Abstract
Several studies have reported the benefits and safety of heterologous vaccination among different approved vaccines; however, there are no specific reports on the effects of vaccination with the Ad5-nCoV and other vaccines of the same or different technologies. In the present study, we evaluated the neutralizing antibodies percentage against SARS-CoV-2 in Mexican patients immunized with the Ad5-nCoV vaccine six months after its application. Moreover, the effect of the heterologous vaccination with the Ad5-nCoV vaccine and a booster dose of ChAdOx1-S-Nov-19, Ad26.COV2.S, BNT162b2, or mRNA-127 were determined. Our results suggest that a heterologous regimen of one dose with Ad5-nCoV vaccine followed by a booster dose of a different vaccine is safe and induces a stronger humoral immune response.
1. Introduction
The development of vaccines against COVID-19 is a preventive public health strategy aimed at avoiding cases of infection and reducing the mortality rate associated with the disease [,]. The rapid technological progress to affront the COVID-19 pandemic has accelerated the development of several vaccine types, including viral vector-based vaccines, mRNA and DNA vaccines, subunit vaccines, nanoparticle-based vaccines, and inactivated-whole virus vaccines [].
The COVID-19 pandemic has forced health authorities around the world to design different vaccination plans; in Mexico, it was decided to start the vaccination in a population considered at risk, including health and educational personnel in which the latter group was exclusively administered the Ad5-nCoV vaccine [,]. The Ad5-nCoV vaccine (developed by the Beijing Institute of Biotechnology, Beijing, China, and CanSino Biologics, Tianjin, China) has only been approved in countries such as Argentina, Chile, China, Ecuador, Hungary, Indonesia, Malaysia, Mexico, Pakistan, and Republic of Moldova [,]. This vaccine contains replication-defective Ad5 vectors expressing the full-length spike gene based on Wuhan-Hu-1. The phase I and II trials results suggest that this vaccine demonstrated a good safety profile [,,]. Despite this information, the lack of authorization by the WHO for the Ad5-nCov vaccine generates uncertainty in the population; thus, it is important to gain more knowledge about the immunogenicity, efficacy, duration of protection, and side effects to contribute to an informed decision-making process for the health authorities.
In a previous study, we evaluated the levels of neutralizing antibodies against SARS-CoV-2 in Mexican individuals immunized with the Ad5-nCoV vaccine. One of the main findings was that 7.4% of the individuals did not present positive titers of neutralizing antibodies after vaccination. Moreover, neutralizing percentages were lower than those observed in the first dose of the BNT162b2 vaccine (Pfizer-BioNTech); therefore, the application of a second booster dose was suggested, especially in older age and immunosuppressed patients []. On this basis, one of the questions posed by the scientific community and the Mexican population is: Is the application of combined vaccines recommended?
Some studies have reported the benefits and security of heterologous vaccination between different approved vaccines [,,]. Regarding the Ad5-nCoV vaccine, it was recently reported that individuals immunized with one dose of Ad5-nCoV and a booster dose of the BNT162b2 vaccine presented higher titers of IgG antibodies against SARS-CoV-2 compared to individuals who received only one dose of Ad5-nCoV []. However, only the presence of IgG antibodies was evaluated and not their neutralizing capacity. Therefore, this study aimed to determine the effect of the heterologous vaccination of the Ad5-nCoV vaccine with ChAdOx1-S-nCoV-19 (AstraZeneca), BNT162b2 (Pfizer-BioNTech), mRNA-127 (Moderna), or the Janssen COVID-19 vaccine.
2. Materials and Methods
2.1. Subjects
From 28 April to 4 May 2021, the Mexican government performed a vaccination campaign against COVID-19 with the Ad5-nCoV vaccine for teachers from Jalisco, Mexico. On the last week of May 2021 (21–24 days after vaccination), some of those professors were invited to voluntarily participate in a study to evaluate reactogenicity to the Ad5-nCoV vaccine, the production of neutralizing antibodies against SARS-CoV-2, and the anti-Ad5 Antibodies in response to this vaccine []. Six months later, the previous study participants were again invited to assess a possible decline in neutralizing titers. Sixty-two people declared that they voluntarily received a heterologous booster 4.5–5 months after applying the Ad5-nCoV vaccine. Those 62 professors and 62 individuals without a booster dose were included in the present study. Both groups were matched for age, gender, treatment, COVID-19 history, and baseline antibody levels 21 days after the first shot.
Two surveys were applied to all participants (N = 124) to obtain clinical and demographic data, history of SARS-CoV-2 infection, and vaccine-associated side effects from the Ad5-nCoV as a single shot or a heterologous booster. The first survey was applied 21–24 days after the first shot and the other one six months after that vaccine application. Additionally, a blood sample was obtained from both study groups to analyze neutralizing antibodies against SARS-CoV-2 in response to vaccination.
Individuals with prior COVID-19 were diagnosed 1–12 months before the study by RT-PCR (reverse transcription-polymerase chain reaction).
2.2. Quantification of Neutralizing Antibodies
The quantification of neutralizing antibodies was performed in the serum collected after six months of the immunization with the Ad5-nCoV vaccine. The cPass™ SARSCoV-2 Neutralization Antibody Detection Kit (GenScript, Piscataway Township, NJ, USA; Cat. L00847-A Kit) was used for this analysis, which is a blocking Enzyme-Linked Immunosorbent Assay (ELISA) designed to detect immunity against the original Wuhan strain of SARS-CoV-2.
This kit is validated for diagnosis with a 30% signal inhibition cut-off point for SARS-CoV-2 neutralizing antibody detection. According to the manufacturer’s instructions, negative and positive sample controls were diluted at 1:10 with the sample dilution buffer and mixed with an equal volume of HRP-conjugated RBD (60 μL and 60 μL). They were then incubated at 37 °C for 30 min. Then, 100 μL of this mixture was transferred to 96-well plates coated with recombinant hACE2 and incubated at 37 °C for 15 min. After the incubation, the supernatant was removed, and the plates were washed four times with the wash solution. Finally, 100 μL of tetramethylbenzidine (TMB) was added and incubated for 15 min at room temperature. The reaction was stopped with 50 μL of stop solution, and plates were read at 450 nm immediately after. The inhibition rate was calculated as follows:
2.3. Statistical Analysis
Statistical analysis was performed using the GraphPad Prism v. 6.01 software (GraphPad company, San Diego, CA, USA) and the R version 4.0.0 statistical software program (R core Team, Vienna, Austria). The significance level was set at p < 0.05. For simple comparisons, we used the Fisher exact test or Student’s t-test. Data with nonparametric distribution were represented as median with interquartile range (IQR). The Mann–Whitney U-test was applied for comparing two groups and the Kruskal–Wallis test was applied for three or more group comparisons.
3. Results
3.1. Clinical and Demographic Characteristics
The clinical and demographic characteristics of both study groups are described in Table 1. A total of 124 individuals vaccinated with the Ad5-nCoV vaccine were considered for the analysis, of which 62 received a booster dose with one of the following vaccines: AstraZeneca, Moderna, Pfizer, or Johnson & Johnson. The age and gender of the individuals were similar in both study groups.

Table 1.
Clinical and demographic characteristics of the study groups.
Both groups were similar in comorbidities and treatments (p > 0.05). Moreover, no differences were found for clinical and demographic characteristics when stratifying by different booster vaccines (Table 2).

Table 2.
Clinical and demographic characteristics of individuals with a booster.
In the baseline determination carried out 21 days after the individuals received the Ad5-nCoV vaccine, the median percentage of neutralizing antibodies was similar in both groups (78.16% vs. 78.65%) (p > 0.05). However, six months later, the median percentage of neutralizing antibodies was significantly higher in individuals who received a booster dose (96.41% vs. 89.33%, p = 0.0004) (Figure 1a). The 62 individuals with a booster were grouped according to the vaccine applied to assess whether any combination showed a greater capacity to generate neutralizing antibodies; however, no differences were observed in this regard (Figure 1b).
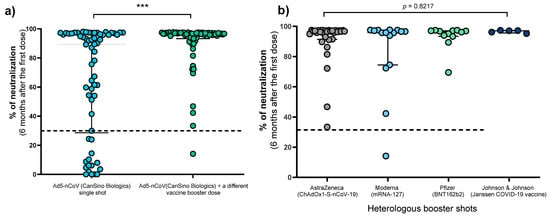
Figure 1.
Comparison of antibody neutralizing signals between individuals with a single dose of Ad5-nCoV vaccine and individuals with a heterologous booster dose. (a) Individuals with a single dose of Ad5-nCoV vaccine vs. individuals with Ad5-nCoV dose and heterologous booster doses (AstraZeneca, Moderna, Pfizer, or Johnson & Johnson), differences were calculated by the Mann—Whitney’s U test. (b) Schemes of individuals with heterologous booster doses (Ad5-nCoV and AstraZeneca, Moderna, Pfizer, or Johnson & Johnson), differences were calculated by the Kruskal–Wallis test. The dotted line indicates the cut-off point for the neutralization test (>30%). ***, p < 0.0001.
The neutralization percentage did not correlate with the time elapsed between the first vaccine and the booster (data not shown, r = −0.06, p = 0.62),
3.2. Adverse Effects in Individuals with or without Booster Doses
Table 3 shows the adverse effects (reactogenicity) of the Ad5-nCoV vaccine with or without a booster dose with a different vaccine. The presence of myalgia and fatigue was more frequent in individuals after receiving the Ad5-nCoV vaccine than in those who received a booster dose with a different vaccine (p = 0.0441 and p < 0.000, respectively). No differences were found regarding adverse effects when comparing the different booster vaccines (Table 4).

Table 3.
Associated side effects comparison with booster presence.

Table 4.
Booster vaccine-associated side effects comparison.
4. Discussion
Progress in the development and emergency approval for vaccines against the SARS-CoV-2 virus is being fast-tracked globally, mainly due to the need to control the COVID-19 lethality. The shortage of COVID-19 vaccines and the low purchasing power of developing countries have led some countries to approve vaccines that have not completed phase III clinical trials at the time of their application, such as the Ad5-nCoV vaccine (commercial name “Convidicea”), which was approved for emergency use in the populations of Argentina, Chile, China, Ecuador, Hungary, Indonesia, Malaysia, Pakistan, Republic of Moldova, and Mexico [].
The phase III study of this vaccine has just been reported; 28 days after vaccination, efficacy against PCR-confirmed COVID-19 was found to be 57.5% and 91.7% protective against severe COVID-19. Moreover, seroconversion of neutralizing antibodies was shown in 75.9% of Ad5-nCoV recipients. It is also noteworthy that efficacy was substantially lower (17.5%) in participants aged ≥ 60 years than participants <60 years, suggesting that additional vaccine doses might be necessary for this age group []. On the other hand, that phase III study presents some limitations, such as the short follow-up time (<2 months for most people) and that individuals with compromised immune systems, unstable medical conditions, and other potential risks were excluded. Therefore, it is necessary to wait until real-world effectiveness studies are carried out to ascertain the ability of the vaccine to protect these vulnerable groups. Moreover, the short follow-up means that the current report cannot provide additional information to address current concerns about the longevity of vaccine-induced protection [].
Our research group previously carried out the first study outside the clinical trials to evaluate the capacity of the Ad5-nCoV vaccine to induce the production of neutralizing antibodies as well as the side effects associated with it. In the present study, we compare the decline of neutralizing antibodies’ percentage against SARS-CoV-2 six-months after immunization with the Ad5-nCoV vaccine in individuals with a single shot or with a heterologous vaccination regimen (Ad5-nCoV and a booster dose of ChAdOx1-S-nCoV-19, Ad26.COV2.S, BNT162b2, or mRNA-127).
Our results indicated that recipients of the heterologous booster regimen had higher percentages of antibodies with neutralizing capacity than those who only received the Ad5-nCoV vaccine. These results are consistent with previous studies where the effects of other heterologous vaccination regimens were evaluated [,,,,,,]. In fact, according to these reports, the effectiveness of a heterologous vaccination scheme was significantly higher compared to homologous schemes with ChAdOx1 nCoV-19 or BNT162b2 [,,,,].
The mRNA technologies have proven to be excellent candidates to be combined with vaccines of different technology and thus broaden the spectrum of protection against SARS-CoV-2 variants. A combined regimen can offer complementary stimulation to different immune pathways and thus induce more effective humoral and cellular responses [].
Heterologous prime-boost doses of COVID-19 vaccines are being studied in two principal clinical trials (‘Com-Cov2 trial’ in the UK and ‘CombivacS trial’ in Spain) [,]. Together, both trials include the analysis of the effectiveness of combinations with Oxford (AZD1222), Pfizer (BNT162b2), Moderna (mRNA-1273), and Novavax (NVX-CoV2373) vaccines for COVID-19 [].
One of the major limitations of the COVID-19 viral vector technologies such as the Ad5-nCoV vaccine is the likely presence of pre-existing antibodies against strains of adenovirus. Zhu et al. reported that the Ad5-vectored vaccine might have lower immunogenicity in populations with a high prevalence of pre-existing anti-Ad5 immunity []. The ChAdOx1-S-nCoV-19 vaccine partly overcomes this limitation since the latter was designed with a chimpanzee adenovirus to which there is no common exposure [].
In Mexico, there are no reports on the seroprevalence of anti-Ad5 antibodies in the general population; however, a study reported that around 23% of cases associated with acute respiratory infections in children were related to adenovirus []. In addition to this, in our previous study [], we reported a slight increase in the presence of anti-Ad5 antibodies after vaccination with Ad5-now. All this suggests that a detailed analysis should be carried out to examine whether it is convenient to use a booster dose with the same vaccine for those who have received the Ade5-nCoV vaccine or whether, the heterologous vaccination scheme, using ChAdOx1 nCoV-19, for example could be most beneficial.
In the present study, which supported the idea for the safety of a heterologous vaccination for Ade5-nCoV, it was not observed that the application of a booster with a heterologous vaccine had aroused greater side effects when compared to those reported in the first/single dose. The frequency in myalgia and fatigue was significantly lower (p < 0.005) in individuals who received a booster dose. These results may be directly related to the fact that the initial dose of the Ad5-nCoV vaccine represents a “primer” for the immune system, this first encounter triggers the production of antibodies as well as a cellular response that is directly associated with side effects such as fatigue and myalgia [].
Most of the patients included in the present study received the booster dose 4.5–5 months after the first Ade5-nCoV dose, so this timing could be a starting point to consider as an adequate time for a booster. The neutralization percentage did not correlate with the time elapsed between the first vaccine and the booster, nor was there a difference between the neutralization percentage induced by each of the vaccines used as a booster. Similarly, we did not observe differences in the side effects associated with each booster shot. Therefore, our results suggest a booster heterologous regimen (Ad5-nCoV vaccine followed by ChAdOx1-S-nCoV-19, Ad26.COV2.S, BNT162b2, or mRNA-127z) could be safe and result in a robust humoral immune response. We only observed one patient who, after booster with the Moderna vaccine, did not generate neutralizing antibodies (<30% neutralization). This was a patient with a history of autoimmunity and the use of immunosuppressants who did not generate neutralizing antibodies since the first dose.
The arrival of new variants, such as Delta and Omicron, demands an urgent vaccination strategy that allows the immunization and protection provided by vaccination to be optimized, specifically in those vulnerable groups and those in which the efficacy of the vaccine has not yet been fully proven as for Ade5-nCov vaccine.
Data from Israel and the United Kingdom indicate that a booster dose of one of the widely used mRNA-based vaccines sharply lowers a person’s likelihood in catching SARS-CoV-2 and getting sick []. Heterologous prime-boost trials have shown safety, effectiveness, good tolerability with improved immunogenicity, and flexibility profiles for future vaccinations, especially during acute and global shortages, compared to the homologous counterparts [].
In conclusion, our preliminary data support heterologous booster dose benefits and their possible safety in those previously vaccinated with Ad5-now. One of the most important limitations of the present study is the small sample size used, which is why larger-scale studies are needed to verify our findings. Therefore, the observed findings must be confirmed in cohorts with a larger number of individuals and interpreted with caution.
Likewise, there is no access to individuals vaccinated with two doses of Ade5-nCoV to compare the response between this scheme and heterologous vaccination because the Ade5-nCoV vaccine was designed as a single shot. In addition, future studies will be needed to determine the long-term effectiveness of the booster dose against current and new emerging variants.
Author Contributions
Conceptualization, J.F.M.-V. and J.H.-B.; formal analysis, S.A.D.-P.; methodology, G.A.S.-Z.; supervision, C.J.B.-H. and A.N.V.-M.; visualization, J.F.M.-V., C.J.B.-H., and A.N.V.-M.; writing—original draft, G.A.S.-Z., M.G.M.-F., C.O.H.-R. and F.J.T.-H.; writing—review and editing, J.F.M.-V. and J.H.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the CONCURRENCIAS FINANCIERAS PARA LA INVESTIGACIÓN Y ATENCIÓN DE LA VINCULACIÓN program of the University of Guadalajara, grant 260761.
Institutional Review Board Statement
This study was conducted according to the Declaration of Helsinki, and it was approved by the Committee of Ethics and Biosecurity of the CUCS, Universidad de Guadalajara, Mexico (Registry number 21–10).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sánchez-Zuno, G.A.; Matuz-Flores, M.G.; González-Estevez, G.; Nicoletti, F.; Turrubiates-Hernández, F.J.; Mangano, K.; Muñoz-Valle, J.F. A Review: Antibody-Dependent Enhancement in COVID-19: The Not so Friendly Side of Antibodies. Int. J. Immunopathol. Pharmacol. 2021, 35, 205873842110501. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Batista, C.; Amor, Y.B.; Ergonul, O.; Figueroa, J.P.; Gilbert, S.; Gursel, M.; Hassanain, M.; Kang, G.; Kaslow, D.C.; et al. Global Public Health Security and Justice for Vaccines and Therapeutics in the COVID-19 Pandemic. EClinicalMedicine 2021, 39, 101053. [Google Scholar] [CrossRef] [PubMed]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef]
- Salud, S.D. Estatus Regulatorio de Las Vacunas Contra El Virus SARS-CoV2 En México 2021. Available online: http://vacunacovid.gob.mx/wordpress/informacion-de-la-vacuna/ (accessed on 7 December 2021).
- Salud, S.D. Calendario de Vacunación 2021. Available online: http://vacunacovid.gob.mx/wordpress/informacion-de-la-vacuna/ (accessed on 7 December 2021).
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, Tolerability, and Immunogenicity of a Recombinant Adenovirus Type-5 Vectored COVID-19 Vaccine: A Dose-Escalation, Open-Label, Non-Randomised, First-in-Human Trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Coronavirus Disease (COVID-19) Situation Reports. 2021. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 12 December 2021).
- Daian e Silva, D.S.D.O.; da Fonseca, F.G. The Rise of Vectored Vaccines: A Legacy of the COVID-19 Global Crisis. Vaccines 2021, 9, 1101. [Google Scholar] [CrossRef] [PubMed]
- Godeau, D.; Petit, A.; Richard, I.; Roquelaure, Y.; Descatha, A. Return-to-Work, Disabilities and Occupational Health in the Age of COVID-19. Scand. J. Work. Environ. Health 2021, 47, 408–409. [Google Scholar] [CrossRef]
- Institute of Biotechnology; Academy of Military Medical Sciences; PLA of China. A Phase II Clinical Trial to Evaluate the Recombinant Vaccine for COVID-19 (Adenovirus Vector) (CTII-NCoV); Hubei Provincial Center for Disease Control and Prevention: Wuhan, China, 2022. [Google Scholar]
- Hernández-Bello, J.; Morales-Núñez, J.J.; Machado-Sulbarán, A.C.; Díaz-Pérez, S.A.; Torres-Hernández, P.C.; Balcázar-Félix, P.; Gutiérrez-Brito, J.A.; Lomelí-Nieto, J.A.; Muñoz-Valle, J.F. Neutralizing Antibodies against SARS-CoV-2, Anti-Ad5 Antibodies, and Reactogenicity in Response to Ad5-NCoV (CanSino Biologics) Vaccine in Individuals with and without Prior SARS-CoV-2. Vaccines 2021, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Nordström, P.; Ballin, M.; Nordström, A. Effectiveness of Heterologous ChAdOx1 NCoV-19 and mRNA Prime-Boost Vaccination against Symptomatic Covid-19 Infection in Sweden: A Nationwide Cohort Study. Lancet Reg. Health-Eur. 2021, 11, 100249. [Google Scholar] [CrossRef]
- Pozzetto, B.; Legros, V.; Djebali, S.; Barateau, V.; Guibert, N.; Villard, M.; Peyrot, L.; Allatif, O.; Fassier, J.-B.; Massardier-Pilonchéry, A.; et al. Immunogenicity and Efficacy of Heterologous ChAdOx1–BNT162b2 Vaccination. Nature 2021, 600, 701–706. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and Immunogenicity of Seven COVID-19 Vaccines as a Third Dose (Booster) Following Two Doses of ChAdOx1 Nov-19 or BNT162b2 in the UK (COV-BOOST): A Blinded, Multicentre, Randomised, Controlled, Phase 2 Trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Romero-Ibarguengoitia, M.E.; González Cantú, A.; Hernández-Ruíz, Y.G.; Amerndariz-Vázquez, A.G.; Rivera-Salinas, D.; Montelongo-Cruz, L.P.; del Río-Parra, G.F.; Barco-Flores, I.A.; González-Facio, R.; Sanz-Sánchez, M.Á. Effect of Heterologous Vaccination Regimen with Ad5-NCoV CanSinoBio and BNT162b2 Pfizer in SARS-CoV-2 IgG Antibodies Titers. Vaccines 2021, 10, 392. [Google Scholar] [CrossRef]
- Halperin, S.A.; Ye, L.; MacKinnon-Cameron, D.; Smith, B.; Cahn, P.E.; Ruiz-Palacios, G.M.; Ikram, A.; Lanas, F.; Lourdes Guerrero, M.; Muñoz Navarro, S.R.; et al. Final Efficacy Analysis, Interim Safety Analysis, and Immunogenicity of a Single Dose of Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) in Adults 18 Years and Older: An International, Multicentre, Randomised, Double-Blinded, Placebo-Controlled Phase 3 Trial. Lancet 2022, 399, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.B. Efficacy of an Adenovirus Type 5 Vectored SARS-CoV-2 Vaccine. Lancet 2021, 399, 212–213. [Google Scholar] [CrossRef]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune Responses against SARS-CoV-2 Variants after Heterologous and Homologous ChAdOx1 NCoV-19/BNT162b2 Vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; García Morales, M.T.; et al. Immunogenicity and Reactogenicity of BNT162b2 Booster in ChAdOx1-S-Primed Participants (CombiVacS): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef]
- Schmidt, T.; Kemmis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and Reactogenicity of Heterologous ChAdOx1 NCoV-19/MRNA Vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Xia, H.; Zhang, X.; Fontes-Garfias, C.R.; Swanson, K.A.; Cai, H.; Sarkar, R.; Chen, W.; Cutler, M.; et al. Neutralizing Activity of BNT162b2-Elicited Serum. N. Engl. J. Med. 2021, 384, 1466–1468. [Google Scholar] [CrossRef]
- Sapkota, B.; Saud, B.; Shrestha, R.; Al-Fahad, D.; Sah, R.; Shrestha, S.; Rodriguez-Morales, A.J. Heterologous Prime–Boost Strategies for COVID-19 Vaccines. J. Travel Med. 2021, taab191. [Google Scholar] [CrossRef]
- Sprent, J.; King, C. COVID-19 Vaccine Side Effects: The Positives about Feeling Bad. Sci. Immunol. 2021, 6, eabj9256. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and Immunogenicity of the ChAdOx1 NCoV-19 Vaccine against SARS-CoV-2: A Preliminary Report of a Phase 1/2, Single-Blind, Randomised Controlled Trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Rosete, D.P.; Manjarrez, M.E.; Barrón, B.L. Adenoviruses C in Non-Hospitalized Mexican Children Older than Five Years of Age with Acute Respiratory Infection. Mem. Inst. Oswaldo Cruz 2008, 103, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Dolgin, E. Omicron Is Supercharging the COVID Vaccine Booster Debate. Nature 2021, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).