Abstract
Influenza viruses remain a major global public health concern, causing significant morbidity and mortality annually despite widespread vaccination efforts. The limitations of current seasonal vaccines, including strain-specific efficacy and manufacturing delays, have accelerated the development of next-generation candidates aiming for universal protection. This review comprehensively summarizes the recent progress in universal influenza vaccine research. We first outline the key conserved antigenic targets, such as the hemagglutinin (HA) stem, neuraminidase (NA), and matrix proteins (M2e, NP, and M1), which are crucial for eliciting broad cross-reactive immunity. We then delve into advanced antigen design strategies, including immunofocusing, multi-antigen combinations, computationally optimized broadly reactive antigens (COBRA), and nanoparticle-based platforms. Furthermore, we evaluate evolving vaccine delivery systems, from traditional inactivated and live-attenuated vaccines to modern mRNA and viral vector platforms, alongside the critical role of novel adjuvants in enhancing immune responses. The convergence of these disciplines—structural biology, computational design, and nanotechnology—is driving the field toward a transformative goal. We conclude that the successful development of a universal influenza vaccine will likely depend on the strategic integration of these innovative approaches to overcome existing immunological and logistical challenges, ultimately providing durable and broad-spectrum protection against diverse influenza virus strains.
1. Introduction
Influenza, an acute respiratory infection caused by influenza viruses, remains a persistent global public health threat due to its ability to cause seasonal epidemics and occasional pandemics []. Characterized by high morbidity and transmissibility, the disease spreads primarily through respiratory droplets from infected individuals, as well as via contact with contaminated surfaces []. Seasonal influenza outbreaks result in an estimated 1 billion cases each year worldwide, including 3 to 5 million severe cases and 290,000 to 650,000 respiratory-related deaths [,]. The burden of influenza mortality varies by region and age group: in industrialized nations, most deaths occur among those aged 65 and older [], whereas 99% of deaths in children under 5 years of age with influenza-related lower respiratory tract infections are found in developing countries []. Beyond its health impact, influenza contributes to significant socioeconomic losses through healthcare spending, worker absenteeism and long-term human-capital loss.
Vaccination represents the most effective strategy for influenza prevention. Seasonal influenza vaccines are designed to protect against the virus strains predicted to circulate in a given season []. For the 2024–2025 influenza season in the United States, trivalent formulations—including inactivated (inactivated influenza vaccine, Trivalent, IIV3), recombinant (recombinant influenza vaccine, Trivalent, RIV3), and live-attenuated (live-attenuated influenza vaccine, Trivalent, LAIV3) vaccines—are expected to be available []. Vaccine composition is updated annually based on global surveillance data and may include strains from the H1N1, H3N2, and B/Victoria lineages, depending on the manufacturing method [].
Despite their widespread use, current influenza vaccines face several limitations. Most seasonal and pandemic influenza vaccines are still produced using egg-based systems, which account for the majority of the inactivated influenza vaccine supply []. This production method has notable drawbacks, including vulnerability to supply chain disruptions due to dependence on embryonated eggs, and the potential for vaccine strain mutations during egg adaptation, which can impair effectiveness []. Additionally, due to antigenic drift in circulating viruses, vaccine efficacy can vary widely, ranging from 20% to 60% in some seasons [].
In response to these challenges, efforts are intensifying to develop next-generation influenza vaccines. mRNA-based platforms represent a highly promising alternative []. These vaccines use lipid nanoparticle-encapsulated mRNA encoding specific viral antigens to elicit robust humoral and cellular immune responses []. Another innovative strategy involves proteolysis-targeting (PROTAR) vaccines, which leverage the host ubiquitin–proteasome system to conditionally regulate viral protein stability and degradation []. Clinical studies indicate favorable safety, immunogenicity, and cross-protective potential for such candidates [].
In summary, despite ongoing vaccination efforts, there remains a critical need for more effective, efficient, and adaptable influenza vaccines. A thorough understanding of influenza virology and epidemiology, combined with insights into current vaccine strengths and limitations, is essential to guide the development of next-generation vaccines capable of providing broader and more durable protection for global public health.
2. Antigenic Targets of Universal Influenza Vaccines
Influenza viruses belong to the Orthomy xoviridae family and possess a genome consisting of eight segments of single-stranded negative-sense RNA, encoding at least 11 viral proteins. Based on antigenic differences in nucleoprotein (NP) and matrix protein (M), influenza viruses are classified into four types: A, B, C, and D [] (Figure 1). Among these, influenza A and B viruses are the main causative agents of seasonal influenza in humans. Influenza A viruses are further subdivided into 18 hemagglutinin (HA) and 11 neuraminidase (NA) subtypes according to their surface glycoproteins []. Subtypes H1–H3 and N1–N2 represent the predominant seasonal strains, while avian-origin subtypes such as H5, H7, and H9 have repeatedly crossed species barriers to infect humans [], often causing severe disease with high mortality and posing ongoing threats to global public health.
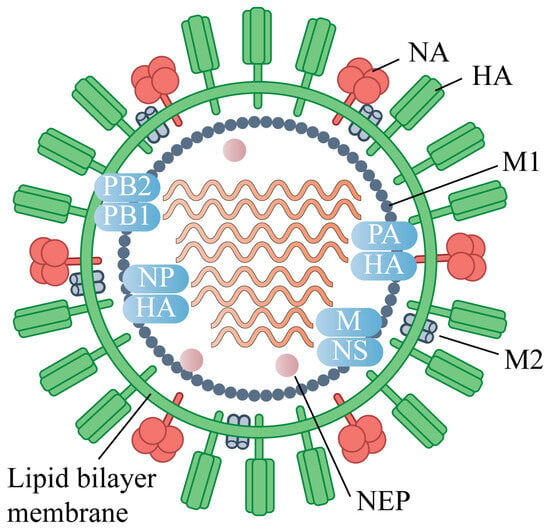
Figure 1.
Virion Structure of the Influenza A virus (IAV).The IAV virion consists of a lipid envelope that contains the glycoproteins hemagglutinin (HA), neuraminidase (NA), and the matrix protein 2 (M2) ion channel. Below the envelope is a layer of matrix protein 1 (M1) and nuclear export protein (NEP). The IAV genome is composed of eight segmented vRNAs: polymerase basic protein 2 (PB2), polymerase basic protein 1 (PB1), polymerase acidic protein (PA), HA, nucleoprotein (NP), NA, M, and non-structural protein (NS).
HA, NA, NP, M1, and M2 represent the principal antigenic targets under investigation for the development of universal influenza vaccines. The HA protein facilitates viral entry by binding to sialic acid receptors and mediating membrane fusion within endosomes []. Structurally, HA forms a homotrimer, with each monomer comprising a globular head domain and a stem region. The globular head is immunodominant and the primary target of strain-specific neutralizing antibodies. However, due to antigenic drift, such antibodies often exhibit limited cross-reactivity [,]. In contrast, the stem region of HA is highly conserved and harbors multiple B- and T-cell epitopes []. Antibodies targeting the HA stem have demonstrated broad neutralizing activity across influenza subtypes [,], highlighting its promise as a universal vaccine candidate. For instance, an equine H3N8 HA-based immunogen recently elicited antibodies reactive against multiple human group 1 and 2 viruses [], underscoring the potential for cross-species protection. Historically, stem-directed antibody responses have been difficult to elicit with conventional vaccine platforms, often requiring stabilized trimeric immunogens and repeated immunizations to achieve robustness []. Overcoming this immune subdominance remains a central challenge in stem-based universal vaccine design. Novel strategies like mRNA delivery are challenging this paradigm.
NA is a tetrameric type II transmembrane glycoprotein that cleaves sialic acid residues from mucins, facilitating viral mobility, release of progeny virions, and prevention of self-aggregation []. Antibodies against NA can confer protection by inhibiting viral release or mediating antibody-dependent cellular cytotoxicity (ADCC). The enzymatic active site of NA (residues 222–230) is highly conserved across influenza A and B viruses [], and anti-NA antibodies have demonstrated both homotypic and heterosubtypic protection [,,]. However, NA is generally less immunogenic than HA and lacks immunodominance in conventional vaccines []. Notably, when mice were vaccinated twice at a 3-week interval intranasally or intramuscularly, mucosal NA vaccination has been shown to induce superior protection compared to intramuscular administration, maybe due to mucosal antibodies including secretory IgA []. Furthermore, broad-inhibition NA-targeting memory B cells are present in healthy adults, representing a plausible target for next-generation vaccines []. Recent advances include the AI-assisted design of genetic algorithm-based mosaic NA antigens aimed at broadening immune coverage.
The M2 protein is a tetrameric proton channel embedded in the viral envelope that facilitates viral uncoating by acidifying the virion interior [,]. Its N-terminal extracellular domain (M2e) is highly conserved—comprising only 23 amino acids—and is unlikely to accumulate escape mutations [,]. Although M2e is poorly immunogenic during natural infection or conventional vaccination, M2e-specific antibodies can mediate ADCC or complement-dependent cytolysis (CDC) of infected cells [,]. As a short peptide, M2e alone suffers from poor stability and weak immunogenicity [], necessitating fusion to carrier platforms or incorporation into multivalent nanoparticle vaccines to enhance immunogenicity. For example, a tandem antigen nanoparticle displaying three M2e domains from influenza A (human and avian/swine strains) and three from influenza B conferred 100% protection against lethal H1N1 challenge and 70% protection against influenza B in mice []. Such carrier-fusion strategies represent a viable path for leveraging M2e in universal vaccine design [,].
NP and M1 are major internal structural proteins of the influenza virus: M1 underpins viral assembly, while NP binds viral RNA to form ribonucleoprotein complexes essential for replication []. Both are relatively conserved and contain epitopes recognized by cross-reactive cytotoxic T lymphocytes (CTLs) [,,]. Although CTL responses can reduce disease severity and mortality, they do not prevent infection and often require synergistic humoral immunity for full protection []. Therefore, NP and M1 are often incorporated into multi-antigen vaccine regimens. In a Phase 2a controlled trial involving healthy adults aged 18–55, a candidate vaccine displaying seven copies of NP induced robust humoral and cellular immunity and showed preliminary efficacy (VE = 84%) against influenza following a single intramuscular dose [], supporting the potential of internal proteins in universal vaccine strategies.
3. Antigen Design Strategies for Universal Influenza Vaccines
The pursuit of a universal influenza vaccine centers on targeting conserved viral regions to elicit broad and durable protection. Key design strategies currently under investigation include immunofocusing, multi-antigen combinations, computationally optimized broadly reactive antigens (COBRA), and nanoparticle-based antigen presentation.
3.1. Immunofocusing Strategy
Immunofocusing aims to direct immune responses toward conserved, functionally critical epitopes while minimizing distraction by variable immunodominant regions. A prominent example involves the use of chimeric hemagglutinin (HA) constructs, where animals immunized with HAs bearing the same conserved stalk domain but different, irrelevant head domains develop potent stalk-directed antibody responses []. Another approach leveraged an avian H5N1 HA backbone by grafting a human influenza HA head domain, successfully inducing broadly cross-reactive antibodies targeting the HA stem in preclinical models []. Early concepts of head-domain removal to unmask conserved regions date back to the 1980s [], and recent advances include vaccine nanoparticles displaying tandem copies of an α-helical stem fragment, which conferred heterosubtypic protection in mice []. Alternatively, conformational masking of the HA head using aluminum adjuvant attachment has been shown to redirect antibody production toward the stem region, broadening reactivity across subtypes []. A central challenge in immunofocusing, however, lies in preserving the native conformation of target epitopes during antigen design—a particular hurdle for structurally complex proteins [].
3.2. Multi-Antigen Combination Strategy
Integrating multiple antigens into a single formulation represents a promising approach to broaden immune coverage. Covalent coupling of HAs from different strains has been shown to enhance breadth of response and reduce biases linked to “original antigenic sin” []. Structure-guided chimeric immunogens—such as grafting an H3 receptor-binding site onto an H1 HA scaffold—have elicited neutralizing antibodies against diverse strains []. Nucleic acid platforms also support this strategy; for example, an mRNA construct co-expressing HA and NA induced cross-reactive antibodies against heterologous viruses []. Fusions of conserved internal and exterior proteins, such as M2e and nucleoprotein, delivered with lipid nanoadjuvants, have provided protection against homologous and heterosubtypic challenges []. Multivalent epitope-based nanoparticles are under active investigation [,], including virus-like particles displaying M2e from human and avian influenza A viruses, which broke host restriction and conferred complete cross-protection in mice []. Despite these advances, multi-antigen vaccines face challenges such as imbalanced immunity toward specific epitopes, increased antigen load raising safety concerns, and difficulties in maintaining the native conformation of each antigen within a complex formulation.
3.3. COBRA Strategy
The COBRA approach leverages bioinformatics to design immunogens based on layered consensus sequences from viral databases, aiming to maximize coverage of past, present, and potential future strains. COBRAs are generated through iterative sequence alignment and consensus-building steps, yielding synthetic proteins with enhanced cross-reactive potential. A COBRA vaccine targeting the H1 HA head was first reported in 2016 and shown to elicit broad antibodies against drifted H1N1 strains []. Similarly, H3-focused COBRA HA immunogens outperformed wild-type HA vaccines in breadth and potency against historical and emerging variants [,], including in studies involving elderly populations []. Extension of COBRA to neuraminidase (NA) has yielded candidates that induce wider cross-reactive antibody responses than wild-type NA []. Adjuvants such as cyclic GMP-AMP (STING agonist), AddaVax, CpG, and Alhydrogel have been tested in combination with COBRA immunogens to further enhance immunogenicity [,]. A limitation of early COBRA designs is their dependence on pre-existing viral sequences, which may not anticipate novel reassortants. Next-generation COBRA strategies are now being developed to improve predictive coverage and responsiveness to emerging strains.
3.4. Nanoparticle
Nanoparticle-based vaccines enable high-density, ordered display of influenza antigens, improving immunogenicity and often incorporating self-adjuvanting properties. Their small size (20–200 nm) facilitates efficient drainage to lymph nodes, enhancing antigen presentation. For example, ferritin nanoparticles displaying conserved epitopes from the HA stem, NP, and M2e induced durable cross-reactive immunity lasting at least six months post-vaccination []. Similarly, Epigraph-designed H9 HA trimers conjugated to mi3 nanoparticles elicited broad antibody and T-cell responses, conferring superior protection against diverse H9N2 viruses []. A baculovirus-derived nanoparticle co-expressing HA, triple-repeat M2e, and an M-cell-targeting ligand provided comprehensive protection across multiple influenza A subtypes []. Other platforms, including self-adjuvanting PLGA, lipid-polymer hybrids, and hydrogel nanoparticles, have also been shown to induce broad and persistent immune responses [,,]. Despite their promise, nanoparticle vaccines face challenges in reproducible size control [], with evidence suggesting that larger particles may be more efficiently internalized by antigen-presenting cells and promote stronger T-cell activation []. Antigen valency and spatial arrangement are also critical; although high density can enhance B-cell activation, overly dense epitope presentation may sterically hinder receptor binding, necessitating careful optimization [].
Future universal influenza vaccine design will likely integrate the strengths of multiple platforms—combining rational epitope selection, structural vaccinology, computational forecasting, and advanced delivery systems. A streamlined, multi-target approach emphasizing conserved regions and balanced humoral and cellular immunity will be essential to achieve broad, durable, and effective protection against diverse influenza viruses.
4. Vaccine Delivery Platform
Vaccination is an important way to achieve herd immunity, which plays an important role in the control of COVID-19. For the influenza virus, making the immunogens available to establish herd immunity is also important. To achieve this goal, in addition to improving vaccination coverage, it is also essential to follow the principle of prioritizing the protection of high-risk populations. Therefore, the development of vaccines for populations including the elderly, children, pregnant women, and patients with chronic disease is crucial. Meanwhile, the tendency of the influenza virus to mutate also hinders the establishment of herd immunity. The platform should also meet the needs of multiple responses to mutant strains.
Conventional influenza vaccine platforms—including inactivated vaccines, live attenuated vaccines, and recombinant protein-based vaccines—have long served as the cornerstone of influenza prophylaxis, providing established safety and stability profiles. Influenza vaccines administered via the nasal route have demonstrated great potential in influenza prevention by inducing strong local and systemic immune responses [,,]. However, these traditional systems face challenges in rapidly addressing the continuous emergence of new viral strains.
Next-generation delivery platforms offer improved adaptability, manufacturing speed, and immunogenicity, positioning them as promising candidates for future influenza vaccines, especially in pandemic settings. Key emerging technologies include recombinant viral vector vaccines, conjugate vaccines, and nucleic acid-based vaccines. The application of nucleic acid-based vaccines, such as mRNA vaccines, during the SARS-CoV-2 pandemic has enriched the evidence of side effects associated with this kind of vaccine, including swelling at the injection site, fatigue, headache, muscle soreness, fever, etc. Serious side effects include myocarditis and allergic reactions. Therefore, the improvement should be made before their widespread application.
The traditional and new vaccines under development were summarized below (Table 1).
4.1. Traditional Vaccines Under Development
4.1.1. Inactivated Vaccines
Traditional inactivated influenza vaccines are produced by propagating the influenza virus in embryonated chicken eggs, followed by chemical inactivation. Although cost-effective, egg-based production is associated with egg-adaptive mutations that may reduce vaccine effectiveness, and reactogenicity such as fever has been reported. Advances in purification, adjuvantation, and the adoption of cell culture-based production systems have improved the safety and immunogenicity of these vaccines [].
Further refinements have led to split-virion and subunit vaccines, which are less reactogenic and offer improved safety profiles. Split vaccines are generated by detergent disruption of whole virions, whereas subunit vaccines are highly purified formulations containing mainly hemagglutinin (HA) and neuraminidase (NA) []. Although the standard production timeline for seasonal influenza vaccines remains around six months, cell culture systems are being increasingly implemented to shorten production time and avoid egg-dependent limitations. Nevertheless, achieving high yield and cost-effectiveness in cell-based systems remains a challenge [].
4.1.2. Live Attenuated Vaccine
Live attenuated influenza vaccines (LAIVs) are generated by reducing viral virulence through methods such as cold adaptation, while retaining the ability to replicate to a limited extent in the respiratory tract. These vaccines induce both humoral and cellular immunity, including mucosal IgA and systemic IgG responses, and have been associated with favorable T-cell responses that confer heterologous protection [,]. However, due to the potential for vaccine-related adverse events, LAIVs are generally not recommended for certain populations, including immunocompromised individuals, pregnant women, and the elderly [].
Novel attenuation strategies are under investigation to improve safety profiles. For example, proteolysis-targeting chimeric (PROTAC) technology has been employed to generate conditionally destabilized influenza viruses, enabling controlled attenuation and enhancing vaccine safety in preclinical studies [,].
4.1.3. Recombinant Protein-Based Vaccine
Recombinant protein vaccines utilize purified viral proteins or epitopes as immunogens, offering a favorable safety profile compared to whole-virus or live attenuated platforms. Their design relies on prior knowledge of antigenic epitopes and genetic information, which can prolong development timelines. However, for influenza, well-established targets such as HA, NA, and M2e allow for flexible and rapid antigen design. HA-based recombinant vaccines can be produced in mammalian or insect cell systems within approximately two months, facilitating a rapid response to emerging strains [].
Innovations in antigen design include mosaic HA (mHA) constructs that integrate conserved epitopes from multiple strains to broaden immune coverage []. Additionally, computationally optimized broadly reactive antigen (COBRA) methodologies, increasingly supported by artificial intelligence (AI), are being used to design HA antigens capable of eliciting cross-reactive immunity against diverse influenza variants [,,,].

Table 1.
The traditional and new vaccines under development for influenza vaccines.
Table 1.
The traditional and new vaccines under development for influenza vaccines.
| Types of Vaccines | Characteristic and Optimization | Delivery Methods | Immune Responses | Advantages | Disadvantages | Application Stage |
|---|---|---|---|---|---|---|
| Inactivated Vaccines [,] | egg-based production | intramuscular injection | humoral and cellular immunity | cost-effective | egg-adaptive mutations | clinically approved |
| cell culture-based production | improved safety and immunogenicity | costly | ||||
| split-virion | ||||||
| viral subunit | ||||||
| Live Attenuated Vaccine [,,,] | cold adaptation | intranasal administration | humoral and cellular immunity | Natural immune responses | vaccine-related adverse events | clinically approved |
| PROTAC | improve safety | enhancing vaccine safety | preclinical studies | |||
| Recombinant Protein-Based Vaccine [,,] | purified viral proteins or epitopes as immunogens | intramuscular injection | enhanced humoral immunity | safety/flexible antigen design | the design relies on prior knowledge of the virus | clinically approved |
| mosaic HA (mHA) | multiple strains coverage | preclinical studies | ||||
| COBRA methodologies | AI-based automation | preclinical studies | ||||
| Recombinant Viral Vector Vaccines [,] | Adenovirus vector | intramuscular/intranasal | TLR-dependent and independent signaling pathways | target specific immune cells | safety concern | preclinical studies |
| MVA vector | ||||||
| NDV vector | ||||||
| Conjugate Vaccine [,,] | links poorly immunogenic antigens to carrier proteins | intramuscular/intradermal | T-cell-dependent immunity and memory B-cell formation | improved immune responses | costly | preclinical studies |
| Nucleic Acid-Based Vaccines | DNA-based [] | intramuscular/Subcutaneous/intradermal/others | both humoral and cellular immunity | rapid, scalable, and cost-effective | safety concern | preclinical studies |
| mRNA-based [,,,,,] | Phase-III-clinical-trial | |||||
| saRNA-based [] | preclinical studies |
PROTAC: proteolysis-targeting chimeric technology; COBRA: computationally optimized broadly reactive antigen; MVA: modified vaccinia Ankara; NDV: Newcastle disease virus; AI: artificial intelligence.
4.2. Recent Vaccine Platform in Progress
Novel vaccine technologies have been addressed for meeting the needs of the development of efficient, safe, and stable vaccines. The next generation of the vaccine platform should respond quickly to the development and manufacture of the vaccine after an epidemic outbreak. For the influenza vaccine, the vaccine also needs to respond quickly against the emerging variant of influenza. To address these challenges, recombinant viral vector vaccines, conjugate vaccines, and NA-based vaccines have been widely studied. Although some of them have not yet reached the application stage, they could potentially induce an appropriate and safe immune response with an efficient delivery system.
4.2.1. Recombinant Viral Vector Vaccines
Recombinant viral vector vaccines employ engineered, replication-deficient or attenuated viruses to deliver influenza antigens. These vectors often possess intrinsic adjuvant properties and can be designed to target specific immune cells, activating both TLR-dependent and independent signaling pathways. The licensure of adenovirus-vectored COVID-19 vaccines (e.g., Vaxzevria®) has validated this platform for respiratory viruses []. For influenza, various vectors—including adenovirus, modified vaccinia Ankara (MVA), and Newcastle disease virus—have been engineered to express antigens such as HA, NP, and M1, eliciting robust T-cell responses in preclinical and clinical studies [].
4.2.2. Conjugate Vaccine
Conjugate vaccine technology, widely used against bacterial pathogens, links poorly immunogenic antigens to carrier proteins to enhance immunogenicity. This approach has recently been applied to viral targets; for example, SARS-CoV-2 antigens conjugated to designer peptides have shown improved immune responses [,]. Conjugate vaccines promote T-cell-dependent immunity and memory B-cell formation []. In the context of influenza, conjugating low-immunogenicity antigens such as NA or M2e to immunogenic carriers can circumvent the limitations of viral antigenic variation. Moreover, mosaic protein designs enable efficient production in bacterial expression systems, supporting scalable manufacturing [].
4.2.3. Nucleic Acid-Based Vaccines
Nucleic acid (NA) vaccines comprise DNA or mRNA encoding target antigens and represent a rapid, scalable, and cost-effective vaccine modality. DNA vaccines introduce plasmid DNA into host cells, enabling in situ antigen expression without the need for live virus culture. Although several influenza DNA vaccines have entered early-stage clinical trials, concerns regarding potential genomic integration have limited their widespread adoption [].
mRNA vaccines have gained prominence as a safe and efficient alternative, with no risk of genomic integration. They elicit both humoral and cellular immunity, with antigen presentation via MHC-I stimulating CD8+ T-cell responses [,]. Preclinical studies of mRNA vaccines encoding multiple HA antigens have demonstrated potent immunity and protection in animal models [,]. Universal influenza mRNA vaccine strategies include targeting conserved internal proteins (e.g., NP, M1, PB1) or combining multiple antigens from different subtypes (e.g., HA, NA, M2) to achieve broad cross-protection [,]. A multivalent mRNA vaccine encoding 20 HA subtypes recently induced broad, subtype-specific antibodies and mitigated influenza symptoms in preclinical models [].
Self-amplifying RNA (saRNA) vaccines represent a further evolution, encoding a viral replicase that enables intracellular RNA amplification and prolonged antigen expression at lower doses. While saRNA platforms have shown promise for influenza and other pathogens, their safety profiles require further evaluation [].
A critical factor for NA vaccine efficacy is the delivery system (Table 2). Lipid nanoparticles (LNPs)—composed of ionizable lipids, cholesterol, PEG-lipids, and phospholipids—have been widely adopted for their ability to protect nucleic acids and enhance cellular uptake. Alternative delivery systems under investigation include lipid-polymer hybrids, exosomes, peptide-based carriers, and inorganic nanoparticles [].

Table 2.
Different methods for the delivery of Nucleic Acids in vaccine development [].
4.3. Adjuvant Systems
Adjuvants are incorporated into vaccine formulations to enhance the magnitude, breadth, and durability of immune responses. Currently licensed adjuvants for influenza vaccines include aluminum salts, emulsions such as MF59 and AS03, and Toll-like receptor (TLR) agonists. These adjuvants, consisting of their active ingredient, were summarized accordingly (Table 3).
4.3.1. Aluminum Adjuvants
Aluminum-based adjuvants (e.g., aluminum hydroxide and aluminum phosphate) are among the most widely used in global vaccination programs. They function by forming antigen depots, promoting phagocytosis, and enhancing Th2-biased humoral immunity. However, they elicit relatively weak cellular immune responses and may cause local reactions, limiting their utility in some contexts [,].
4.3.2. Emulsion Adjuvants
MF59, an oil-in-water emulsion containing squalene, polysorbate 80, and sorbitan trioleate, enhances antigen uptake and promotes a mixed Th1/Th2 response via MyD88-dependent signaling []. Similarly, AS03—composed of squalene, polysorbate 80, and DL-α-tocopherol—strengthens antibody responses and has been used in pandemic influenza vaccines such as Pandemrix™ [,].
4.3.3. TLR-Agonists-Based Adjuvants
TLR agonists activate innate immunity and promote robust adaptive responses. Monophosphoryl lipid A (MPLA), a TLR4 agonist, is included in several licensed vaccine formulations (e.g., AS01 in Shingrix® and AS04 in Fendrix®) and promotes Th1-skewed immunity [,,,,]. Other TLR agonists in clinical use include the TLR7/8 agonist imidazoquinoline, which is used in COVAXIN® and the TLR9 agonist CpG 1018 in Heplisav-B® [,,].
4.3.4. Development of Novel Adjuvants
Emerging adjuvants under investigation include mannan-based compounds, which enhance antigen presentation and have shown promise in allergen immunotherapy trials [,]. New TLR agonists in development for influenza include TLR3 agonists (e.g., poly(I:C12U)), TLR5 agonists (e.g., flagellin in VAX128), and imidazoquinolines(TLR7/8 agonists), which have demonstrated potent adjuvant activity in preclinical and clinical studies [,,].

Table 3.
Adjuvant systems used in influenza vaccines.
Table 3.
Adjuvant systems used in influenza vaccines.
| Types of Adjuvant | Main Components | Immune Enhancement | Application Stage |
|---|---|---|---|
| Aluminum Adjuvants [,] | aluminum hydroxide | Th2-biased humoral immunity | clinically employed |
| aluminum phosphate | |||
| Emulsion Adjuvants [,] | MF59 | mixed Th1/Th2 response | clinically employed |
| AS03 | strengthens antibody responses | ||
| TLR-Agonists-Based Adjuvants | TLR4 agonist (MPLA) [,,,,] | promote innate and robust adaptive responses | clinically employed |
| TLR7/8 agonist (imidazoquinoline) [,,] | clinically employed | ||
| TLR9 agonist (CpG 1018) [,] | clinically employed | ||
| TLR3 agonists (poly(I:C12U)) [] | preclinical studies | ||
| TLR5 agonists (flagellin) [] | preclinical studies | ||
| TLR7/8 agonists (imidazoquinolines) [,] | preclinical studies |
5. Conclusions and Perspective
Despite decades of progress, influenza remains a formidable global health challenge. Current seasonal vaccines, while instrumental in reducing morbidity and mortality, are constrained by their limited breadth of protection and the logistical challenges of annual reformulation. The pursuit of a universal influenza vaccine has thus emerged as a critical scientific priority, driving innovation across multiple fronts.
Substantial advances have been made in identifying conserved antigenic targets, rational antigen design, and novel delivery platforms. Strategies such as immunofocusing on the HA stem, multi-antigen combinations, and computationally optimized antigens (COBRA) are yielding promising candidates with broader reactivity. Concurrently, mRNA and nanoparticle platforms have demonstrated an unprecedented capacity for rapid, scalable production and potent immunogenicity.
Looking forward, the successful development of a universal vaccine will likely depend on the intelligent integration of these strategies. No single approach may suffice; instead, a combination of conserved epitopes, structural vaccinology, and advanced delivery systems will be essential. Key challenges remain, including overcoming immune subdominance of conserved regions, ensuring long-lasting immunity, and establishing robust correlates of protection. Furthermore, the transition of these promising platforms from the laboratory to clinical use will necessitate exhaustive clinical trials to thoroughly demonstrate their effectiveness and safety profiles. Global collaboration and leveraging lessons from COVID-19 vaccine development will be paramount. The ultimate goal is a vaccine that confers durable, broad-spectrum protection against seasonal and pandemic strains, transforming our ability to manage influenza and safeguard public health worldwide.
Author Contributions
Conceptualization, H.J. and S.C.; validation, S.C., C.Y., C.F. and H.J.; investigation, S.C., C.Y., C.F. and H.J.; data curation, S.C., C.Y., C.F. and H.J.; writing—original draft preparation, S.C., C.Y., C.F. and H.J.; writing—review and editing, H.J.; visualization, C.Y.; supervision, C.F. and H.J.; funding acquisition, H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Discipline Innovation and Development Program of Tangdu Hospital: 2021LCYJ025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This review was independently completed by the authors, and no individual requires special acknowledgement.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADCC | Antibody-dependent cellular cytotoxicity |
| AI | Artificial intelligence |
| CDC | Complement-dependent cytolysis |
| COBRA | Computationally optimized broadly reactive antigen |
| CTLs | Cytotoxic T lymphocytes |
| HA | Hemagglutinin |
| IAVs | Influenza A viruses |
| IIV3 | Inactivated influenza vaccines |
| LAIV3 | Live-attenuated influenza vaccine |
| LNPs | Lipid nanoparticles |
| M1 | Matrix protein |
| M2e | M2 extracellular domain |
| MHC | Major histocompatibility complex |
| MPLA | Monophosphoryl lipid A |
| MVA | Modified Ankara |
| NP | Nucleoprotein |
| PB1 | Polymerase basic protein 1 |
| PEG | Polyethylene glycol |
| PLGA | Poly lactic-co-glycolic acid |
| PROTAC | Proteolysis-targeting chimeric |
| PROTAR | Proteolysis-targeting |
| RIV3 | Recombinant influenza vaccine |
| saRNA | Self-amplifying RNA |
| TLR | Toll-like receptor |
| vRNA | Viral RNA |
References
- Peacock, T.P.; Moncla, L.; Dudas, G.; VanInsberghe, D.; Sukhova, K.; Lloyd-Smith, J.O.; Worobey, M.; Lowen, A.C.; Nelson, M.I. The global H5N1 influenza panzootic in mammals. Nature 2025, 637, 304–313. [Google Scholar] [CrossRef]
- Fan, Y.; Nishimura, H.; Katsumi, M.; Yang, J.; Sakata, S.; Kohzuki, M.; Ebihara, S. Minimal Influenza Virus Transmission from Touching Contaminated Floors and Metal Door Levers: Laboratory Study II. Microbiol. Immunol. 2025, 69, 397–406. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Tao, F.; Chen, Y.; Zhou, Y.; Cheng, J.; Wang, X. Global analysis of influenza epidemic characteristics in the first two seasons after lifting the nonpharmaceutical interventions for COVID-19. Int. J. Infect. Dis. 2025, 151, 107372. [Google Scholar] [CrossRef] [PubMed]
- Puente-Massaguer, E.; Beyer, A.; Loganathan, M.; Sapse, I.; Carreño, J.M.; Bajic, G.; Sun, W.; Palese, P.; Krammer, F. Bioprocess development for universal influenza vaccines based on inactivated split chimeric and mosaic hemagglutinin viruses. Front. Bioeng. Biotechnol. 2023, 11, 1097349. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Gong, H.; Zhong, G.; Deng, X.; Tian, Y.; Wang, M.; Yu, H.; Yang, J. Estimating mortality associated with seasonal influenza among adults aged 65 years and above in China from 2011 to 2016: A systematic review and model analysis. Influenza Other Respir. Viruses 2023, 17, e13067. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; O’Brien, K.L.; Madhi, S.A.; Widdowson, M.-A.; Byass, P.; Omer, S.B.; Abbas, Q.; Ali, A.; Amu, A.; et al. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: A systematic review and modelling study. Lancet Glob. Health 2020, 8, e497–e510. [Google Scholar] [CrossRef]
- Clark, T.W.; Tregoning, J.S.; Lister, H.; Poletti, T.; Amin, F.; Nguyen-Van-Tam, J.S. Recent advances in the influenza virus vaccine landscape: A comprehensive overview of technologies and trials. Clin. Microbiol. Rev. 2024, 37, e0002524. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Blanton, L.H.; Fry, A.M.; Jernigan, D.B.; Atmar, R.L. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020–2021 Influenza Season. Recomm. Rep. 2020, 69, 1–24. [Google Scholar]
- Kim, K.-H.; Li, Z.; Bhatnagar, N.; Subbiah, J.; Park, B.R.; Shin, C.H.; Pushko, P.; Wang, B.-Z.; Kang, S.-M. Universal protection against influenza viruses by multi-subtype neuraminidase and M2 ectodomain virus-like particle. PLoS Pathog. 2022, 18, e1010755. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ambrose, K.; Canaday, D.H.; Delair, S.; Ezike, N.; Huber, V.C.; Jhaveri, R.; Nyquist, A.-C.; Sporer, A.; Varman, M.; et al. The association between influenza vaccine effectiveness and egg-based manufacturing technology: Literature review and US expert consensus. Curr. Med. Res. Opin. 2024, 40, 335–343. [Google Scholar] [CrossRef]
- Hamamoto, I. Developments and current challenges in the process of cell culture-based seasonal influenza vaccine manufacture in Japan. Glob. Health Med. 2024, 6, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, C.M.; Kistner, O.; Montomoli, E.; Viviani, S.; Marchi, S. Influenza Viruses and Vaccines: The Role of Vaccine Effectiveness Studies for Evaluation of the Benefits of Influenza Vaccines. Vaccines 2022, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Chivukula, S.; Plitnik, T.; Tibbitts, T.; Karve, S.; Dias, A.; Zhang, D.; Goldman, R.; Gopani, H.; Khanmohammed, A.; Sarode, A.; et al. Development of multivalent mRNA vaccine candidates for seasonal or pandemic influenza. NPJ Vaccines 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Kawai, A.; Shimizu, T.; Tanaka, H.; Shichinohe, S.; Anindita, J.; Hirose, M.; Kawahara, E.; Senpuku, K.; Shimooka, M.; Mai, L.T.Q.; et al. Low-inflammatory lipid nanoparticle-based mRNA vaccine elicits protective immunity against H5N1 influenza virus with reduced adverse reactions. Mol. Ther. 2025, 33, 529–547. [Google Scholar] [CrossRef]
- Zhang, C.; Hou, J.; Li, Z.; Shen, Q.; Bai, H.; Chen, L.; Shen, J.; Wang, P.; Su, Y.; Li, J.; et al. PROTAR Vaccine 2.0 generates influenza vaccines by degrading multiple viral proteins. Nat. Chem. Biol. 2025, 21, 1330–1340. [Google Scholar] [CrossRef]
- Soens, M.; Ananworanich, J.; Hicks, B.; Lucas, K.J.; Cardona, J.; Sher, L.; Livermore, G.; Schaefers, K.; Henry, C.; Choi, A.; et al. A phase 3 randomized safety and immunogenicity trial of mRNA-1010 seasonal influenza vaccine in adults. Vaccine 2025, 50, 126847. [Google Scholar] [CrossRef]
- Han, A.X.; de Jong, S.P.J.; Russell, C.A. Co-evolution of immunity and seasonal influenza viruses. Nat. Rev. Microbiol. 2023, 21, 805–817. [Google Scholar] [CrossRef]
- Luczo, J.M.; Spackman, E. Epitopes in the HA and NA of H5 and H7 avian influenza viruses that are important for antigenic drift. FEMS Microbiol. Rev. 2024, 48, fuae014. [Google Scholar] [CrossRef]
- Al-Eitan, L.; Khair, I.; Shakhatreh, Z.; Almahdawi, D.; Alahmad, S. Epidemiology, biosafety, and biosecurity of Avian Influenza: Insights from the East Mediterranean region. Rev. Med. Virol. 2024, 34, e2559. [Google Scholar] [CrossRef]
- Russell, C.J.; Hu, M.; Okda, F.A. Influenza Hemagglutinin Protein Stability, Activation, and Pandemic Risk. Trends Microbiol. 2018, 26, 841–853. [Google Scholar] [CrossRef]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.M.G.; Fenton, M.J. Immunobiology of influenza vaccines. Chest 2013, 143, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Khalaj-Hedayati, A.; Moosavi, S.; Manta, O.; Helal, M.H.; Ibrahim, M.M.; El-Bahy, Z.M.; Supriyanto, G. Identification and In Silico Characterization of a Conserved Peptide on Influenza Hemagglutinin Protein: A New Potential Antigen for Universal Influenza Vaccine Development. Nanomaterials 2023, 13, 2796. [Google Scholar] [CrossRef] [PubMed]
- Impagliazzo, A.; Milder, F.; Kuipers, H.; Wagner, M.V.; Zhu, X.; Hoffman, R.M.; Van Meersbergen, R.; Huizingh, J.; Wanningen, P.; Verspuij, J.; et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015, 349, 1301–1306. [Google Scholar] [CrossRef]
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.-J.; Kanekiyo, M.; Kong, W.-P.; Gallagher, J.R.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef]
- Verhoeven, D.; Sponseller, B.A.; Crowe, J.E., Jr.; Bangaru, S.; Webby, R.J.; Lee, B.M. Use of equine H3N8 hemagglutinin as a broadly protective influenza vaccine immunogen. NPJ Vaccines 2024, 9, 247. [Google Scholar] [CrossRef]
- Kirchenbaum, G.A.; Carter, D.M.; Ross, T.M. Sequential Infection in Ferrets with Antigenically Distinct Seasonal H1N1 Influenza Viruses Boosts Hemagglutinin Stalk-Specific Antibodies. J. Virol. 2016, 90, 1116–1128. [Google Scholar] [CrossRef]
- Zhu, X.; Turner, H.L.; Lang, S.; McBride, R.; Bangaru, S.; Gilchuk, I.M.; Yu, W.; Paulson, J.C.; Crowe, J.E.; Ward, A.B.; et al. Structural Basis of Protection against H7N9 Influenza Virus by Human Anti-N9 Neuraminidase Antibodies. Cell Host Microbe 2019, 26, 729–738.E4. [Google Scholar] [CrossRef]
- Gravel, C.; Li, C.; Wang, J.; Hashem, A.M.; Jaentschke, B.; Xu, K.-W.; Lorbetskie, B.; Gingras, G.; Aubin, Y.; Van Domselaar, G.; et al. Qualitative and quantitative analyses of virtually all subtypes of influenza A and B viral neuraminidases using antibodies targeting the universally conserved sequences. Vaccine 2010, 28, 5774–5784. [Google Scholar] [CrossRef]
- Wohlbold, T.J.; Nachbagauer, R.; Xu, H.; Tan, G.S.; Hirsh, A.; A Brokstad, K.; Cox, R.J.; Palese, P.; Krammer, F. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. mBio 2015, 6, e02556. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Skarlupka, A.L.; Bebin-Blackwell, A.-G.; Sumner, S.F.; Ross, T.M. Universal Influenza Virus Neuraminidase Vaccine Elicits Protective Immune Responses against Human Seasonal and Pre-pandemic Strains. J. Virol. 2021, 95, e0075921. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kong, H.; Chu, B.; Yang, Q.; Lin, C.; Liu, R.; Chen, C.; Gao, Y.; Wang, G.; Wang, D.; et al. Identification of a broad-inhibition influenza neuraminidase antibody from pre-existing memory B cells. Cell Host Microbe 2025, 33, 151–166.E8. [Google Scholar] [CrossRef] [PubMed]
- Schnell, J.R.; Chou, J.J. Structure and mechanism of the M2 proton channel of influenza A virus. Nature 2008, 451, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Ciampor, F.; Thompson, C.; Grambas, S.; Hay, A. Regulation of pH by the M2 protein of influenza A viruses. Virus Res. 1992, 22, 247–258. [Google Scholar] [CrossRef]
- Schulte, M.C.; Barcellona, A.T.; Wang, X.; Schrum, A.G.; Ulery, B.D. M2e-Derived Peptidyl and Peptide Amphiphile Micelles as Novel Influenza Vaccines. Pharmaceuticals 2024, 17, 1503. [Google Scholar] [CrossRef]
- Gerhard, W.; Mozdzanowska, K.; Zharikova, D. Prospects for universal influenza virus vaccine. Emerg. Infect. Dis. 2006, 12, 569–574. [Google Scholar] [CrossRef]
- Jegerlehner, A.; Schmitz, N.; Storni, T.; Bachmann, M.F. Influenza A vaccine based on the extracellular domain of M2: Weak protection mediated via antibody-dependent NK cell activity. J. Immunol. 2004, 172, 5598–5605. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, M.; Mozdzanowska, K.; Zharikova, D.; Hoff, H.; Wunner, W.; Couch, R.B.; Gerhard, W. Influenza A virus infection engenders a poor antibody response against the ectodomain of matrix protein 2. Virol. J. 2006, 3, 102. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Gomes, K.B.; Lee, Y.-Z.; Ward, G.; Xie, B.; Auclair, S.; He, L.; Zhu, J. A Single-Component Multilayered Self-Assembling Protein Nanoparticle Vaccine Based on Extracellular Domains of Matrix Protein 2 against Both Influenza A and B. Vaccines 2024, 12, 975. [Google Scholar] [CrossRef]
- Fiers, W.; De Filette, M.; El Bakkouri, K.; Schepens, B.; Roose, K.; Schotsaert, M.; Birkett, A.; Saelens, X. M2e-based universal influenza A vaccine. Vaccine 2009, 27, 6280–6283. [Google Scholar] [CrossRef]
- Tao, W.; Hurst, B.L.; Shakya, A.K.; Uddin, J.; Ingrole, R.S.; Hernandez-Sanabria, M.; Arya, R.P.; Bimler, L.; Paust, S.; Tarbet, E.B.; et al. Consensus M2e peptide conjugated to gold nanoparticles confers protection against H1N1, H3N2 and H5N1 influenza A viruses. Antivir. Res. 2017, 141, 62–72. [Google Scholar] [CrossRef]
- Eisfeld, A.J.; Neumann, G.; Kawaoka, Y. At the centre: Influenza A virus ribonucleoproteins. Nat. Rev. Microbiol. 2015, 13, 28–41. [Google Scholar] [CrossRef]
- Townsend, A.R.; Gotch, F.M.; Davey, J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell 1985, 42, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Yewdell, J.W.; Bennink, J.R.; Smith, G.L.; Moss, B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 1985, 82, 1785–1789. [Google Scholar] [CrossRef] [PubMed]
- Voeten, J.T.M.; Bestebroer, T.M.; Nieuwkoop, N.J.; Fouchier, R.A.M.; Osterhaus, A.D.M.E.; Rimmelzwaan, G.F. Antigenic drift in the influenza A virus (H3N2) nucleoprotein and escape from recognition by cytotoxic T lymphocytes. J. Virol. 2000, 74, 6800–6807. [Google Scholar] [CrossRef]
- Nüssing, S.; Sant, S.; Koutsakos, M.; Subbarao, K.; Nguyen, T.H.O.; Kedzierska, K. Innate and adaptive T cells in influenza disease. Front. Med. 2018, 12, 34–47. [Google Scholar] [CrossRef]
- Jacobs, B.; Leroux-Roels, I.; Bruhwyler, J.; Groth, N.; Waerlop, G.; Janssens, Y.; Tourneur, J.; De Boever, F.; Alhatemi, A.; Moris, P.; et al. Evaluation of Safety, Immunogenicity and Cross-Reactive Immunity of OVX836, a Nucleoprotein-Based Universal Influenza Vaccine, in Older Adults. Vaccines 2024, 12, 1391. [Google Scholar] [CrossRef]
- Krammer, F.; Pica, N.; Hai, R.; Margine, I.; Palese, P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 2013, 87, 6542–6550. [Google Scholar] [CrossRef]
- Ellebedy, A.H.; Krammer, F.; Li, G.-M.; Miller, M.S.; Chiu, C.; Wrammert, J.; Chang, C.Y.; Davis, C.W.; McCausland, M.; Elbein, R.; et al. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 13133–13138. [Google Scholar] [CrossRef]
- Graves, P.; Schulman, J.; Young, J.; Palese, P. Preparation of influenza virus subviral particles lacking the HA1 subunit of hemagglutinin: Unmasking of cross-reactive HA2 determinants. Virology 1983, 126, 106–116. [Google Scholar] [CrossRef]
- McCraw, D.M.; Myers, M.L.; Gulati, N.M.; Prabhakaran, M.; Brand, J.; Andrews, S.; Gallagher, J.R.; Maldonado-Puga, S.; Kim, A.J.; Torian, U.; et al. Designed nanoparticles elicit cross-reactive antibody responses to conserved influenza virus hemagglutinin stem epitopes. PLoS Pathog. 2023, 19, e1011514. [Google Scholar] [CrossRef]
- Xu, D.; Carter, J.J.; Li, C.; Utz, A.; Weidenbacher, P.A.B.; Tang, S.; Sanyal, M.; Pulendran, B.; Barnes, C.O.; Kim, P.S. Vaccine design via antigen reorientation. Nat. Chem. Biol. 2024, 20, 1012–1021. [Google Scholar] [CrossRef]
- Mallajosyula, V.; Chakraborty, S.; Sola, E.; Fong, R.F.; Shankar, V.; Gao, F.; Burrell, A.R.; Gupta, N.; Wagar, L.E.; Mischel, P.S.; et al. Coupling antigens from multiple subtypes of influenza can broaden antibody and T cell responses. Science 2024, 386, 1389–1395. [Google Scholar] [CrossRef]
- Castro, K.M.; Ayardulabi, R.; Wehrle, S.; Cui, H.; Georgeon, S.; Schmidt, J.; Xiao, S.; Seraj, N.; Harshbarger, W.; Mallett, C.P.; et al. Structure-based Design of Chimeric Influenza Hemagglutinins to Elicit Cross-group Immunity. bioRxiv 2025. [Google Scholar] [CrossRef]
- Leonard, R.A.; Burke, K.N.; Spreng, R.L.; Macintyre, A.N.; Tam, Y.; Alameh, M.-G.; Weissman, D.; Heaton, N.S. Improved influenza vaccine responses after expression of multiple viral glycoproteins from a single mRNA. Nat. Commun. 2024, 15, 8712. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Han, S.; Lu, F.; Liu, Q.; Yang, J.; Wang, W.; Wang, Y.; Zhang, J.; Ju, R.; Shen, X.; et al. Dimethyl-Dioctadecyl-Ammonium Bromide/Poly(lactic acid) Nanoadjuvant Enhances the Immunity and Cross-Protection of an NM2e-Based Universal Influenza Vaccine. ACS Nano 2024, 18, 12905–12916. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, Q.; Qi, M.; Chen, J.; Wu, X.; Zhang, X.; Li, W.; Zhang, X.-E.; Cui, Z. An Intranasal Multivalent Epitope-Based Nanoparticle Vaccine Confers Broad Protection against Divergent Influenza Viruses. ACS Nano 2023, 17, 13474–13487. [Google Scholar] [CrossRef]
- Xiong, F.; Zhang, C.; Shang, B.; Zheng, M.; Wang, Q.; Ding, Y.; Luo, J.; Li, X. An mRNA-based broad-spectrum vaccine candidate confers cross-protection against heterosubtypic influenza A viruses. Emerg. Microbes Infect. 2023, 12, 2256422. [Google Scholar] [CrossRef]
- Fatimah, M.N.N.; Thian, B.Y.Z.; Wong, C.L.; Ong, H.K.; Hussin, H.; Mariatulqabtiah, A.R.; Ho, K.L.; Omar, A.R.; Tan, W.S. Chimeric virus-like particles of nodavirus displaying M2e of human and avian influenza A viruses as a potential dual-use vaccine: Inducing a broader immune response and protecting mice against viral infections. Vaccine 2025, 56, 127165. [Google Scholar] [CrossRef]
- Carter, D.M.; Darby, C.A.; Lefoley, B.C.; Crevar, C.J.; Alefantis, T.; Oomen, R.; Anderson, S.F.; Strugnell, T.; Cortés-Garcia, G.; Vogel, T.U.; et al. Design and Characterization of a Computationally Optimized Broadly Reactive Hemagglutinin Vaccine for H1N1 Influenza Viruses. J. Virol. 2016, 90, 4720–4734. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Ross, T.M. Next generation methodology for updating HA vaccines against emerging human seasonal influenza A(H3N2) viruses. Sci. Rep. 2021, 11, 4554. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, X.; Ge, P.; Meliopoulos, V.; Freiden, P.; Livingston, B.; Schultz-Cherry, S.; Ross, T.M. Inactivated influenza virus vaccines expressing COBRA hemagglutinin elicited broadly reactive, long-lived protective antibodies. Hum. Vaccines Immunother. 2024, 20, 2356269. [Google Scholar] [CrossRef] [PubMed]
- Olawade, D.B.; Teke, J.; Fapohunda, O.; Weerasinghe, K.; Usman, S.O.; Ige, A.O.; David-Olawade, A.C. Leveraging artificial intelligence in vaccine development: A narrative review. J. Microbiol. Methods 2024, 224, 106998. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Hendy, D.A.; Bachelder, E.M.; Ainslie, K.M.; Ross, T.M. Multi-COBRA hemagglutinin formulated with cGAMP microparticles elicits protective immune responses against influenza viruses. mSphere 2024, 9, e0016024. [Google Scholar] [CrossRef]
- Nagashima, K.; Abbadi, N.; Vyas, V.; Roegner, A.; Ross, T.M.; Mousa, J.J. Adjuvant-Mediated Differences in Antibody Responses to Computationally Optimized Hemagglutinin and Neuraminidase Vaccines. Viruses 2023, 15, 347. [Google Scholar] [CrossRef]
- Hao, M.; Wang, Y.; Yang, W.; Xu, M.; Guan, Y.; Zhang, Y.; Chen, J. Epitope-Optimized Influenza Hemagglutinin Nanoparticle Vaccine Provides Broad Cross-Reactive Immunity against H9N2 Influenza Virus. ACS Nano 2025, 19, 20824–20840. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Peng, C.; Guo, S.; Wang, B.; Chen, L.; Wang, Y.; Tang, H.; Liu, L.; Pan, Q.; et al. Cross-protection against homo and heterologous influenza viruses via intranasal administration of an HA chimeric multiepitope nanoparticle vaccine. J. Nanobiotechnol. 2025, 23, 77. [Google Scholar] [CrossRef]
- Heng, W.T.; Lim, H.X.; Tan, K.O.; Poh, C.L. Validation of Multi-epitope Peptides Encapsulated in PLGA Nanoparticles Against Influenza A Virus. Pharm. Res. 2023, 40, 1999–2025. [Google Scholar] [CrossRef]
- Saouaf, O.M.; Ou, B.S.; Song, Y.E.; Carter, J.J.; Yan, J.; Jons, C.K.; Barnes, C.O.; Appel, E.A. Sustained Vaccine Exposure Elicits More Rapid, Consistent, and Broad Humoral Immune Responses to Multivalent Influenza Vaccines. Adv. Sci. 2025, 12, e2404498. [Google Scholar] [CrossRef]
- Bailey-Hytholt, C.M.; Ghosh, P.; Dugas, J.; Zarraga, I.E.; Bandekar, A. Formulating and Characterizing Lipid Nanoparticles for Gene Delivery using a Microfluidic Mixing Platform. J. Vis. Exp. 2021, 168, e62226. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef] [PubMed]
- Uno, N.; Ebensen, T.; Guzman, C.A.; Ross, T.M. Intranasal administration of octavalent next-generation influenza vaccine elicits protective immune responses against seasonal and pre-pandemic viruses. J. Virol. 2024, 98, e0035424. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Ohno, M.; Nomura, N.; Handabile, C.; Shingai, M.; Jackson, D.C.; Brown, L.E.; Kida, H. Selecting and Using the Appropriate Influenza Vaccine for Each Individual. Viruses 2021, 13, 971. [Google Scholar] [CrossRef]
- Kavian, N.; Hachim, A.; Li, A.P.; A Cohen, C.; Chin, A.W.; Poon, L.L.; Fang, V.J.; Leung, N.H.; Cowling, B.J.; A Valkenburg, S. Assessment of enhanced influenza vaccination finds that FluAd conveys an advantage in mice and older adults. Clin. Transl. Immunol. 2020, 9, e1107. [Google Scholar] [CrossRef]
- Mohn, K.G.-I.; Smith, I.; Sjursen, H.; Cox, R.J. Immune responses after live attenuated influenza vaccination. Hum. Vaccines Immunother. 2018, 14, 571–578. [Google Scholar] [CrossRef]
- Mohn, K.G.-I.; A Brokstad, K.; Islam, S.; Oftung, F.; Tøndel, C.; Aarstad, H.J.; Cox, R.J. Early Induction of Cross-Reactive CD8+ T-Cell Responses in Tonsils After Live-Attenuated Influenza Vaccination in Children. J. Infect. Dis. 2020, 221, 1528–1537. [Google Scholar] [CrossRef]
- Si, L.; Shen, Q.; Li, J.; Chen, L.; Shen, J.; Xiao, X.; Bai, H.; Feng, T.; Ye, A.Y.; Li, L.; et al. Generation of a live attenuated influenza A vaccine by proteolysis targeting. Nat. Biotechnol. 2022, 40, 1370–1377. [Google Scholar] [CrossRef]
- Mukerjee, N.; Maitra, S.; Mukherjee, D.; Ghosh, A.; Alexiou, A.T.; Thorat, N.D. Harnessing PROTACs to combat H5N1 influenza: A new frontier in viral destruction. J. Med. Virol. 2024, 96, e29926. [Google Scholar] [CrossRef]
- Feng, J.; Du, Y.; Chen, L.; Su, W.; Wei, H.; Liu, A.; Jiang, X.; Guo, J.; Dai, C.; Xu, Y.; et al. A quadrivalent recombinant influenza Hemagglutinin vaccine induced strong protective immune responses in animal models. Vaccine 2024, 42, 126008. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, I.; Puente-Massaguer, E.; Abdeljawad, A.; Lai, T.Y.; Liu, Y.; Loganathan, M.; Francis, B.; Lemus, N.; Dolange, V.; Boza, M.; et al. Preclinical evaluation of a universal inactivated influenza B vaccine based on the mosaic hemagglutinin-approach. NPJ Vaccines 2024, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Zhang, X.; Medina, J.M.; Thomas, M.H.; Lynch, A.; Nelson, R.; Aguirre, J.; Ross, T.M. Computationally Optimized Hemagglutinin Proteins Adjuvanted with Infectimune Generate Broadly Protective Antibody Responses in Mice and Ferrets. Vaccines 2024, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Knoll, M.D.; Wonodi, C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef]
- Blanas, A.; Karsjens, H.; de Ligt, A.; Huijbers, E.J.; van Loon, K.; Denisov, S.S.; Durukan, C.; Engbersen, D.J.; Groen, J.; Hennig, S.; et al. Vaccination with a bacterial peptide conjugated to SARS-CoV-2 receptor-binding domain accelerates immunity and protects against COVID-19. iScience 2022, 25, 104719. [Google Scholar] [CrossRef]
- Myburgh, L.; Karsjens, H.; Blanas, A.; de Ligt, A.; van Loon, K.; Huijbers, E.J.; van Beijnum, J.R.; Engbersen, D.J.; Rekiki, A.; Mignon, C.; et al. Targeting the early life stages of SARS-CoV-2 using a multi-peptide conjugate vaccine. Vaccine 2025, 54, 126989. [Google Scholar] [CrossRef]
- Clemente, B.; Denis, M.; Silveira, C.P.; Schiavetti, F.; Brazzoli, M.; Stranges, D. Straight to the point: Targeted mRNA-delivery to immune cells for improved vaccine design. Front. Immunol. 2023, 14, 1294929. [Google Scholar] [CrossRef]
- Myburgh, L.; van Loon, K.; Huijbers, E.J.; van Beijnum, J.R.; Russell, C.A.; Griffioen, A.W. Guided design for the development of an evolution-proof influenza vaccine. Vaccine 2025, 59, 127281. [Google Scholar] [CrossRef]
- Petsch, B.; Schnee, M.; Vogel, A.B.; Lange, E.; Hoffmann, B.; Voss, D.; Schlake, T.; Thess, A.; Kallen, K.-J.; Stitz, L.; et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012, 30, 1210–1216. [Google Scholar] [CrossRef]
- Russell, C.A.; Fouchier, R.A.M.; Ghaswalla, P.; Park, Y.; Vicic, N.; Ananworanich, J.; Nachbagauer, R.; Rudin, D. Seasonal influenza vaccine performance and the potential benefits of mRNA vaccines. Hum. Vaccines Immunother. 2024, 20, 2336357. [Google Scholar] [CrossRef]
- Van de Ven, K.; Lanfermeijer, J.; van Dijken, H.; Muramatsu, H.; de Melo, C.V.B.; Lenz, S.; Peters, F.; Beattie, M.B.; Lin, P.J.C.; Ferreira, J.A.; et al. A universal influenza mRNA vaccine candidate boosts T cell responses and reduces zoonotic influenza virus disease in ferrets. Sci. Adv. 2022, 8, eadc9937. [Google Scholar] [CrossRef]
- Freyn, A.W.; da Silva, J.R.; Rosado, V.C.; Bliss, C.M.; Pine, M.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Ferreira, L.C.d.S.; Weissman, D.; et al. A Multi-Targeting, Nucleoside-Modified mRNA Influenza Virus Vaccine Provides Broad Protection in Mice. Mol. Ther. 2020, 28, 1569–1584. [Google Scholar] [CrossRef]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.-G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef]
- Casmil, I.C.; Jin, J.; Won, E.-J.; Huang, C.; Liao, S.; Cha-Molstad, H.; Blakney, A.K. The advent of clinical self-amplifying RNA vaccines. Mol. Ther. 2025, 33, 2565–2582. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.Y.; Tham, S.K.; Poh, C.L. Revolutionizing immunization: A comprehensive review of mRNA vaccine technology and applications. Virol. J. 2025, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Thotathil, N.; Zhao, Z.; Mitragotri, S. Vaccine adjuvants for infectious disease in the clinic. Bioeng. Transl. Med. 2024, 9, e10663. [Google Scholar] [CrossRef] [PubMed]
- Moyer, T.J.; Kato, Y.; Abraham, W.; Chang, J.Y.H.; Kulp, D.W.; Watson, N.; Turner, H.L.; Menis, S.; Abbott, R.K.; Bhiman, J.N.; et al. Engineered immunogen binding to alum adjuvant enhances humoral immunity. Nat. Med. 2020, 26, 430–440. [Google Scholar] [CrossRef]
- Huang, Z.; Gong, H.; Sun, Q.; Yang, J.; Yan, X.; Xu, F. Research progress on emulsion vaccine adjuvants. Heliyon 2024, 10, e24662. [Google Scholar] [CrossRef]
- Chen, X. Emerging adjuvants for intradermal vaccination. Int. J. Pharm. 2023, 632, 122559. [Google Scholar] [CrossRef]
- Caserta, L.C.; Frye, E.A.; Butt, S.L.; Laverack, M.; Nooruzzaman, M.; Covaleda, L.M.; Thompson, A.C.; Koscielny, M.P.; Cronk, B.; Johnson, A.; et al. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature 2024, 634, 669–676. [Google Scholar] [CrossRef]
- Xing, J.; Zhao, X.; Li, X.; Fang, R.; Sun, M.; Zhang, Y.; Song, N. The recent advances in vaccine adjuvants. Front. Immunol. 2025, 16, 1557415. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-X.; Tseng, J.-C.; Yu, G.-Y.; Luo, Y.; Huang, C.-Y.F.; Hong, Y.-R.; Chuang, T.-H. Recent Advances in the Development of Toll-like Receptor Agonist-Based Vaccine Adjuvants for Infectious Diseases. Pharmaceutics 2022, 14, 423. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.B.B.; Kikuchi, S.; Rejzek, M.; Owen, C.; Reed, J.; Orme, A.; Misra, R.C.; El-Demerdash, A.; Hill, L.; Hodgson, H.; et al. Complete biosynthesis of the potent vaccine adjuvant QS-21. Nat. Chem. Biol. 2024, 20, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Roman, F.; Burny, W.; Ceregido, M.A.; Laupèze, B.; Temmerman, S.T.; Warter, L.; Coccia, M. Adjuvant system AS01: From mode of action to effective vaccines. Expert Rev. Vaccines 2024, 23, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef]
- Li, J.-X.; Zhu, F.-C. Adjuvantation helps to optimise COVID-19 vaccine candidate. Lancet Infect. Dis. 2021, 21, 891–893. [Google Scholar] [CrossRef]
- Van Haren, S.D.; Ganapathi, L.; Bergelson, I.; Dowling, D.J.; Banks, M.; Samuels, R.C.; Reed, S.G.; Marshall, J.D.; Levy, O. In vitro cytokine induction by TLR-activating vaccine adjuvants in human blood varies by age and adjuvant. Cytokine 2016, 83, 99–109. [Google Scholar] [CrossRef]
- The Medical Letter. A two-dose hepatitis B vaccine for adults (Heplisav-B). Med. Lett. Drugs Ther. 2018, 60, 17–18. [Google Scholar]
- Ojeda, P.; Barjau, M.C.; Subiza, J.; Moreno, A.; Ojeda, I.; Solano, E.; Alonso, A.; Caballero, R.; Del Pozo, S.; Gómez-Perosanz, M.; et al. Grass pollen allergoids conjugated with mannan for subcutaneous and sublingual immunotherapy: A dose-finding study. Front. Immunol. 2024, 15, 1431351. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, G.S. Applications of mannose-binding lectins and mannan glycoconjugates in nanomedicine. J. Nanopart. Res. 2022, 24, 228. [Google Scholar] [CrossRef]
- Kaushik, D.; Kaur, A.; Petrovsky, N.; Salunke, D.B. Structural evolution of toll-like receptor 7/8 agonists from imidazoquinolines to imidazoles. RSC Med. Chem. 2021, 12, 1065–1120. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Cui, C.; Wang, Y.; Sun, X.; Wang, S.; Yang, M.; Yu, Y.; Wang, L. CpG ODN as an adjuvant arouses the vigor of B cells by relieving the negative regulation of surface TLR9 to enhance the antibody response to vaccine. Appl. Microbiol. Biotechnol. 2021, 105, 4213–4224. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).