Abstract
Collagen accumulation in sub-conjunctival tissue at the surgical wound is one of the major complications associated with glaucoma filtration surgery (GFS). This process often leads to unwanted fibrotic scar formation at the lesion site and dysfunction of tissues. Previously, we demonstrated that NADPH oxidase 4 (Nox4) is implicated in transforming growth factor-beta (TGFβ)-induced collagen production in ocular fibroblasts and scarring responses in a mouse model of corneal injury. Here, we propose that Nox4 is an important facilitator of TGFβ-induced responses. We tested this hypothesis in human Tenon’s fibroblasts (HTF) and also assessed a role of Nox4 in an experimental mouse model of GFS. TGFβ1 induced Nox4 mRNA expression but downregulated Nox5 in HTF. Targeting Nox4 gene expression with an adenovirus carrying a Nox4 small interfering RNA (siRNA) (Ad-Nox4i) or removal of hydrogen peroxide (H2O2) with EUK-134 (25 μM) in HTFs significantly reduced TGFβ1-induced Nox4 expression, H2O2 production, and collagen synthesis (p < 0.05, n = 3–6). SIS3 (5 μM) that prevents Smad3 phosphorylation is found to suppress TGFβ1-induced collagen production in HTFs. Furthermore, Ad-Nox4i and EUK-134 both abolished TGFβ1-stimulated proliferation of HTFs. We also compared collagen deposition at the wound arising from GFS between wildtype (WT) and Nox4 knockout (KO) mice. Both collagen deposition and fibrovascularization at the wound were significantly decreased in Nox4 KO mice at 14 days after GFS. Our results provide comprehensive evidence that Nox4 is an important mediator for TGFβ1-induced responses in HTFs and collagen deposition in surgical wound following GFS in mice. As such, pharmacological inhibition of Nox4 would be a viable therapeutic strategy for the control of scarring after glaucoma surgery.
1. Introduction
Glaucoma is the leading cause of vision impairment and blindness [1], and it results from degeneration of the optic nerve, often exacerbated by increasing pressure within the eye (intraocular pressure (IOP)). If IOP lowering drugs or laser treatment fails, glaucoma filtration surgery (GFS) is the gold standard operation widely used to treat glaucoma and manage IOP by creating an opening that bypasses the trabecular meshwork and allows aqueous to drain into the sub-conjunctival space [2]. However, trabeculectomy procedure can sometimes stimulate excessive scarring and fibrosis in the sub-conjunctival tissue, which is the principal cause of GFS failure and inadequate control of IOP [3]. To reduce the risk of bleb scarring, anti-mitotic agents mitomycin C (MMC) and fluorouracil (5-FU) are often applied intra-operatively or post-operatively [4,5] to inhibit fibroblasts activation and improve surgical outcomes. However, the cytotoxic nature of these drugs poses limitation and risk on their applications as an adjunct to GFS. A safer and more effective means has always been explored to improve the long-term success of GFS.
GFS is known to activate a key cell type identified as Tenon’s fibroblasts in the conjunctiva. Activation of these cells enhances the proliferation and production of fibrotic proteins, leading to excess scar formation in the bleb [6]. One of the major factors involved in bleb scarring is transforming growth factor (TGF) β, and increased levels of TGFβ1 and β2 have indeed been detected in the filtering bleb from patients after GFS [7,8]. Anti-TGFβ2 strategy with monoclonal antibody has failed to prevent ocular scarring in patients with glaucoma surgery [9]. Moreover, TGFβ is a key profibrotic factor, it has crucial physiological functions and hence, direct inhibition of TGFβ may have severe off-target effects. As such, intervention at signaling proteins/molecules downstream of TGFβ may be a better therapeutic approach for preventing post-surgical scarring.
Recently, we and others have shown that TGFβ-dependent fibrotic responses in cultured fibroblasts or animal models of fibrosis involved NADPH oxidase 4 (Nox4) [10,11,12,13]. Nox4 is an isoform of the NADPH oxidase enzyme that is known to solely produce reactive oxygen species (ROS), such as superoxide and hydrogen peroxide (H2O2) [14]. An oral treatment with a Nox4 inhibitor substantially reduced the extent of fibrosis and expression of TGFβ-activated fibrotic markers, such as collagen and fibronectin, in a preclinical model of bleomycin-induced lung fibrosis [15], hence highlighting a role of Nox4 in TGFβ and lung fibrosis. Although the mechanisms underlining TGFβ-dependent fibrotic responses are common in lung and ocular fibrosis, it remains unclear if Nox4 contributes to collagen deposition in wound healing following GFS surgery. Therefore, we hypothesized that TGFβ induces scarring responses via a Nox4-mediated signaling pathway, and inhibition of Nox4 activity will suppress fibrotic responses in human Tenon’s fibroblasts (HTFs). We have, therefore, tested this hypothesis in vitro using primary cultures of human Tenon’s fibroblasts (HTF). Using Nox4 knockout (KO) mice, we have further assessed the role of Nox4 in post-surgical scarring in an experimental model of GFS.
2. Materials and Methods
2.1. Human Tenon’s Fibroblast Culture
As previously described, HTFs were derived from explanted subconjunctival Tenon’s capsule collected during GFS performed in patients [16]. The patients included for collecting the human biopsy specimens were aged between 18–65 years who were booked for the first glaucoma filtration procedure with no history of a clinically significant inflammatory eye condition and no recent anterior surgery, such as cataract extraction and lens implantation. Explanted tissue derived from patients (n = 3) were attached to the bottom of a six-well plate with a sterile coverslip and overlaid with Roswell Park Memorial Institute Medium (RPMI)-1640 supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL) and streptomycin (100 μg/mL) (all from Invitrogen, Victoria, Australia). Once the monolayers reached confluence, fibroblasts were then propagated, passaged, and subcultured in 75-cm2 tissue culture flasks. Cells were incubated at 37 °C/5% CO2 in a humidified incubator. Independent experiments were performed with different passages of cells. The collection of human biopsy specimens was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). Institutional human ethics committee (St. Vincent’s Hospital Human Research Ethics Committee (HREC 07/724H) approval was granted and written informed consent was obtained from all participating patients.
2.2. Pharmacological Drug Treatment
Unless otherwise specified, HTFs were serum starved for 24 h, followed by TGFβ1 induction (5 ng/mL) for 6 h for H2O2 measurement, gene expression, and Western blot analysis, and 24 h for total collagen assay. In some cases, the cells were pre-treated with Smad3 inhibitor SIS3 (5 μM; Sigma-Aldrich, New South Wales, Australia) or superoxide dismutase (SOD)/catalase mimetic EUK-134 (25 μM; Cayman Chemical, Ann Arbor, MI, USA) for 30 min prior to adding TGFβ1 (5 ng/mL; Sigma-Aldrich, New South Wales, Australia).
2.3. Adenovirus Infection
As described in our previous studies, expression of Nox4 gene was silenced using adenoviral vectors expressing small interfering RNA (siRNA). In brief, siRNA that has been designed to target human Nox4 nucleotides 418–436 from the start codon (Ad-Nox4i) [17] was used to infect cells to suppress the ROS production capacity of Nox4. Adenovirus expressing green fluorescent protein siRNA (Ad-CtrlRNAi-GFP) was used as a control. HTFs (105 cells/well) were seeded in 6-well plate at one day prior to infection. The HTFs were then infected with 2500 multiplicity of infection (MOI) of either Ad-CtrlRNAi or Adv-Nox4i in reduced serum Opti-MEM medium (Life Technologies, Waltham, MA, USA) for 24 h. HTFs were then allowed to recover in complete RPMI medium for another 24 h. All experiments were then carried out at 48 h after infection.
2.4. Determination of Extracellular H2O2
Extracellular level of H2O2 from cells were analyzed using Amplex® Red assay kit (Molecular Probes, Life Technologies, Victoria, Australia) according to manufacturer’s instructions as described previously [10]. Briefly, cells (2 × 104 cells/well) were seeded in a 24-well plate and incubated overnight at 37 °C/5% CO2. Cells were treated with or without TGFβ1 (5 ng/mL) and in combination with EUK-134 (10 µM) or adenoviruses for 24 h in Krebs-Ringer Bicarbonate Buffer containing 0.1% serum, Amplex® Red reagent (50 µM), and horseradish peroxidase (HRP; 0.1 U/mL). Fluorescence was then measured for 30 min at 37 °C using a Polarstar microplate reader (BMG Labtech, Ortenberg, Germany) with excitation and emission wavelengths at 550 nm and 590 nm, respectively.
2.5. Gene Expression Analysis
HTFs (105 cells/well) were plated in 6-well plates and incubated overnight at 37 °C/5% CO2. Cells were serum deprived for 24 h, followed by treatment with inhibitors and/or TGFβ1. Total RNA was extracted from treated cells using the TRI Reagent according to manufacturer’s protocol (Ambion, Waltham, MA, USA). cDNA was prepared from 200 ng of total RNA using high capacity performance reverse transcription reagents (Applied Biosystems, Waltham, MA, USA) at 25 °C for 10 min, at 37 °C for 2 h, and followed by 85 °C for 5 s in a Thermal cycler (BioRad-DNA Engine, Bio-Rad, Hercules, CA, USA). The quantitative real-time PCR (RT-PCR) reactions were performed (7300 system, Applied Biosystems, Life Technologies, Carlsbad, CA, USA) using TaqMan Universal PCR master mix and commercially available predesigned gene-specific probes and primer sets (TaqMan Gene Expression Assay, Life Technologies, Victoria, Australia) for Nox1, Nox2, Nox4, and Nox5 and GAPDH (Glyceraldehyde 3-phosphate dehydrogenase ) sequence (Table 1). Diethyl pyrocarbonate (DEPC)-treated water was used as a negative control. The cycle threshold (CT) values from all quantitative rt-PCR experiments were analyzed using ΔΔCT method. Data were normalized to housekeeping gene GAPDH and expressed as fold changes over that in the control group.

Table 1.
Human Taqman primer sequences used to amplify NADPH oxidase (Nox) isoforms for human Tenon’s fibroblasts (HTFs).
2.6. Western Blot Analysis
HTFs (1.5 × 105 cells/well) were cultured in 6-well tissue culture plates, serum deprived for 24 h, and followed by TGFβ1 induction. Proteins was extracted in radioimmunoprecipitation assay buffer RIPA lysis buffer and equal amounts of protein were then separated by electrophoresis using gradient SDS-PAGE gels and transferred to nitrocellulose membranes (Amersham Pharmacia, GE Healthcare Biosciences Pty. Ltd., New South Wales, Australia). After blocking non-specific proteins with 5% non-fat skim milk in Tris–HCl (20 mM, pH 7.5), NaCl (100 mM) and Tween 20 (0.1%) buffer respective membranes were incubated at 4 °C overnight with either primary rabbit monoclonal anti-NOX4 (No. ab60940, lot# GR18955-1; 1:1000 dilution, Abcam, Victoria, Australia), rabbit monoclonal anti-phospho-Smad2/Smad3 (No. 8828, 1:1000; Cell Signaling Technology, Danvers, MA, USA), or rabbit monoclonal anti-total Smad2/3 (No. 8685, 1:1000; Cell Signaling Technology, Danvers, MA, USA) and normalized with housekeeping mouse monoclonal anti-β-actin (No. ab8224, 1:10,000, Merck Millipore, Darmstadt, Germany) antibodies. Proteins were detected using an enhanced chemiluminescence detection kit (GE Healthcare, New South Wales, Australia) with horseradish peroxidase conjugated to appropriate secondary antibodies (Bio-Rad, New South Wales, Australia). The image was captured and processed using CanoScan 8800F/PhotoStudio 5.5 software (New South Wales, Australia).
2.7. Picro-Sirius Red Spectrophotometric Assay
Total collagen content was measured using Sirius red based high-throughput assay. Briefly, HTF cells (2.5 × 104) seeded in 96-well plates followed by various treatments of inhibitors with or without TGFβ1 were fixed using methanol for 1 h at −20 °C, followed by a gentle wash with phosphate-buffered saline PBS to prevent cell loss, and then incubated in Picro-Sirius red (0.1%; Sigma-Aldrich, Australia) for 1 h at room temperature. Picro-Sirius red was then removed, and cells were washed three times with 0.1% acetic acid. Picro-Sirius red was then eluted in 0.1 N sodium hydroxide (NaOH), 200 µL/well, the plates were placed on a rocking platform at room temperature for 1 h, and the optical density at 540 nm was determined using a Bio-Tek spectrophotometer (BioTek, Winooski, VT, USA).
2.8. Cell Proliferation Assay
Cells (104 cells/well) were plated in a 24-well plate. Serum-deprived cells were treated with or without EUK-134 (10 µM) for 24 h. Cell proliferation was then induced by replacing serum-free media with RPMI containing 0.5% serum. After 48 h, cell numbers were analyzed using Alamar blue assay kit. Each well was incubated with Alamar blue assay solution (1:10 dilution with RPMI media; Life Technologies, Victoria, Australia) for 1 h at 37 °C, 5% CO2. Fluorescence was then measured with excitation and emission wavelengths of 480 nm and 520 nm, respectively, using a Polarstar microplate reader at 37 °C.
2.9. Mouse Model of Glaucoma Filtration Surgery
All procedures were performed as per National Institutes of Health guide for the care and use of Laboratory animals and were approved by the institutional animal care and use committee (St. Vincent’s Animal Ethics Committee Protocol AEC#027/14). C57BL/6J mice (derived from Jacksons Lab, CT USA) were obtained from ARC (Western Australia, Australia) and bred at the EMSU mouse facility (Fitzroy, Victoria, Australia). GFS was performed on C57BL/6J WT and Nox4 KO male mice (10 weeks old) as previously described [3]. Briefly, mice were anesthetized using intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). The conjunctiva of one eye was dissected to create a small filtration space in the subconjunctival. An incision was then made with a 30-gauge needle through the sclera into the anterior chamber of the eye to create a fistula, which allows aqueous humor to exit from the anterior chamber and into the subconjunctival space. The dissected conjunctiva was then sutured over the newly created fistula. Topical antibiotic ointment Chlorsig (0.5%) was applied to the operated eye to avoid any infection after surgery. The non-operated fellow eye did not receive topical Chlorsig and served as the control. Eyes were then harvested at day-14 post-surgery to evaluate collagen deposition around the bleb. Eyes were enucleated and fixed in 4% paraformaldehyde (PFA; ProSciTech, Kirwan, Queensland, Australia) overnight, followed by agar and paraffin-embedding. Four-μm sections were then stained with Picro-Sirius red for quantification and assessment of collagen matrix in Nox4 KO and WT mice. Images were captured with a microscope (20X objective magnification, Olympus, South Australia, Australia). The area of collagen accumulation at the surgical wound was delineated and measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA), by a researcher who is masked to the mouse genotype.
2.10. Statistical Analysis
All values are expressed as mean ± S.E.M. The mean results were analyzed using one-way analysis of variance (ANOVA) followed by post-hoc Tukey analysis. A p value < 0.05 was considered as statistically significant.
3. Results
3.1. TGFβ1 Cause Increase in Nox4 mRNA, H2O2 Generation, and Collagen Synthesis in HTFs
We treated HTFs with TGFβ1 (5 ng/mL) and assayed the mRNA expression of Nox1, Nox2, Nox4 and Nox5 by rt-PCR at 3, 6, and 24 h after TGFβ1 application. Of the four Nox isoforms, TGFβ1 induced Nox4 mRNA and protein expressions at all timepoints and peak responses were seen at 6 and 24 h (Figure 1A, p < 0.05, n = 4). TGFβ1 did not alter the mRNA expression of Nox1 and Nox2 but downregulated Nox5 (data not shown). In parallel to an upregulation of Nox4 mRNA and protein, the level of H2O2 generation was elevated at all timepoints (Figure 1B, p < 0.05, n = 3–5). We assessed collagen production by evaluating the absorbance readings of the eluents from HTFs stained with picrosirius red. There was statistically significant accumulation of collagen in HTFs at both 6 and 24 h following TGFβ1 stimulation (Figure 1C, p < 0.05, n = 3–5).
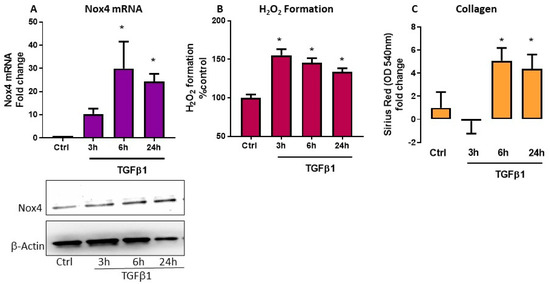
Figure 1.
Effect of transforming growth factor (TGF)β1 in human Tenon’s fibroblasts (HTFs). Responses were determined in control fibroblasts without and with TGFβ1 (5 ng/mL) treatment for 3, 6, and 24 h. TGFβ1 caused increase in (A) Nox4 mRNA and protein expression and (B) H2O2 generation. (A) A representative blot and corresponding bar graph showing expression of Nox4 and internal control β-actin. (C) Collagen accumulation was determined by the absorbance values of Picrosirius red determined at 540 nm and TGFβ1 increases absorbances at 6 and 24 h. * p < 0.05 (n = 3–5). All data are mean ± SEM from three to five independent experiments, * p < 0.05 from Ctrl. without treatment.
3.2. TGFβ1-Induced Nox4 Expression Requires Smad3-Activation in HTFs
We have previously demonstrated that the phosphorylation of Smad3 is involved in the TGFβ1-induced Nox4 gene expression and collagen production in rabbit conjunctival fibroblasts [10]. We analyzed the effect of TGFβ1 on protein expression of Smad3 (total Smad) and its phosphorylated form (p-Smad3) in HTFs using Western blots at 10, 30, and 60 min after treatment. As expected, TGFβ1 induced phosphorylation of Smad3 within 10 min in these cells (Figure 2A), and treatment with a known Smad3 inhibitor SIS3 was found to abolish TGFβ1-induced phosphorylation of Smad3 (Figure 2A). HTFs pre-treated with SIS3 abolished TGFβ1-induced Nox4 mRNA expression (Figure 2B) and total collagen production (Figure 2C), confirming that Smad3 is required for both responses.
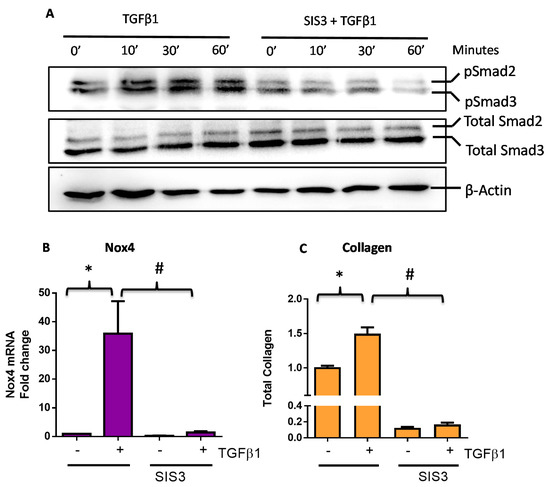
Figure 2.
TGFβ1 responses are mediated via a Smad3-dependent pathway in human Tenon’s fibroblasts (HTFs). (A) A representative Western blot showing TGFβ1 (5 ng/mL) induced Smad3 phosphorylation in the absence and presence of a Smad3 inhibitor SIS3 (5 μM). (B) Pre-treatment of Smad3 inhibitor SIS3 suppressed TGFβ1-induced (B) Nox4 gene expression (6 h) and (C) total collagen expression (24 h). All data are mean ± SEM from three to four independent experiments, * p < 0.05 from Ctrl without treatment; # p < 0.05 from cells treated with TGFβ1.
3.3. Suppression of Nox4 and H2O2 Generation Decreased TGFβ1-Induced Responses in HTFs
To demonstrate the functional importance of TGFβ1-induced Nox4 expression, we used an adenoviral vector carrying siRNA targeting human Nox4 (Ad-Nox4i) to silence the expression of Nox4 in HTFs. As expected, Ad-Nox4i significantly reduced TGFβ1 induction of Nox4 mRNA (Figure 3A) and protein (Figure 3B). Importantly, we also showed that Ad-Nox4i suppressed the production of H2O2 (Figure 3C) and total collagen synthesis (Figure 3D) in the presence of TGFβ1 stimulation. Similarly, removal of H2O2 using SOD/catalase mimetic EUK-134 also suppressed TGFβ1-induced H2O2 formation (Figure 3E) and total collagen production (Figure 3F), suggesting that Nox4-derived H2O2 is required for collagen synthesis by HTFs.
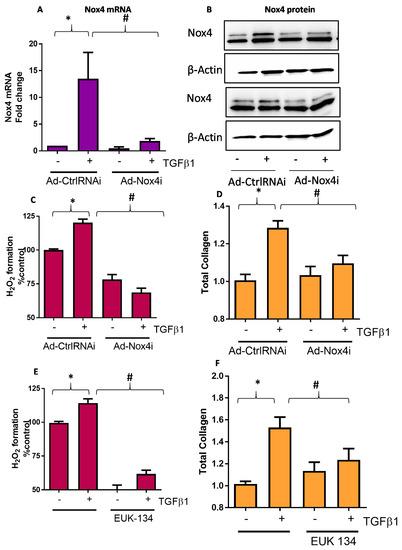
Figure 3.
Effect of Nox4 inhibition and EUK-134 on H2O2 and collagen production in human Tenon’s fibroblasts (HTFs). Treatment of HTF with Ad-Nox4i inhibited the stimulatory effects of TGFβ1 (5 ng/mL) on expressions of both Nox4 (A) gene and (B) protein at 6 h, (C) generation of H2O2 at 6 h, and (D) total collagen at 24 h. Similarly, H2O2 scavenger EUK-134 (25 μM) inhibited TGFβ1-induced (E) H2O2 formation and (F) total collagen production. All data are mean ± SEM from three to six independent experiments, * p < 0.05 from control without treatment; # p < 0.05 from treated cells with TGFβ1.
3.4. Nox4 and H2O2 Generation Is Involved in TGFβ1-Induced Proliferation of HTFs
Activation of Tenon’s fibroblasts is known to enhance its proliferation capacity [18] and production of fibrotic proteins, which promotes scar formation and fibrosis. We, therefore, explored Nox4 regulation on TGFβ1-induced cell proliferation of HTFs. Treatment with Ad-Nox4i markedly abolished TGFβ1 stimulated cell proliferation (Figure 4A). Ad-Nox4i was also found to inhibit cell proliferation in the absence of TGFβ1, suggesting Nox4 might be involved in cell proliferation during normal growth (Figure 4A). Likewise, scavenging H2O2 with EUK-134 suppressed proliferation of HTFs in the presence of TGFβ1 stimulation (Figure 4B).
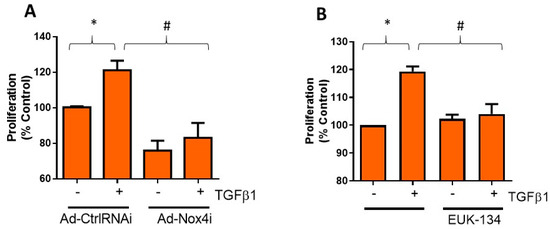
Figure 4.
Effect of Nox4 inhibition and EUK-134 on proliferation of human Tenon’s fibroblasts (HTFs). (A) Treatment of HTFs with Ad-Nox4i inhibited the stimulatory effects of TGFβ1 (5 ng/mL) on cell proliferation. (B) Similarly, H2O2 scavenger EUK-134 (25 μM) inhibited TGFβ1-induced cell proliferation. All data are mean ± SEM from three independent experiments, * p < 0.05 from control without TGFβ1 treatment; # p < 0.05 from treated cells with TGFβ1.
3.5. Collagen Deposition at the Wound Is Reduced in Nox4-Deficient Mice with Glaucoma Filtration Surgery
As expected, there are increases in both collagen accumulation (Figure 5A) and area of fibrovascularization at the wound (Figure 5B) at 2 weeks after GFS in wildtype (WT) mice. Mice deficient in Nox4 (Nox4 KO) showed a significant reduction in collagen accumulation and fibrovascular area at the wound (Figure 5A–C, p < 0.05, n = 4–5).
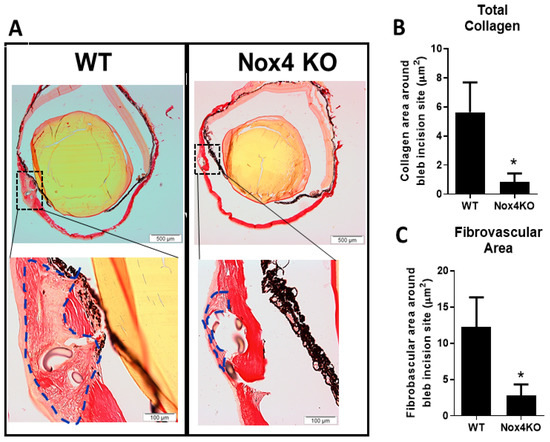
Figure 5.
Reduced deposition of collagen at the wound in Nox4 knockout (KO) mice with glaucoma filtration surgery (GFS). (A) Representative images of eye cross sections from wildtype (WT) and Nox4 KO mice at 2 weeks after GFS. (B) A reduction in the area of both total collagen accumulation and (C) fibrovascularization at the wound is demonstrated in Nox4 KO mice. All data are mean ± SEM from four to five animals, * p < 0.05 from WT.
4. Discussion
Excessive post-operative scarring is a major cause of failure in GFS [19]. In this study, we demonstrate that TGFβ1-induced Nox4 expression plays an important role in collagen synthesis by using HTFs. Furthermore, we showed for the first time that accumulation of collagen at the surgical site was reduced in Nox4 KO mice at day 14 following GFS. Given the limited option for controlling post-operative scarring, targeting Nox4 could be a potential therapeutic strategy for improving long-term surgical success.
The involvement of Nox4 in TGFβ1-mediated fibrotic responses has been characterized in a variety of human fibroblasts, including cardiac [20], lung [21], and dermal [22]. To date, not much is known about Nox4 and TGFβ signaling in ocular fibroblasts in the context of eye fibrosis. Collagen synthesis by HTFs is one of the responses induced by TGFβ1 that contributes to GFS post-operative fibrosis [23,24]. We, therefore, explored the involvement of Nox4 in TGFβ1 signaling by evaluating collagen accumulation in HTFs with Sirius red assay. Sirius red assay has been validated against another collagen quantification method, like hydroxyproline assay [25], and it has previously been used to determine collagen content in rabbit Tenon [25] and fibroblast cultures [10,26]. The present study, for the first time, demonstrated that the effect of TGFβ1 on collagen accumulation in HTFs involved Nox4 expression and H2O2 generation. These findings align with our previous study that shows Nox4 is implicated in TGFβ1-mediated collagen synthesis in rabbit conjunctival fibroblasts [10] and Nox4.
Nox4 belongs to the Nox family of ROS-generating enzymes, and seven homologues, namely Nox1, Nox2, Nox3, Nox4, Nox5, and Duox1 and 2, have been identified [27]. Human fibroblasts from various tissues, such as heart [20], lungs [28], and skin [22,29], express more than one subtype of Nox. For example, pulmonary fibroblasts express both Nox1 and Nox4 [28], cardiac fibroblasts express Nox4 and Nox5 [20], and dermal fibroblasts express Nox1, Nox2, Nox4, and Nox5 [22,29]. Despite expressing different Nox isoforms, Nox4 appears to be the isoform involved in TGFβ1-mediated fibrotic responses at least in heart and lung fibroblasts [20,28]. Likewise, we found that HTFs expressed Nox4 and Nox5, and TGFβ1 stimulated Nox4 gene expression but decreased Nox5 mRNA level. This distinct effect of TGFβ1 on Nox isoforms in HTFs is similar to the findings in human cardiac fibroblasts [20].
We demonstrate that the stimulatory effect of TGFβ1 on collagen production in HTFs involved phosphorylation of Smad3. Our findings agree with the study by Xiao et al. [30], who demonstrated that inhibitor of Smad3 phosphorylation abrogated TGFβ-induced fibroblast trans differentiation and gene upregulation of collagen I in HTFs. By inhibiting Smad3 phosphorylation with SIS3, the effect of TGFβ1 on both Nox4 mRNA expression and H2O2 generation was abolished. Moreover, knocking down Nox4 gene expression with siRNA or scavenging H2O2 inhibited TGFβ1-induced collagen accumulation in HTFs. We, therefore, propose that TGFβ1 activates Nox4 expression and enhances H2O2 production through Smad3 phosphorylation and subsequently leads to collagen production. The proposed pathway is consistent with our previous findings that show TGFβ1/Smad3/Nox4 signaling mechanism in collagen synthesis in rabbit conjunctival fibroblasts [10].
To validate that Nox4 is involved in scarring responses in an in vivo model, we employed the mouse model of GFS to examine the contribution of Nox4 to collagen deposition at the surgical wound. It has been shown that the accumulation of collagen at the wound occurs in 14 days after GFS [3,31]. Further the surgery instigates a wound healing response that involves TGFβ signaling [32] and this aligns with the cell culture assays that employs TGFβ as an inducer. We demonstrated that the wound at the surgical site is packed with dense collagen fibers in WT mice with GFS. The surgical site of operated eye shows loosely organized collagen fibers and the deposition of collagen is significantly suppressed in mice lacking Nox4. This novel finding agrees with the contribution of Nox4 to TGFβ1 mediated collagen production by HTFs.
TGFβ is known to be a crucial profibrotic factor implicated in wound scarring after GFS and an antisense oligonucleotides to TGFβ has been shown to improve surgical outcome in a rabbit model of GFS [32]. Although anti-TGFβ therapy appears to be an effective way to control scarring, TGFβ has other important physiological functions, and incomplete inhibition of TGFβ likely due to insufficient dosage regimen or its receptor activation would result in severe off-target effects [9,33]. Therefore, targeting TGFβ downstream pathway may be an alternative strategy. Indeed, inhibition of TGFβ messenger protein, like Smad, has already been explored as an avenue to control conjunctival fibrosis [33]. TGFβ is found to activate Nox4 through Smad in HTF and Nox4 KO mice with GFS also show less wound scarring, our findings thus highlight the potential of Nox4 targeting in the treatment of post-surgical scarring. Further study would be required to assess if Nox4 inhibition would be efficacious in suppressing scarring responses in vivo.
5. Conclusions
In conclusion, we have highlighted a novel role of Nox4 in the stimulatory effect of TGFβ1 on collagen production and cell proliferation in HTFs (Figure 6). We further confirm that collagen deposition at the surgical site is significantly reduced in GFS operated mice lacking Nox4. Given the limited option for controlling post-operative scarring in GFS, targeting Nox4 would be a potential therapeutic strategy or an adjunct therapy for improving the long-term success of surgical outcome.
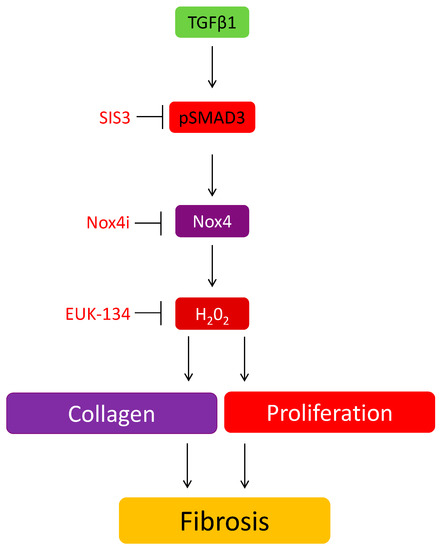
Figure 6.
The involvement of Nox4 in the induction of TGFβ on collagen and cell proliferation in HTF.
Author Contributions
Conceptualization, M.H.S., J.G.C. and H.M.P.; methodology, M.H.S, E.C.C., N.J.V.B., S.S.P., S.N. and H.M.P.; validation, M.H.S., E.C.C., N.J.V.B., S.S.P and H.M.P.; formal analysis, M.H.S., E.C.C, S.N. and H.M.P.; data curation, M.H.S. and H.M.P.; writing—original draft preparation, M.H.S., E.C.C. and HM.P.; writing—review and editing, M.H.S., E.C.C. and J.G.C.; supervision, M.H.S, and H.M.P.; project administration, M.H.S., J.G.C. and H.M.P.; funding acquisition, H.M.P. and J.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by National Health and Medical Research Council Research Project Grant GNT1102092, Edols Trust and The Eye Research Australia Foundation. The Centre for Eye Research Australia received Operational Infrastructure Support from the Victorian Government.
Acknowledgments
We thank Karl-Heinz Krause for providing the Nox4 KO mice.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Adv-Ctrl siRNA | adenovirus expressing scrambled siRNA |
| Adv-Nox4i | adenovirus carrying siRNA targeting Nox |
| GFS | glaucoma filtration surgery |
| H2O2 | hydrogen peroxide |
| HTF | human Tenon’s fibroblasts |
| MOI | multiplicity of infection |
| mRNA | messenger ribonucleic acid |
| NADPH oxidase 4 | Nox4 |
| PCR | polymerase chain reaction |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| SIS3 | specific inhibitor of Smad3 |
| TGFβ | transforming growth factor β |
References
- Silvio, P.M.; World Health Organization. Global Data on Visual Impairments 2010; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Kirwan, J.F.; Lockwood, A.J.; Shah, P.; Macleod, A.; Broadway, D.C.; King, A.J.; McNaught, A.I.; Agrawal, P.; Trabeculectomy Outcomes Group Audit Study Group. Trabeculectomy in the 21st century: A multicenter analysis. Ophthalmology 2013, 120, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Seet, L.F.; Lee, W.S.; Su, R.; Finger, S.N.; Crowston, J.G.; Wong, T.T. Validation of the glaucoma filtration surgical mouse model for antifibrotic drug evaluation. Mol. Med. 2011, 17, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Malla, P.; Karki, P.; Das, H. Effectiveness of intra-operative and post-operative use of 5-fluorouracil in trabeculectomy—A randomized clinical trial. Nepal. J. Ophthalmol. 2010, 2, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Arvind, H.; Wechsler, D. Outcomes: Trabeculectomy Bleb Needle Revision with 5-Fluorouracil. J. Glaucoma 2016, 25, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Fan Gaskin, J.C.; Nguyen, D.Q.; Ang, G.S.; O’Connor, J.; Crowston, J.G. Wound Healing Modulation in Glaucoma Filtration Surgery-Conventional Practices and New Perspectives: The Role of Antifibrotic Agents (Part I). J. Curr. Glaucoma Pract. 2014, 8, 37–45. [Google Scholar] [PubMed]
- Min, S.H.; Lee, T.I.; Chung, Y.S.; Kim, H.K. Transforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uveitic eyes. Korean J. Ophthalmol. 2006, 20, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Yamanaka, O.; Baba, Y.; Kawashima, Y.; Shirai, K.; Miyamoto, T.; Okada, Y.; Ohnishi, Y.; Ooshima, A. Accumulation of latent transforming growth factor-beta binding protein-1 and TGF beta 1 in extracellular matrix of filtering bleb and of cultured human subconjunctival fibroblasts. Graefes Arch. Clin. Exp. Ophthalmol. 2001, 239, 234–241. [Google Scholar] [CrossRef]
- CAT-152 0102 Trabeculectomy Study Group; Khaw, P.; Grehn, F.; Holló, G.; Overton, B.; Wilson, R.; Vogel, R.; Smith, Z. A phase III study of subconjunctival human anti-transforming growth factor beta(2) monoclonal antibody (CAT-152) to prevent scarring after first-time trabeculectomy. Ophthalmology 2007, 114, 1822–1830. [Google Scholar] [CrossRef]
- Brown, K.D.; Shah, M.H.; Liu, G.S.; Chan, E.C.; Crowston, J.G.; Peshavariya, H.M. Transforming Growth Factor beta1-Induced NADPH Oxidase-4 Expression and Fibrotic Response in Conjunctival Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3011–3017. [Google Scholar] [CrossRef]
- Carnesecchi, S.; Deffert, C.; Donati, Y.; Basset, O.; Hinz, B.; Preynat-Seauve, O.; Guichard, C.; Arbiser, J.L.; Banfi, B.; Pache, J.-C.; et al. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid. Redox Signal. 2011, 15, 607–619. [Google Scholar] [CrossRef]
- Chan, E.C.; Peshavariya, H.M.; Liu, G.S.; Jiang, F.; Lim, S.Y.; Dusting, G.J. Nox4 modulates collagen production stimulated by transforming growth factor beta1 in vivo and in vitro. Biochem. Biophys. Res. Commun. 2012, 430, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Hecker, L.; Vittal, R.; Jones, T.; Jagirdar, R.; Luckhardt, T.R.; Horowitz, J.C.; Pennathur, S.; Martinez, F.J.; Thannickal, V.J. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009, 15, 1077–1081. [Google Scholar] [CrossRef]
- Chan, E.C.; Jiang, F.; Peshavariya, H.M.; Dusting, G.J. Regulation of cell proliferation by NADPH oxidase-mediated signaling: Potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol. Ther. 2009, 122, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Jarman, E.R.; Khambata, V.S.; Cope, C.; Jones, P.; Roger, J.; Ye, L.Y.; Duggan, N.; Head, D.; Pearce, A.; Press, N.J.; et al. An inhibitor of NADPH oxidase-4 attenuates established pulmonary fibrosis in a rodent disease model. Am. J. Respir. Cell Mol. Biol. 2014, 50, 158–169. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.C.; Qin, Q.; Van Bergen, N.J.; Connell, P.P.; Vasudevan, S.; Coote, M.A.; Trounce, I.A.; Wong, T.T.L.; Crowston, G.J. Antifibrotic activity of bevacizumab on human Tenon’s fibroblasts in vitro. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6524–6532. [Google Scholar] [CrossRef]
- Peshavariya, H.M.; Liu, G.S.; Chang, C.W.; Jiang, F.; Chan, E.C.; Dusting, G.J. Prostacyclin signalling boosts NADPH oxidase 4 in the endothelium promoting cytoprotection and angiogenesis. Antioxid. Redox Signal. 2014, 20, 2710–2725. [Google Scholar] [CrossRef] [PubMed]
- Stahnke, T.; Kowtharapu, B.S.; Stachs, O.; Schmitz, K.P.; Wurm, J.; Wree, A.; Guthoff, R.F.; Hovakimyan, M. Suppression of TGF-beta pathway by pirfenidone decreases extracellular matrix deposition in ocular fibroblasts in vitro. PLoS ONE 2017, 12, e0172592. [Google Scholar] [CrossRef] [PubMed]
- Fan Gaskin, J.C.; Nguyen, D.Q.; Ang, G.S.; O’Connor, J.; Crowston, J.G. Wound Healing Modulation in Glaucoma Filtration Surgery- Conventional Practices and New Perspectives: Antivascular Endothelial Growth Factor and Novel Agents (Part II). J. Curr. Glaucoma Pract. 2014, 8, 46–53. [Google Scholar]
- Cucoranu, I.; Clempus, R.; Dikalova, A.; Phelan, P.J.; Ariyan, S.; Dikalov, S.; Sorescu, D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 2005, 97, 900–907. [Google Scholar] [CrossRef]
- Ghatak, S.; Hascall, V.C.; Markwald, R.R.; Feghali-Bostwick, C.; Artlett, C.M.; Gooz, M.; Bogatkevich, G.S.; Atanelishvili, I.; Silver, R.M.; Wood, J.; et al. Transforming growth factor beta1 (TGFbeta1)-induced CD44V6-NOX4 signaling in pathogenesis of idiopathic pulmonary fibrosis. J. Biol. Chem. 2017, 292, 10490–10519. [Google Scholar] [CrossRef]
- Dosoki, H.; Stegemann, A.; Taha, M.; Schnittler, H.; Luger, T.A.; Schröder, K.; Distler, J.H.W.; Kerkhoff, C.; Böhm, M. Targeting of NADPH oxidase in vitro and in vivo suppresses fibroblast activation and experimental skin fibrosis. Exp. Dermatol. 2017, 26, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Sun, L.; Zhang, X.; Shi, H.; Xu, K.; Xiao, Y.; Ye, W. 5-Aza-2′-deoxycytidine induces human Tenon’s capsule fibroblasts differentiation and fibrosis by up-regulating TGF-beta type I receptor. Exp. Eye Res. 2017, 165, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.H.; Wang, H.Y.; Zhang, J.Y.; Shi, H.M.; Zhang, N.; Ye, W.; Xiao, Y.Q. Overexpression of ALK5 Induces Human Tenon’s Capsule Fibroblasts Transdifferentiation and Fibrosis In Vitro. Curr. Eye Res. 2017, 42, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Taskiran, D.; Taşkiran, E.; Yercan, H.; Kutay, F.Z. Quantification of total collagen in rabbit tendon by the sirius red method. Turk. J. Med. Sci. 1999, 29, 7–9. [Google Scholar]
- Trackman, P.C.; Saxena, D.; Bais, M.V. TGF-beta1- and CCN2-Stimulated Sirius Red Assay for Collagen Accumulation in Cultured Cells. Methods Mol. Biol. 2017, 1489, 481–485. [Google Scholar]
- Chan, E.C.; Liu, G.S.; Dusting, G.J. Redox mechanisms in pathological angiogenesis in the retina: Roles for NADPH oxidase. Curr. Pharm. Des. 2015, 21, 5988–5998. [Google Scholar] [CrossRef] [PubMed]
- Amara, N.; Goven, D.; Prost, F.; Muloway, R.; Crestani, B.; Boczkowski, J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax 2010, 65, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Wu, L.C.; Dai, T.; Chen, S.Y.; Wang, A.Y.; Lin, K.; Lin, D.M.; Yang, J.Q.; Cheng, B.; Zhang, L.; et al. NADPH oxidase-2 is a key regulator of human dermal fibroblasts: A potential therapeutic strategy for the treatment of skin fibrosis. Exp. Dermatol. 2014, 23, 639–644. [Google Scholar] [CrossRef]
- Xiao, Y.Q.; Liu, K.; Shen, J.F.; Xu, G.T.; Ye, W. SB-431542 inhibition of scar formation after filtration surgery and its potential mechanism. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Seet, L.F.; Su, R.; Barathi, V.A.; Lee, W.S.; Poh, R.; Heng, Y.M.; Manser, E.; Vithana, E.N.; Aung, T.; Weaver, M.; et al. SPARC deficiency results in improved surgical survival in a novel mouse model of glaucoma filtration surgery. PLoS ONE 2010, 5, e9415. [Google Scholar] [CrossRef][Green Version]
- Cordeiro, M.F.; Mead, A.; Ali, R.R.; Alexander, R.A.; Murray, S.; Chen, C.; York-Defalco, C.; Dean, N.M.; Schultz, G.S.; Khaw, P.T. Novel antisense oligonucleotides targeting TGF-beta inhibit in vivo scarring and improve surgical outcome. Gene Ther. 2003, 10, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, O.; Kitano-Izutani, A.; Tomoyose, K.; Reinach, P.S. Pathobiology of wound healing after glaucoma filtration surgery. BMC Ophthalmol. 2015, 15 (Suppl. 1), 157. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).