COVID-19 Vaccines Confer Protection in Hospitalized Pregnant and Postpartum Women with Severe COVID-19: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analyses
3. Results
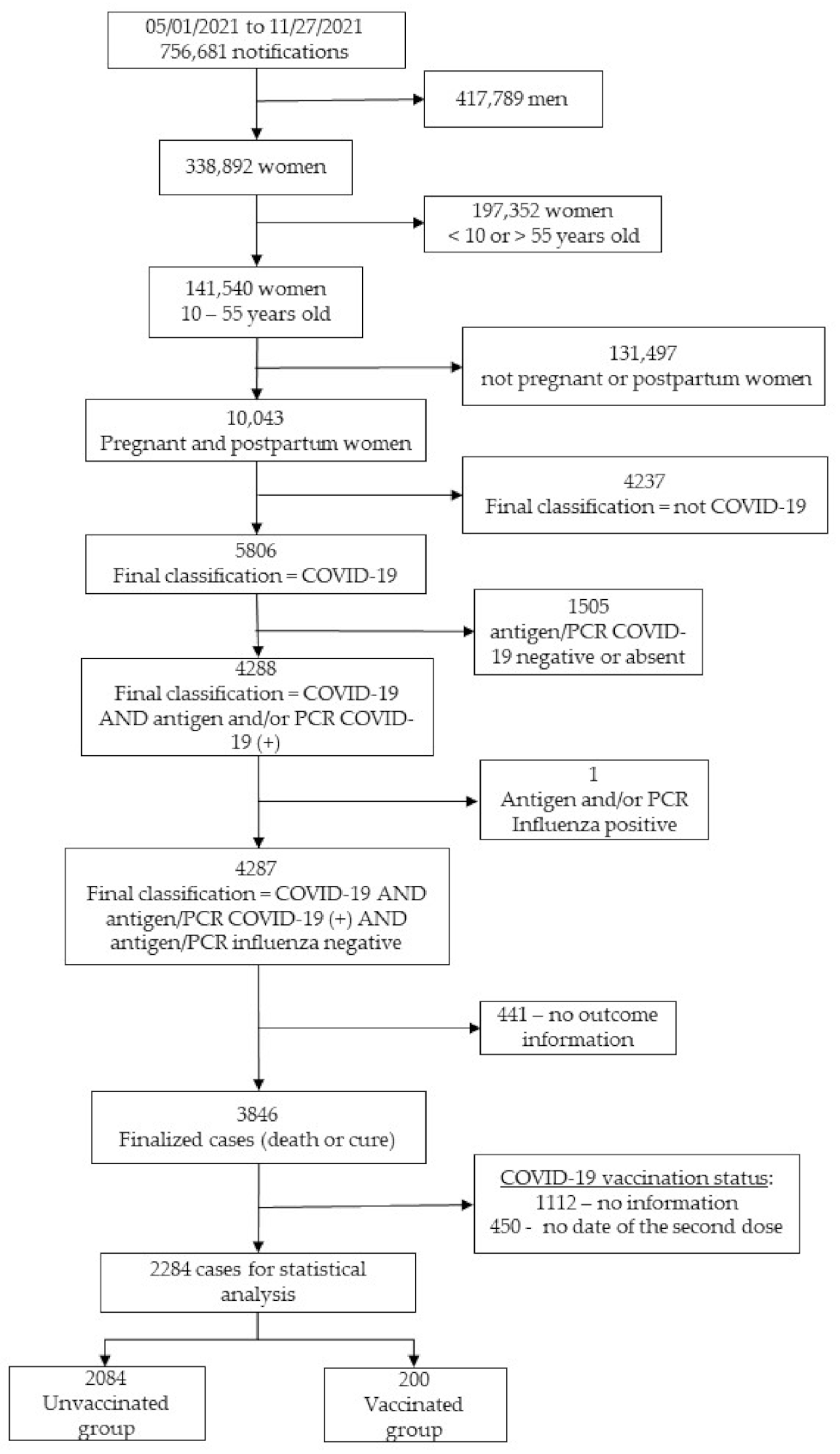
3.1. Study Population
3.2. Baseline Characteristics of Enrolled Subjects
3.3. Clinical Manifestations of COVID-19
3.4. COVID-19 Adverse Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection—The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Roberton, T.; Carter, E.D.; Chou, V.B.; Stegmuller, A.R.; Jackson, B.D.; Tam, Y.; Sawadogo-Lewis, T.; Walker, N. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: A modelling study. Lancet Glob. Health 2020, 8, e901–e908. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Rasmussen, S.A. An update on COVID-19 and pregnancy. Am. J. Obstet. Gynecol. 2022, 226, 177–186. [Google Scholar] [CrossRef]
- Lokken, E.M.; Taylor, G.G.; Huebner, E.M.; Vanderhoeven, J.; Hendrickson, S.; Coler, B.; Sheng, J.S.; Walker, C.L.; McCartney, S.A.; Kretzer, N.M.; et al. Higher severe acute respiratory syndrome coronavirus 2 infection rate in pregnant patients. Am. J. Obstet. Gynecol. 2021, 225, 75.e1–75.e16. [Google Scholar] [CrossRef]
- Rodrigues, A.; Lacerda, L.; Francisco, R.P. Brazilian Obstetric Observatory. arXiv 2021, arXiv:2105.06534. Available online: https://observatorioobstetrico.shinyapps.io/covid_gesta_puerp_br/ (accessed on 8 February 2022).
- Francisco, R.P.V.; Lacerda, L.; Rodrigues, A.S. Obstetric Observatory BRAZIL—COVID-19: 1031 maternal deaths because of COVID-19 and the unequal access to health care service. Clinics 2021, 76, e3120. [Google Scholar] [CrossRef]
- Beigi, R.H.; Krubiner, C.; Jamieson, D.J.; Lyerly, A.D.; Hughes, B.; Riley, L.; Faden, R.; Karron, R. The need for inclusion of pregnant women in COVID-19 vaccine trials. Vaccine 2021, 39, 868–870. [Google Scholar] [CrossRef]
- Shook, L.L.; Fallah, P.N.; Silberman, J.N.; Edlow, A.G. COVID-19 Vaccination in Pregnancy and Lactation: Current Research and Gaps in Understanding. Front. Cell Infect. Microbiol. 2021, 11, 735394. [Google Scholar] [CrossRef]
- Garg, I.; Shekhar, R.; Sheikh, A.B.; Pal, S. COVID-19 vaccine in pregnant and lactating women: A review of existing evidence and practice guidelines. Infect. Dis. Rep. 2021, 13, 685–699. [Google Scholar] [CrossRef]
- Krubiner, C.B.; Faden, R.R.; Karron, R.A.; Little, M.O.; Lyerly, A.D.; Abramson, J.S.; Beigi, R.H.; Cravioto, A.R.; Durbin, A.P.; Gellin, B.G.; et al. Pregnant women & vaccines against emerging epidemic threats: Ethics guidance for preparedness, research, and response. Vaccine 2021, 39, 85–120. [Google Scholar] [PubMed]
- Beeler, J.A.; Lambach, P.; Fulton, T.R.; Narayanan, D.; Ortiz, J.R.; Omer, S.B. A systematic review of ethical issues in vaccine studies involving pregnant women. Hum. Vaccines Immunother. 2016, 12, 1952–1959. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crane, M.A.; Jaffe, E.; Beigi, R.H.; Karron, R.A.; Krubiner, C.B.; Wonodi, C.B.; Faden, R.R. Priorization of pregnant individuals in state plans for coronavirus disease 2019 vaccination. Am. J. Obstet. Gynecol. 2021, 225, 95–99. [Google Scholar] [CrossRef]
- Ministério da Saúde. Secretaria Extraordinária de Enfrentamento à COVID-19. Gabinete. 6 July 2021. NOTA TÉCNICA No 2/2021-SECOVID/GAB/SECOVID/MS. Available online: https://portaldeboaspraticas.iff.fiocruz.br/wp-content/uploads/2021/09/NotaTecnica_vacinacaocovid-19gestantespuerperas.pdf (accessed on 1 October 2021).
- International Federation Gynecology and Obstetrics. COVID-19 Vaccination for Pregnant and Breastfeeding Women. 2021. Available online: https://www.figo.org/covid-19-vaccination-pregnant-and-breastfeeding-women (accessed on 16 December 2021).
- Royal College of Obstetricians and Gynaecologists. Updated Advice on COVID-19 Vaccination in Pregnancy and Women Who Are Breastfeeding. 2020, pp. 1–45. Available online: https://www.rcm.org.uk/news-views/news/2020/december/updated-advice-on-covid-19-vaccination-in-pregnancy-and-women-who-are-breastfeeding/ (accessed on 16 December 2021).
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Male, V. Are COVID-19 vaccines safe in pregnancy? Nat. Rev. Immunol. 2021, 21, 200–201. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Biron-Shental, T.; Makov-Assif, M.; Key, C.; Kohane, I.S.; Hernán, M.A.; Lipsitch, M.; Hernandez-Diaz, S.; Reis, B.Y.; et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat. Med. 2021, 27, 1693–1695. [Google Scholar] [CrossRef]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar] [CrossRef]
- Trostle, M.E.; Limaye, M.A.; Avtushka, V.; Lighter, J.L.; Penfield, C.A.; Roman, A.S. COVID-19 vaccination in pregnancy: Early experience from a single institution. Am. J. Obstet. Gynecol. MFM 2021, 3, 100464. [Google Scholar] [CrossRef]
- Mackin, D.W.; Walker, S.P. The historical aspects of vaccination in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 76, 13–22. [Google Scholar] [CrossRef]
- Sebghati, M.; Khalil, A. Uptake of vaccination in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 76, 53–65. [Google Scholar] [CrossRef]
- Stock, S.J.; Carruthers, J.; Calvert, C.; Denny, C.; Donaghy, J.; Goulding, A.; Hopcroft, L.E.; Hopkins, L.; McLaughlin, T.; Pan, J.; et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat. Med. 2022, 28, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Engjom, H.; van den Akker, T.; Aabakke, A.; Ayras, O.; Bloemenkamp, K.; Donati, S.; Cereda, D.; Overtoom, E.; Knight, M. Severe COVID-19 in pregnancy is almost exclusively limited to unvaccinated women—Time for policies to change. Lancet Reg. Health-Eur. 2022, 13, 100313. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde; Conselho Nacional de Saúde. Resolução no 510, de 7 de abril de 2016. Diário Oficial da União. Brasília, Brasil. 24 May 2016. Available online: https://www.in.gov.br/materia/-%0A/asset_publisher/Kujrw0TZC2Mb/content/id/22917581 (accessed on 16 December 2021).
- Austin, P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.0.3; R Foundation for 353 Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Ho, D.E.; Imai, K.; Gary King, E.A.S. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011, 42, 1–28. Available online: https://www.jstatsoft.org/v42/i08/ (accessed on 1 February 2022). [CrossRef]
- CNN Brasil. Covaxin Tem Maior Preço por Vacina Negociado pelo Brasil; Veja Comparativo. 23 June 2021. Available online: https://www.cnnbrasil.com.br/saude/covaxin-tem-maior-preco-por-vacina-negociado-pelo-brasil-veja-comparativo/ (accessed on 16 December 2021).
- Brasil Ministério da Saúde; Secretaria de Atenção Especializada à Saúde. PORTARIA No 237, DE 18 DE MARÇO DE 2020—Tabela de Procedimentos, Medicamentos, Órteses, Próteses e Materiais Especiais do (SUS), Para Atendimento Exclusivo de Pacientes com Diagnóstico Clínico de COVID-19. 2020. Available online: https://www.in.gov.br/en/web/dou/-/portaria-n-237-de-18-de-marco-de-2020-*-261494685 (accessed on 1 October 2021).
- Blakeway, H.; Prasad, S.; Kalafat, E.; Heath, P.T.; Ladhani, S.N.; Le Doare, K.; Magee, L.A.; O’brien, P.; Rezvani, A.; von Dadelszen, P.; et al. COVID-19 vaccination during pregnancy: Coverage and safety. Am. J. Obstet. Gynecol. 2022, 226, 236.e1-14. [Google Scholar] [CrossRef] [PubMed]
- Modi, N.; Ayres-de-Campos, D.; Bancalari, E.; Benders, M.; Briana, D.; Di Renzo, G.C.; Fonseca, E.B.; Hod, M.; Poon, L.; Cortes, M.S.; et al. Equity in coronavirus disease 2019 vaccine development and deployment. Am. J. Obstet. Gynecol. 2021, 224, 423–427. [Google Scholar] [CrossRef]
- Riad, A.; Schünemann, H.; Attia, S.; Peričić, T.P.; Žuljević, M.F.; Jürisson, M.; Kalda, R.; Lang, K.; Morankar, S.; Yesuf, E.A.; et al. COVID-19 vaccines safety tracking (CoVaST): Protocol of a multi-center prospective cohort study for active surveillance of COVID-19 vaccines’ side effects. Int. J. Environ. Res. Public Health 2021, 18, 7859. [Google Scholar] [CrossRef]
- Krause, P.R.; Gruber, M.F. Emergency use authorization of COVID-19 vaccines—safety and efficacy follow-up considerations. N. Engl. J. Med. 2020, 383, e107. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde; Agência Nacional de Vigilância Sanitária (ANVISA). Plano de Monitoramento de Eventos Adversos de Vacinas. Available online: https://www.gov.br/anvisa/pt-br/assuntos/fiscalizacao-e-monitoramento/notificacoes/medicamentos-e-vacinas (accessed on 26 April 2022).
- Geoghegan, S.; Stephens, L.C.; Feemster, K.A.; Drew, R.J.; Eogan, M.; Butler, K.M. “This choice does not just affect me.” Attitudes of pregnant women toward COVID-19 vaccines: A mixed-methods study. Hum. Vaccines Immunother. 2021, 17, 3371–3376. [Google Scholar] [CrossRef]
- Lipkind, H.S.; Vazquez-Benitez, G.; DeSilva, M.; Vesco, K.K.; Ackerman-Banks, C.; Zhu, J.; Boyce, T.G.; Daley, M.F.; Fuller, C.C.; Getahun, D.; et al. Receipt of COVID-19 Vaccine During Pregnancy and Preterm or Small-for-Gestational-Age at Birth—Eight Integrated Health Care Organizations, United States, 15 December 2020–22 July 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 26–30. [Google Scholar] [CrossRef]
- Theiler, R.N.; Wick, M.; Mehta, R.; Weaver, A.L.; Virk, A.; Swift, M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am. J. Obs. Gynecol. MFM 2021, 3, 100467. [Google Scholar] [CrossRef] [PubMed]
- Magnus, M.C.; Örtqvist, A.K.; Dahlqwist, E.; Ljung, R.; Skår, F.; Oakley, L.; Macsali, F.; Pasternak, B.; Gjessing, H.K.; Håberg, S.E.; et al. Association of SARS-CoV-2 Vaccination during Pregnancy with Pregnancy Outcomes. JAMA 2022, 327, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Fell, D.B.; Dhinsa, T.; Alton, G.D.; Török, E.; Dimanlig-Cruz, S.; Regan, A.K.; Sprague, A.E.; Buchan, S.A.; Kwong, J.C.; Wilson, S.E.; et al. Association of COVID-19 Vaccination in Pregnancy with Adverse Peripartum Outcomes. JAMA 2022, 327, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
| Variables | Vaccinated (n = 200) | Unvaccinated (n = 2084) | p-Value |
|---|---|---|---|
| Age (years); mean ± sd $ | 31.44 ± 7.72 | 29.72 ± 7.09 | 0.0031 |
| Self-reported ethnicity, n (%) White Nonwhite | 0.106 2 | ||
| 111/183 (60.7) | 1022/1888 (54.1) | ||
| 72/183 (39.3) | 866/1888 (45.9) | ||
| Comorbidities, n (%): | |||
| Cardiac | 22/93 (23.7) | 104/842 (12.4) | 0.0042 |
| Diabetes mellitus | 21/94 (22.3) | 144/852 (16.9) | 0.239 2 |
| Hematologic | 2/89 (2.2) | 7/814 (0.9) | 0.219 3 |
| Obesity | 14/90 (15.6) | 191/871 (21.9) | 0.204 2 |
| Asthma | 9/90 (10.0) | 65/832 (7.8) | 0.602 2 |
| Hepatic | 1/89 (1.1) | 3/810 (0.4) | 0.341 3 |
| Neurologic | 2/88 (2.3) | 9/811 (1.1) | 0.293 3 |
| Other lung diseases | 3/89 (3.4) | 9/814 (1.1) | 0.105 3 |
| Immunosuppression | 5/89 (5.6) | 11/812 (1.4) | 0.0153 |
| Renal | 2/89 (2.2) | 9/806 (1.1) | 0.300 3 |
| Education, n (%) | 0.116 2 | ||
| Up to 9 years | 20/101 (19.8) | 279/1044 (26.7) | |
| From 9 to 12 years | 55/101 (54.5) | 573/1044 (54.9) | |
| Over 12 years | 26/101 (25.7) | 192/1044 (18.4) | |
| Residence area, n (%) | 0.850 3 | ||
| Urban | 179/188 (95.2) | 1838/1957 (93.9) | |
| Periurban | 0 (0.0) | 6/1957 (0.3) | |
| Rural | 9/188 (4.8) | 113/1957 (5.8) |
| Variables | Vaccinated (n = 200) | Unvaccinated (n = 2084) | OR (95% CI) |
|---|---|---|---|
| Symptoms, n (%) | |||
| Fever | 82/176 (46.6) | 1077/1860 (58.9) | 0.63 (0.47–0.86) |
| Cough | 137/182 (75.3) | 1532/1938 (79.1) | 0.81 (0.57–1.15) |
| Sore throat | 33/159 (20.8) | 447/1710 (26.1) | 0.74 (0.50–1.10) |
| Dyspnea | 97/173 (56.1) | 1349/1914 (70.5) | 0.53 (0.39–0.73) |
| Respiratory discomfort | 77/172 (44.8) | 1028/1820 (56.5) | 0.62 (0.46–0.86) |
| Desaturation | 69/172 (40.1) | 1025/1840 (55.7) | 0.53 (0.39–0.73) |
| Diarrhea | 16/159 (10.1) | 189/1677 (11.3) | 0.88 (0.51–1.51) |
| Vomiting | 16/159 (10.1) | 202/1682 (12.0) | 0.82 (0.48–1.40) |
| Abdominal pain | 18/156 (11.5) | 164/1658 (9.9) | 1.19 (0.71–1.99) |
| Fatigue | 53/163 (32.5) | 590/1737 (34.0) | 0.94 (0.67–1.32) |
| Loss of smell | 33/160 (20.6) | 310/1682 (18.4) | 1.15 (0.77–1.72) |
| Loss of taste | 30/159 (18.9) | 287/1687 (16.8) | 1.15 (0.76–1.74) |
| Any respiratory symptom | 127/183 (69.4) | 1618/1979 (81.8) | 0.51 (0.36–0.71) |
| Any symptom | 178/194 (91.8) | 1980/2058 (96.2) | 0.44 (0.25–0.77) |
| Variables n (%) | Vaccinated (n = 200) | Unvaccinated (n = 2084) | OR (95%CI) | Vaccinated (n = 200) PSM | Unvaccinated (n = 200) PSM | OR (95% CI) PSM |
|---|---|---|---|---|---|---|
| ICU admission | 44/187 (23.5) | 740/1979 (37.4) | 0.52 (0.36–0.73) | 44/187 (23.5) | 69/190 (36.3) | 0.54 (0.34–0.85) |
| Intubation | 9/189 (4.8) | 368/1959 (18.8) | 0.22 (0.11–0.43) | 9/189 (4.8) | 39/189 (20.6) | 0.19 (0.09–0.41) |
| Final outcome, | ||||||
| Cure Death | 194/200 (97.0) 6/200 (3.0) | 1790/2084 (85.9) 294/2084 (14.1) | 0.188 (0.083–0.428) | 194/200 (97.0) 6/200 (3.0) | 174/200 (87.0) 26/200 (13.0) | 0.207 (0.0.83–0.515) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Freitas Paganoti, C.; Alkmin da Costa, R.; Papageorghiou, A.T.; da Silva Costa, F.; Quintana, S.M.; Graziela de Godoi, L.; Adriana Jiménez Monroy, N.; Sacramento Rodrigues, A.; Pulcineli Vieira Francisco, R. COVID-19 Vaccines Confer Protection in Hospitalized Pregnant and Postpartum Women with Severe COVID-19: A Retrospective Cohort Study. Vaccines 2022, 10, 749. https://doi.org/10.3390/vaccines10050749
de Freitas Paganoti C, Alkmin da Costa R, Papageorghiou AT, da Silva Costa F, Quintana SM, Graziela de Godoi L, Adriana Jiménez Monroy N, Sacramento Rodrigues A, Pulcineli Vieira Francisco R. COVID-19 Vaccines Confer Protection in Hospitalized Pregnant and Postpartum Women with Severe COVID-19: A Retrospective Cohort Study. Vaccines. 2022; 10(5):749. https://doi.org/10.3390/vaccines10050749
Chicago/Turabian Stylede Freitas Paganoti, Cristiane, Rafaela Alkmin da Costa, Aris T. Papageorghiou, Fabrício da Silva Costa, Silvana Maria Quintana, Luciana Graziela de Godoi, Nátaly Adriana Jiménez Monroy, Agatha Sacramento Rodrigues, and Rossana Pulcineli Vieira Francisco. 2022. "COVID-19 Vaccines Confer Protection in Hospitalized Pregnant and Postpartum Women with Severe COVID-19: A Retrospective Cohort Study" Vaccines 10, no. 5: 749. https://doi.org/10.3390/vaccines10050749
APA Stylede Freitas Paganoti, C., Alkmin da Costa, R., Papageorghiou, A. T., da Silva Costa, F., Quintana, S. M., Graziela de Godoi, L., Adriana Jiménez Monroy, N., Sacramento Rodrigues, A., & Pulcineli Vieira Francisco, R. (2022). COVID-19 Vaccines Confer Protection in Hospitalized Pregnant and Postpartum Women with Severe COVID-19: A Retrospective Cohort Study. Vaccines, 10(5), 749. https://doi.org/10.3390/vaccines10050749







