Influenza Virus: Global Health Impact, Strategies, Challenges, Role of Nanotechnolgy in Influenza Vaccine Development
Abstract
1. Introduction
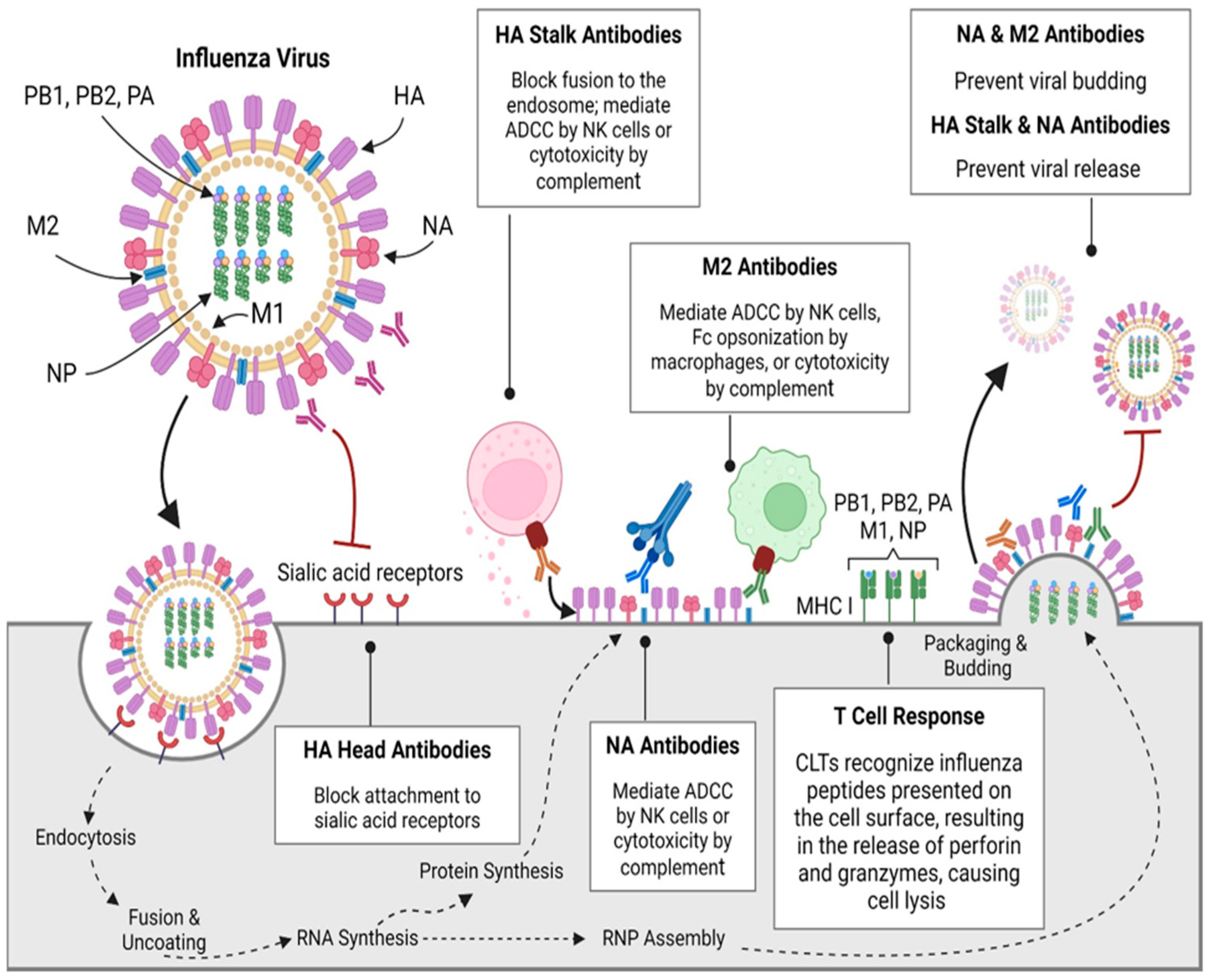
2. Influenza Immune Response
2.1. Antibody-Mediated (Humoral) Response
2.2. Cell-Mediated Immunity
2.3. Mucosal Immunity
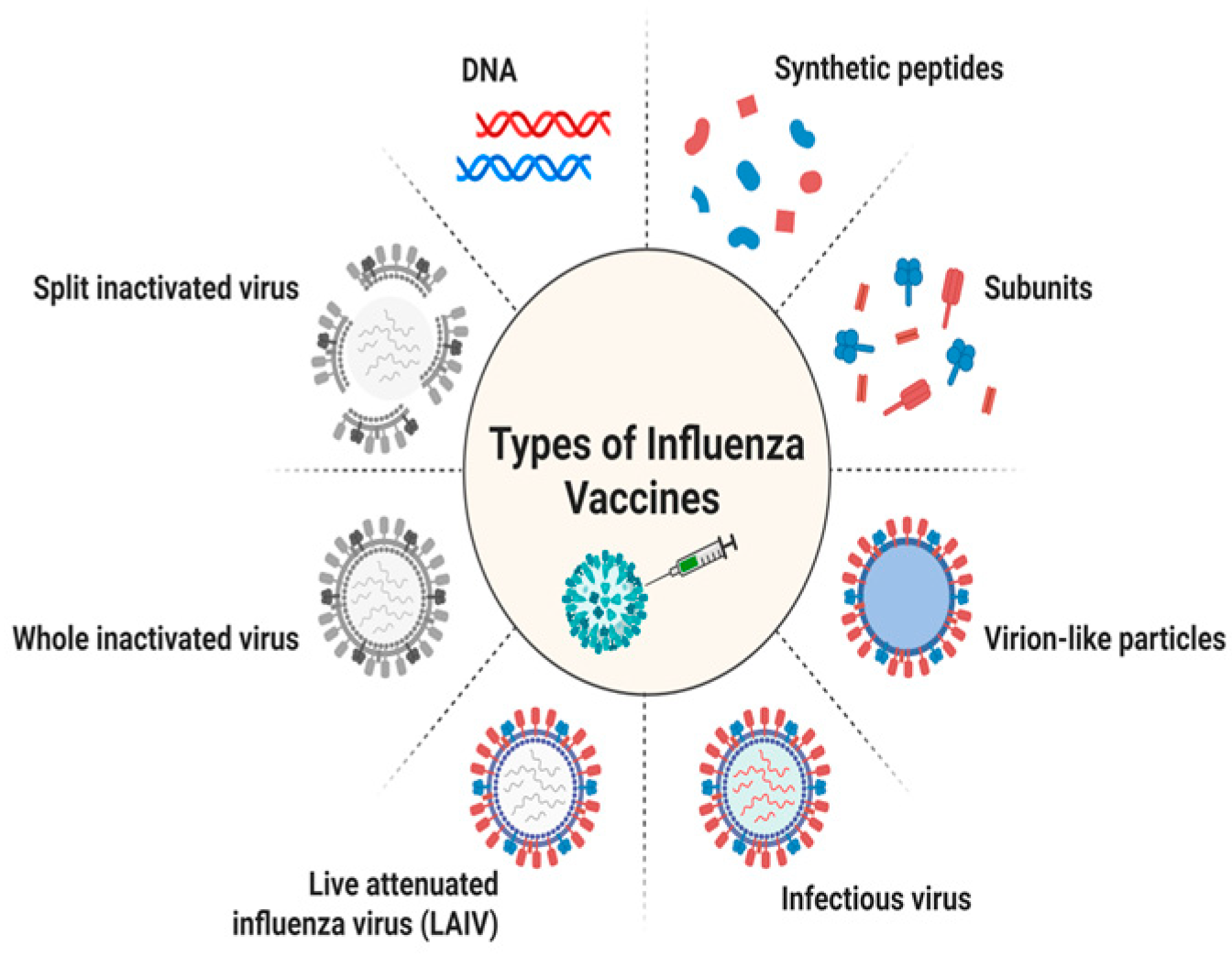
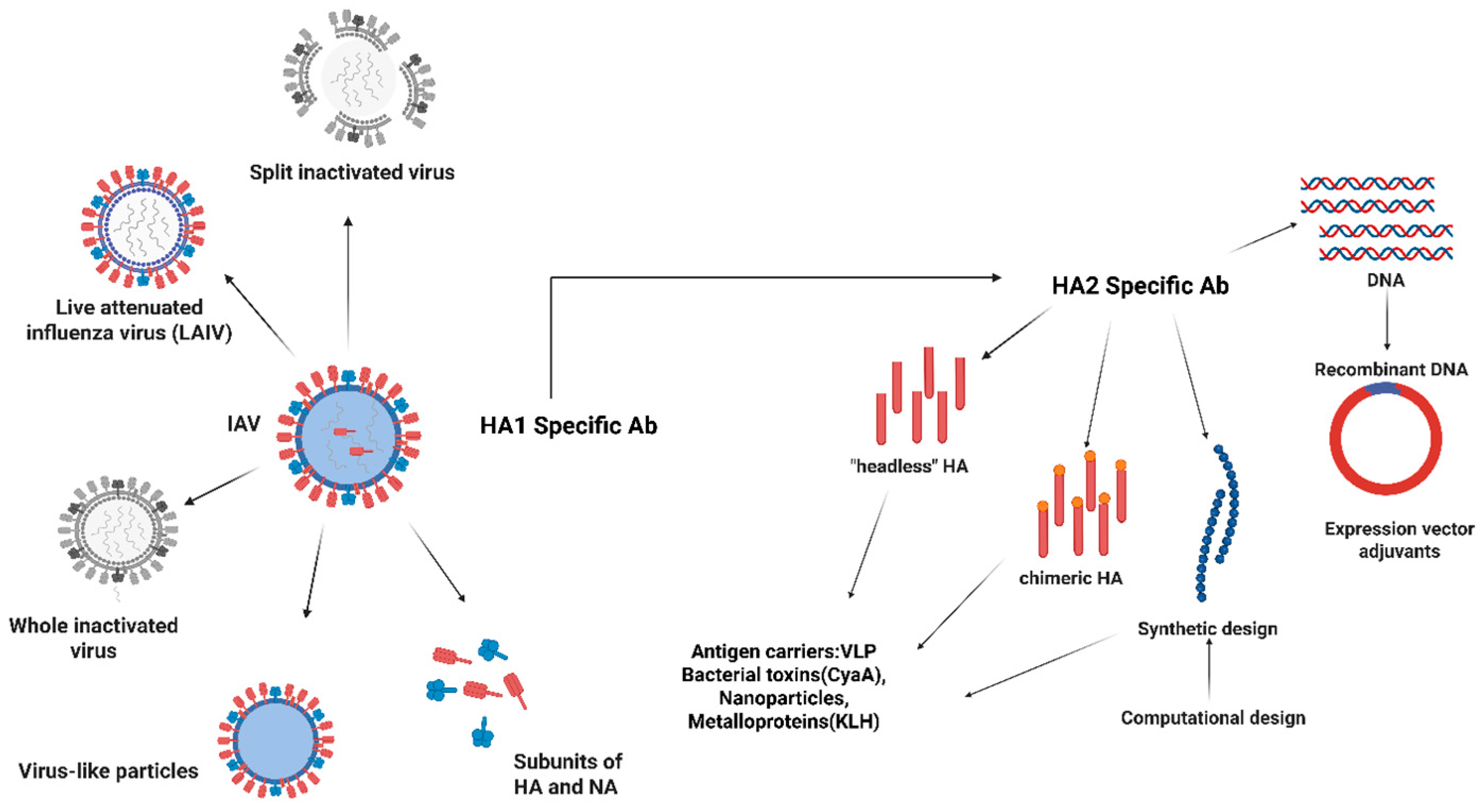
3. Vaccination Strategies for Influenza
3.1. Inactivated Vaccines
3.2. Live Attenuated Vaccines
3.3. Recombinant Vaccines
4. Influenza Vaccine Effectiveness
| Season | Vaccine Type | Target Strain(s) | Effectiveness (%) | Age Group | Study Type | References |
|---|---|---|---|---|---|---|
| 2019–2020 | Inactivated (IIV4) | H1N1pdm09, H3N2, B/Victoria, B/Yamagata | ~45% | All adults | TND (CDC) | [56] |
| 2020–2021 | Recombinant (RIV4) | Same as above | ~51% | 18–49 yrs | Clinical trial | [57] |
| 2021–2022 | Live Attenuated (LAIV) | H3N2 mismatch season | ~25% | 2–17 yrs | TND (US/UK) | [58] |
| 2022–2023 | Cell-based IIV | H1N1, H3N2 | ~47% | Elderly | TND (CDC) | [59] |
| 2023–2024 | mRNA Influenza (Trial) | H3N2, B/Vic | ~63% | 18–64 yrs | Phase II/III trial | [60] |
5. Challenges in Vaccine Development
5.1. Antigen Drift and Shift
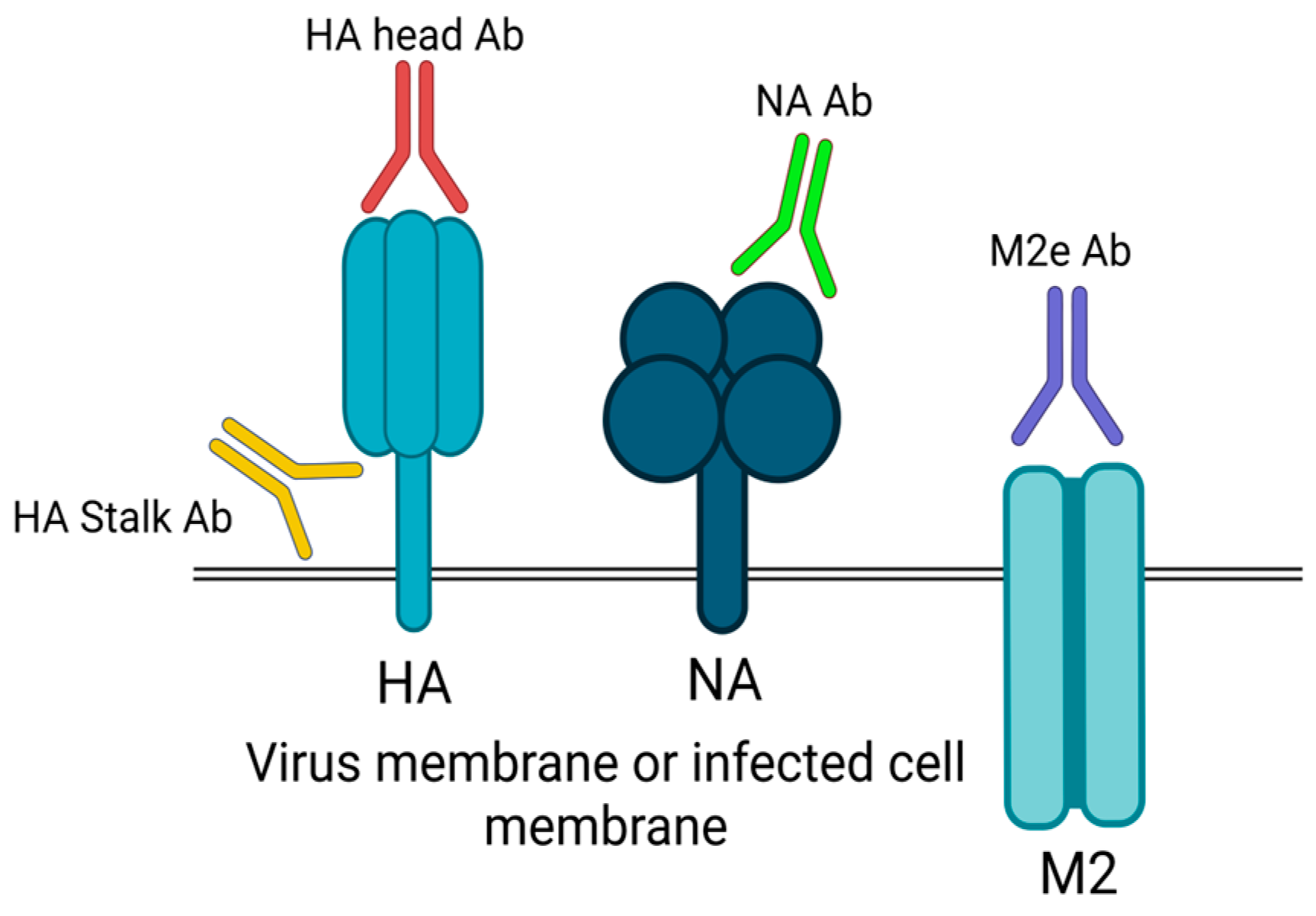
5.2. Vaccine Strain Selection
5.3. Production Limitation
6. Impact of COVID-19 on Influenza Vaccination
7. Role of Nanotechnology in Vaccine Development
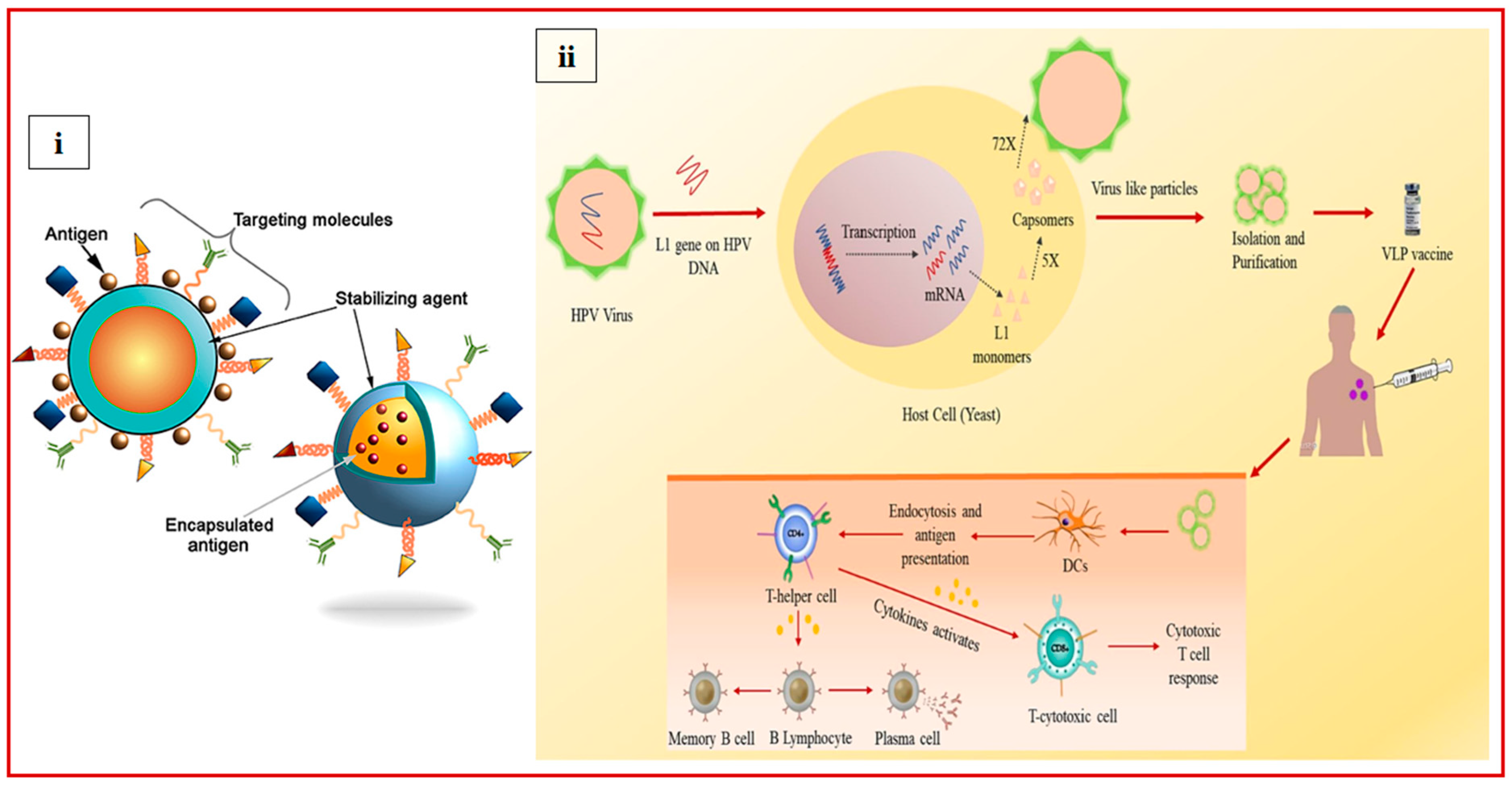
8. Various Types of Nanoparticles
8.1. Natural Nanoparticles
8.1.1. Virus-like Particles (VLPs)
8.1.2. Bacterial Spore
8.1.3. Polysaccharide
8.1.4. Outer Membrane Vesicles (OMVs)
8.1.5. Extracellular Vesicle Vaccines (EVs)
8.2. Synthetic Nanoparticles
8.2.1. Inorganic NPs
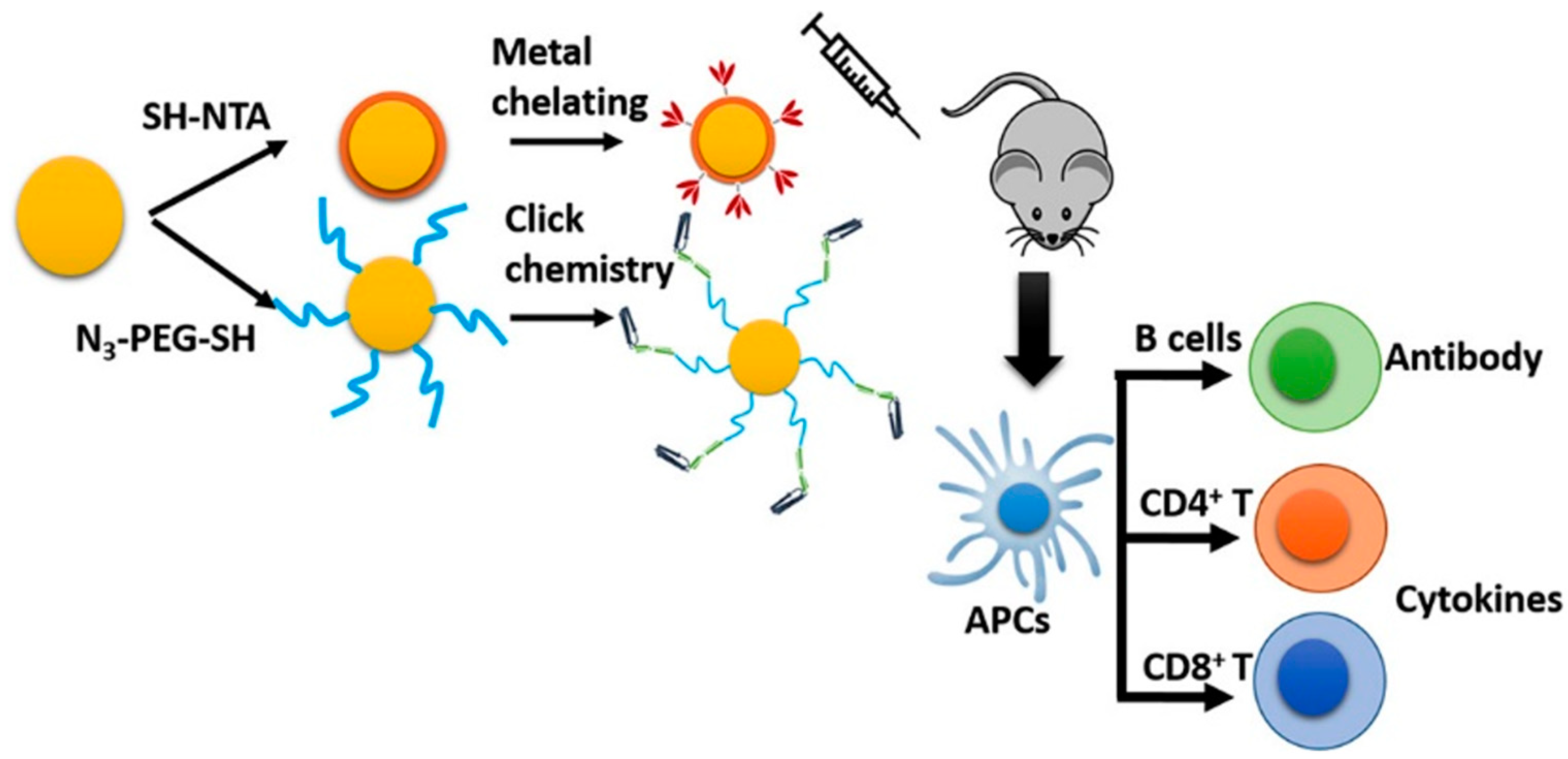
8.2.2. Liposomes
8.2.3. Polymer NPs
| Type of Nanoparticle | Antigen Used | Adjuvant | Route of Administration | Targeted Influenza Subtype | Trial Phase/Outcome | References |
|---|---|---|---|---|---|---|
| VLP (Virus-Like Particle) | Hemagglutinin (HA) from H1N1 | Matrix-M™ | Intramuscular (IM) | H1N1 | Phase III (NanoFlu, Novavax)—Safe, strong immunogenicity | [45] |
| Lipid Nanoparticle (LNP) | mRNA-encoding HA (H1, H3, B-Yamagata, B-Victoria) | None (self-adjuvanting) | Intramuscular (IM) | Multivalent | Phase I/II (Moderna mRNA-1010)—Under evaluation | [49] |
| Polymeric Nanoparticle (PLGA) | Recombinant HA (rHA) from H5N1 | MPLA | Intranasal (IN) | H5N1 | Preclinical—Strong mucosal and systemic response | [81] |
| Gold Nanoparticles (AuNPs) | Full-length HA from H3N2 | CpG ODN | Intranasal (IN) | H3N2 | Preclinical—Enhanced Th1-biased immune response | [127] |
| Chitosan Nanoparticle | Conserved M2e peptide | TLR agonist | Intranasal (IN) | Universal target | Preclinical—Cross-protective, improved mucosal IgA | [128] |
| Liposome-Based | HA and NP proteins | Monophosphoryl lipid A | Subcutaneous (SC) | H5N1 | Preclinical—High T-cell responses | [129] |
| Ferritin Nanoparticle | HA from H1, H3, B strains | None | Intramuscular (IM) | Multivalent | Phase I (NIH)—Ongoing; induces broadly neutralizing antibodies | [130] |
| Micelle (Self-assembling) | M2e peptide | TLR7 agonist | Intranasal (IN) | Broad/universal | Preclinical—Strong mucosal immunity, IgA, and IFN-γ | [131] |
| Polyanhydride Nanoparticle | Inactivated whole virus | None | Subcutaneous (SC) | H1N1 | Preclinical—Prolonged antigen release | [132] |
9. Challenges
9.1. Antigen Design Approaches (Universal Influenza Vaccine)
9.2. Innovative Delivery Methods
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Influenza: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 751–758. [Google Scholar]
- Stöhr, K. Influenza—WHO cares. Lancet Infect. Dis. 2002, 2, 517. [Google Scholar] [CrossRef]
- Ferdinands, J.M.; Thompson, M.G.; Blanton, L.; Spencer, S.; Grant, L.; Fry, A.M. Does influenza vaccination attenuate the severity of breakthrough infections? A narrative review and recommendations for further research. Vaccine 2021, 39, 3678–3695. [Google Scholar] [CrossRef]
- To, J.; Torres, J. Viroporins in the Influenza Virus. Cells 2019, 8, 654. [Google Scholar] [CrossRef]
- Kreijtz, J.H.C.M.; Fouchier, R.A.M.; Rimmelzwaan, G.F. Immune responses to influenza virus infection. Virus Res. 2011, 162, 19–30. [Google Scholar] [CrossRef]
- Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 6 June 2025).
- Pandemic Influenza—Overview|Occupational Safety and Health Administration. Available online: https://www.osha.gov/pandemic-influenza (accessed on 6 June 2025).
- World Health Organization Emergency Cycle—Pandemic Influenza—A Threat That All Countries Need to Prepare for. Available online: https://www.who.int/europe/emergencies/emergency-cycle/prepare/pandemic-influenza (accessed on 6 June 2025).
- Ashraf, M.A.; Raza, M.A.; Amjad, M.N.; ud Din, G.; Yue, L.; Shen, B.; Chen, L.; Dong, W.; Xu, H.; Hu, Y. A comprehensive review of influenza B virus, its biological and clinical aspects. Front. Microbiol. 2024, 15, 1467029. [Google Scholar] [CrossRef]
- Emerging Influenza D Virus Threat: What We Know so Far! Available online: https://www.mdpi.com/2077-0383/8/2/192 (accessed on 6 June 2025).
- Liu, B.; Cao, B.; Wang, C.; Han, B.; Sun, T.; Miao, Y.; Lu, Q.; Cui, F. Immunogenicity and Safety of Childhood Combination Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Vaccines. Available online: https://www.who.int/teams/global-influenza-programme/vaccines (accessed on 6 June 2025).
- Influenza Vaccination for Elderly, Vulnerable and High-Risk Subjects: A Narrative Review and Expert Opinion—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC11039544/ (accessed on 6 June 2025).
- CDC H5 Bird Flu: Current Situation. Available online: https://www.cdc.gov/bird-flu/situation-summary/index.html (accessed on 6 August 2025).
- CDC CDC Confirms First Severe Case of H5N1 Bird Flu in the United States. Available online: https://www.cdc.gov/media/releases/2024/m1218-h5n1-flu.html (accessed on 6 August 2025).
- Cordeiro, A.S.; Patil-Sen, Y.; Shivkumar, M.; Patel, R.; Khedr, A.; Elsawy, M.A. Nanovaccine Delivery Approaches and Advanced Delivery Systems for the Prevention of Viral Infections: From Development to Clinical Application. Pharmaceutics 2021, 13, 2091. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.H.; Hauguel, T.; Beitelshees, M.; Davitt, M.; Welch, V.; Lindert, K.; Allen, P.; True, J.M.; Dolsten, M. Deciphering immune responses: A comparative analysis of influenza vaccination platforms. Drug Discov. Today 2024, 29, 104125. [Google Scholar] [CrossRef]
- Mancini, N.; Solforosi, L.; Clementi, N.; De Marco, D.; Clementi, M.; Burioni, R. A potential role for monoclonal antibodies in prophylactic and therapeutic treatment of influenza. Antivir. Res. 2011, 92, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, W.A.; Offit, P.A.; Edwards, K.M.; Plotkin, S.A. Plotkin’s Vaccines, E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2022; ISBN 978-0-323-79059-8. [Google Scholar]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef]
- Nuwarda, R.F.; Alharbi, A.A.; Kayser, V. An overview of influenza viruses and vaccines. Vaccines 2021, 9, 1032. [Google Scholar] [CrossRef]
- Cox, R.J. Correlates of protection to influenza virus, where do we go from here? Hum. Vaccines Immunother. 2013, 9, 405–408. [Google Scholar] [CrossRef]
- Margine, I.; Krammer, F.; Hai, R.; Heaton, N.S.; Tan, G.S.; Andrews, S.A.; Runstadler, J.A.; Wilson, P.C.; Albrecht, R.A.; García-Sastre, A.; et al. Hemagglutinin Stalk-Based Universal Vaccine Constructs Protect against Group 2 Influenza A Viruses. J. Virol. 2013, 87, 10435–10446. [Google Scholar] [CrossRef]
- Gao, R.; Sheng, Z.; Sreenivasan, C.C.; Wang, D.; Li, F. Influenza A Virus Antibodies with Antibody-Dependent Cellular Cytotoxicity Function. Viruses 2020, 12, 276. [Google Scholar] [CrossRef]
- Zarnitsyna, V.I.; Ellebedy, A.H.; Davis, C.; Jacob, J.; Ahmed, R.; Antia, R. Masking of antigenic epitopes by antibodies shapes the humoral immune response to influenza. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140248. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Wohlbold, T.J.; Zheng, N.-Y.; Huang, M.; Huang, Y.; Neu, K.E.; Lee, J.; Wan, H.; Rojas, K.T.; Kirkpatrick, E.; et al. Influenza Infection in Humans Induces Broadly Cross-Reactive and Protective Neuraminidase-Reactive Antibodies. Cell 2018, 173, 417–429.e10. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.E.; Sun, X.; Bai, Y.; Liu, Y.V.; Massare, M.J.; Pearce, M.B.; Belser, J.A.; Maines, T.R.; Creager, H.M.; Glenn, G.M.; et al. Neuraminidase-based recombinant virus-like particles protect against lethal avian influenza A(H5N1) virus infection in ferrets. Virology 2017, 509, 90–97. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.J.; Gotch, F.M.; Noble, G.R.; Beare, P.A.S. Cytotoxic T-Cell Immunity to Influenza. N. Engl. J. Med. 1983, 309, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.E.; Kelso, A. Prospects for an influenza vaccine that induces cross-protective cytotoxic T lymphocytes. Immunol. Cell Biol. 2009, 87, 300–308. [Google Scholar] [CrossRef]
- Brown, D.M.; Lampe, A.T.; Workman, A.M. The Differentiation and Protective Function of Cytolytic CD4 T Cells in Influenza Infection. Front. Immunol. 2016, 7, 93. [Google Scholar] [CrossRef]
- Romagnani, S. Induction of TH1 and TH2 responses: A key role for the “natural” immune response? Immunol. Today 1992, 13, 379–381. [Google Scholar] [CrossRef]
- Aleebrahim-Dehkordi, E.; Molavi, B.; Mokhtari, M.; Deravi, N.; Fathi, M.; Fazel, T.; Mohebalizadeh, M.; Koochaki, P.; Shobeiri, P.; Hasanpour-Dehkordi, A. T helper type (Th1/Th2) responses to SARS-CoV-2 and influenza A (H1N1) virus: From cytokines produced to immune responses. Transpl. Immunol. 2022, 70, 101495. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.M.; Gerlach, T.; Elbahesh, H.; Rimmelzwaan, G.F.; Saletti, G. Influenza virus-specific CD4+ and CD8+ T cell-mediated immunity induced by infection and vaccination. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2019, 119, 44–52. [Google Scholar] [CrossRef] [PubMed]
- van Riet, E.; Ainai, A.; Suzuki, T.; Hasegawa, H. Mucosal IgA responses in influenza virus infections; thoughts for vaccine design. Vaccine 2012, 30, 5893–5900. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.Z.M.; Wakim, L.M. Tissue resident memory T cells in the respiratory tract. Mucosal Immunol. 2022, 15, 379–388. [Google Scholar] [CrossRef]
- Sridhar, S.; Brokstad, K.A.; Cox, R.J. Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines. Vaccines 2015, 3, 373–389. [Google Scholar] [CrossRef]
- Types of Seasonal Influenza Vaccine. Available online: https://www.who.int/europe/news-room/fact-sheets/item/types-of-seasonal-influenza-vaccine (accessed on 6 June 2025).
- Dolan, G.P.; Harris, R.C.; Clarkson, M.; Sokal, R.; Morgan, G.; Mukaigawara, M.; Horiuchi, H.; Hale, R.; Stormont, L.; Béchard-Evans, L.; et al. Vaccination of healthcare workers to protect patients at increased risk of acute respiratory disease: Summary of a systematic review. Influenza Other Respir. Viruses 2013, 7, 93–96. [Google Scholar] [CrossRef]
- The Human Antibody Response to Influenza a Virus Infection and Vaccination | Nature Reviews Immunology. Available online: https://www.nature.com/articles/s41577-019-0143-6 (accessed on 6 August 2025).
- Conners, E.E. Screening and Testing for Hepatitis B Virus Infection: CDC Recommendations—United States, 2023. MMWR Recomm. Rep. 2023, 72, 1–25. [Google Scholar] [CrossRef]
- Ambrose, C.S.; Levin, M.J. The Rationale for Quadrivalent Influenza Vaccines. Hum. Vaccines Immunother. 2012, 8, 81–88. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Pepin, S.; Tabar, C.; Fries, K.; Talanova, O.; See, S.; Essink, B.; Bertoch, T.; Drazan, D.; Natalini Martínez, S.; et al. Comparative assessment of immunogenicity and safety of recombinant influenza vaccine in children, adolescents, and adults: Results from a phase 3, immunobridging, open-label, non-randomised study. Lancet Infect. Dis. 2025. [Google Scholar] [CrossRef]
- Krammer, F.; Palese, P. Universal Influenza Virus Vaccines That Target the Conserved Hemagglutinin Stalk and Conserved Sites in the Head Domain. J. Infect. Dis. 2019, 219, S62–S67. [Google Scholar] [CrossRef]
- Sedova, E.S.; Shcherbinin, D.N.; Migunov, A.I.; Smirnov, I.A.; Logunov, D.I.; Shmarov, M.M.; Tsybalova, L.M.; Naroditskiĭ, B.S.; Kiselev, O.I.; Gintsburg, A.L. Recombinant Influenza Vaccines. Acta Naturae 2012, 4, 17–27. [Google Scholar] [CrossRef]
- Novavax. A Phase 3, Randomized, Observer-Blinded, Active-Controlled Trial to Evaluate Immunogenicity; Safety of a Recombinant Quadrivalent Nanoparticle Influenza Vaccine (Quad-NIV) with Matrix-M1TM Adjuvant Against Fluzone® Quadrivalent in Clinically Stable Adults ≥ 65 Years of Age. 2023. Available online: https://clinicaltrials.gov/study/NCT04120194 (accessed on 6 August 2025).
- National Institute of Allergy and Infectious Diseases (NIAID). Randomized, Double-Blinded, Placebo-Controlled, Phase 1 Study of the Safety and Immunogenicity of BPL-1357, A BPL-Inactivated, Whole-Virus, Universal Influenza Vaccine. 2025. Available online: https://clinicaltrials.gov/study/NCT05027932 (accessed on 6 August 2025).
- Radboud University Medical Center. First-in-Human Trial of the Coronavirus Virus-like Particle Subunit Vaccine ABNCoV2 in SARS-CoV-2-Naïve Adult Volunteers in Good Health. 2023. Available online: https://onderzoekmetmensen.nl/en/node/50898/pdf (accessed on 6 August 2025).
- BioNTech SE. A PHASE 1/2/3, Placebo-Controlled, Randomized, Observer-Blind, Dose-Finding Study to Evaluate the Safety, Tolerability, Immunogenicity, and Efficacy of SARS-COV-2 RNA Vaccine Candidates Against COVID-19 in Healthy Individuals. 2023. Available online: https://clinicaltrials.gov/study/NCT04368728 (accessed on 6 August 2025).
- ModernaTX, Inc. A Phase 1/2, Randomized, Stratified, Observer-Blind, Dose-Ranging Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1010 Seasonal Influenza Vaccine in Healthy Adults 18 Years and Older. 2023. Available online: https://clinicaltrials.gov/study/NCT04956575 (accessed on 6 August 2025).
- National Institute of Allergy and Infectious Diseases (NIAID). VRC 321: A Phase I Open-Label Clinical Trial to Evaluate Dose, Safety, Tolerability, and Immunogenicity of an Influenza H1 Stabilized Stem Ferritin Vaccine, VRCFLUNPF099-00-VP, in Healthy Adults. 2022. Available online: https://clinicaltrials.gov/study/NCT03814720 (accessed on 6 August 2025).
- Hartikka, J.; Bozoukova, V.; Yang, C.K.; Ye, M.; Rusalov, D.; Shlapobersky, M.; Vilalta, A.; Wei, Q.; Rolland, A.; Smith, L.R. Vaxfectin®, a cationic lipid-based adjuvant for protein-based influenza vaccines. Vaccine 2009, 27, 6399–6403. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Kistner, O.; Montomoli, E.; Viviani, S.; Marchi, S. Influenza Viruses and Vaccines: The Role of Vaccine Effectiveness Studies for Evaluation of the Benefits of Influenza Vaccines. Vaccines 2022, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Evaluation of Influenza Vaccine Effectiveness—A Guide to the Design and Interpretation of Observational Studies. Available online: https://www.who.int/publications/i/item/9789241512121 (accessed on 6 June 2025).
- Rajaram, S.; Wojcik, R.; Moore, C.; Ortiz de Lejarazu, R.; de Lusignan, S.; Montomoli, E.; Rossi, A.; Pérez-Rubio, A.; Trilla, A.; Baldo, V.; et al. The impact of candidate influenza virus and egg-based manufacture on vaccine effectiveness: Literature review and expert consensus. Vaccine 2020, 38, 6047–6056. [Google Scholar] [CrossRef] [PubMed]
- Jones-Gray, E.; Robinson, E.J.; Kucharski, A.J.; Fox, A.; Sullivan, S.G. Does repeated influenza vaccination attenuate effectiveness? A systematic review and meta-analysis. Lancet Respir. Med. 2023, 11, 27–44. [Google Scholar] [CrossRef]
- CDC Benefits of the Flu Vaccine. Available online: https://www.cdc.gov/flu-vaccines-work/benefits/index.html (accessed on 6 August 2025).
- Dunkle, L.M.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Muse, D.; Callahan, J.; Cox, M.M. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N. Engl. J. Med. 2017, 376, 2427–2436. [Google Scholar] [CrossRef]
- CDC. Flu Vaccine Effectiveness (VE) Data for 2022–2023. Available online: https://www.cdc.gov/flu-vaccines-work/php/effectiveness-studies/2022-2023.html (accessed on 6 August 2025).
- CDC. U.S. Flu Vaccine Effectiveness (VE) Data for 2021–2022. Available online: https://www.cdc.gov/flu-vaccines-work/php/effectiveness-studies/2021-2022.html (accessed on 6 August 2025).
- News Release. Available online: https://feeds.issuerdirect.com/news-release.html?newsid=4899326521164266&symbol=MRNA (accessed on 6 August 2025).
- Skehel, J.J.; Wiley, D.C. Receptor Binding and Membrane Fusion in Virus Entry: The Influenza Hemagglutinin. Annu. Rev. Biochem. 2000, 69, 531–569. [Google Scholar] [CrossRef]
- Gao, Z.; Robinson, K.; Skowronski, D.M.; De Serres, G.; Withers, S.G. Quantification of the total neuraminidase content of recent commercially-available influenza vaccines: Introducing a neuraminidase titration reagent. Vaccine 2020, 38, 715–718. [Google Scholar] [CrossRef]
- Gouma, S.; Anderson, E.M.; Hensley, S.E. Challenges of Making Effective Influenza Vaccines. Annu. Rev. Virol. 2020, 7, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Treanor, J. Influenza vaccine--outmaneuvering antigenic shift and drift. N. Engl. J. Med. 2004, 350, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Influenza | Nature Reviews Disease Primers. Available online: https://www.nature.com/articles/s41572-018-0002-y (accessed on 7 August 2025).
- Ziegler, T.; Mamahit, A.; Cox, N.J. 65 years of influenza surveillance by a World Health Organization-coordinated global network. Influenza Other Respir. Viruses 2018, 12, 558–565. [Google Scholar] [CrossRef]
- Barr, I.G.; McCauley, J.; Cox, N.; Daniels, R.; Engelhardt, O.G.; Fukuda, K.; Grohmann, G.; Hay, A.; Kelso, A.; Klimov, A.; et al. Epidemiological, antigenic and genetic characteristics of seasonal influenza A(H1N1), A(H3N2) and B influenza viruses: Basis for the WHO recommendation on the composition of influenza vaccines for use in the 2009–2010 northern hemisphere season. Vaccine 2010, 28, 1156–1167. [Google Scholar] [CrossRef]
- Ravina; Manjeet; Mohan, H.; Narang, J.; Pundir, S.; Pundir, C.S. A changing trend in diagnostic methods of Influenza A (H3N2) virus in human: A review. 3 Biotech 2021, 11, 87. [Google Scholar] [CrossRef]
- Smith, D.J.; Lapedes, A.S.; de Jong, J.C.; Bestebroer, T.M.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Mapping the antigenic and genetic evolution of influenza virus. Science 2004, 305, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Cobey, S.; Hensley, S.E. Immune history and influenza virus susceptibility. Curr. Opin. Virol. 2017, 22, 105–111. [Google Scholar] [CrossRef]
- Gerdil, C. The annual production cycle for influenza vaccine. Vaccine 2003, 21, 1776–1779. [Google Scholar] [CrossRef]
- Hu, L.; Lao, G.; Liu, R.; Feng, J.; Long, F.; Peng, T. The race toward a universal influenza vaccine: Front runners and the future directions. Antiviral Res. 2023, 210, 105505. [Google Scholar] [CrossRef]
- Despite Global Influenza Vaccine Production Remaining Steady, Production and Distribution Challenges Remain. Available online: https://www.who.int/news/item/25-03-2025-despite-global-influenza-vaccine-production-remaining-steady--production-and-distribution-challenges-remain (accessed on 6 June 2025).
- Ashraf, M.A.; Raza, M.A.; Imran, A.; Amjad, M.N. Next-generation vaccines for influenza B virus: Advancements and challenges. Arch. Virol. 2025, 170, 25. [Google Scholar] [CrossRef]
- Alshagrawi, S. Impact of COVID-19 pandemic on influenza vaccination rate among health care workers. Hum. Vaccines Immunother. 2024, 20, 2426284. [Google Scholar] [CrossRef]
- Alshagrawi, S.; Hazazi, A. Impact of COVID-19 pandemic on influenza vaccination rates among healthcare workers and the general population in Saudi Arabia: A meta-analysis. Hum. Vaccines Immunother. 2025, 21, 2477954. [Google Scholar] [CrossRef]
- Influenza Vaccination During the COVID-19 Pandemic. IFPMA. Available online: https://www.ifpma.org/wp-content/uploads/2023/01/i2023_IFPMA_HPP_Influenza_vaccination_during_COVID-19_pandemic.pdf (accessed on 6 June 2025).
- Debbag, R.; Rudin, D.; Ceddia, F.; Watkins, J. The Impact of Vaccination on COVID-19, Influenza, and Respiratory Syncytial Virus-Related Outcomes: A Narrative Review. Infect. Dis. Ther. 2025, 14, 63–97. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef]
- Vicente, S.; Prego, C.; Csaba, N.; Alonso, M.J. From single-dose vaccine delivery systems to nanovaccines. J. Drug Deliv. Sci. Technol. 2010, 20, 267–276. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.-X.; Mitter, N.; Yu, C.; Middelberg, A.P.J. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef]
- Nandedkar, T.D. Nanovaccines: Recent developments in vaccination. J. Biosci. 2009, 34, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.E.; Dyer, D.P.; Allen, J.E. The extracellular matrix and the immune system: A mutually dependent relationship. Science 2023, 379, eabp8964. [Google Scholar] [CrossRef] [PubMed]
- Priyanka; Abusalah, M.A.H.; Chopra, H.; Sharma, A.; Mustafa, S.A.; Choudhary, O.P.; Sharma, M.; Dhawan, M.; Khosla, R.; Loshali, A.; et al. Nanovaccines: A game changing approach in the fight against infectious diseases. Biomed. Pharmacother. 2023, 167, 115597. [Google Scholar] [CrossRef]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines Against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef]
- Poria, R.; Kala, D.; Nagraik, R.; Dhir, Y.; Dhir, S.; Singh, B.; Kaushik, N.K.; Noorani, M.S.; Kaushal, A.; Gupta, S. Vaccine development: Current trends and technologies. Life Sci. 2024, 336, 122331. [Google Scholar] [CrossRef]
- Grgacic, E.V.L.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods San Diego Calif 2006, 40, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Young, K.R.; Ross, T.M. Particle-based vaccines for HIV-1 infection. Curr. Drug Targets Infect. Disord. 2003, 3, 151–169. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, S.; Yu, H.; Xia, N.; Modis, Y. Virus-like particle-based human vaccines: Quality assessment based on structural and functional properties. Trends Biotechnol. 2013, 31, 654–663. [Google Scholar] [CrossRef]
- Bright, R.A.; Carter, D.M.; Daniluk, S.; Toapanta, F.R.; Ahmad, A.; Gavrilov, V.; Massare, M.; Pushko, P.; Mytle, N.; Rowe, T.; et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 2007, 25, 3871–3878. [Google Scholar] [CrossRef]
- Taylor, D.N.; Treanor, J.J.; Strout, C.; Johnson, C.; Fitzgerald, T.; Kavita, U.; Ozer, K.; Tussey, L.; Shaw, A. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI). Vaccine 2011, 29, 4897–4902. [Google Scholar] [CrossRef]
- Wang, B.-Z.; Quan, F.-S.; Kang, S.-M.; Bozja, J.; Skountzou, I.; Compans, R.W. Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. J. Virol. 2008, 82, 11813–11823. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Zhu, J.; Mahalingam, M.; Batra, H.; Rao, V.B. Bacteriophage T4 nanoparticles for vaccine delivery against infectious diseases. Adv. Drug Deliv. Rev. 2019, 145, 57–72. [Google Scholar] [CrossRef]
- Patterson, D.P.; Rynda-Apple, A.; Harmsen, A.L.; Harmsen, A.G.; Douglas, T. Biomimetic antigenic nanoparticles elicit controlled protective immune response to influenza. ACS Nano 2013, 7, 3036–3044. [Google Scholar] [CrossRef] [PubMed]
- Fritze, D. Taxonomy of the genus bacillus and related genera: The aerobic endospore-forming bacteria. Phytopathology 2004, 94, 1245–1248. [Google Scholar] [CrossRef]
- McKenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis endospore: Assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 2013, 11, 33–44. [Google Scholar] [CrossRef]
- Ciabattini, A.; Parigi, R.; Isticato, R.; Oggioni, M.R.; Pozzi, G. Oral priming of mice by recombinant spores of Bacillus subtilis. Vaccine 2004, 22, 4139–4143. [Google Scholar] [CrossRef]
- Duc, L.H.; Hong, H.A.; Fairweather, N.; Ricca, E.; Cutting, S.M. Bacterial spores as vaccine vehicles. Infect. Immun. 2003, 71, 2810–2818. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Miao, Y.; Guo, Y.; Qiu, H.; Sun, S.; Kou, Z.; Yu, H.; Li, J.; Chen, Y.; Jiang, S.; et al. Development of a heat-stable and orally delivered recombinant M2e-expressing B. subtilis spore-based influenza vaccine. Hum. Vaccines Immunother. 2014, 10, 3649–3658. [Google Scholar] [CrossRef]
- Song, M.; Hong, H.A.; Huang, J.-M.; Colenutt, C.; Khang, D.D.; Nguyen, T.V.A.; Park, S.-M.; Shim, B.-S.; Song, H.H.; Cheon, I.S.; et al. Killed Bacillus subtilis spores as a mucosal adjuvant for an H5N1 vaccine. Vaccine 2012, 30, 3266–3277. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Mbow, M.L.; De Gregorio, E.; Valiante, N.M.; Rappuoli, R. New adjuvants for human vaccines. Curr. Opin. Immunol. 2010, 22, 411–416. [Google Scholar] [CrossRef]
- Zhao, K.; Shi, X.; Zhao, Y.; Wei, H.; Sun, Q.; Huang, T.; Zhang, X.; Wang, Y. Preparation and immunological effectiveness of a swine influenza DNA vaccine encapsulated in chitosan nanoparticles. Vaccine 2011, 29, 8549–8556. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; von Ehrlich-Treuenstätt, V.; Schardey, J.; Wirth, U.; Zimmermann, P.; Andrassy, J.; Bazhin, A.V.; Werner, J.; Kühn, F. Gut Barrier Dysfunction and Bacterial Lipopolysaccharides in Colorectal Cancer. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2023, 27, 1466–1472. [Google Scholar] [CrossRef]
- Ahmed, A.A.Q.; Besio, R.; Xiao, L.; Forlino, A. Outer Membrane Vesicles (OMVs) as Biomedical Tools and Their Relevance as Immune-Modulating Agents against H. pylori Infections: Current Status and Future Prospects. Int. J. Mol. Sci. 2023, 24, 8542. [Google Scholar] [CrossRef]
- Schick, J.; Altunay, M.; Lacorcia, M.; Marschner, N.; Westermann, S.; Schluckebier, J.; Schubart, C.; Bodendorfer, B.; Christensen, D.; Alexander, C.; et al. IL-4 and helminth infection downregulate MINCLE-dependent macrophage response to mycobacteria and Th17 adjuvanticity. eLife 2023, 12, e72923. [Google Scholar] [CrossRef]
- Shehata, M.M.; Mostafa, A.; Teubner, L.; Mahmoud, S.H.; Kandeil, A.; Elshesheny, R.; Boubak, T.A.; Frantz, R.; Pietra, L.L.; Pleschka, S.; et al. Bacterial Outer Membrane Vesicles (OMVs)-Based Dual Vaccine for Influenza A H1N1 Virus and MERS-CoV. Vaccines 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cai, X.; Yao, J.; Guo, H.; Yin, L.; Leung, W.; Xu, C. Role of Extracellular Vesicles in Influenza Virus Infection. Front. Cell. Infect. Microbiol. 2020, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Pordanjani, P.M.; Bolhassani, A.; Milani, A.; Pouriayevali, M.H. Extracellular vesicles in vaccine development and therapeutic approaches for viral diseases. Process Biochem. 2023, 128, 167–180. [Google Scholar] [CrossRef]
- Sabanovic, B.; Piva, F.; Cecati, M.; Giulietti, M. Promising Extracellular Vesicle-Based Vaccines against Viruses, Including SARS-CoV-2. Biology 2021, 10, 94. [Google Scholar] [CrossRef]
- Santos, P.; Almeida, F. Exosome-Based Vaccines: History, Current State, and Clinical Trials. Front. Immunol. 2021, 12, 711565. [Google Scholar] [CrossRef]
- Turner, C.T.; McInnes, S.J.P.; Voelcker, N.H.; Cowin, A.J. Therapeutic Potential of Inorganic Nanoparticles for the Delivery of Monoclonal Antibodies. J. Nanomater. 2015, 2015, 309602. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, W.; Luo, Y.; Wang, B.-Z. Gold nanoparticles conjugating recombinant influenza hemagglutinin trimers and flagellin enhanced mucosal cellular immunity. Nanomedicine Nanotechnol. Biol. Med. 2018, 14, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Gill, H.S. M2e-immobilized gold nanoparticles as influenza A vaccine: Role of soluble M2e and longevity of protection. Vaccine 2015, 33, 2307–2315. [Google Scholar] [CrossRef]
- Neto, L.M.M.; Zufelato, N.; de Sousa-Júnior, A.A.; Trentini, M.M.; da Costa, A.C.; Bakuzis, A.F.; Kipnis, A.; Junqueira-Kipnis, A.P. Specific T cell induction using iron oxide based nanoparticles as subunit vaccine adjuvant. Hum. Vaccines Immunother. 2018, 14, 2786–2801. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.M.; Sanz-Ortega, L.; Mulens-Arias, V.; Gutiérrez, L.; Pérez-Yagüe, S.; Barber, D.F. Superparamagnetic iron oxide nanoparticle uptake alters M2 macrophage phenotype, iron metabolism, migration and invasion. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1127–1138. [Google Scholar] [CrossRef]
- Mody, K.T.; Popat, A.; Mahony, D.; Cavallaro, A.S.; Yu, C.; Mitter, N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale 2013, 5, 5167–5179. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.V.; de Ruiz, R.C.; Scaramuzzi, K.; Marengo, E.B.; Matos, J.R.; Tambourgi, D.V.; Fantini, M.C.A.; Sant’Anna, O.A. Immunological parameters related to the adjuvant effect of the ordered mesoporous silica SBA-15. Vaccine 2010, 28, 7829–7836. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Fries, C.N.; Curvino, E.J.; Chen, J.-L.; Permar, S.R.; Fouda, G.G.; Collier, J.H. Advances in nanomaterial vaccine strategies to address infectious diseases impacting global health. Nat. Nanotechnol. 2021, 16, 1–14. [Google Scholar] [CrossRef]
- Jain, S.; O’Hagan, D.T.; Singh, M. The long-term potential of biodegradable poly(lactide-co-glycolide) microparticles as the next-generation vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 1731–1742. [Google Scholar] [CrossRef]
- Thomas, C.; Rawat, A.; Hope-Weeks, L.; Ahsan, F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol. Pharm. 2011, 8, 405–415. [Google Scholar] [CrossRef]
- Pawar, D.; Mangal, S.; Goswami, R.; Jaganathan, K.S. Development and characterization of surface modified PLGA nanoparticles for nasal vaccine delivery: Effect of mucoadhesive coating on antigen uptake and immune adjuvant activity. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgem. Pharm. Verfahrenstech. EV 2013, 85, 550–559. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kirtane, A.R.; Kim, N.Y.; Rajesh, N.U.; Tang, C.; Ishida, K.; Hayward, A.M.; Langer, R.; Traverso, G. Gastrointestinal Delivery of an mRNA Vaccine Using Immunostimulatory Polymeric Nanoparticles. AAPS J. 2023, 25, 81. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, B.-Z. A perspective of nanoparticle universal influenza vaccines. ACS Infect. Dis. 2018, 4, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Jitoboam, K.; Phaonakrop, N.; Libsittikul, S.; Thepparit, C.; Roytrakul, S.; Smith, D.R. Actin Interacts with Dengue Virus 2 and 4 Envelope Proteins. PLoS ONE 2016, 11, e0151951. [Google Scholar] [CrossRef]
- Petousis-Harris, H.; Poole, T.; Stewart, J.; Turner, N.; Goodyear-Smith, F.; Coster, G.; Lennon, D. An investigation of three injections techniques in reducing local injection pain with a human papillomavirus vaccine: A randomized trial. Vaccine 2013, 31, 1157–1162. [Google Scholar] [CrossRef]
- Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection|Nature Medicine. Available online: https://www.nature.com/articles/nm.3927 (accessed on 7 August 2025).
- Wöll, S.; Schiller, S.; Bachran, C.; Swee, L.K.; Scherließ, R. Pentaglycine lipid derivates—rp-HPLC analytics for bioorthogonal anchor molecules in targeted, multiple-composite liposomal drug delivery systems. Int. J. Pharm. 2018, 547, 602–610. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, T.; Xu, C.; Li, J.; Liu, G.; Yin, K.; Cui, Y.; Wei, Q.; Huang, B.; Sun, H. Protective immune response against Toxoplasma gondii elicited by a novel yeast-based vaccine with microneme protein 16. Vaccine 2018, 36, 3943–3948. [Google Scholar] [CrossRef]
- Pielak, R.M.; Chou, J.J. Influenza M2 proton channels. Biochim. Biophys. Acta 2011, 1808, 522–529. [Google Scholar] [CrossRef]
- Stauffer, S.; Feng, Y.; Nebioglu, F.; Heilig, R.; Picotti, P.; Helenius, A. Stepwise priming by acidic pH and a high K+ concentration is required for efficient uncoating of influenza A virus cores after penetration. J. Virol. 2014, 88, 13029–13046. [Google Scholar] [CrossRef]
- De Jong, N.M.C.; Aartse, A.; Van Gils, M.J.; Eggink, D. Development of broadly reactive influenza vaccines by targeting the conserved regions of the hemagglutinin stem and head domains. Expert Rev. Vaccines 2020, 19, 563–577. [Google Scholar] [CrossRef]
- Nguyen, Q.-T.; Choi, Y.-K. Targeting Antigens for Universal Influenza Vaccine Development. Viruses 2021, 13, 973. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-C.; Sayedahmed, E.E.; Sambhara, S.; Mittal, S.K. Progress towards the Development of a Universal Influenza Vaccine. Viruses 2022, 14, 1684. [Google Scholar] [CrossRef]
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.-J.; Kanekiyo, M.; Kong, W.-P.; Gallagher, J.R.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef]
- Akingbola, A.; Adegbesan, A.; Adegoke, K.; Idahor, C.; Mariaria, P.; Peters, F.; Salami, R.A.; Ojo, O.; Nwaeze, E.; Abdullahi, O.; et al. Comparing Moderna’s mRNA-1083 and Pfizer’s dual-target mRNA vaccines for influenza and COVID-19. NPJ Vaccines 2025, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Mohan, S. Influenza vaccine: A review on current scenario and future prospects. J. Genet. Eng. Biotechnol. 2023, 21, 154. [Google Scholar] [CrossRef]
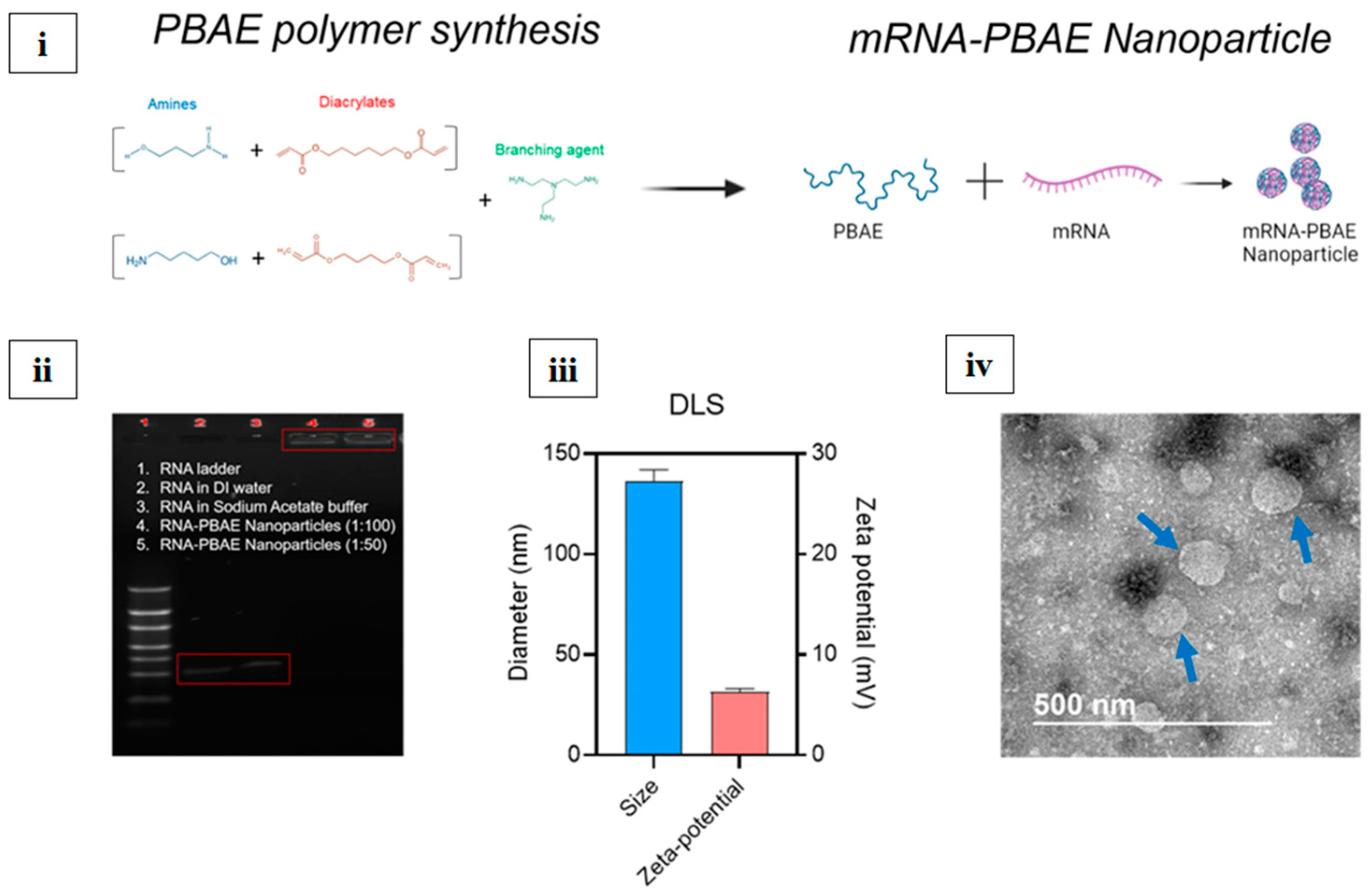
| Product Name | Developer Company | Disease | Nanocarrier System | Viral Antigen Cargo | Clinical Trial (Registration Number) | Marketing Authorization | Ref. |
|---|---|---|---|---|---|---|---|
| NanoFlu | Novavax | Influenza | Virus-like particle (VLP) | rHA (H1N1, H3N2, B-Yamagata, B-Victoria) | Phase III (NCT04120194) | No | [45] |
| IVX-411 | Icosavax | SARS-CoV-2 | VLP (I53-50 scaffold) | SARS-CoV-2 RBD trimer | Phase I/II (NCT05027932) | No | [46] |
| ABNCoV2 | AdaptVac | SARS-CoV-2 | Capsid VLP (cVLP) | SARS-CoV-2 RBD | Phase II (NCT04839146) | No | [47] |
| BNT162b2 | Pfizer/BioNTech | SARS-CoV-2 | Lipid Nanoparticle (LNP) | mRNA-encoding SARS-CoV-2 spike protein | Phase III (NCT04368728) | Yes (Emergency Use) | [48] |
| mRNA-1010 | Moderna | Influenza | Lipid Nanoparticle (LNP) | mRNA-encoding HA (H1, H3, B-Yam, B-Vic) | Phase I/II (NCT04956575) | No | [49] |
| Ferritin-HA | NIH/VRC | Influenza | Ferritin Nanoparticle | Hemagglutinin (H1, H3, B) | Phase I (NCT03814720) | No | [50] |
| Vaxfectin® DNA vaccine | Vical Inc. | Influenza | Cationic lipid (Vaxfectin®) | DNA plasmid encoding HA | Phase I (NCT00709877) | No | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvez, S.; Pathrathota, A.; Uppar, A.L.; Yadagiri, G.; Mudavath, S.L. Influenza Virus: Global Health Impact, Strategies, Challenges, Role of Nanotechnolgy in Influenza Vaccine Development. Vaccines 2025, 13, 890. https://doi.org/10.3390/vaccines13090890
Parvez S, Pathrathota A, Uppar AL, Yadagiri G, Mudavath SL. Influenza Virus: Global Health Impact, Strategies, Challenges, Role of Nanotechnolgy in Influenza Vaccine Development. Vaccines. 2025; 13(9):890. https://doi.org/10.3390/vaccines13090890
Chicago/Turabian StyleParvez, Shabi, Anushree Pathrathota, Arjun L. Uppar, Ganesh Yadagiri, and Shyam Lal Mudavath. 2025. "Influenza Virus: Global Health Impact, Strategies, Challenges, Role of Nanotechnolgy in Influenza Vaccine Development" Vaccines 13, no. 9: 890. https://doi.org/10.3390/vaccines13090890
APA StyleParvez, S., Pathrathota, A., Uppar, A. L., Yadagiri, G., & Mudavath, S. L. (2025). Influenza Virus: Global Health Impact, Strategies, Challenges, Role of Nanotechnolgy in Influenza Vaccine Development. Vaccines, 13(9), 890. https://doi.org/10.3390/vaccines13090890






