternal Antibodies. Vaccines 2021, 9, 236. https://doi.org/10.3390/vaccines9030236
Valarcher JF, Hägglund S, Näslund K, Jouneau L, Malmström E, Boulesteix O, Pinard A, Leguéré D, Deslis A, Gauthier D, et al. Single-Shot Vaccines against Bovine Respiratory Syncytial Virus (BRSV): Comparative Evaluation of Long-Term Protection after Immunization in the Presence of BRSV-Specific Maternal Antibodies. Vaccines. 2021; 9(3):236. https://doi.org/10.3390/vaccines9030236
Chicago/Turabian StyleValarcher, Jean François, Sara Hägglund, Katarina Näslund, Luc Jouneau, Ester Malmström, Olivier Boulesteix, Anne Pinard, Dany Leguéré, Alain Deslis, David Gauthier, and et al. 2021. "Single-Shot Vaccines against Bovine Respiratory Syncytial Virus (BRSV): Comparative Evaluation of Long-Term Protection after Immunization in the Presence of BRSV-Specific Maternal Antibodies" Vaccines 9, no. 3: 236. https://doi.org/10.3390/vaccines9030236
APA StyleValarcher, J. F., Hägglund, S., Näslund, K., Jouneau, L., Malmström, E., Boulesteix, O., Pinard, A., Leguéré, D., Deslis, A., Gauthier, D., Dubuquoy, C., Pietralunga, V., Rémot, A., Falk, A., Shevchenko, G., Bergström Lind, S., Von Brömssen, C., Vargmar, K., Zhang, B., ... Riffault, S. (2021). Single-Shot Vaccines against Bovine Respiratory Syncytial Virus (BRSV): Comparative Evaluation of Long-Term Protection after Immunization in the Presence of BRSV-Specific Maternal Antibodies. Vaccines, 9(3), 236. https://doi.org/10.3390/vaccines9030236






