Abstract
Background/Objectives: Streptococcus pneumoniae with, or following, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been associated with increased mortality, particularly in older adults. However, vaccination can be an effective preventative measure. This Phase 3 study (NCT05158140) assessed the immunogenicity and safety of co-administering the SARS-CoV-2 vaccine mRNA-1273 with the 23-valent pneumococcal polysaccharide vaccine (PPSV23) or the 15-valent pneumococcal conjugate vaccine (PCV15). Methods: Participants were healthy adults ≥50 years of age who had previously received a two-dose primary series of mRNA-1273 ≥5 months before the first study visit and may have received a booster dose of mRNA-1273 ≥4 months prior to the first study visit. Participants were randomized (1:1:1:1) to receive mRNA-1273 concomitantly with PPSV23 or PCV15 on Day 1 followed by placebo on Day 30, or sequentially with mRNA-1273 and placebo on Day 1 and PPSV23 or PCV15 on Day 30. The primary study endpoints were pneumococcal-serotype-specific opsonophagocytic activity (OPA) geometric mean titers (GMTs) and SARS-CoV-2-specific binding antibody GMTs at 30 days after vaccination, as well as safety and tolerability following vaccination. Results: In total, 850 adults participated in the study. Serotype-specific OPA GMTs at 30 days post-vaccination with PPSV23 or PCV15 were generally comparable between the concomitant and sequential groups. SARS-CoV-2-specific GMTs increased in all groups from pre-vaccination to 30 days post-vaccination with mRNA-1273, with a consistent response between concomitant and sequential groups. Safety profiles were comparable across study groups. Conclusions: Co-administration of mRNA-1273 with PPSV23 or PCV15 in healthy adults ≥50 years of age was immunogenic and well tolerated.
1. Introduction
The burden of disease caused by Streptococcus pneumoniae is high among adults ≥50 years of age and is highest in older adults ≥65 years of age [1,2,3]. Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which results in coronavirus disease 2019 (COVID-19), can predispose individuals to bacterial infections and diseases, such as pneumococcal disease (PD) [4]. Pneumococcal infection can occur at the same time as an infection with SARS-CoV-2 (co-infection) or can occur afterwards as a secondary infection [4,5]; both have been associated with increased mortality compared with invasive PD (IPD) or COVID-19 alone [6]. This increased mortality can often be observed among at-risk populations, such as older adults or those with certain underlying chronic medical conditions [7]. In addition, studies have shown that individuals who experience milder symptoms of SARS-CoV-2 infection, such as fever, mild or moderate coughing, and shortness of breath, can have higher carriage rates of S. pneumoniae than those not infected with SARS-CoV-2 [8]. Furthermore, infection with SARS-CoV-2 can compromise the immune system and is considered a primary risk factor for IPD [8,9].
Pneumococcal polysaccharide and conjugate vaccines with varying serotype coverage are recommended by the US Centers for Disease Control and Prevention (CDC) for children ≤5 years of age, adults ≥50 years of age, and children and adults at increased risk of PD [10,11]. Pneumococcal vaccines have a long history of use and have been demonstrated to be immunogenic and effective, with a favorable safety profile [12]. These vaccines are the mainstay of disease prevention and have led to a substantial decrease in cases of IPD, respiratory disease burden, and healthcare utilization [13,14,15]. A widely used pneumococcal vaccine is the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax® 23, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA [MSD]). PPSV23 is indicated for the prevention of PD in older adults ≥50 years of age and individuals ≥2 years of age with certain medical conditions that can lead to an increased risk of PD. PPSV23 contains 23 different serotypes of S. pneumoniae (serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F) [13,16,17,18,19]. A 15-valent pneumococcal conjugate vaccine (PCV15; VAXNEUVANCE™, MSD) is also available in many countries and is indicated in individuals ≥6 weeks of age for the prevention of IPD and pneumonia, and in individuals 6 weeks to ≤18 years of age for acute otitis media, caused by S. pneumoniae serotypes contained in the vaccine (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F) [15]. The CDC recommends that, in adults, administration of PCV15 should be followed by a single dose of PPSV23 1 year later [10,20].
After the novel SARS-CoV-2 virus was identified in 2019, an urgent need arose for vaccines to curb the rapidly spreading pandemic, reduce severe illness and mortality, and address the global health crisis [21]. The mRNA-1273 COVID-19 vaccine (SPIKEVAX®, Moderna Inc., Cambridge, MA, USA) received emergency use authorization (EUA) from the US Food and Drug Administration on 18 December 2020, for the immunization of adults ≥18 years of age. Almost a year later, the EUA was amended to include the use of an mRNA-1273 booster dose, administered ≥6 months after completion of the primary series [22]. mRNA-1273 is a nucleoside-modified messenger RNA (mRNA) vaccine containing single mRNAs encoding the prefusion-stabilized spike glycoprotein of ancestral SARS-CoV-2 (Wuhan-Hu-1). During the study period, the monovalent mRNA-1273 was indicated for active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals ≥12 years of age (SPIKEVAX® [COVID-19 Vaccine, mRNA, 2024–2025 Formula], Moderna TX, Cambridge, MA, USA) [23].
The US Advisory Committee on Immunization Practices (ACIP) recommends that SARS-CoV-2 vaccines be co-administered with other vaccines [24]. The feasibility of co-administration of vaccines in older adults has been previously demonstrated [25,26]. Co-administration of vaccines helps increase vaccination coverage by reducing the number of vaccination visits, lowering costs, minimizing missed opportunities, improving compliance, and ensuring timely vaccine administration [27,28,29]. In a meta-analysis of 17 studies, co-administration of influenza and pneumococcal vaccines resulted in an additional 24% reduction in pneumonia and an additional 28% reduction in deaths in older adults compared with pneumococcal vaccination alone [30]. Furthermore, in a study of US adults who had received 13-valent pneumococcal conjugate vaccine (Prevnar13®, Pfizer Inc., New York, NY, USA) with or without PPSV23, based on the intervals recommended in the 2015 ACIP guidelines for those ≥65 years of age, pneumococcal vaccination was associated with a reduced risk of COVID-19 diagnosis, hospitalization, or fatal hospitalization [31].
This study was conducted to assess the safety, tolerability, and immunogenicity of mRNA-1273 administered concomitantly with a single dose of a pneumococcal vaccine (PPSV23 or PCV15), compared with sequential administration of the same vaccines, in healthy adults ≥50 years of age.
2. Methods
This Phase 3, randomized, placebo-controlled, parallel-group, multicenter, double-blind study was conducted between 12 January 2022 and 27 October 2023, during the COVID-19 pandemic (Protocol V110-911; NCT05158140; Figure 1). Participants were enrolled at 44 sites across the United States and Puerto Rico (Table S1).
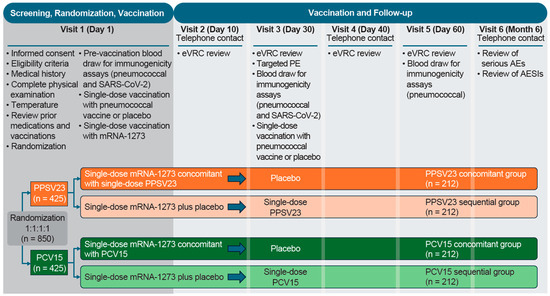
Figure 1.
Study design. AE: adverse event; AESI: adverse event of special interest; eVRC: electronic vaccination report card; mRNA-1273: monovalent mRNA COVID-19 vaccine; PCV15: 15-valent pneumococcal conjugate vaccine; PE: physical examination; PPSV23: 23-valent pneumococcal polysaccharide vaccine; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
2.1. Participants, Randomization, and Blinding
Eligible participants were adults ≥50 years of age who had previously completed a two-dose primary series of mRNA-1273 ≥5 months before the receipt of the study vaccine at the first study visit. Participants with historic SARS-CoV-2 infection and those who had received a prior booster dose of mRNA-1273 were also included. The study was conducted amidst an evolving SARS-CoV-2 vaccine landscape and the protocol was amended to reflect changes in vaccine recommendations. Participants were excluded if they had a current SARS-CoV-2 infection, a known history of SARS-CoV-2 infection <3 months before receipt of the study vaccine at the first study visit, or if they had a history of myocarditis and/or pericarditis. Participants with underlying chronic conditions were assessed by the investigator to be in a stable condition. Detailed inclusion and exclusion criteria are provided in Table S2.
Participants were randomly assigned in a 1:1:1:1 ratio via a centralized interactive response technology system. Participants who received mRNA-1273 (50 µg/0.25 mL) concomitantly with either PPSV23 (refer to product label for dosage) or PCV15 (refer to product label for dosage) at the first study visit, followed by placebo at Visit 3, were in the PPSV23 concomitant group or PCV15 concomitant group, respectively. Participants who received mRNA-1273 with placebo at the first study visit, followed by either PPSV23 or PCV15 at Visit 3, were in the PPSV23 sequential group or PCV15 sequential group, respectively (Figure 1). Stratification factors included age (50–64 years, 65–74 years, and ≥75 years of age), history of pneumococcal vaccination (yes or no), receipt of prior mRNA-1273 booster dose following the two-dose primary series (yes or no), and history of prior SARS-CoV-2 infection (yes or no).
PPSV23, PCV15, and placebo were prepared and administered by an unblinded pharmacist or qualified personnel. The participant, investigator, and other personnel involved in study conduct or clinical evaluation were blinded. mRNA-1273 was provided open-label and was also prepared and administered by unblinded personnel.
This study was conducted in accordance with principles of Good Clinical Practice guidelines and was approved by the appropriate institutional review boards and regulatory agencies [32]. The institutional review board approved the protocol, protocol amendments, and informed consent form. All participants provided written informed consent before enrollment. The study protocol and statistical analysis plan are available online [33].
2.2. Immunogenicity Assessments
There were two primary immunogenicity endpoints in this study. The first was to evaluate the pneumococcal-serotype-specific opsonophagocytic activity (OPA) geometric mean titers (GMTs) at 30 days post-vaccination with PPSV23 or PCV15 in each of the four groups. The other primary endpoint was to evaluate SARS-CoV-2-specific binding antibody GMTs at 30 days post-vaccination with mRNA-1273 in each of the four groups. Data from both sequential groups were combined for the SARS-CoV-2 immunogenicity analysis.
Secondary endpoints included assessing the serotype-specific geometric mean fold rises (GMFRs) and the proportions of participants with a ≥4-fold rise from baseline (pre-vaccination) to 30 days post-vaccination for OPA responses in each group. For SARS-CoV-2-specific binding antibody responses, the GMFRs and proportions of participants with a ≥4-fold rise from baseline to 30 days post-vaccination with mRNA-1273 were assessed in each group. The immunogenicity analyses were based on the per-protocol population, which comprised all randomized participants without deviations from the protocol that could have substantially affected immunogenicity measurements.
Immune responses were measured for the PCV15 serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F) in a validated multiplex opsonophagocytic assay. This assay measured 14 of the 23 pneumococcal serotypes in PPSV23 (1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F). A validated ligand-binding assay specific to the SARS-CoV-2 spike protein was used to measure vaccine-induced responses to mRNA-1273.
2.3. Safety Assessments
The primary safety objective was the assessment of safety and tolerability of a booster dose of mRNA-1273 administered concomitantly or sequentially with a single dose of pneumococcal vaccine (PPSV23 or PCV15). Primary safety endpoints included solicited injection-site adverse events (AEs), solicited systemic AEs, and vaccine-related serious AEs (SAEs). Participants were followed for 7 days post-vaccination for the number of solicited injection-site and systemic AEs. All solicited injection-site AEs (from Day 1 through Day 7 post-vaccination) were considered to be vaccine-related. All SAEs and AEs of special interest (AESIs), discontinuations of study intervention due to an AE, and deaths were collected from Day 1 through to study completion. AESIs were consistent with the regulatory guidance for the safety evaluation of mRNA-1273 and are listed in Table S3. Post-vaccination body temperature measurement was the only vital sign collected, and the temperatures were recorded after each vaccination on Days 1–7 using an electronic vaccination report card. All AEs and SAEs were assessed for overall intensity grade by the investigator and were recorded along with AE duration in days. Instances of injection-site erythema and swelling occurring on Days 1–7 were categorized as mild (2.5–5.0 cm), moderate (5.1–10.0 cm), or severe (>10 cm). Safety analyses were based on the all-participants-as-treated population, which included all randomized participants who received at least one dose of the study vaccine.
2.4. Statistical Analyses
On 31 August 2022, EUA for monovalent COVID-19 vaccines was withdrawn in favor of bivalent COVID-19 vaccine boosters. This change resulted in prematurely concluding enrollment [34,35]. Therefore, the planned enrollment of 1300 participants was changed to approximately 850 participants. Owing to this change, the study was descriptive in nature and not based on formal statistical hypothesis testing.
Evaluation of the OPA GMTs and the binding antibody GMTs at 30 days post-vaccination in each group included descriptive summaries and within-group 95% confidence intervals (CIs) for each vaccination group. The point estimates were calculated by exponentiating the estimates of the mean of the natural log values, and the within-group CIs were derived by exponentiating the CIs of the mean of the natural log values based on the t-distribution.
Reverse cumulative distribution curves were used to show the distribution of serotype-specific OPA titers and SARS-CoV-2-specific binding antibody titers for PPSV23 and PCV15 concomitant and sequential groups.
The safety analysis followed a tiered approach. For the Tier 2 safety endpoints, point estimates, with corresponding within-group 95% CIs based on the exact binomial method proposed by Clopper and Pearson [36], were provided for the proportions of participants with events following any vaccination. Clinical laboratory evaluations were not prespecified for this study. The Medical Dictionary for Regulatory Activities (MedDRA) version 26.0 was used for this study.
3. Results
3.1. Study Population
A total of 850 participants were randomized: 212 participants in the PPSV23 sequential group, 210 participants in the PCV15 sequential group, and 214 participants in each of the PPSV23 and PCV15 concomitant groups (Figure 2). Most randomized participants received at least one dose of study vaccine and completed the study (≥93.9%). The most common reasons for discontinuing the study across all groups were participant withdrawal (2.4%) and loss to follow-up (1.9%). Most randomized participants were included in the immunogenicity analyses; in the PPSV23 sequential and PCV15 sequential groups, ≥87.3% and ≥87.6% were included at one or more timepoints, respectively. In addition, ≥85.8% and ≥87.6% were included for both baseline and post-vaccination timepoints, respectively.
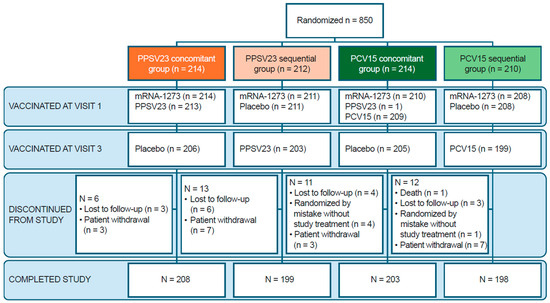
Figure 2.
CONSORT flow diagram. Each participant was counted once. Two participants received study interventions other than what they were randomized to receive. In the PPSV23 concomitant group, one participant received two doses of mRNA-1273 at the first study visit. In the PCV15 concomitant group, one participant received PPSV23 and mRNA-1273 at the first study visit. Enrollment was closed on 31 August 2022, following EUA of the bivalent COVID-19 vaccine boosters and revocation of the EUA for monovalent COVID-19 vaccine boosters. At the time enrollment was closed, 850 participants were enrolled. As of the final database lock, 850 participants were randomized. COVID-19: coronavirus disease 2019; EUA: emergency use authorization; mRNA-1273: monovalent mRNA COVID-19 vaccine; PCV15: 15-valent pneumococcal conjugate vaccine; PPSV23: 23-valent pneumococcal polysaccharide vaccine.
Baseline demographic characteristics were comparable across all groups (Table 1 and Table S4). The mean age of participants was 60.4 years, with 73.4% of participants being in the 50–64-years-old age category; 56.9% were female, 77.9% were white, and 43.2% were of Hispanic or Latino ethnicity. Participants across all four groups generally had comparable pre-existing medical conditions (Table S5). No participants had a prior history of infection with SARS-CoV-2.

Table 1.
Baseline characteristics and demographics.
3.2. Immunogenicity
Serotype-specific OPA GMTs at 30 days post-vaccination were generally comparable between the PPSV23 concomitant and sequential groups and PCV15 concomitant and sequential groups (Table 2). A trend was observed in which the serotype-specific OPA GMTs for some serotypes were numerically lower in the PPSV23 and PCV15 concomitant groups compared with the PPSV23 or PCV15 sequential groups, respectively. The distribution of serotype-specific OPA titers was generally comparable between all groups, as demonstrated by the reverse cumulative distribution curves (Figures S1 and S2).

Table 2.
Primary immunogenicity endpoint: serotype-specific OPA responses at 30 days post-vaccination.
The observed serotype-specific GMFRs and the proportions of participants with a ≥4-fold rise in GMFRs from baseline to 30 days post-vaccination with PPSV23 and PCV15 were generally comparable for most serotypes in the PPSV23 and PCV15 concomitant groups compared with the PPSV23 and PCV15 sequential groups (Tables S6 and S7).
SARS-CoV-2-specific binding antibody GMTs at 30 days post-vaccination with mRNA-1273 were numerically lower in the PPSV23 and PCV15 concomitant groups compared with the PPSV23 and PCV15 sequential groups (Table 3). The distribution of SARS-CoV-2-specific binding antibody titers was similar between the PPSV23 sequential and concomitant groups, as well as the PCV15 sequential and concomitant groups, as shown by reverse cumulative distribution curves (Figure S3).

Table 3.
Primary immunogenicity endpoint: summary of SARS-CoV-2-specific binding antibody GMTs responses with mRNA-1273.
The observed SARS-CoV-2 binding antibody titer GMFRs and the proportions of participants with a ≥4-fold rise in GMFRs from baseline to 30 days post-vaccination with mRNA-1273 were generally comparable in the PPSV23 and PCV15 concomitant groups compared with the PPSV23 and PCV15 sequential groups (Tables S6 and S7).
3.3. Safety
The majority of participants in the PPSV23 groups (81.4%) and PCV15 groups (81.1%) reported at least one AE following any vaccination (Table 4). The proportions of participants with AEs, including injection-site AEs, systemic AEs, vaccine-related AEs, and SAEs, were generally comparable across all groups. Post-vaccination, the most common AEs in the PPSV23 and PCV15 groups were solicited AEs (≥79% and ≥78%, respectively; Figure 3), which were similar in frequency in all groups, and most events were classified as mild (Grade 1) or moderate (Grade 2) in intensity. The incidence of severe solicited AEs (Grade 3) was low in the PPSV23 and PCV15 groups (≤4.3% and ≤5.8%, respectively). Injection-site pain was the most frequently reported solicited AE in the PPSV23 and PCV15 groups (74.6% and 73.9%, respectively; Table S8).

Table 4.
Safety summary.
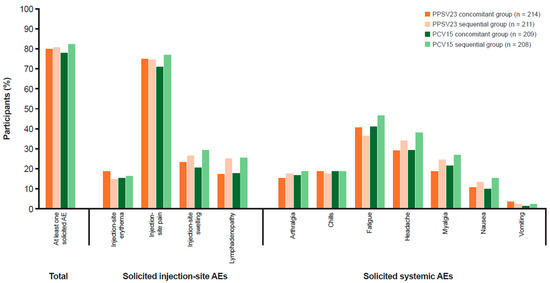
Figure 3.
Participants with solicited AEs (incidence >0% in one or more group) in the all-participants-as-treated population following any vaccination. Every participant was counted a single time for each AE. Injection-site erythema, injection-site pain, injection-site swelling, lymphadenopathy, arthralgia, fatigue, headache, myalgia, nausea, vomiting, and chills were solicited from Day 1 to Day 7 following vaccination. MedDRA version 26.0 was used. AE: adverse event; MedDRA: Medical Dictionary for Regulatory Activities; PCV15: 15-valent pneumococcal conjugate vaccine; PPSV23: 23-valent pneumococcal polysaccharide vaccine.
The proportions of participants with systemic AEs were generally comparable between the PPSV23 concomitant (57.9%) and sequential (54.2%) groups, and between the PCV15 concomitant (53.1%) and sequential (54.2%) groups. The proportions of participants with vaccine-related systemic AEs were similar between the PPSV23 concomitant (55.6%) and sequential (51.2%) groups, and between the PCV15 concomitant (53.1%) and sequential (59.6%) groups, following any vaccination. The incidence of SAEs was low in both the PPSV23 (<1%) and PCV15 (<3%) groups, and none were considered by the investigator to be related to study vaccine.
One participant (0.2%) discontinued from the PPSV23 concomitant group due to a non-serious AE of injection-site induration, which was considered by the investigator to be vaccine-related. Two (0.5%) participants (both from the PCV15 sequential group) discontinued due to SAEs, which were not considered by the investigator to be vaccine-related; one participant had an SAE of pancreatic carcinoma and the other had an SAE, which was also classified as an AESI, of cardiac arrest on Day 8, resulting in death. There were no AESIs or deaths due to AEs in the PPSV23 groups. The frequency of solicited maximum body temperature measurements was similar between the PPSV23 groups and the PCV15 groups, with <100.4 °F (38.0 °C) being reported in >94% of participants in each group.
4. Discussion
The results from this study support the co-administration of mRNA-1273 with either PPSV23 or PCV15. Serotype-specific OPA GMTs at 30 days post-vaccination were generally comparable when PPSV23 or PCV15 were administered concomitantly or sequentially with mRNA-1273. Concomitant administration of mRNA-1273 with either PPSV23 or PCV15 could alter antigen uptake and the function of antigen-presenting cells, potentially influencing the immunogenicity of one or both vaccines compared with administering mRNA-1273 alone [37,38]. The SARS-CoV-2 antibody responses were lower when mRNA-1273 was administered concomitantly with PPSV23 or PCV15, compared with mRNA-1273 + placebo. However, this difference is unlikely to be clinically meaningful, given the robust GMTs observed overall. Furthermore, reverse cumulative distribution curves for SARS-CoV-2 binding antibody titers, binding antibody GMFRs, and the proportions of participants with ≥4-fold rises in binding antibody GMTs from baseline to 30 days post-vaccination with mRNA-1273 were generally comparable between the groups.
The safety results from this study showed that co-administration of PPSV23 or PCV15 with mRNA-1273 was well tolerated, and the safety profiles of concomitant administration were consistent with those of the individual vaccines, as observed in other studies [39,40,41,42,43]. The overall proportions of participants with AEs were generally comparable between the groups.
Further evidence to support the feasibility and potential benefits of co-administration of a pneumococcal vaccine with a COVID-19 vaccine was demonstrated in a Phase 3 study [44]. The safety and immunogenicity profiles of concomitant administration of 20-valent pneumococcal conjugate vaccine (Prevnar20®, Pfizer Inc., New York, NY, USA) and BNT162b2 (Comirnaty®; Pfizer-BioNTech) were comparable to those observed when each vaccine was administered alone [44]. The co-administration of pneumococcal and COVID-19 vaccines could be a strategy to increase vaccination rates by maximizing opportunities to vaccinate populations at-risk and by helping to increase the likelihood that individuals are fully vaccinated at the appropriate age, ultimately having a positive impact on public health [26,29,30]. Several studies have assessed the co-administration of COVID-19 vaccines and influenza vaccines [25,29,45,46]. The studies reported no major safety issues associated with co-administration, and AEs were typically of mild or moderate severity and were self-limiting. Co-administration did not significantly affect the immunogenicity of either the influenza or COVID-19 vaccines [25,29,45,46]. A systematic literature review reported that, in studies that assessed the acceptability of co-administration, the majority of individuals expressed willingness to receive both vaccines [29]. This growing body of evidence supports the benefits of administering COVID-19 vaccines alongside other vaccines. Therefore, there is a strong rationale for raising awareness about the safety of vaccine co-administration, which could enhance compliance with vaccine recommendations [27]. Furthermore, concomitant administration of vaccines ensures that individuals facing challenges in accessing care receive full protection [27,29]. Co-administration decreases the number of consultations and avoids scheduling multiple vaccination appointments that can often be missed and lead to higher rates of attrition [28,29].
The limitations of the study included that it was descriptive in nature and not based on formal statistical hypothesis testing, as enrollment closed early [34,35]. Although formal statistical comparisons were not made, serotype-specific OPA GMTs and SARS-CoV-2-specific GMTs for concomitant and sequential groups demonstrated generally comparable immunogenicity, with overlapping 95% CIs for most serotypes tested, suggesting limited changes in immunogenicity when administered concomitantly. While it is not expected that any numerical differences observed between groups are clinically meaningful, real-world evidence is required to confirm. As this study evaluated the original mRNA-1273 formulation, it is important to note the findings may not fully apply to the updated mRNA-1273 formulations or other pneumococcal vaccines not tested. However, clinical trial and safety surveillance data support the concomitant administration of pneumococcal vaccines with these updated formulations, showing comparable safety and immunologic profiles to the original mRNA-1273 vaccine [47,48,49]. Another limitation was that the study assessed individuals who previously received the mRNA-1273 vaccine, while, in practice, many individuals receive a mix of SARS-CoV-2 vaccines from different manufacturers. These factors restrict the generalizability of the findings to broader vaccine strategies [30,50].
5. Conclusions
This study provides evidence that the co-administration of mRNA-1273 with PPSV23 or PCV15 in healthy adults ≥50 years of age is immunogenic, with a safety profile comparable to administering mRNA-1273 with a placebo. These results add to the growing evidence that COVID-19 vaccines have a good safety profile and are effective when administered concomitantly with other vaccines [44]. These findings may inform future vaccination recommendations or vaccination guidelines, enhance vaccine program implementation, and potentially help to increase coverage rates for other licensed vaccines.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines13020192/s1, Table S1: Investigators. The following investigators randomized at least one participant in the clinical study; Table S2: Eligibility criteria; Table S3: Definition of adverse events of special interest; Table S4: Baseline characteristics and demographics by strata; Table S5: Pre-existing medical conditions occurring in ≥5% of participants (any group); Table S6: Secondary immunogenicity endpoint: Serotype-specific GMFRs and proportions of participants with ≥4-fold rise for OPA responses at 30 days post-vaccination with PPSV23; Table S7: Secondary immunogenicity endpoints: GMFRs and proportions of participants with ≥4-fold rise for OPA antibody responses at 30 days post-vaccination with PCV15; Table S8: Most frequently reported AEs (≥5% incidence in any group); Figure S1: Reverse cumulative distribution curve of OPA titers at baseline and 30 days post-vaccination in the PPSV23 groups; Figure S2: Reverse cumulative distribution curve of OPA titers at baseline and 30 days post-vaccination in the PCV15 groups; Figure S3: Reverse cumulative distribution curve of SARS-CoV-2-specific binding titers at baseline and 30 days post-vaccination with mRNA-1273.
Author Contributions
T.O., S.C., Z.E., G.T., T.M.S., T.S., N.B. and U.K.B. were responsible for the conception, design, and planning of the study. E.P., T.S. and M.J. were responsible for the acquisition of data. M.J., N.B. and A.E.-J. were responsible for analysis of data. A.S.W., L.G., T.S., M.J., N.B., U.K.B. and A.E.-J. were responsible for interpretation of results. T.S., M.J., N.B., U.K.B. and A.E.-J. were responsible for original draft preparation. E.P., A.S.W., S.C., Z.E., G.T., T.M.S., L.G., T.S., M.J., N.B., U.K.B. and A.E.-J. were responsible for critically reviewing and revising the manuscript for important intellectual content. A.E.-J. was responsible for preparation and creation of the published work, specifically visualization/data presentation. T.O., A.S.W., G.T., T.M.S., T.S., U.K.B. and A.E.-J. were responsible for overseeing research activity planning and execution. T.O. and T.S. were responsible for project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Institutional Review Board Statement
This study (NCT05158140) was conducted in accordance with principles of Good Clinical Practice guidelines and was approved by the appropriate institutional review boards and regulatory agencies. The approach of Merck & Co., Inc., Rahway, NJ, USA to the conduct of clinical trials is in accordance with the ethical principles that have their origin in the Declaration of Helsinki and is consistent with Good Clinical Practice and applicable regulatory requirement(s).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data sharing policy, including restrictions, of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), is available at https://trialstransparency.msdclinicaltrials.com/policies-perspectives.aspx (accessed on 13 February 2025). Requests for access to the clinical study data can be submitted via email to the Data Access mailbox (mailto:dataaccess@msd.com).
Acknowledgments
The authors would like to thank the participants, their families, and all investigators involved in this study. Medical writing support, including assisting authors with the development of the outline and initial draft and incorporation of comments, was provided by Sara Shaw, CMPP, and Lauren Moreton, MRes, and editorial support, including fact checking, referencing, figure preparation, formatting, proofreading, and submission, was provided by Ian Norton, all of Scion, a Division of Prime, London, UK, supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M22-1460; accessed on 13 February 2025).
Conflicts of Interest
T.O., G.T., T.M.S., L.G., T.S., M.J., N.B., U.K.B. and A.E.-J. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. E.P. reports serving as a principal investigator for this study. A.S.W. may own shares or stock options in Carbon Health, North Hollywood, CA, USA. S.C. and Z.E. are employees of Moderna, Inc. and may own stock/stock options in the company. Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
References
- Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Report: Streptococcus pneumoniae. 2019. Available online: https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2019.pdf (accessed on 26 November 2024).
- Grant, L.R.; Meche, A.; McGrath, L.; Miles, A.; Alfred, T.; Yan, Q.; Chilson, E. Risk of pneumococcal disease in US adults by age and risk profile. Open Forum Infect. Dis. 2023, 10, ofad192. [Google Scholar] [CrossRef]
- Kobayashi, M.; Leidner, A.J.; Gierke, R.; Farrar, J.L.; Morgan, R.L.; Campos-Outcalt, D.; Schechter, R.; Poehling, K.A.; Long, S.S.; Loehr, J.; et al. Use of 21-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Recommendations of the Advisory Committee on Immunization Practices—United States, 2024; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2024; pp. 793–798. [Google Scholar]
- Feldman, C.; Anderson, R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia 2021, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L. Respiratory co-and superinfections in COVID-19. Rev. Esp. Quimioter. 2021, 34 (Suppl. 1), 69–71. [Google Scholar] [CrossRef] [PubMed]
- Amin-Chowdhury, Z.; Aiano, F.; Mensah, A.; Sheppard, C.L.; Litt, D.; Fry, N.K.; Andrews, N.; Ramsay, M.E.; Ladhani, S.N. Impact of the Coronavirus Disease 2019 (COVID-19) Pandemic on Invasive Pneumococcal Disease and Risk of Pneumococcal Coinfection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Prospective National Cohort Study, England. Clin. Infect. Dis. 2021, 72, e65–e75. [Google Scholar] [CrossRef]
- Sultana, J.; Mazzaglia, G.; Luxi, N.; Cancellieri, A.; Capuano, A.; Ferrajolo, C.; de Waure, C.; Ferlazzo, G.; Trifirò, G. Potential effects of vaccinations on the prevention of COVID-19: Rationale, clinical evidence, risks, and public health considerations. Expert Rev. Vaccines 2020, 19, 919–936. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Ser, J.; Sim, U.; Cho, H. Promising expectations for pneumococcal vaccination during COVID-19. Vaccines 2021, 9, 1507. [Google Scholar] [CrossRef] [PubMed]
- Newell, K.; Fischer, M.; Massey, S.; Orell, L.; Steinberg, J.; Tompkins, M.; Castrodale, L.; McLaughlin, J. Temporally Associated Invasive Pneumococcal Disease and SARS-CoV-2 Infection, Alaska, USA, 2020–2021. Emerg. Infect. Dis. 2023, 29, 1765–1771. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Pneumococcal Vaccine Recommendations. Available online: https://www.cdc.gov/pneumococcal/hcp/vaccine-recommendations/index.html (accessed on 23 January 2025).
- Kobayashi, M.; Leidner, A.; Gierke, R.; Xing, W.; Accorsi, E.; Moro, P.; Kamboj, M.; Kuchel, G.; Schechter, R.; Loehr, J.; et al. Expanded Recommendations for Use of Pneumococcal Conjugate Vaccines Among Adults Aged ≥50 Years: Recommendations of the Advisory Committee on Immunization Practices—United States, 2024. MMWR 2025, 74, 1–8. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention (CDC). Types of Pneumococcal Vaccines. Available online: https://www.cdc.gov/pneumococcal/vaccines/types.html (accessed on 26 November 2024).
- Food and Drug Administration. PNEUMOVAX 23 Prescribing Information. Available online: https://www.fda.gov/media/80547/download (accessed on 4 December 2024).
- Tereziu, S.; Minter, D.A. Pneumococcal Vaccine; [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Food and Drug Administration. VAXNEUVANCE™ (Pneumococcal 15-Valent Conjugate Vaccine) Prescribing Information. Available online: https://www.fda.gov/media/150819/download (accessed on 26 November 2024).
- World Health Organization. 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly. Epidemiol. Rec. 2008, 83, 373–384. [Google Scholar]
- Centers for Disease Control and Prevention. Chapter 17: Pneumococcal Disease. In The Pink Book: Epidemiology and Prevention of Vaccine-Preventable Diseases, 14th ed.; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2015. Available online: https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-17-pneumococcal-disease.html (accessed on 26 November 2024).
- Centers for Disease Control and Prevention. Pneumococcal Polysaccharide Vaccine Information Statement. Available online: https://www.cdc.gov/vaccines/hcp/current-vis/pneumococcal-polysaccharide.html (accessed on 26 November 2024).
- Centers for Disease Control and Prevention (CDC). Meeting of the Advisory Committee on Immunization Practices (ACIP). Available online: https://www.cdc.gov/faca/committees/acip.html (accessed on 26 November 2024).
- Center for Disease Control and Prevention (CDC). Summary of Risk-Based Pneumococcal Vaccination Recommendations. Available online: https://www.cdc.gov/pneumococcal/hcp/vaccine-recommendations/risk-indications.html (accessed on 26 November 2024).
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Emergency Use Authorization (EUA) Amendment for an Unapproved Product Review Memorandum: EUA 27073. Available online: https://www.fda.gov/media/153912/download (accessed on 26 November 2024).
- Food and Drug Administration. SPIKEVAX Package Insert. Available online: https://www.fda.gov/media/155675/download?attachment (accessed on 26 November 2024).
- Center for Disease Control and Prevention (CDC). Interim Clinical Considerations for Use of COVID-19 Vaccines in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html (accessed on 26 November 2024).
- Toback, S.; Galiza, E.; Cosgrove, C.; Galloway, J.; Goodman, A.L.; Swift, P.A.; Rajaram, S.; Graves-Jones, A.; Edelman, J.; Burns, F.; et al. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: An exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2022, 10, 167–179. [Google Scholar] [CrossRef]
- Center for Disease Control Prevention (CDC). Vaccine Recommendations Guidelines of the A.C.I.P. Timing and Spacing of Immunobiologics. Available online: https://www.cdc.gov/vaccines/hcp/imz-best-practices/timing-spacing-immunobiologics.html (accessed on 26 November 2024).
- Bonanni, P.; Steffen, R.; Schelling, J.; Balaisyte-Jazone, L.; Posiuniene, I.; Zatoński, M.; Van Damme, P. Vaccine co-administration in adults: An effective way to improve vaccination coverage. Hum. Vaccines Immunother. 2023, 19, 2195786. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.A.; Chachlani, P.; Hayes, K.N.; McCarthy, E.P.; Wen, K.J.; Deng, Y.; Zullo, A.R.; Djibo, D.A.; McMahill-Walraven, C.N.; Smith-Ray, R.L.; et al. COVID-19 and influenza vaccine coadministration among older U.S. adults. Am. J. Prev. Med. 2024, 67, 67–78. [Google Scholar] [CrossRef]
- Janssen, C.; Mosnier, A.; Gavazzi, G.; Combadière, B.; Crépey, P.; Gaillat, J.; Launay, O.; Botelho-Nevers, E. Coadministration of seasonal influenza and COVID-19 vaccines: A systematic review of clinical studies. Hum. Vaccines Immunother. 2022, 18, 2131166. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Huang, L.; Zhang, Y.; Yu, N.; Xu, X.; Liang, Y.; Ni, J. Effectiveness and safety of dual influenza and pneumococcal vaccination versus separate administration or no vaccination in older adults: A meta-analysis. Expert Rev. Vaccines 2018, 17, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Lewnard, J.A.; Bruxvoort, K.J.; Fischer, H.; Hong, V.X.; Grant, L.R.; Jodar, L.; Gessner, B.D.; Tartof, S.Y. Prevention of coronavirus disease 2019 among older adults receiving pneumococcal conjugate vaccine suggests interactions between Streptococcus pneumoniae and severe acute respiratory syndrome coronavirus 2 in the respiratory tract. J. Infect. Dis. 2022, 225, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.R., Jr. The International Conference on Harmonization Good Clinical Practice guideline. Qual. Assur. 1998, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Safety, Tolerability, and Immunogenicity of V110 or V114 Co-Administered with a Booster Dose of mRNA-1273 in Healthy Adults (V110-911) (NCT05158140). Available online: https://clinicaltrials.gov/study/NCT05158140?term=V110-911&rank=1 (accessed on 23 January 2024).
- Pfizer. Pfizer and BioNTech Granted FDA Emergency Use Authorization of Omicron BA.4/BA.5-Adapted Bivalent COVID-19 Vaccine Booster for Ages 12 Years and Older. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-granted-fda-emergency-use-authorization (accessed on 26 November 2024).
- Rosenblum, H.G.; Wallace, M.; Godfrey, M.; Roper, L.E.; Hall, E.; Fleming-Dutra, K.E.; Link-Gelles, R.; Pilishvili, T.; Williams, J.; Moulia, D.L.; et al. Interim recommendations from the advisory committee on immunization practices for the use of bivalent booster doses of COVID-19 vaccines—United States, October 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Clopper, C.J.; Pearson, E.S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934, 26, 404–413. [Google Scholar] [CrossRef]
- Pattinson, D.; Jester, P.; Gu, C.; Guan, L.; Armbrust, T.; Petrie, J.G.; King, J.P.; Nguyen, H.Q.; Belongia, E.A.; Halfmann, P.; et al. Ipsilateral and contralateral coadministration of influenza and COVID-19 vaccines produce similar antibody responses. EBioMedicine 2024, 103, 105103. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, M.U. Does co-administration of vaccines interfere with immune responses? The jury is still out. Clin. Microbiol. Infect. 2023, 29, 1482–1484. [Google Scholar] [CrossRef] [PubMed]
- Stacey, H.L.; Rosen, J.; Peterson, J.T.; Williams-Diaz, A.; Gakhar, V.; Sterling, T.M.; Acosta, C.J.; Nolan, K.M.; Li, J.; Pedley, A.; et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV-15) compared to PCV-13 in healthy older adults. Hum. Vaccines Immunother. 2019, 15, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Chang, C.J.; Andrews, C.; Diez-Domingo, J.; Oh, M.D.; Dagan, R.; Hartzel, J.; Pedley, A.; Li, J.; Sterling, T.; et al. Safety, tolerability, and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, followed by sequential PPSV23 vaccination in healthy adults aged ≥50years: A randomized phase III trial (PNEU-PATH). Vaccine 2021, 39, 6422–6436. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, U.K.; Andrews, C.P.; Ervin, J.; Peterson, J.T.; Tamms, G.M.; Krupa, D.; Ajiboye, P.; Roalfe, L.; Krick, A.L.; Sterling, T.M.; et al. Sequential administration of Prevnar 13 and PNEUMOVAX 23 in healthy participants 50 years of age and older. Hum. Vaccines Immunother. 2021, 17, 2678–2690. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Vrbicky, K.; Montefiori, D.; Huang, W.; Nestorova, B.; Chang, Y.; Carfi, A.; Edwards, D.K.; Oestreicher, J.; Legault, H.; et al. Immune response to SARS-CoV-2 after a booster of mRNA-1273: An open-label phase 2 trial. Nat. Med. 2022, 28, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Follmann, D.; Hachigian, G.; Strout, C.; Overcash, J.S.; Doblecki-Lewis, S.; Whitaker, J.A.; Anderson, E.J.; et al. Long-term safety and effectiveness of mRNA-1273 vaccine in adults: COVE trial open-label and booster phases. Nat. Commun. 2024, 15, 7469. [Google Scholar] [CrossRef] [PubMed]
- Fitz-Patrick, D.; Young, M.; Yacisin, K.; McElwee, K.; Belanger, T.; Belanger, K.; Peng, Y.; Lee, D.Y.; Gruber, W.C.; Scott, D.A.; et al. Randomized trial to evaluate the safety, tolerability, and immunogenicity of a booster (third dose) of BNT162b2 COVID-19 vaccine coadministered with 20-valent pneumococcal conjugate vaccine in adults ≥65 years old. Vaccine 2023, 41, 4190–4198. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, R.; Baos, S.; Cappel-Porter, H.; Carson-Stevens, A.; Clout, M.; Culliford, L.; Emmett, S.R.; Garstang, J.; Gbadamoshi, L.; Hallis, B.; et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): A multicentre, randomised, controlled, phase 4 trial. Lancet 2021, 398, 2277–2287. [Google Scholar] [CrossRef]
- Izikson, R.; Brune, D.; Bolduc, J.S.; Bourron, P.; Fournier, M.; Moore, T.M.; Pandey, A.; Perez, L.; Sater, N.; Shrestha, A.; et al. Safety and immunogenicity of a high-dose quadrivalent influenza vaccine administered concomitantly with a third dose of the mRNA-1273 SARS-CoV-2 vaccine in adults aged ≥65 years: A phase 2, randomised, open-label study. Lancet Respir. Med. 2022, 10, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; Chang, Y.; et al. A bivalent Omicron-containing booster vaccine against Covid-19. N. Engl. J. Med. 2022, 387, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, S.; Whatley, J.L.; Eder, F.; Essink, B.; Khetan, S.; Bradley, P.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; et al. Original SARS-CoV-2 monovalent and Omicron BA.4/BA.5 bivalent COVID-19 mRNA vaccines: Phase 2/3 trial interim results. Nat. Med. 2023, 29, 2325–2333. [Google Scholar] [CrossRef]
- Chalkias, S.; McGhee, N.; Whatley, J.L.; Essink, B.; Brosz, A.; Tomassini, J.E.; Girard, B.; Edwards, D.K.; Wu, K.; Nasir, A.; et al. Interim report of the reactogenicity and immunogenicity of severe acute respiratory syndrome coronavirus 2 XBB-containing vaccines. J. Infect. Dis. 2024, 230, e279–e286. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention (CDC). Vaccine Recommendations and Guidelines of the ACIP—Special Situations. Available online: https://www.cdc.gov/vaccines/hcp/imz-best-practices/special-situations.html (accessed on 26 November 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).