Antiviral Inactivated Vaccines: Looking to the Past to Face the Future—A Narrative Review
Abstract
1. Introduction
2. Pandemics/Endemics: Historical Background, the Development of Vaccines and the Impact on Global Health
3. Classical Vaccines: Mitigation of Infectious Diseases
A Brief Comparative Overview of Live-Attenuated and Inactivated Vaccines
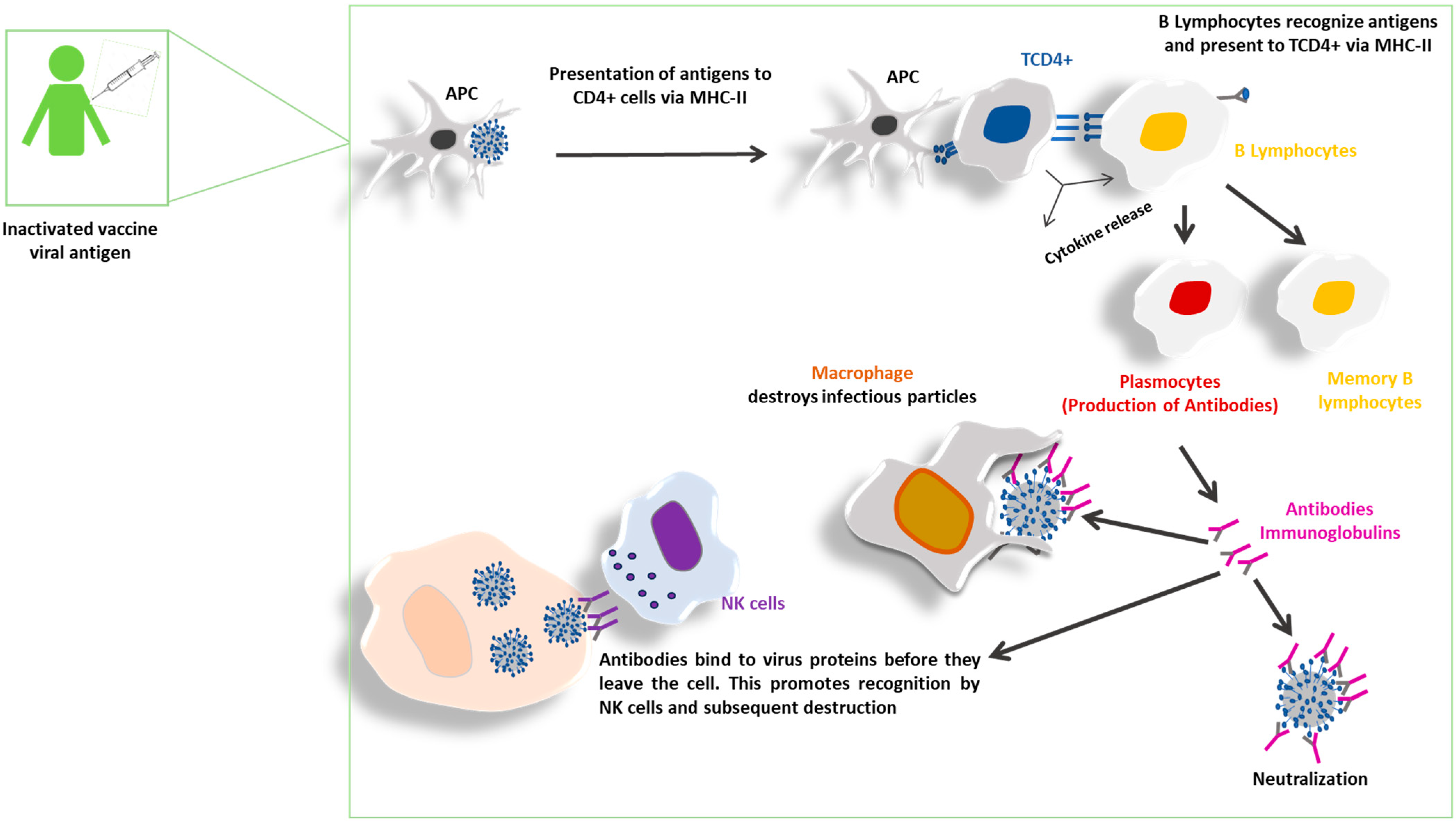
4. Inactivated Vaccines: Potential to Induce Immune Response
5. High Hydrostatic Pressure (HHP): A Potential Platform for the Production of Inactivated Viral Vaccines
6. Safety: Inactivated Vaccines Versus Modern Platforms (Recombinant, mRNA, DNA and Vectored)
6.1. Inactivated and Recombinant Vaccines
6.2. mRNA and DNA Vaccines
6.3. Viral Vector Vaccines
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HHP | High Hydrostatic Pressure |
| WHO | World Health Organization |
| COVID-19 | Coronavirus Disease 2019 |
| HIV | human immunodeficiency virus |
| HCV | Hepatitis C virus |
| IPV | Inactivated Poliomyelitis Vaccine |
| OPV | Oral Poliomyelitis Vaccine |
| (VAPP) | vaccine-associated paralytic polio |
| (cVDPV) | circulating vaccine-derived poliovirus |
| BPL | β-Propiolactone |
| APCs | Antigen-Presenting Cells |
| MHC | Major Histocompatibility Complex |
| Th1 | T helper 1 |
| Th2 | T helper 2 |
| IgG | Immunoglobulin G |
| FMDV | Foot-and-Mouth Disease Virus |
| VSV | Vesicular Stomatitis Virus |
| CpG | Cytosine-phosphate-Guanine |
| Poly I:C | Polyinosinic:polycytidylic Acid |
| PAMP | Pathogen-Associated Molecular Pattern |
| Fc | Fragment crystallizable (antibody region) |
| Bis-ANS | 4,4′-Dianilino-1,1′-binaphthyl-5,5′-disulfonic Acid |
| ChAdOx1 | Chimpanzee Adenovirus Oxford 1 |
| Ad26.CoV2.S | Adenovirus serotype 26-vectored COVID-19 vaccine |
References
- Enders, J.F.; Weller, T.H.; Robbins, F.C. Cultivation of the Lansing Strain of Poliomyelitis Virus in Cultures of Various Human Embryonic Tissues. Science 1949, 109, 85–87. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Pandemics Throughout History. Front. Microbiol. 2020, 11, 631736. [Google Scholar] [CrossRef]
- Lindahl, J.F.; Grace, D. The consequences of human actions on risks for infectious diseases: A review. Infect. Ecol. Epidemiol. 2015, 5, 30048. [Google Scholar] [CrossRef]
- Littman, R.J.; Littman, M.L. Galen and the Antonine plague. Am. J. Philol. 1973, 94, 243–255. [Google Scholar] [CrossRef]
- Mordechai, L.; Eisenberg, M.; Newfield, T.P.; Izdebski, A.; Kay, J.E.; Poinar, H. The Justinianic Plague: An inconsequential pandemic? Proc. Natl. Acad. Sci. USA 2019, 116, 25546–25554. [Google Scholar] [CrossRef] [PubMed]
- Zietz, B.P.; Dunkelberg, H. The history of the plague and the research on the causative agent Yersinia pestis. Int. J. Hyg. Environ. Health 2004, 207, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Faruque, S.M.; Siddique, A.K.; Saha, M.N.; Rahman, M.M.; Zaman, K.; Albert, M.J.; Sack, D.A.; Sack, R.B. Molecular characterization of a new ribotype of Vibrio cholerae O139 Bengal associated with an outbreak of cholera in Bangladesh. J. Clin. Microbiol. 1999, 37, 1313–1318. [Google Scholar] [CrossRef]
- Safa, A.; Nair, G.B.; Kong, R.Y. Evolution of new variants of Vibrio cholerae O1. Trends Microbiol. 2010, 18, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.E.; Semple, D. On the Employment of Dead Bacteria in the Serum Diagnosis of Typhoid and Malta Fever, and on an Easy Method of Extemporising a Blowpipe Flame for Making Capillary Sero-Sedimentation Tubes. Br. Med. J. 1897, 1, 1214–1215. [Google Scholar] [CrossRef]
- Wright, A.E.; Semple, D. Remarks on Vaccination Against Typhoid Fever. Br. Med. J. 1897, 1, 256–259. [Google Scholar] [CrossRef][Green Version]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef]
- Berche, P. Life and death of smallpox. Presse Medicale 2022, 51, 104117. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. History of Small Pox Vaccine. 2025. Available online: https://www-who-int.translate.goog/news-room/spotlight/history-of-vaccination/history-of-smallpox-vaccination?_x_tr_sl=en&_x_tr_tl=pt&_x_tr_hl=pt&_x_tr_pto=tc (accessed on 17 June 2025).
- Control, C.F.D. History of Smallpox. 2024. Available online: https://www.cdc.gov/smallpox/about/history.html (accessed on 17 June 2025).
- Jenner, E. On the Origin of the Vaccine Inoculation. Med. Phys. J. 1801, 5, 505–508. [Google Scholar]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef]
- Worobey, M.; Han, G.Z.; Rambaut, A. Genesis and pathogenesis of the 1918 pandemic H1N1 influenza A virus. Proc. Natl. Acad. Sci. USA 2014, 111, 8107–8112. [Google Scholar] [CrossRef]
- Patterson, K.D. Pandemic Influenza, 1700–1900: A Study in Historical Epidemiology; Rowan & Littlefield: Totowa, NJ, USA, 1986; ISBN: 978-0847675128. [Google Scholar]
- Kousoulis, A.A. The 1889-90 flu pandemic in Greece: A social, cultural and economic history with lessons for the 21(st) century. Infez. Med. 2023, 31, 411–420. [Google Scholar] [PubMed]
- Brussow, H.; Brussow, L. Clinical evidence that the pandemic from 1889 to 1891 commonly called the Russian flu might have been an earlier coronavirus pandemic. Microb. Biotechnol. 2021, 14, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Erkoreka, A.; Hernando-Perez, J.; Ayllon, J. Coronavirus as the Possible Causative Agent of the 1889–1894 Pandemic. Infect Dis. Rep. 2022, 14, 453–469. [Google Scholar] [CrossRef]
- Valleron, A.-J.; Cori, A.; Valtat, S.; Meurisse, S.; Carrat, F.; Boelle, P.-Y. Transmissibility and geographic spread of the 1889 influenza pandemic. Proc. Natl. Acad. Sci. USA 2010, 107, 8778–8781. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Kash, J.C.; Morens, D.M. The 1918 influenza pandemic: 100 years of questions answered and unanswered. Sci. Transl. Med. 2019, 11, eeaau5485. [Google Scholar] [CrossRef]
- Kawaoka, Y.; Krauss, S.; Webster, R.G. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J. Virol. 1989, 63, 4603–4608. [Google Scholar] [CrossRef] [PubMed]
- Viboud, C.; Simonsen, L.; Fuentes, R.; Flores, J.; Miller, M.A.; Chowell, G. Global Mortality Impact of the 1957–1959 Influenza Pandemic. J. Infect. Dis. 2016, 213, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. 1968 Pandemic (H3N2 Virus). 2 January 2019. Available online: https://archive.cdc.gov/www_cdc_gov/flu/pandemic-resources/1968-pandemic.html (accessed on 2 October 2025).
- Jester, B.J.; Uyeki, T.M.; Jernigan, D.B. Fifty Years of Influenza A(H3N2) Following the Pandemic of 1968. Am. J. Public Heal. 2020, 110, 669–676. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Data on the Size of the HIV Epidemic. 2025. Available online: https://www.who.int/data/gho/data/themes/hiv-aids/data-on-the-size-of-the-hiv-aids-epidemic (accessed on 2 October 2025).
- Merson, M.H.; O’MAlley, J.; Serwadda, D.; Apisuk, C. The history and challenge of HIV prevention. Lancet 2008, 372, 475–488. [Google Scholar] [CrossRef]
- Choo, Q.L.; Kuo, G.; Weiner, A.J.; Overby, L.R.; Bradley, D.W.; Houghton, M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 1989, 244, 359–362. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis C. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 2 October 2025).
- Krekulova, L.; Honzak, R.; Riley, L.W. Viral hepatitis C pandemic: Challenges and threats to its elimination. J. Viral Hepat. 2021, 28, 694–698. [Google Scholar] [CrossRef]
- Easterbrook, J.D.; Kash, J.C.; Sheng, Z.-M.; Qi, L.; Gao, J.; Kilbourne, E.D.; Eichelberger, M.C.; Taubenberger, J.K. Immunization with 1976 swine H1N1- or 2009 pandemic H1N1-inactivated vaccines protects mice from a lethal 1918 influenza infection. Influenza Other Respir. Viruses 2011, 5, 198–205. [Google Scholar] [CrossRef]
- Li, Y.T.; Linster, M.; Mendenhall, I.H.; Su, Y.C.F.; Smith, G.J.D. Avian influenza viruses in humans: Lessons from past outbreaks. Br. Med. Bull. 2019, 132, 81–95. [Google Scholar] [CrossRef]
- Saúde, O.P.-A.D. Perguntas e Respostas: SARS-CoV-2 na América Latina e no Caribe, 4 Anos Depois. 2024. Available online: https://www.paho.org/pt/noticias/23-2-2024-perguntas-e-respostas-sars-cov-2-na-america-latina-e-no-caribe-4-anos-depois#:~:text=Qual%20%C3%A9%20a%20probabilidade%20de,constante%20e%20a%20resposta%20r%C3%A1pida (accessed on 17 June 2025).
- World Health Organization. Pathogens Prioritization: A Scientific Framework for Epidemic and Pandemic Research Preparedness. 2024. Available online: https://www.who.int/publications/m/item/pathogens-prioritization-a-scientific-framework-for-epidemic-and-pandemic-research-preparedness (accessed on 23 September 2025).
- Salk, J.E.; Bennett, B.L.; Lewis, L.J.; Ward, E.N.; Youngner, J.S. Studies in human subjects on active immunization against poliomyelitis. I. A preliminary report of experiments in progress. J. Am. Med. Assoc. 1953, 151, 1081–1098. [Google Scholar] [CrossRef]
- Salk, J.E. Recent studies on immunization against poliomyelitis. Pediatrics 1953, 12, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Juskewitch, J.E.; Tapia, C.J.; Windebank, A.J. Lessons from the Salk polio vaccine: Methods for and risks of rapid translation. Clin. Transl. Sci. 2010, 3, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Offit, P.A. The Cutter incident, 50 years later. N. Engl. J. Med. 2005, 352, 1411–1412. [Google Scholar] [CrossRef]
- Global Polio Eradication Initiative. Polio Eradication & Endgame Strategy Plan 2013–2018; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Kew, O.M.; Wright, P.F.; Agol, V.I.; Delpeyroux, F.; Shimizu, H.; Nathanson, N.; A Pallansch, M. Circulating vaccine-derived polioviruses: Current state of knowledge. Bull. World Health Organ. 2004, 82, 16–23. [Google Scholar]
- Eze, O.V.; Meyer, J.C.; Campbell, S.M. Poliomyelitis in Nigeria: Impact of Vaccination Services and Polio Intervention and Eradication Efforts. Vaccines 2025, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Grotto, I.; Agha, H.; Abu Al-Halaweh, A.; Davidovitch, N.; McKee, M.; Nitzan, D. Public health, war and cross-border challenges: The recent cVDPV2 polio outbreak in Gaza. eClinicalMedicine 2025, 81, 103136. [Google Scholar] [CrossRef]
- Platt, L.R.; Estivariz, C.F.; Sutter, R.W. Vaccine-associated paralytic poliomyelitis: A review of the epidemiology and estimation of the global burden. J. Infect. Dis. 2014, 210 (Suppl. S1), S380–S389. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Polio Vaccination; CDC: Bethesda, MD, USA, 2024.
- Barrett, P.N.; Mundt, W.; Kistner, O.; Howard, M.K. Vero cell platform in vaccine production: Moving towards cell culture-based viral vaccines. Expert Rev. Vaccines 2009, 8, 607–618. [Google Scholar] [CrossRef]
- Fang, Z.; Lyu, J.; Li, J.; Li, C.; Zhang, Y.; Guo, Y.; Wang, Y.; Zhang, Y.; Chen, K. Application of bioreactor technology for cell culture-based viral vaccine production: Present status and future prospects. Front. Bioeng. Biotechnol. 2022, 10, 921755. [Google Scholar] [CrossRef]
- Fermi, C. Uber die Immunisierung gegen Wutkrankheit. Z. Hyg. Infectionskrankh. 1908, 58, 233–276. [Google Scholar] [CrossRef]
- Madhusudana, S.N.; Shamsundar, R.; Seetharaman, S. In Vitro inactivation of the rabies virus by ascorbic acid. Int. J. Infect. Dis. 2004, 8, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Larghi, O.P.; Nebel, A.E. Rabies virus inactivation by binary ethylenimine: New method for inactivated vaccine production. J. Clin. Microbiol. 1980, 11, 120–122. [Google Scholar] [CrossRef]
- Amanna, I.J.; Raue, H.P.; Slifka, M.K. Development of a new hydrogen peroxide-based vaccine platform. Nat. Med. 2012, 18, 974–979. [Google Scholar] [CrossRef]
- Budowsky, E.I.; Bresler, S.E.; Friedman, E.A.; Zheleznova, N.V. Principles of selective inactivation of viral genome. I. UV-induced inactivation of influenza virus. Arch. Virol. 1981, 68, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kochel, T.J.; Maves, R.C.; Porter, K. Psoralen-Inactivated Viral Vaccine and Method of Preparation. U.S. Patent 9005633B2, 19 March 2015. [Google Scholar]
- Schneider, K.; Wronka-Edwards, L.; Leggett-Embrey, M.; Walker, E.; Sun, P.; Ondov, B.; Wyman, T.H.; Rosovitz, M.; Bohn, S.S.; Burans, J. Psoralen Inactivation of Viruses: A Process for the Safe Manipulation of Viral Antigen and Nucleic Acid. Viruses 2015, 7, 5875–5888. [Google Scholar] [CrossRef] [PubMed]
- Eckels, K.H.; Putnak, R. Formalin-inactivated whole virus and recombinant subunit flavivirus vaccines. Adv. Virus Res. 2003, 61, 395–418. [Google Scholar]
- Elveborg, S.; Monteil, V.M.; Mirazimi, A. Methods of Inactivation of Highly Pathogenic Viruses for Molecular, Serology or Vaccine Development Purposes. Pathogens 2022, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.R.; Jiang, Y.W.; Li, F.X.; Liu, D.; Lin, T.F.; Zhao, Z.Y.; Wei, C.; Jin, Q.Y.; Li, X.M.; Jia, Y.X.; et al. Efficacy of SARS-CoV-2 vaccines and the dose-response relationship with three major antibodies: A systematic review and meta-analysis of randomised controlled trials. Lancet Microbe 2023, 4, e236–e246. [Google Scholar] [CrossRef]
- Bull, J.J. Evolutionary reversion of live viral vaccines: Can genetic engineering subdue it? Virus Evol. 2015, 1, vev005. [Google Scholar] [CrossRef]
- Ljungman, P. Vaccination of immunocompromised patients. Clin. Microbiol. Infect. 2012, 18 (Suppl. S5), 93–99. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Saúde, O.P.-A.D. OMS Valida Vacina Contra COVID-19 da Sinovac Para Uso Emergencial e Emite Recomendações de Políticas Provisórias. 2021. Available online: https://www.paho.org/pt/noticias/1-6-2021-oms-valida-vacina-contra-covid-19-da-sinovac-para-uso-emergencial-e-emite (accessed on 28 July 2025).
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.Ş.; Akalın, E.H.; Tabak, Ö.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.A.R.; Correa, A.S.; de Jesus, T.A.; Bortolini, M.J.S.; Taketomi, E.A.; Resende, R.d.O. Distinct Adverse Reactions to mRNA, Inactivated Virus, and Adenovirus Vector COVID-19 Vaccines: Insights from a Cohort Study on Atopic and Non-Atopic Subjects in Brazil. Vaccines 2024, 12, 408. [Google Scholar] [CrossRef]
- Lounis, M.; Aouissi, H.A.; Abdelhadi, S.; Rais, M.A.; Belkessa, S.; Bencherit, D. Short-Term Adverse Effects Following Booster Dose of Inactivated-Virus vs. Adenoviral-Vector COVID-19 Vaccines in Algeria: A Cross-Sectional Study of the General Population. Vaccines 2022, 10, 1781. [Google Scholar] [CrossRef]
- Chong, C.Y.; Kam, K.-Q.; Zhang, J.; Bertoletti, A.; Hariharaputran, S.; Sultana, R.; Piragasam, R.; Mah, Y.-Y.; Tan, C.-W.; Wang, L.; et al. Immunogenicity and safety of Sinovac-CoronaVac booster vaccinations in 12–17- year-olds with clinically significant reactions from Pfizer-BNT162b2 vaccination. Vaccine 2024, 42, 2951–2954. [Google Scholar] [CrossRef]
- Ge, H.; Cao, H.; Lv, J.; Li, X.; Lee, A.; Zou, J.; Jiang, M.; Xiao, L.; Gan, Y.; Shen, M.; et al. Efficacy of influenza vaccines and its relationship with immunological surrogate endpoints: A systematic review and meta-analysis of RCT. Clin. Microbiol. Infect. 2025, in press. [Google Scholar]
- Lee Ho, P.; Medina-Armenteros, Y.; Dati, L.M.M.; Cajado-Carvalho, D.; Silva, C.S.; Campos, P.F.; Abreu, P.A.E.; de Castro, J.T.; Tonolli, P.N.; Fujimori, M.; et al. Production and Immune Response Against Pandemic Influenza Candidate Vaccines as Preparedness Against the Circulating H5N1 Influenza Viruses. Vaccines 2025, 13, 620. [Google Scholar] [CrossRef] [PubMed]
- Nuwarda, R.F.; Alharbi, A.A.; Kayser, V. An Overview of Influenza Viruses and Vaccines. Vaccines 2021, 9, 1032. [Google Scholar] [CrossRef]
- Oude Blenke, E.; Örnskov, E.; Schöneich, C.; Nilsson, G.A.; Volkin, D.B.; Mastrobattista, E.; Almarsson, Ö.; Crommelin, D.J. The Storage and In-Use Stability of mRNA Vaccines and Therapeutics: Not A Cold Case. J. Pharm. Sci. 2023, 112, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [PubMed]
- Firmino-Cruz, L.; Dos-Santos, J.S.; da Fonseca-Martins, A.M.; Oliveira-Maciel, D.; Guadagnini-Perez, G.; Roncaglia-Pereira, V.A.; Dumard, C.H.; Guedes-Da-Silva, F.H.; Santos, A.C.V.; Alvim, R.G.F.; et al. Intradermal Immunization of SARS-CoV-2 Original Strain Trimeric Spike Protein Associated to CpG and AddaS03 Adjuvants, but Not MPL, Provide Strong Humoral and Cellular Response in Mice. Vaccines 2022, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Dos-Santos, J.S.; Firmino-Cruz, L.; da Fonseca-Martins, A.M.; Oliveira-Maciel, D.; Perez, G.G.; Roncaglia-Pereira, V.A.; Dumard, C.H.; Guedes-Da-Silva, F.H.; Santos, A.C.V.; Leandro, M.D.S.; et al. Immunogenicity of SARS-CoV-2 Trimeric Spike Protein Associated to Poly(I:C) Plus Alum. Front. Immunol. 2022, 13, 884760. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef]
- Kanuri, S.H.; Sirrkay, P.J. Adjuvants in COVID-19 vaccines: Innocent bystanders or culpable abettors for stirring up COVID-heart syndrome. Ther. Adv. Vaccines Immunother. 2024, 12, 25151355241228439. [Google Scholar] [CrossRef]
- Clem, A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.P.; Jimeno, J.; Han, H.H.; Lin, S.; Hartmann, K.; Borkowski, A.; Sáez-Llorens, X. Safety and immunogenicity of experimental stand-alone trivalent, inactivated Sabin-strain polio vaccine formulations in healthy infants: A randomized, observer-blind, controlled phase 1/2 trial. Vaccine 2020, 38, 5313–5323. [Google Scholar] [CrossRef]
- Wilde, J.A.; McMillan, J.A.; Serwint, J.; Butta, J.; O’Riordan, M.A.; Steinhoff, M.C. Effectiveness of influenza vaccine in health care professionals: A randomized trial. JAMA. 1999, 281, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, K.M.; Dupont, W.D.; Wright, P.F.; Edwards, K.M. Efficacy of inactivated and cold-adapted vaccines against influenza A infection, 1985 to 1990: The pediatric experience. Pediatr. Infect. Dis. J. 2001, 20, 733–740. [Google Scholar] [CrossRef]
- Edwards, K.M.; Dupont, W.D.; Westrich, M.K.; Plummer, W.D.; Palmer, P.S.; Wright, P.F. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J. Infect. Dis. 1994, 169, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.E.; D’HOndt, E.; Delem, A.; Safary, A. Clinical assessment of the safety and efficacy of an inactivated hepatitis A vaccine: Rationale and summary of findings. Vaccine 1992, 10 (Suppl. S1), S160–S168. [Google Scholar] [CrossRef]
- Yadav, P.D.; Ella, R.; Kumar, S.; Patil, D.R.; Mohandas, S.; Shete, A.M.; Vadrevu, K.M.; Bhati, G.; Sapkal, G.; Kaushal, H.; et al. Immunogenicity and protective efficacy of inactivated SARS-CoV-2 vaccine candidate, BBV152 in rhesus macaques. Nat. Commun. 2021, 12, 1386. [Google Scholar] [CrossRef]
- Li, Z.; Xiang, T.; Liang, B.; Liu, J.; Deng, H.; Yang, X.; Wang, H.; Feng, X.; Zelinskyy, G.; Trilling, M.; et al. SARS-CoV-2-specific T cell responses wane profoundly in convalescent individuals 10 months after primary infection. Virol. Sin. 2023, 38, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, Y.; Yang, R.; Tan, W. SARS-CoV-2-specific T cell immunity to structural proteins in inactivated COVID-19 vaccine recipients. Cell. Mol. Immunol. 2021, 18, 2040–2041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Liu, D.; Zeng, Q.; Li, L.; Zhou, Q.; Li, M.; Mei, J.; Yang, N.; Mo, S.; et al. Time of day influences immune response to an inactivated vaccine against SARS-CoV-2. Cell Res. 2021, 31, 1215–1217. [Google Scholar] [CrossRef] [PubMed]
- DeBenedictis, J.N.; de Kok, T.M.; van Breda, S.G. Impact of Processing Method and Storage Time on Phytochemical Concentrations in an Antioxidant-Rich Food Mixture. Antioxidants 2023, 12, 1252. [Google Scholar] [CrossRef]
- Silva, J.L.; Oliveira, A.C.; Vieira, T.C.R.G.; de Oliveira, G.A.P.; Suarez, M.C.; Foguel, D. High-pressure chemical biology and biotechnology. Chem. Rev. 2014, 114, 7239–7267. [Google Scholar] [CrossRef]
- Weber, G.; Drickamer, H.G. The effect of high pressure upon proteins and other biomolecules. Q. Rev. Biophys. 1983, 16, 89–112. [Google Scholar] [CrossRef]
- Dumard, C.H.; Barroso, S.P.C.; Oliveira, G.A.P.D.; Carvalho, C.A.M.; Gomes, A.M.O.; Couceiro, J.N.S.S.; Ferreira, D.F.; Nico, D.; Oliveira, A.C.; Silva, J.L.; et al. Full inactivation of human influenza virus by high hydrostatic pressure preserves virus structure and membrane fusion while conferring protection to mice against infection. PLoS ONE 2013, 8, e80785. [Google Scholar] [CrossRef][Green Version]
- Freitas, M.; Da Poian, A.T.; Barth, O.M.; Rebello, M.A.; Silva, J.L.; Gaspar, L.P. The fusogenic state of Mayaro virus induced by low pH and by hydrostatic pressure. Cell Biochem. Biophys. 2006, 44, 325–335. [Google Scholar] [CrossRef]
- Gomes, A.M.; Pinheiro, A.S.; Bonafe, C.F.S.; Silva, J.L. Pressure-induced fusogenic conformation of vesicular stomatitis virus glycoprotein. Biochemistry 2003, 42, 5540–5546. [Google Scholar] [CrossRef] [PubMed]
- Pontes, L.; Cordeiro, Y.; Giongo, V.; Villas-Boas, M.; Barreto, A.; Araújo, J.R.; Silva, J.L. Pressure-induced formation of inactive triple-shelled rotavirus particles is associated with changes in the spike protein Vp4. J. Mol. Biol. 2001, 307, 1171–1179. [Google Scholar] [CrossRef]
- Basset, J.; Lepine, P.; Chaumont, L. Effects of high pressures on the poliomyelitis virus (Lansing strain). Ann. Inst. Pasteur 1956, 90, 575–593. [Google Scholar]
- Chen, Z.M.; Tian, S.M.; Ruan, K.C. A Vaccine to Coxsackievirus Prepared by High Pressure. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao 2001, 33, 128–130. [Google Scholar] [PubMed]
- Ishimaru, D.; Sa-Carvalho, D.; Silva, J.L. Pressure-inactivated FMDV: A potential vaccine. Vaccine 2004, 22, 2334–2339. [Google Scholar] [CrossRef]
- Ferreira, E.; Mendes, Y.S.; Silva, J.L.; Galler, R.; Oliveira, A.C.; Freire, M.S.; Gaspar, L.P. Effects of hydrostatic pressure on the stability and thermostability of poliovirus: A new method for vaccine preservation. Vaccine 2009, 27, 5332–5337. [Google Scholar] [CrossRef][Green Version]
- Tian, S.M.; Ruan, K.; Qian, J.; Shao, G.; Balny, C. Effects of hydrostatic pressure on the structure and biological activity of infectious bursal disease virus. Eur. J. Biochem. 2000, 267, 4486–4494. [Google Scholar] [CrossRef] [PubMed]
- Da Poian, A.T.; Gomes, A.M.; Oliveira, R.J.; Silva, J.L. Migration of vesicular stomatitis virus glycoprotein to the nucleus of infected cells. Proc. Natl. Acad. Sci. USA 1996, 93, 8268–8273. [Google Scholar] [CrossRef]
- Silva, J.L.; Luan, P.; Glaser, M.; Voss, E.W.; Weber, G. Effects of hydrostatic pressure on a membrane-enveloped virus: High immunogenicity of the pressure-inactivated virus. J. Virol. 1992, 66, 2111–2117. [Google Scholar] [CrossRef]
- Barroso, S.P.; Nico, D.; Nascimento, D.; Santos, A.C.V.; Couceiro, J.N.S.S.; A Bozza, F.; A Ferreira, A.M.; Ferreira, D.F.; Palatnik-De-Sousa, C.B.; Souza, T.M.L.; et al. Intranasal Immunization with Pressure Inactivated Avian Influenza Elicits Cellular and Humoral Responses in Mice. PLoS ONE 2015, 10, e0128785. [Google Scholar] [CrossRef][Green Version]
- Gaspar, L.P.; Mendes, Y.S.; Yamamura, A.M.; Almeida, L.F.; Caride, E.; Gonçalves, R.B.; Silva, J.L.; Oliveira, A.C.; Galler, R.; Freire, M.S. Pressure-inactivated yellow fever 17DD virus: Implications for vaccine development. J. Virol. Methods 2008, 150, 57–62. [Google Scholar] [CrossRef]
- Dumard, C.H.; Barroso, S.P.; Santos, A.C.V.; Alves, N.S.; Couceiro, J.N.S.; Gomes, A.M.; Santos, P.S.; Silva, J.L.; Oliveira, A.C. Stability of different influenza subtypes: How can high hydrostatic pressure be a useful tool for vaccine development? Biophys. Chem. 2017, 231, 116–124. [Google Scholar] [CrossRef]
- Brandolini, M.; Rocculi, P.; Morbarigazzi, M.; De Pascali, A.M.; Dirani, G.; Zannoli, S.; Lelli, D.; Lavazza, A.; Battioni, F.; Grumiro, L.; et al. Development and in vivo evaluation of a SARS-CoV-2 inactivated vaccine using high hydrostatic pressure. NPJ Vaccines 2025, 10, 83. [Google Scholar] [CrossRef]
- Lim, W.W.; Mak, L.; Leung, G.M.; Cowling, B.J.; Peiris, M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe 2021, 2, e423. [Google Scholar] [CrossRef] [PubMed]
- Bouazzaoui, A.; Abdellatif, A.A.H.; Al-Allaf, F.A.; Bogari, N.M.; Al-Dehlawi, S.; Qari, S.H. Strategies for Vaccination: Conventional Vaccine Approaches Versus New-Generation Strategies in Combination with Adjuvants. Pharmaceutics 2021, 13, 140. [Google Scholar] [CrossRef]
- Wang, K.; Guo, Z.; Zeng, T.; Sun, S.; Lu, Y.; Wang, J.; Li, S.; Luan, Z.; Li, H.; Zhang, J.; et al. Transmission Characteristics and Inactivated Vaccine Effectiveness Against Transmission of SARS-CoV-2 Omicron BA.5 Variants in Urumqi, China. JAMA Netw. Open 2023, 6, e235755. [Google Scholar] [CrossRef]
- Blumberg, D.; Sridhar, A.; Lakshminrusimha, S.; Higgins, R.D.; Saade, G. COVID-19 Vaccine Considerations during Pregnancy and Lactation. Am. J. Perinatol. 2021, 38, 523–528. [Google Scholar] [CrossRef]
- Parikh, R.; Singer, D.; Chmielewski-Yee, E.; Dessart, C. Effectiveness and safety of recombinant zoster vaccine: A review of real-world evidence. Hum. Vaccines Immunother. 2023, 19, 2263979. [Google Scholar] [CrossRef] [PubMed]
- Giles, M.L.; Krishnaswamy, S.; Macartney, K.; Cheng, A. The safety of inactivated influenza vaccines in pregnancy for birth outcomes: A systematic review. Hum. Vaccines Immunother. 2019, 15, 687–699. [Google Scholar] [CrossRef] [PubMed]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef]
- Ambrose, C.S.; Levin, M.J.; Belshe, R.B. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respir. Viruses 2011, 5, 67–75. [Google Scholar] [CrossRef]
- van den Ende, C.; Marano, C.; van Ahee, A.; Bunge, E.M.; De Moerlooze, L. The immunogenicity of GSK’s recombinant hepatitis B vaccine in children: A systematic review of 30 years of experience. Expert Rev. Vaccines 2017, 16, 789–809. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Ribeiro, A.C.; Aikawa, N.E.; Saad, C.G.S.; Yuki, E.F.N.; Pedrosa, T.; Fusco, S.R.G.; Rojo, P.T.; Pereira, R.M.R.; Shinjo, S.K.; Andrade, D.C.O.; et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: A phase 4 trial. Nat. Med. 2021, 27, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Kroehl, M.E.; Johnson, M.J.; Hammes, A.; Reinhold, D.; Lang, N.; Weinberg, A. Th1 memory differentiates recombinant from live herpes zoster vaccines. J. Clin. Investig. 2018, 128, 4429–4440. [Google Scholar] [CrossRef]
- Ogawa, T.; Yamada, T.; Matsumoto, Y.; Minami, K.; Kawanishi, F.; Nakano, T.; Ukimura, A. Adverse events after administration of the first and second doses of messenger RNA-based COVID-19 vaccines in Japanese subjects aged 12–18 years. J. Int. Med. Res. 2022, 50, 3000605221127518. [Google Scholar] [CrossRef] [PubMed]
- Dadras, O.; Mehraeen, E.; Karimi, A.; Tantuoyir, M.M.; Afzalian, A.; Nazarian, N.; Mojdeganlou, H.; Mirzapour, P.; Shamsabadi, A.; Dashti, M.; et al. Safety and Adverse Events Related to Inactivated COVID-19 Vaccines and Novavax;a Systematic Review. Arch. Acad. Emerg. Med. 2022, 10, e54. [Google Scholar]
- Demongeot, J.; Fougere, C. mRNA COVID-19 Vaccines-Facts and Hypotheses on Fragmentation and Encapsulation. Vaccines 2022, 11, 40. [Google Scholar] [CrossRef]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US from December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef]
- Avci, E.; Abasiyanik, F. Autoimmune hepatitis after SARS-CoV-2 vaccine: New-onset or flare-up? J. Autoimmun. 2021, 125, 102745. [Google Scholar] [CrossRef]
- Khan, K.H. DNA vaccines: Roles against diseases. Germs 2013, 3, 26–35. [Google Scholar] [CrossRef]
- Kozak, M.; Hu, J. DNA Vaccines: Their Formulations, Engineering and Delivery. Vaccines 2024, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.; Poh, C.L. The development of DNA vaccines against SARS-CoV-2. Adv. Med. Sci. 2023, 68, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Kariko, K.; Tureci, O. mRNA-based therapeutics-developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Nguyen, M.; Martin, D.; DeStefano, F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2015, 33, 4398–4405. [Google Scholar] [CrossRef]
- Garg, I.; Shekhar, R.; Sheikh, A.B.; Pal, S. COVID-19 Vaccine in Pregnant and Lactating Women: A Review of Existing Evidence and Practice Guidelines. Infect. Dis. Rep. 2021, 13, 685–699. [Google Scholar] [CrossRef]
- Abrignani, M.G.; Murrone, A.; De Luca, L.; Roncon, L.; Di Lenarda, A.; Valente, S.; Caldarola, P.; Riccio, C.; Oliva, F.; Gulizia, M.M.; et al. COVID-19, Vaccines, and Thrombotic Events: A Narrative Review. J. Clin. Med. 2022, 11, 948. [Google Scholar] [CrossRef]
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): The interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Pandya, R.; Apostolopoulos, V. DNA vaccines for SARS-CoV-2: Toward third-generation vaccination era. Expert Rev. Vaccines 2021, 20, 1549–1560. [Google Scholar] [CrossRef]
- Lee, J.; Kumar, S.A.; Jhan, Y.Y.; Bishop, C.J. Engineering DNA vaccines against infectious diseases. Acta Biomater. 2018, 80, 31–47. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Valu, D.; Patel, B.; Kumar, A.; Prasad, S.; Kakoti, B.B.; Kaushik, A.; Jesawadawala, M. Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccines 2023, 11, 432. [Google Scholar] [CrossRef]
- Deng, S.; Liang, H.; Chen, P.; Li, Y.; Li, Z.; Fan, S.; Wu, K.; Li, X.; Chen, W.; Qin, Y.; et al. Viral Vector Vaccine Development and Application During the COVID-19 Pandemic. Microorganisms 2022, 10, 1450. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef]
- Saxena, M.; Van, T.T.H.; Baird, F.J.; Coloe, P.J.; Smooker, P.M. Pre-existing immunity against vaccine vectors—Friend or foe? Microbiology 2013, 159 Pt 1, 1–11. [Google Scholar] [CrossRef]
- Ura, T.; Okuda, K.; Shimada, M. Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624–641. [Google Scholar] [CrossRef] [PubMed]
- Frasca, L.; Ocone, G.; Palazzo, R. Safety of COVID-19 Vaccines in Patients with Autoimmune Diseases, in Patients with Cardiac Issues, and in the Healthy Population. Pathogens 2023, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- See, I.; Su, J.R.; Lale, A.; Woo, E.J.; Guh, A.Y.; Shimabukuro, T.T.; Streiff, M.B.; Rao, A.K.; Wheeler, A.P.; Beavers, S.F.; et al. US Case Reports of Cerebral Venous Sinus Thrombosis with Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA 2021, 325, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Yaamika, H.; Muralidas, D.; Elumalai, K. Review of adverse events associated with COVID-19 vaccines, highlighting their frequencies and reported cases. J. Taibah Univ. Med. Sci. 2023, 18, 1646–1661. [Google Scholar] [CrossRef]
- Graham, B.S. Rapid COVID-19 vaccine development. Science 2020, 368, 945–946. [Google Scholar] [CrossRef]

| Method | Mechanism | Advantages | Limitations/Risks |
|---|---|---|---|
| Formaldehyde inactivation | Covalently cross-links viral proteins and nucleic acids, blocking replication. |
|
|
| β-propiolactone (BPL) | Alkylates nucleic acids and some amino acid residues, rapidly inactivating viral genomes. |
|
|
| High Hydrostatic Pressure (HHP) | Disrupts non-covalent interactions in viral capsid/envelope, preserving many structural epitopes. |
|
|
| Vaccine | Definition | Security | Infection | Adverse Effect | Adjuvant | Stability | Immunogenicity | Vulnerability |
|---|---|---|---|---|---|---|---|---|
| Inactivated | Inactivated pathogen | Mild reactions | Null, pathogens are dead and do not replicate | Local reactions, mild fever, fatigue and muscle pain | Requires adjuvants | High stability, simple storage (2–8 °C) | Need of booster doses | Safe for vulnerable groups |
| RNA | Messenger RNA that encodes viral proteins | Adverse events are rare | Null, RNA is rapidly degraded | Fever, fatigue, local pain, headache, mild lymphadenopathy | Require adjuvants | Lower stability; requires deep freezing (−70 °C) | Superior to inactivated and viral vector. Need of booster doses | Ongoing studies in vulnerable groups |
| DNA | DNA plasmids that encode viral antigens | Discussion about risks of integration into genome | Null, plasmid DNA does not cause infection | Local reactions, mild fever, fatigue | Requires or not adjuvants | Greater thermal stability than RNA (−70 °C) | Relatively low | Deficiencies in vulnerable groups |
| Viral Vector | Non-pathogenic virus used to deliver genetic material | Possible response to the viral vector | Null in non-replicative vectors | Local reactions, fever, fatigue, thrombocytopenia in severe cases | Does not require adjuvants | High stability, simple storage (2–8 °C) | High without the need for adjuvants | Evaluated for prior immunity to the vector |
| Recombinant | Purified viral proteins produced by genetic engineering | Low risk of adverse events | Null, proteins are not infectious | Mild reactions, local pain, fatigue, mild fever | Requires adjuvants | High stability, simple storage (2–8 °C) | Elevated if accompanied by adjuvant | Generally safe for vulnerable groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guedes-da-Silva, F.H.; Roncaglia-Pereira, V.A.; Torres, S.; García, M.C.E.; Viana, K.F.; Silva, J.L.; Oliveira, A.C.; Gomes, A.M.O. Antiviral Inactivated Vaccines: Looking to the Past to Face the Future—A Narrative Review. Vaccines 2025, 13, 1140. https://doi.org/10.3390/vaccines13111140
Guedes-da-Silva FH, Roncaglia-Pereira VA, Torres S, García MCE, Viana KF, Silva JL, Oliveira AC, Gomes AMO. Antiviral Inactivated Vaccines: Looking to the Past to Face the Future—A Narrative Review. Vaccines. 2025; 13(11):1140. https://doi.org/10.3390/vaccines13111140
Chicago/Turabian StyleGuedes-da-Silva, Francisca Hildemagna, Victor Augusto Roncaglia-Pereira, Sara Torres, María Camila Escobar García, Kelvinson Fernandes Viana, Jerson Lima Silva, Andréa Cheble Oliveira, and Andre Marco Oliveira Gomes. 2025. "Antiviral Inactivated Vaccines: Looking to the Past to Face the Future—A Narrative Review" Vaccines 13, no. 11: 1140. https://doi.org/10.3390/vaccines13111140
APA StyleGuedes-da-Silva, F. H., Roncaglia-Pereira, V. A., Torres, S., García, M. C. E., Viana, K. F., Silva, J. L., Oliveira, A. C., & Gomes, A. M. O. (2025). Antiviral Inactivated Vaccines: Looking to the Past to Face the Future—A Narrative Review. Vaccines, 13(11), 1140. https://doi.org/10.3390/vaccines13111140






