Deficits in Long-Term Vaccine Immunity Among Childhood Cancer Survivors Despite Revaccination Programs
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Sample and Data Collection
2.3. Serological Assays
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
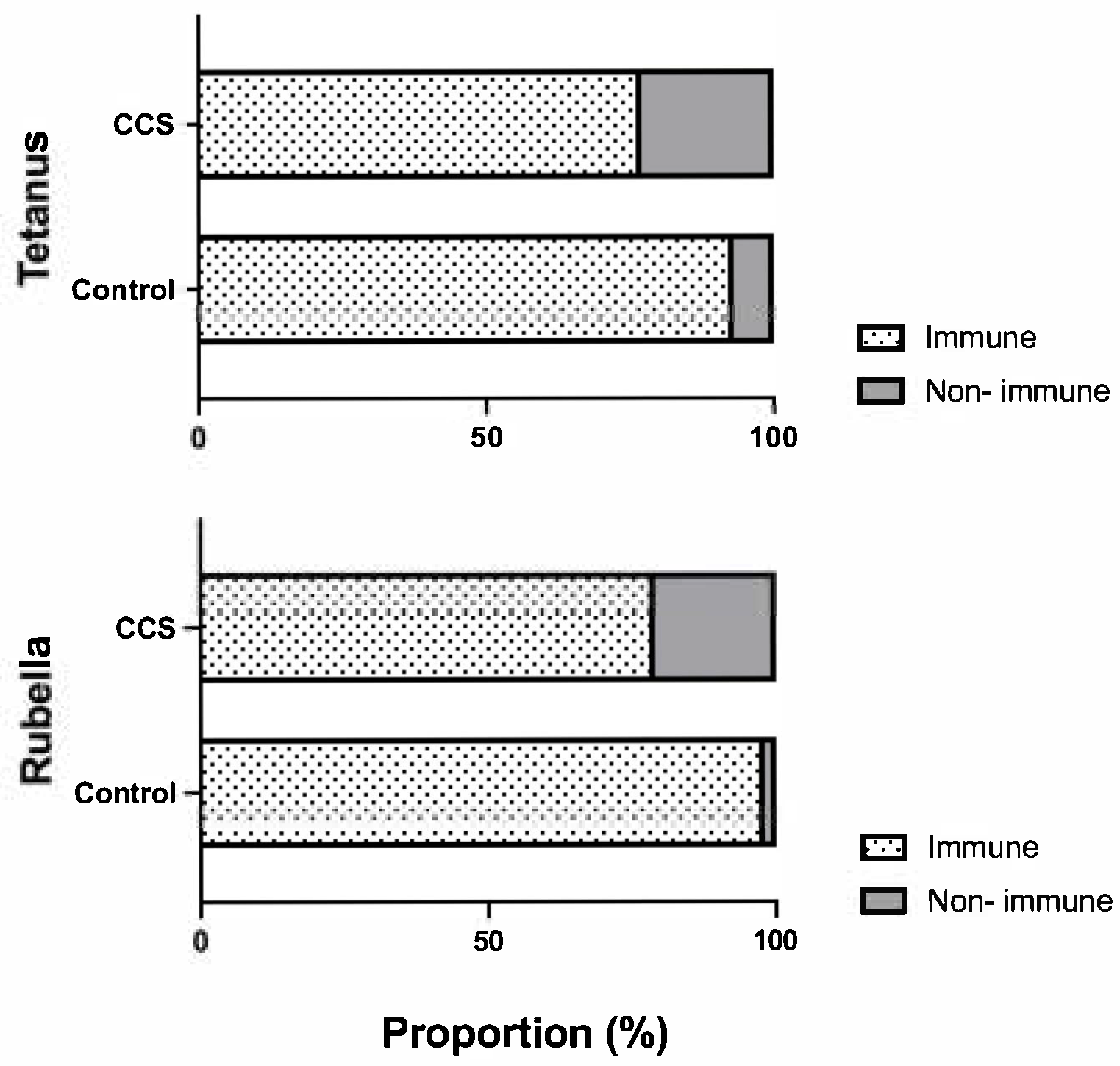
3.2. Seroprevalence of Tetanus IgG and Rubella IgG
3.3. Status of Revaccination and Protective Antibodies Depending on Degree of Immunosuppression
3.4. Association of Antibody Prevalence with Different Background Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCSs | childhood cancer survivors |
| Abs | antibodies |
| NIP | national immunization program |
| DTP | diphtheria–tetanus–pertussis |
| MMR | measles–mumps–rubella |
| IgG | immunoglobulin G |
| CNS | central nervous system |
| ALL | acute lymphoblastic leukemia |
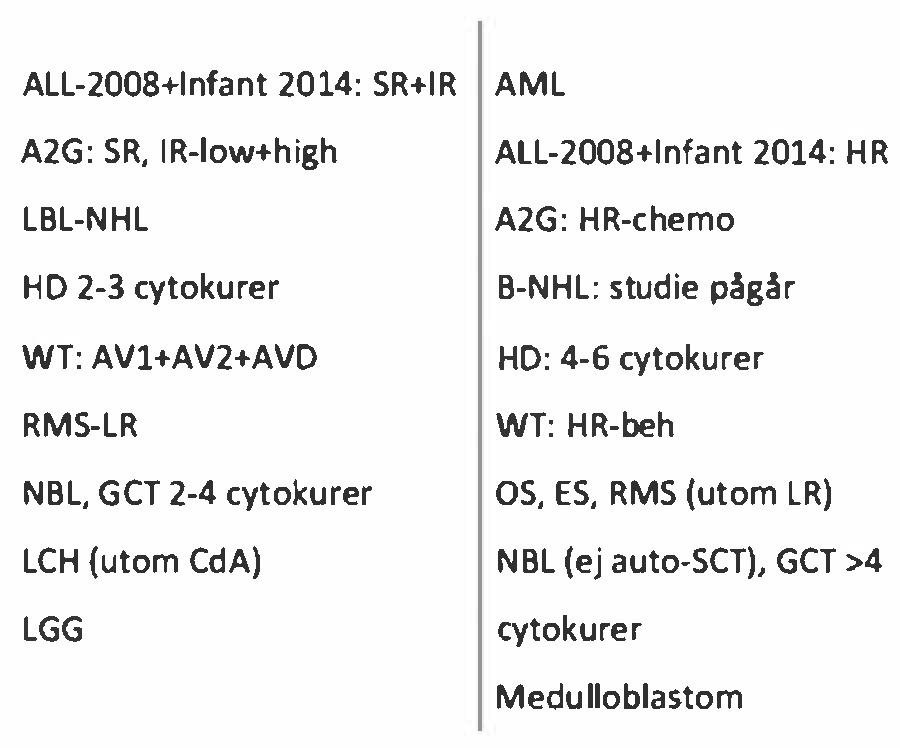
Appendix A
References
- Bhakta, N.; Force, L.M.; Allemani, C.; Atun, R.; Bray, F.; Coleman, M.P.; Steliarova-Foucher, E.; Frazier, A.L.; Robison, L.L.; Rodriguez-Galindo, C.; et al. Childhood cancer burden: A review of global estimates. Lancet Oncol. 2019, 20, e42–e53. [Google Scholar] [CrossRef]
- Ohlsen, T.J.; Martos, M.R.; Hawkins, D.S. Recent advances in the treatment of childhood cancers. Curr. Opin. Pediatr. 2023, 36, 57–63. [Google Scholar] [CrossRef]
- Guilcher, G.M.T.; Rivard, L.; Huang, J.T.; Wright, N.A.M.; Anderson, L.; Eissa, H.; Pelletier, W.; Ramachandran, S.; Schechter, T.; Shah, A.J.; et al. Immune function in childhood cancer survivors: A Children’s Oncology Group review. Lancet Child Adolesc. Health 2021, 5, 284–294. [Google Scholar] [CrossRef]
- Saghafian-Hedengren, S.; Soderstrom, I.; Sverremark-Ekstrom, E.; Nilsson, A. Insights into defective sero-logical memory after acute lymphoblastic leukaemia treatment: The role of the plasma cell survival niche, memory B-cells and gut microbiota in vaccine responses. Blood Rev. 2018, 32, 71–80. [Google Scholar] [CrossRef]
- Bochennek, K.; Allwinn, R.; Langer, R.; Becker, M.; Keppler, O.T.; Klingebiel, T.; Lehrnbecher, T. Differential loss of humoral immunity against measles, mumps, rubella and varicella-zoster virus in children treated for cancer. Vaccine 2014, 32, 3357–3361. [Google Scholar] [CrossRef]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin. Infect. Dis. 2014, 58, 309–318. [Google Scholar] [CrossRef]
- Mikulska, M.; Cesaro, S.; de Lavallade, H.; Di Blasi, R.; Einarsdottir, S.; Gallo, G.; Rieger, C.; Engelhard, D.; Lehrnbecher, T.; Ljungman, P.; et al. Vaccination of patients with haematological malignancies who did not have transplantations: Guidelines from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, e188–e199. [Google Scholar] [CrossRef]
- Kampouri, E.; Walti, C.S.; Gauthier, J.; Hill, J.A. Managing hypogammaglobulinemia in patients treated with CAR-T-cell therapy: Key points for clinicians. Expert Rev. Hematol. 2022, 15, 305–320. [Google Scholar] [CrossRef]
- Public Health Agency, Sweden. Vaccination Programmes for Children. Available online: https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/communicable-disease-control/vaccinations/vaccination-programmes/2022 (accessed on 23 April 2025).
- Public Health Agency, Sweden. Barnvaccinationsprogrammet i Sverige 2023-Årsrapport. 2024. Available online: https://www.folkhalsomyndigheten.se/contentassets/39e211b5dbc449de8574d81f1a416a26/barnvaccinationsprogrammet-sverige-2023.pdf (accessed on 23 April 2025).
- Ek, T. Riktlinjer för Vaccinationer vid Barncancer. 2020. Available online: https://pho.barnlakarforeningen.se/wp-content/uploads/sites/20/2020/11/PM-PHO-Vaccination-rev-201106.pdf (accessed on 23 April 2025).
- Sundberg, E.; Hoffman, T.; Nilsson, A.; Pahnke, S.; Enblad, G.; Kolstad, L.; Rönnberg, B.; Lundkvist, Å.; Torkki, M.; Zhou, O.; et al. COVID-19 seroprevalence and clinical picture in Swedish pediatric oncology and hematology patients. Pediatr. Blood Cancer 2022, 69, e29773. [Google Scholar] [CrossRef]
- Euroimmun. Euroimmun® Anti-Rubella Virus ELISA (IgG) Kit In: AG EML. 2024. Available online: https://www.euroimmun.com/documents/Indications/Infections/Rubella-virus/EI_2590_D_UK_A.pdf (accessed on 23 April 2025).
- van Gageldonk, P.G.; van Schaijk, F.G.; van der Klis, F.R.; Berbers, G.A. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J. Immunol. Methods 2008, 335, 79–89. [Google Scholar] [CrossRef]
- Chehab, L.; Doody, D.R.; Esbenshade, A.J.; Guilcher, G.M.; Dvorak, C.C.; Fisher, B.T.; Mueller, B.A.; Chow, E.J.; Rossoff, J. A Population-Based Study of the Long-Term Risk of Infections Associated with Hospitalization in Childhood Cancer Survivors. J. Clin. Oncol. 2023, 41, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Thornton, C.P.; Ruble, K.; Cooper, S.L. Post-Chemotherapy Titer Status and Need for Revaccination After Treatment for Childhood Cancer. Clin. Pediatr. 2020, 59, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Keskin Yildirim, Z.; Buyukavci, M. Assessment of Humoral Immunity to Hepatitis B, Measles, Rubella, and Mumps in Children After Chemotherapy. J. Pediatr. Hematol. Oncol. 2018, 40, e99–e102. [Google Scholar] [CrossRef] [PubMed]
- Brodtman, D.H.; Rosenthal, D.W.; Redner, A.; Lanzkowsky, P.; Bonagura, V.R. Immunodeficiency in children with acute lymphoblastic leukemia after completion of modern aggressive chemotherapeutic regimens. J. Pediatr. 2005, 146, 654–661. [Google Scholar] [CrossRef]
- Speckhart, S.A. MMR vaccination timing and long-term immunity among childhood cancer survivors. Pediatr. Blood Cancer 2023, 70, e30133. [Google Scholar] [CrossRef]
- Cetin, M.; Gumy-Pause, F.; Gualtieri, R.; Posfay-Barbe, K.M.; Blanchard-Rohner, G. Vaccine Immunity in Children After Hematologic Cancer Treatment: A Retrospective Single-center Study. J. Pediatr. Hematol. 2023, 46, e51–e59. [Google Scholar] [CrossRef]
- Crawford, N.W.; Heath, J.A.; Ashley, D.; Downie, P.; Buttery, J.P. Survivors of childhood cancer: An Australian audit of vaccination status after treatment. Pediatr. Blood Cancer 2009, 54, 128–133. [Google Scholar] [CrossRef]
- Choi, D.K.; Strzepka, J.T.; Hunt, S.R.; Tannenbaum, V.L.; Jang, I.E. Vaccination in pediatric cancer survivors: Vaccination rates, immune status, and knowledge regarding compliance. Pediatr. Blood Cancer 2020, 67, e28565. [Google Scholar] [CrossRef]
- Renduchintala, K.; Arevalo, M.; Fonseca, G.; Haver, M.K.; Gwede, C.K.; Pabbathi, S.; Christy, S.M. Vaccination uptake among post-treatment cancer survivors: A multi-vaccine scoping review. Vaccine 2024, 42, 125995. [Google Scholar] [CrossRef]
- Schenk, J.; Abrams, S.; Theeten, H.; Van Damme, P.; Beutels, P.; Hens, N. Immunogenicity and persistence of trivalent measles, mumps, and rubella vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2020, 21, 286–295. [Google Scholar] [CrossRef]
- de de la Fuente Garcia, I.; Coïc, L.; Leclerc, J.-M.; Laverdière, C.; Rousseau, C.; Ovetchkine, P.; Tapiéro, B. Protection against vaccine preventable diseases in children treated for acute lymphoblastic leukemia. Pediatr. Blood Cancer 2016, 64, 315–320. [Google Scholar] [CrossRef]
- Nilsson, A.; De Milito, A.; Engström, P.; Nordin, M.; Narita, M.; Grillner, L.; Chiodi, F.; Björk, O. Current Chemotherapy Protocols for Childhood Acute Lymphoblastic Leukemia Induce Loss of Humoral Immunity to Viral Vaccination Antigens. Pediatrics 2002, 109, e91. [Google Scholar] [CrossRef] [PubMed]
- van Tilburg, C.M.; Sanders, E.A.M.; Rovers, M.M.; Wolfs, T.F.W.; Bierings, M.B. Loss of antibodies and response to (re-)vaccination in children after treatment for acute lymphocytic leukemia: A systematic review. Leukemia 2006, 20, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Wiegering, V.; Frank, J.; Freudenberg, S.; Morbach, H.; Schlegel, P.G.; Eyrich, M.; Winkler, B. Impaired B-cell reconstitution in children after chemotherapy for standard or medium risk acute precursor B-lymphoblastic leukemia. Leuk. Lymphoma 2013, 55, 870–875. [Google Scholar] [CrossRef] [PubMed]
| Solid Tumors | CNS Tumors | Leukemia/Lymphoma * | ||
|---|---|---|---|---|
| n = 51 | n = 23 | n = 106 | ||
| Age at diagnosis (years) | 8 (0–17 ) | 5 (0–14) | 7.5 (0–17) | p = 0.056 |
| median (range) | ||||
| Preschool (0–5 yrs) n (%) | 22 (43) | 12 (61) | 83 (46) | |
| School (6–12 yrs) n (%) | 17 (33) | 8 (35) | 54 (30) | |
| Teenagers (13–18 yrs) n (%) | 12 (24) | 1 (4) | 43 (24) | |
| Follow-up time (years) | 9 (1–17.5) | 13 (2–18) | 10.5 (1–18.5) | p = 0.078 |
| median (range) | ||||
| Estimated treatment intensity n (%) | p < 0.001 | |||
| Mild | 1 (2) | 1 (4) | 5 (5) | |
| Moderate | 16 (32) | 11 (48) | 0 (0) | |
| Intensive | 34 (66) | 11 (48) | 101 (95) | |
| Relapse n (%) | 6 (12) | 3 (13) | 8 (7) | p = 0.496 |
| Revaccination n (%) | ||||
| DTP | 25/51 (49%) | 9/23 (39%) | 72/106 (68%) | p = 0.009 |
| MMR | 19/51 (37%) | 5/23 (22%) | 56/106 (53%) | p = 0.011 |
| Intensity of Treatment | Mild | Moderate | Intense |
|---|---|---|---|
| n = 7 | n = 27 | n = 146 | |
| DTP n (%) | |||
| Revaccination | n = 1 | n = 9 | n = 98 * |
| Tetanus IgG > cut-off | 1 (100.0) | 9 (100.0) | 81 (82.6) |
| Tetanus IgG < cut-off | 0 (0.0) | 0 (0.0) | 13 (13.2) |
| No revaccination * | n = 6 | n = 18 * | n = 48 |
| Tetanus IgG > cut-off | 5 (83.3) | 16 (88.8) | 24 (50.0) |
| Tetanus IgG < cut-off | 1 (17.7) | 1 (5.6) | 24 (50.0) |
| MMR n (%) | |||
| Revaccination | n = 2 # | n = 5 # | n = 73 # |
| Rubella IgG > cut-off | 1 (50.0) | 4 (80.0) | 57 (78.1) |
| Rubella IgG < cut-off | 0 (0.0) | 0 (0.0) | 14 (19.2) |
| No revaccination | n = 5 | n = 22 # | n = 73 # |
| Rubella IgG > cut-off | 5 (100.0) | 16 (72.7) | 53 (72.6) |
| Rubella IgG < cut-off | 0 (0.0) | 5 (22.7) | 17 (23.2) |
| Tetanus | Rubella | |||||
|---|---|---|---|---|---|---|
| IgG < Cut-off (n = 39) | IgG > Cut-off (n = 136) | p-Value | IgG < Cut-off (n = 36) | IgG > Cut-off (n = 136) | p-Value | |
| Age at diagnosis, n | 0.160 | 0.036 | ||||
| 0–5 years | 22 | 60 | 21 | 57 | ||
| 6–12 years | 7 | 46 | 5 | 48 | ||
| 13–18 years | 10 | 30 | 10 | 31 | ||
| Diagnosis group, n | 0.175 | 0.587 | ||||
| Solid tumors | 7 | 42 | 8 | 41 | ||
| CNS tumors | 4 | 19 | 4 | 17 | ||
| Leukemia/lymphoma | 28 | 75 | 24 | 78 | ||
| Treatment intensity, n | 0.023 | 0.667 | ||||
| Mild | 1 | 6 | 0 | 6 | ||
| Moderate | 1 | 25 | 5 | 20 | ||
| Intense | 37 | 105 | 31 | 110 | ||
| DTP revaccination *, n | <0.001 | |||||
| Yes | 13 | 91 | n/A | n/A | ||
| No | 26 | 45 | n/A | n/A | ||
| MMR revaccination #, n | 0.572 | |||||
| Yes | n/A | n/A | 14 | 62 | ||
| No | n/A | n/A | 22 | 74 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zadruzny, A.; Tiselius, E.; Lepp, T.; Aktas, T.; Tecleab, T.; Hellman, S.; Jahnmatz, M.; Nilsson, A. Deficits in Long-Term Vaccine Immunity Among Childhood Cancer Survivors Despite Revaccination Programs. Vaccines 2025, 13, 617. https://doi.org/10.3390/vaccines13060617
Zadruzny A, Tiselius E, Lepp T, Aktas T, Tecleab T, Hellman S, Jahnmatz M, Nilsson A. Deficits in Long-Term Vaccine Immunity Among Childhood Cancer Survivors Despite Revaccination Programs. Vaccines. 2025; 13(6):617. https://doi.org/10.3390/vaccines13060617
Chicago/Turabian StyleZadruzny, Alexander, Eva Tiselius, Tiia Lepp, Teodora Aktas, Teghesti Tecleab, Samuel Hellman, Maja Jahnmatz, and Anna Nilsson. 2025. "Deficits in Long-Term Vaccine Immunity Among Childhood Cancer Survivors Despite Revaccination Programs" Vaccines 13, no. 6: 617. https://doi.org/10.3390/vaccines13060617
APA StyleZadruzny, A., Tiselius, E., Lepp, T., Aktas, T., Tecleab, T., Hellman, S., Jahnmatz, M., & Nilsson, A. (2025). Deficits in Long-Term Vaccine Immunity Among Childhood Cancer Survivors Despite Revaccination Programs. Vaccines, 13(6), 617. https://doi.org/10.3390/vaccines13060617






