Comparison of CLEIA and ELISA for SARS-CoV-2 Virus Antibodies after First and Second Dose Vaccinations with the BNT162b2 mRNA Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement, Participants, and Sample Processing
2.1.1. Study Design and Participants
2.1.2. Serological Tests
2.2. Antibody Measurements
2.2.1. Chemiluminescent Enzyme Immunoassay (CLEIA)
2.2.2. Enzyme-Linked Immunosorbent Assays (ELISA)
2.3. Statistical Analyses
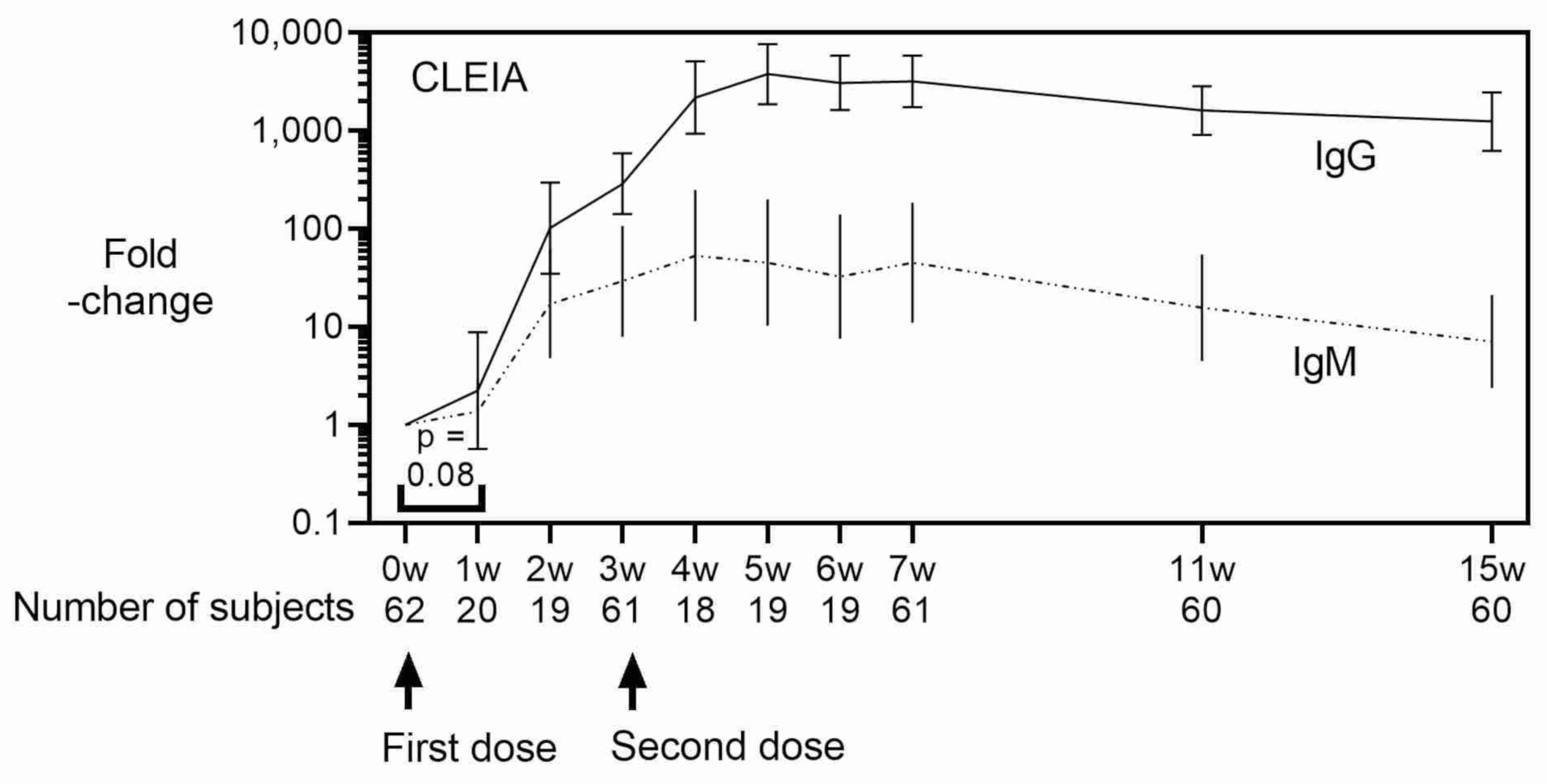
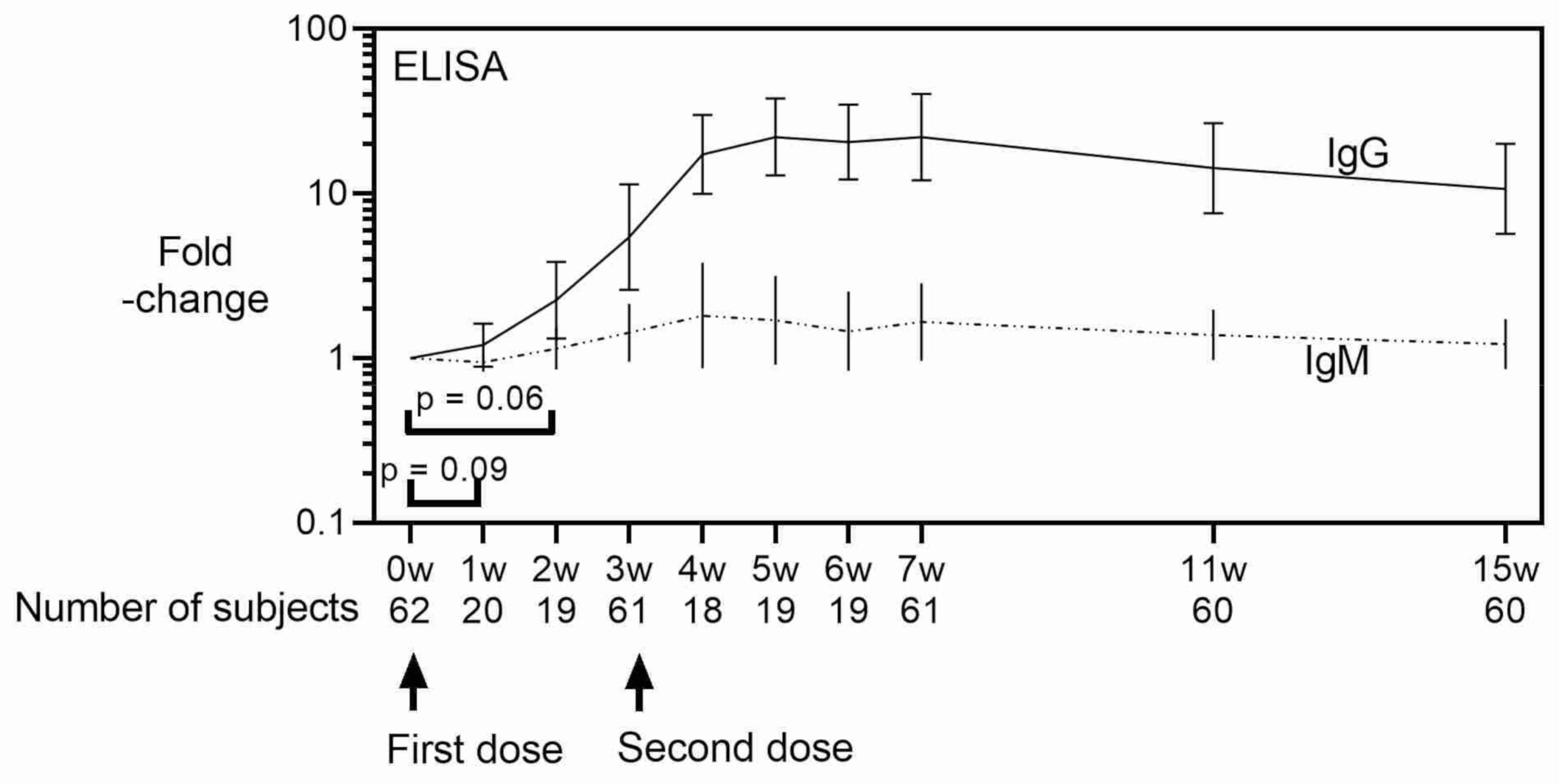
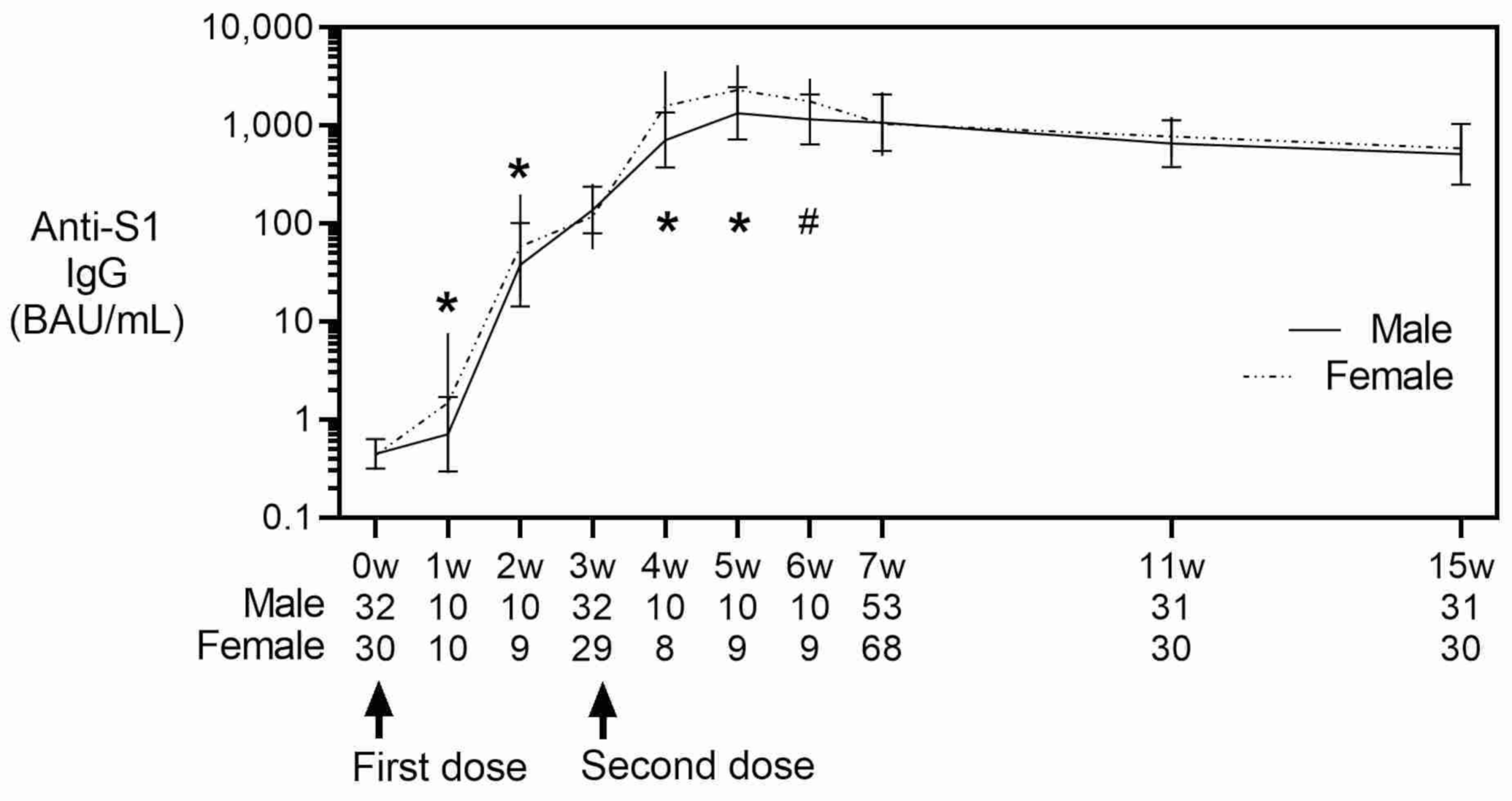
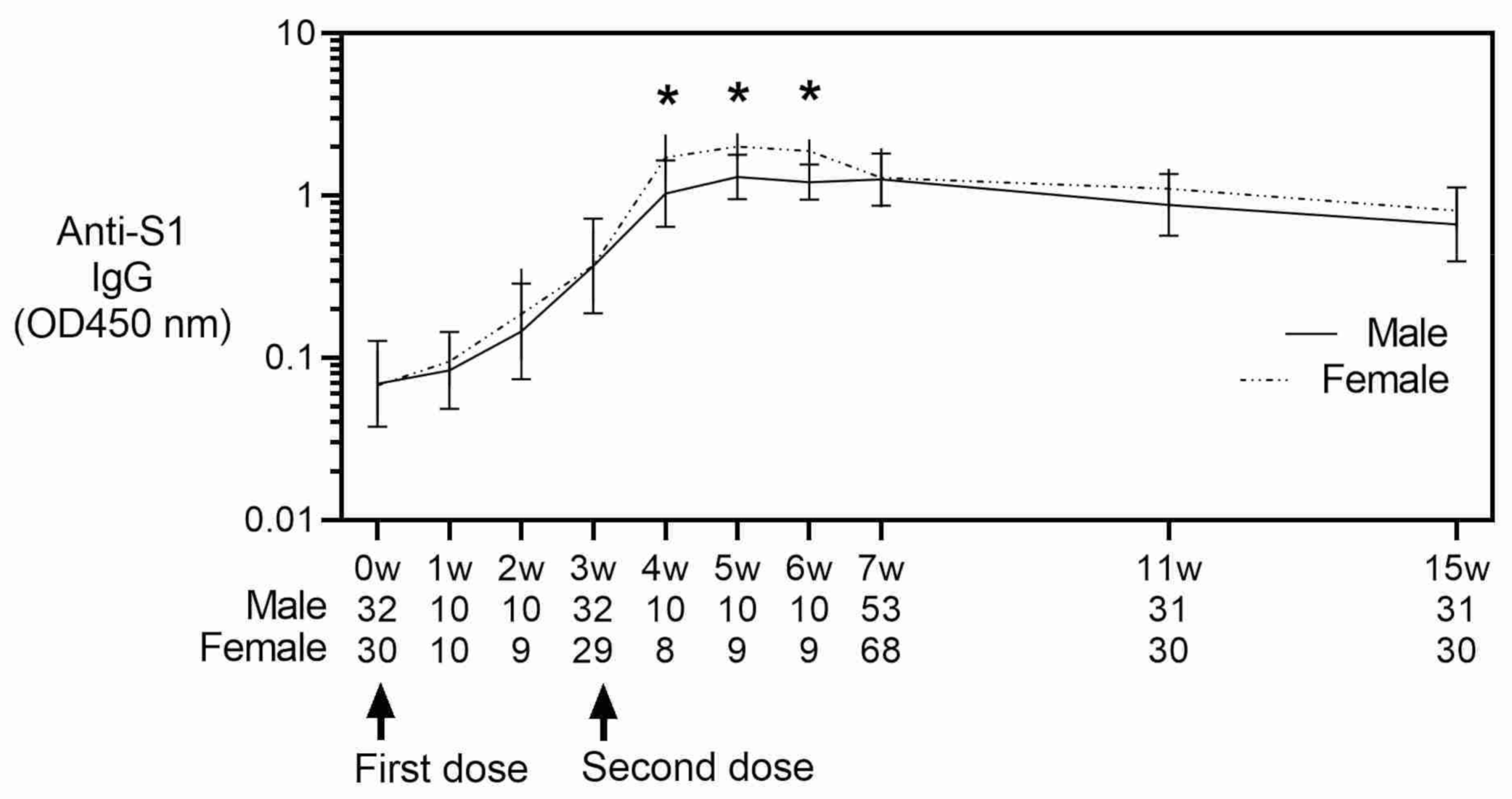
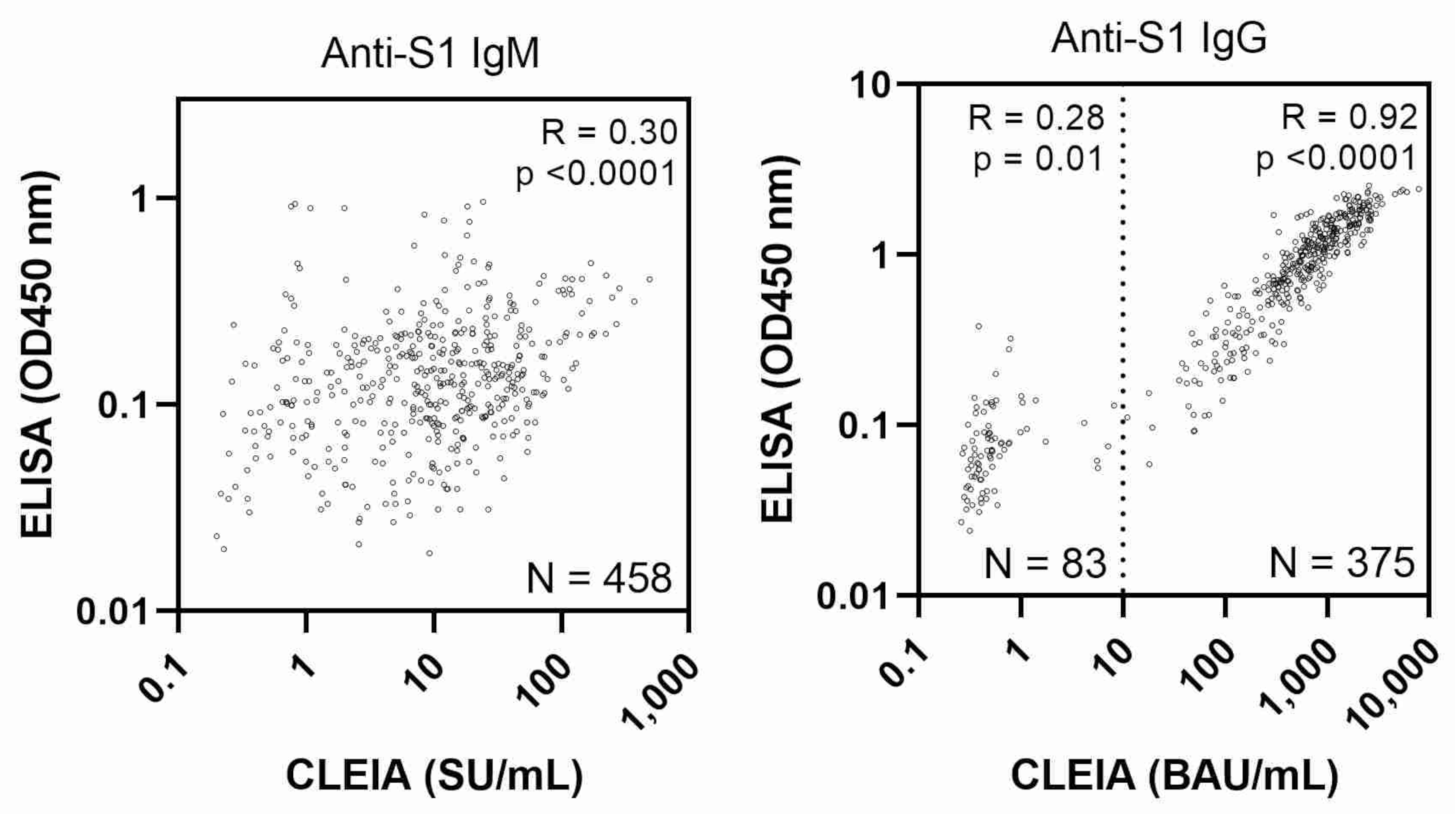
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO-Director General’s Opening Remarks at the Media Briefing on COVID-19. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 11 March 2020).
- Moxon, E.R. Applications of molecular microbiology to vaccinology. Lancet 1997, 350, 1240–1244. [Google Scholar] [CrossRef]
- World Health Organization (COVID-19) Dashboard 2022. Available online: https://covid19.who.int/ (accessed on 6 February 2022).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int/region/wpro/country/jp (accessed on 26 January 2022).
- COVID 19 Vaccine Tracker. Available online: https://covid19.trackvaccines.org/country/japan/ (accessed on 12 November 2021).
- Ashour, H.M.; Elkhatib, W.F.; Rahman, M.M.; Elshabrawy, H.A. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens 2020, 9, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, H.J.; Bickerton, E.; Britton, P. (Eds.) Coronaviruses. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2015; Volume 1282. [Google Scholar] [CrossRef]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Asthagiri Arunkumar, G.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. medRxiv 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Li, D.D.; Li, Q.H. SARS-CoV-2: Vaccines in the pandemic era. Mil. Med. Res. 2021, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Wei, J.; Pouwels, K.B.; Stoesser, N.; Matthews, P.C.; Diamond, I.; Studley, R.; Rourke, E.; Cook, D.; Bell, J.I.; Newton, J.N.; et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat. Med. 2022, 1–11. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, Y.R.; Heo, S.T.; Oh, H.; Kim, M.; Lee, H.R.; Yoo, J.R. Healthcare Workers in South Korea Maintain a SARS-CoV-2 Antibody Response Six Months After Receiving a Second Dose of the BNT162b2 mRNA Vaccine. Front. Immunol. 2022, 13, 82730. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schaffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-Scale Study of Antibody Titer Decay following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection. Vaccines 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Puranik, A.; Lenehan, P.J.; Silvert, E.; Niesen, M.J.M.; Corchado-Garcia, J.; O’Horo, J.C.; Virk, A.; Swift, M.D.; Gordon, J.E.; Speicher, L.L.; et al. Comparative effectiveness of mRNA-1273 and BNT162b2 against symptomatic SARS-CoV-2 infection. Medicine 2022, 3, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by COVID-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Henry, B.M.; di Piazza, G.; Pighi, L.; de Nitto, S.; Bragantini, D.; Gianfilippi, G.L.; Lippi, G. Anti-spike S1 IgA, anti-spike trimeric IgG, and anti-spike RBD IgG response after BNT162b2 COVID-19 mRNA vaccination in healthcare workers. J. Med. Biochem. 2021, 40, 327–334. [Google Scholar] [CrossRef]
- Fujigaki, H.; Inaba, M.; Osawa, M.; Moriyama, S.; Takahashi, Y.; Suzuki, T.; Yamase, K.; Yoshida, Y.; Yagura, Y.; Oyamada, T.; et al. Comparative Analysis of Antigen-Specific Anti-SARS-CoV-2 Antibody Isotypes in COVID-19 Patients. J. Immunol. 2021, 206, 2393–2401. [Google Scholar] [CrossRef]
- Edridge, A.W.D.; Kaczorowska, J.; Hoste, A.C.R.; Bakker, M.; Klein, M.; Loens, K.; Jebbink, M.F.; Matser, A.; Kinsella, C.M.; Rueda, P.; et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020, 26, 1691–1693. [Google Scholar] [CrossRef]
- Algaissi, A.; Alfaleh, M.A.; Hala, S.; Abujamel, T.S.; Alamri, S.S.; Almahboub, S.A.; Alluhaybi, K.A.; Hobani, H.I.; Alsulaiman, R.M.; AlHarbi, R.H.; et al. SARS-CoV-2 S1 and N-based serological assays reveal rapid seroconversion and induction of specific antibody response in COVID-19 patients. Sci. Rep. 2020, 10, 16561. [Google Scholar] [CrossRef]
- Noda, K.; Matsuda, K.; Yagishita, S.; Maeda, K.; Akiyama, Y.; Terada-Hirashima, J.; Matsushita, H.; Iwata, S.; Yamashita, K.; Atarashi, Y.; et al. A novel highly quantitative and reproducible assay for the detection of anti-SARS-CoV-2 IgG and IgM antibodies. Sci. Rep. 2021, 11, 5198. [Google Scholar] [CrossRef]
- Abu Jabal, K.; Ben-Amram, H.; Beiruti, K.; Batheesh, Y.; Sussan, C.; Zarka, S.; Edelstein, M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Eurosurveillance 2021, 26, 2100096. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine 2021, 36, 100928. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Para, O.; Giordano, M. Immune system and COVID-19 by sex differences and age. Womens Health 2021, 17, 17455065211022262. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; Shurin, G.V.; Yost, M.; Anderson, A.; Pinto, L.; Wells, A.; Shurin, M.R. Differential antibody response to mRNA COVID-19 vaccines in healthy subjects. Microbiol. Spectr. 2021, 9, e0034121. [Google Scholar] [CrossRef] [PubMed]
| Healthcare Workers * | Hospital Staff * | Students * | |
|---|---|---|---|
| n = 20 | n = 59 | n = 42 | |
| Study design | |||
| Vaccination | |||
| First dose | April, 2021 | February, 2021 | May, 2021 |
| Second dose | Three weeks after the first dose, except one participant received the second dose six weeks after the first dose | Three weeks after the first dose | Three weeks after the first dose |
| Blood samples | Prior to first dose, every other week after vaccination up to three weeks after second vaccination | Four weeks after the second dose | Prior to first dose, three weeks after the first dose, four weeks after the second dose |
| Characteristics | |||
| Male % | 50% | 37% | 52% |
| Age | |||
| Median | 38 | 43 | 22 |
| Interquartile range | 30.3–43.5 | 36.8–51.3 | 22–23 |
| Disease history | |||
| Sleep disorder | 1 | 1 | 0 |
| O Hypertension | 0 | 13 | 1 |
| Diabetes | 0 | 1 | 0 |
| Dyslipidemia | 1 | 9 | 0 |
| Kidney disease | 0 | 1 | 1 |
| Liver disease | 0 | 3 | 0 |
| Heart disease | 0 | 2 | 1 |
| COPD | 0 | 5 | 2 |
| Steroid use | 0 | 4 | 2 |
| Others | 0 | 4 | 1 |
| Healthcare Workers | Hospital Staff | Students | |
|---|---|---|---|
| n = 20 | n = 59 | n = 42 | |
| Number of measurements | 7–8 | 1 | 3 |
| CLEIA (SU/mL) | |||
| Median (range) | 0.038 (0.01–0.709) | 0.037 (0.003–0.228) | 0.049 (0.009–0.194) |
| Intra-individual CV, median (range) | 9% (5–30%) | - | 11% (0–116%) |
| ELISA (O.D. 450 nm) | |||
| Median (range) | 0.208 (0.063–0.464) | 0.09 (0.011–0.626) | 0.115 (0.018–0.424) |
| Intra-individual CV, median (range) | 10% (3–31%) | - | 11% (1–86%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashenagar, M.S.; Matsumoto, A.; Sakai, H.; Tokiya, M.; Hara, M.; Hirota, Y. Comparison of CLEIA and ELISA for SARS-CoV-2 Virus Antibodies after First and Second Dose Vaccinations with the BNT162b2 mRNA Vaccine. Vaccines 2022, 10, 487. https://doi.org/10.3390/vaccines10040487
Ashenagar MS, Matsumoto A, Sakai H, Tokiya M, Hara M, Hirota Y. Comparison of CLEIA and ELISA for SARS-CoV-2 Virus Antibodies after First and Second Dose Vaccinations with the BNT162b2 mRNA Vaccine. Vaccines. 2022; 10(4):487. https://doi.org/10.3390/vaccines10040487
Chicago/Turabian StyleAshenagar, Mohammad Said, Akiko Matsumoto, Hironori Sakai, Mikiko Tokiya, Megumi Hara, and Yoshio Hirota. 2022. "Comparison of CLEIA and ELISA for SARS-CoV-2 Virus Antibodies after First and Second Dose Vaccinations with the BNT162b2 mRNA Vaccine" Vaccines 10, no. 4: 487. https://doi.org/10.3390/vaccines10040487
APA StyleAshenagar, M. S., Matsumoto, A., Sakai, H., Tokiya, M., Hara, M., & Hirota, Y. (2022). Comparison of CLEIA and ELISA for SARS-CoV-2 Virus Antibodies after First and Second Dose Vaccinations with the BNT162b2 mRNA Vaccine. Vaccines, 10(4), 487. https://doi.org/10.3390/vaccines10040487







