Neuroprotective Effect of Tricyclic Pyridine Alkaloids from Fusarium lateritium SSF2, against Glutamate-Induced Oxidative Stress and Apoptosis in the HT22 Hippocampal Neuronal Cell Line
Abstract
1. Introduction
2. Materials and Methods
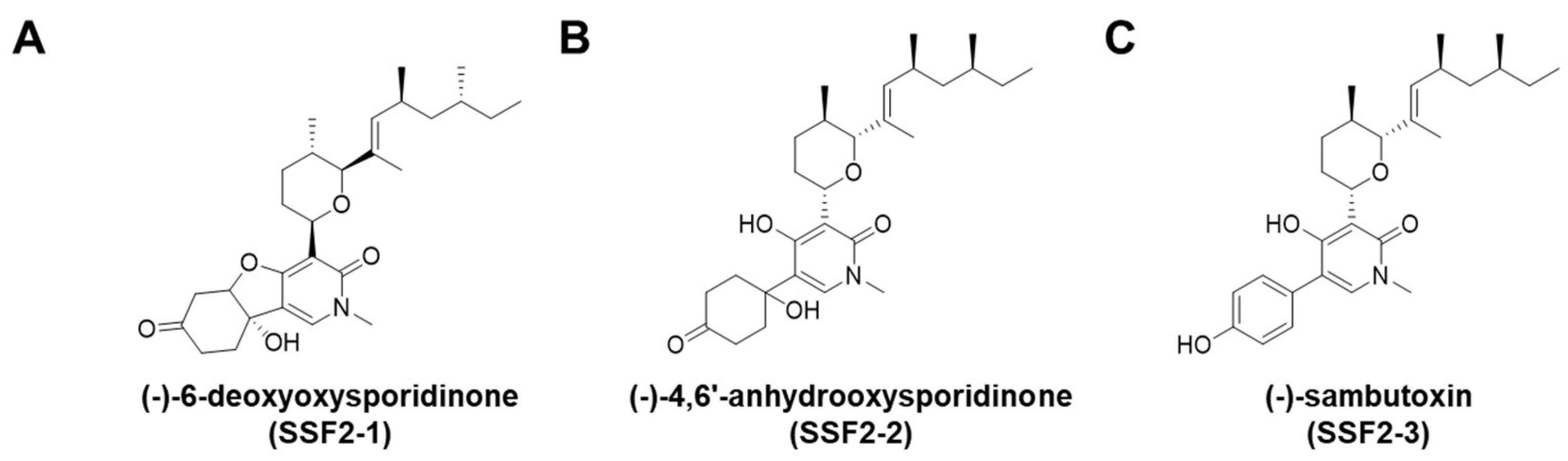
2.1. Extraction and Isolation
2.2. Cell Culture
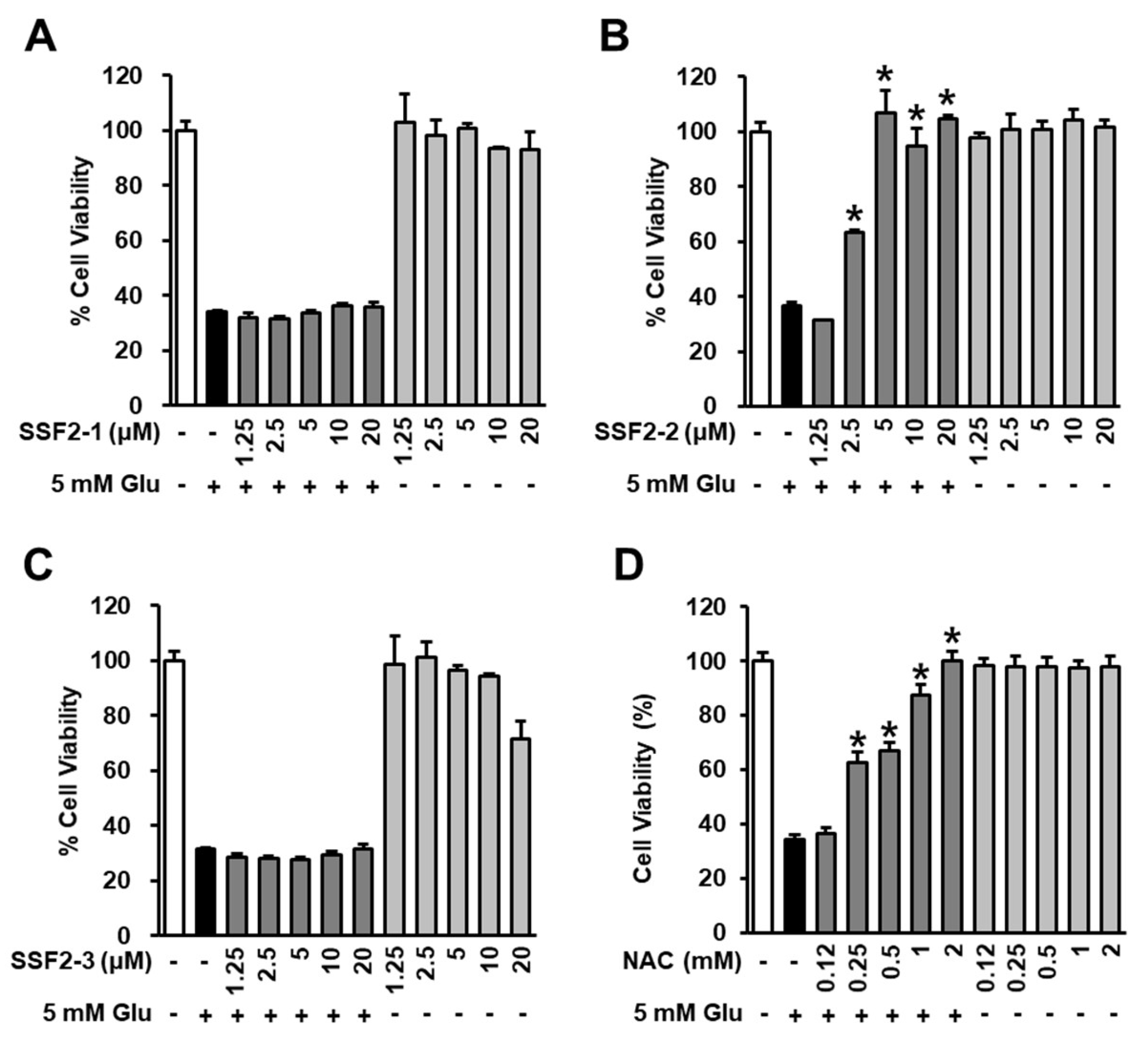
2.3. Measurement of Cell Viability
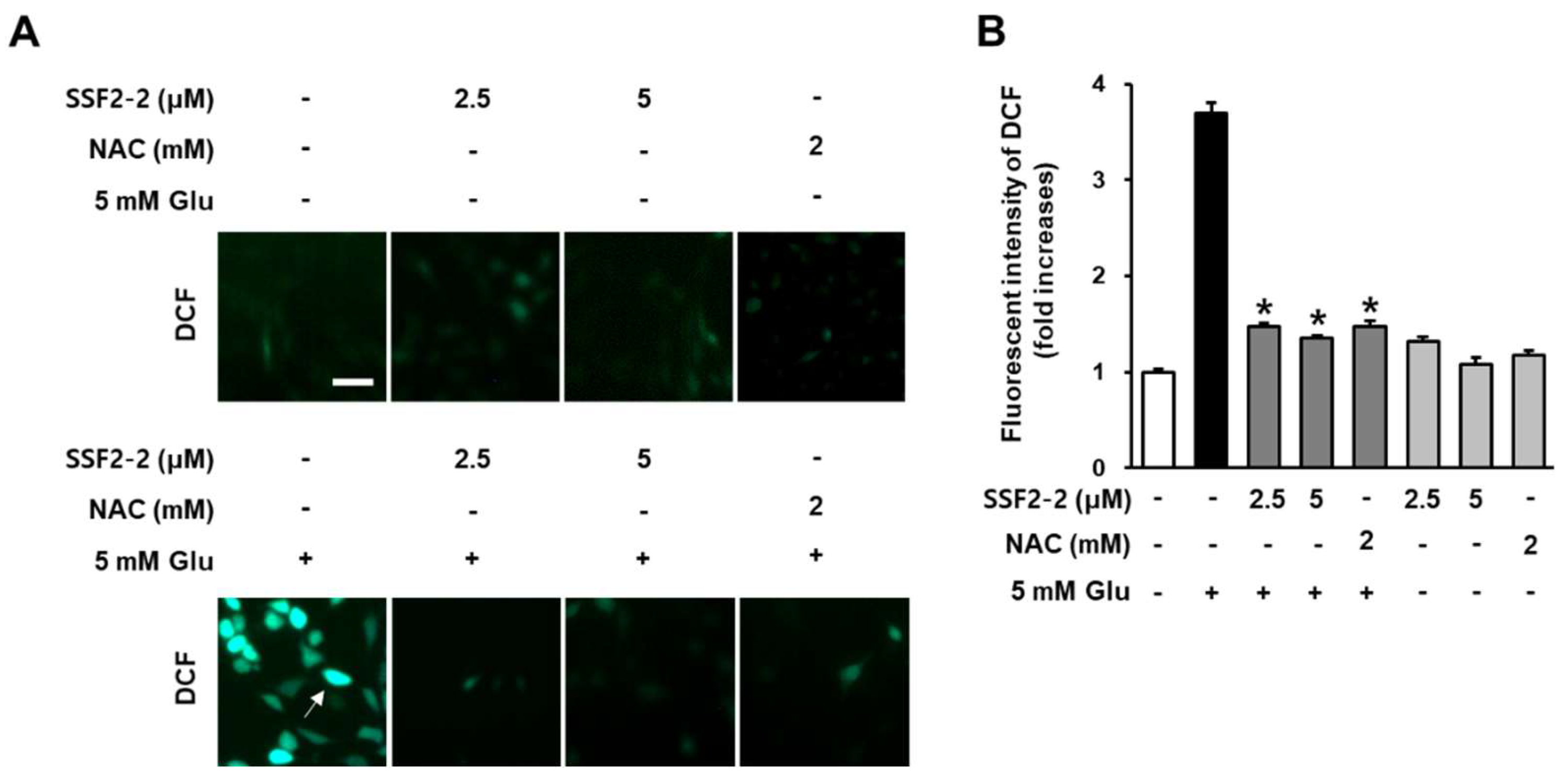
2.4. Measurement of Intracellular Reactive Oxygen Species
2.5. Measurement of Intracellular Ca2+
2.6. Measurement of Superoxide Radical
2.7. Measurement of Mitochondrial Membrane Potential
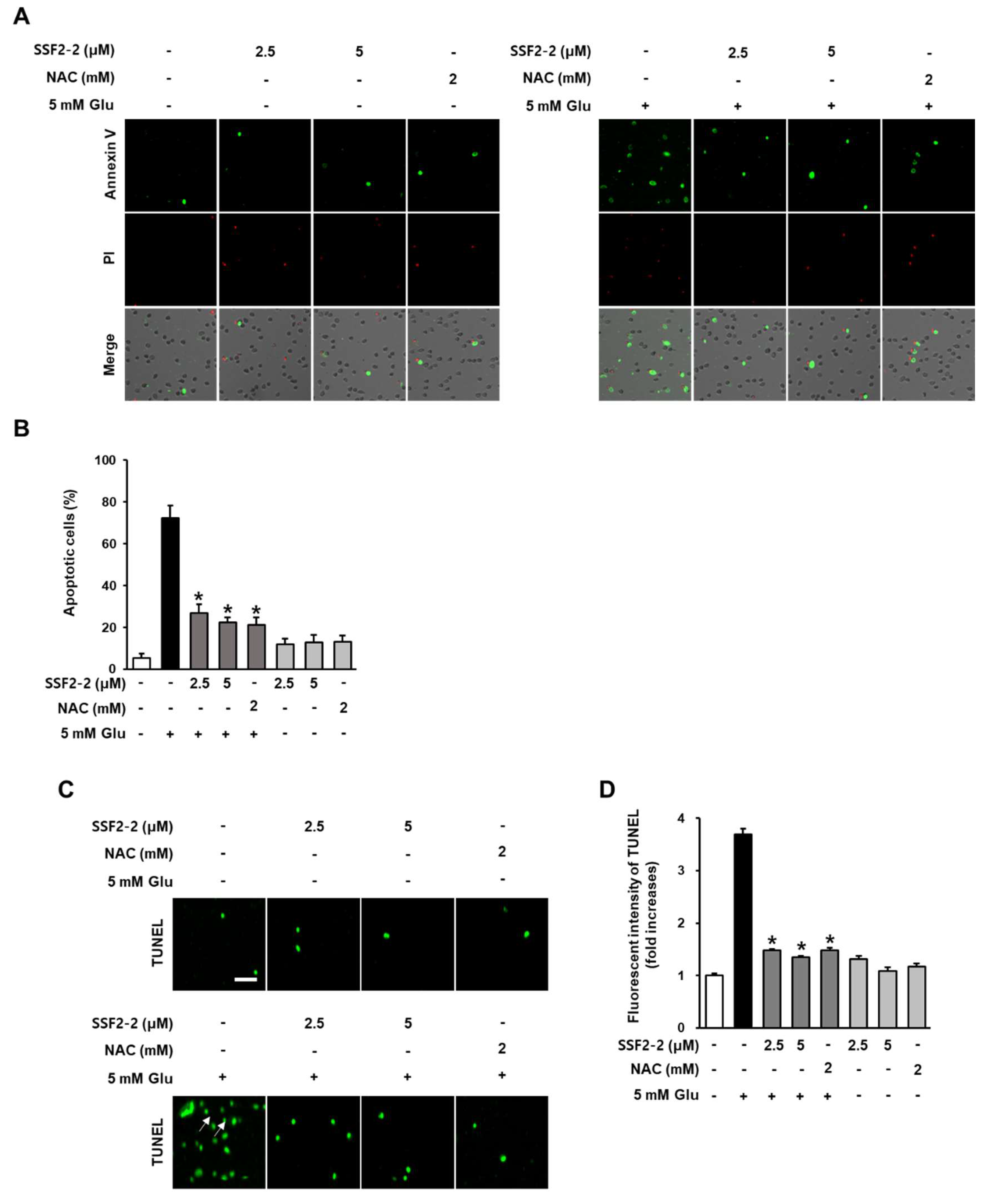
2.8. Measurement of Apoptotic Cell Death
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Effects of SSF2-1, SSF2-2, and SSF2-3 on Cell Viability after Glutamate Treatment
3.2. Inhibitory Effects of SSF2-2 on Glutamate-Induced ROS Generation
3.3. Inhibitory Effects of SSF2-2 on Glutamate-induced Excessive Levels of Ca2+
3.4. The inhibitory Effects of SSF2-2 on Glutamate-induced Depolarization of Mitochondrial Membrane Potential
3.5. The Inhibitory Effects of SSF2-2 on Glutamate-Induced Apoptosis
3.6. Effects of SSF2-2 on Glutamate-induced Reductions in the Nrf2 and HO-1.
3.7. Inhibitory Effects of SSF2-2 on Glutamate-Mediated Cytochrome c Release and Cleaved Caspase-9, -3
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chi, H.; Chang, H.Y.; Sang, T.K. Neuronal cell death mechanisms in major neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 3082. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Weon, J.B.; Yang, W.S.; Ryu, G.; Ma, C.J. Neuroprotective effects of Magnoliae Flos extract in mouse hippocampal neuronal cells. Sci. Rep. 2018, 8, 9693. [Google Scholar] [CrossRef] [PubMed]
- Zündorf, G.; Reiser, G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid. Redox Signal 2011, 14, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Gleichmann, M.; Collis, L.P.; Smith, P.J.; Mattson, M.P. Simultaneous single neuron recording of O2 consumption, [Ca2+]i and mitochondrial membrane potential in glutamate toxicity. J. Neurochem. 2009, 109, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Duchen, M.R. Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochim. Biophys. Acta 2008, 1777, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Lalkovičová, M.; Danielisová, V. Neuroprotection and antioxidants. Neural Regen. Res. 2016, 11, 865. [Google Scholar] [CrossRef]
- Sukprasansap, M.; Chanvorachote, P.; Tencomnao, T. Cyanidin-3-glucoside activates Nrf2-antioxidant response element and protects against glutamate-induced oxidative and endoplasmic reticulum stress in HT22 hippocampal neuronal cells. BMC Complement. Med. Ther. 2020, 20, 46. [Google Scholar] [CrossRef]
- Lin, S.P.; Li, W.; Winters, A.; Liu, R.; Yang, S.H. Artemisinin prevents glutamate-induced neuronal cell death via Akt pathway activation. Front. Cell. Neurosci. 2018, 12, 108. [Google Scholar] [CrossRef]
- Yang, E.J.; Lee, J.Y.; Park, S.H.; Lee, T.; Song, K.S. Neuroprotective effects of neolignans isolated from Magnoliae Cortex against glutamate-induced apoptotic stimuli in HT22 cells. Food Chem. Toxicol. 2013, 56, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Kim, G.S.; Jun, M.; Song, K.S. Kaempferol attenuates the glutamate-induced oxidative stress in mouse-derived hippocampal neuronal HT22 cells. Food Funct. 2014, 5, 1395–1402. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Ouyang, Z.; Su, Z.; Wang, D.; Yu, X. Neuroprotective effects of adenosine isolated from Cordyceps cicadae against oxidative and ER stress damages induced by glutamate in PC12 cells. Environ. Toxicol. Pharmacol. 2016, 44, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Song, J.H.; Song, J.H.; Ko, H.J.; Baek, J.Y.; Trinh, T.A.; Beemelmanns, C.; Yamabe, N.; Kim, K.H. Chemical identification of isoflavonoids from a termite-associated Streptomyces sp. RB1 and their neuroprotective effects in murine hippocampal HT22 cell line. Int. J. Mol. Sci. 2018, 19, 2640. [Google Scholar] [CrossRef]
- Kang, H.S.; Kim, J.P. Butenolide derivatives from the fungus Aspergillus terreus and their radical scavenging activity and protective activity against glutamate-induced excitotoxicity. Appl. Biol. Chem. 2019, 62, 43. [Google Scholar] [CrossRef]
- Trinh, T.A.; Park, E.J.; Lee, D.; Song, J.H.; Lee, H.L.; Kim, K.H.; Kim, Y.; Jung, K.; Kang, K.S.; Yoo, J.E.; et al. Estrogenic activity of sanguiin H-6 through activation of estrogen receptor α coactivator-binding site. Nat. Prod. Sci. 2019, 25, 28–33. [Google Scholar] [CrossRef][Green Version]
- Lim, L.; Ju, S.; Song, H. Dendropanax morbifera extract protects cardiomyocytes against hypoxia/reoxygenation injury by inhibition of reactive oxygen species generation and calcium perturbation. Nat. Prod. Sci. 2019, 25, 136–142. [Google Scholar] [CrossRef]
- Biel, T.G.; Rao, V.A. Mitochondrial dysfunction activates lysosomal-dependent mitophagy selectively in cancer cells. Oncotarget 2018, 9, 995. [Google Scholar] [CrossRef]
- Choi, J.H.; Choi, A.Y.; Yoon, H.; Choe, W.; Yoon, K.S.; Ha, J.; Yeo, E.J.; Kang, I. Baicalein protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis through inhibition of reactive oxygen species production and CHOP induction. Exp. Mol. Med. 2010, 42, 811–822. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, W.Y.; Jeon, Y.J.; Lee, S.K.; Son, C.G. Aquilariae Lignum extract attenuates glutamate-induced neuroexcitotoxicity in HT22 hippocampal cells. Biomed. Pharmacother. 2018, 106, 1031–1038. [Google Scholar] [CrossRef]
- Atlante, A.; Calissano, P.; Bobba, A.; Giannattasio, S.; Marra, E.; Passarella, S. Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett. 2001, 497, 1–5. [Google Scholar] [CrossRef]
- Aschner, M.; Syversen, T.; Souza, D.; Rocha, J.B.T.D.; Farina, M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz. J. Med. Biol. Res. 2007, 40, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Abd Jalil, A.; Khaza’ai, H.; Nordin, N.; Mansor, N.I.; Zaulkffali, A.S. Vitamin E-mediated modulation of glutamate receptor expression in an oxidative stress model of neural cells derived from embryonic stem cell cultures. Evid. Based Complement. Altern. Med. 2017, 2017, 6048936. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Kim, G.S.; Kim, J.A.; Song, K.S. Protective effects of onion-derived quercetin on glutamate-mediated hippocampal neuronal cell death. Pharmacogn. Mag. 2013, 9, 302. [Google Scholar] [PubMed]
- Lee, H.Y.; Weon, J.B.; Ryu, G.; Yang, W.S.; Kim, N.Y.; Kim, M.K.; Ma, C.J. Neuroprotective effect of Aronia melanocarpa extract against glutamate-induced oxidative stress in HT22 cells. BMC Complement. Altern. Med. 2017, 17, 207. [Google Scholar] [CrossRef]
- Park, J.Y.; Amarsanaa, K.; Cui, Y.; Lee, J.H.; Wu, J.; Yang, Y.S.; Eun, S.Y.; Jung, S.C. Methyl lucidone exhibits neuroprotective effects on glutamate-induced oxidative stress in HT-22 cells via Nrf-2/HO-1 signaling. Appl. Biol. Chem. 2019, 62, 67. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, H.J.; Lee, S.R.; Lee, H.S.; Huh, J.W.; Bae, Y.C.; Lee, D.S. Peroxiredoxin 5 inhibits glutamate-induced neuronal cell death through the regulation of calcineurin-dependent mitochondrial dynamics in HT22 cells. Mol. Cell. Biol. 2019, 39, e00148-19. [Google Scholar] [CrossRef]
- Peng, T.I.; Jou, M.J. Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci. 2010, 1201, 183–188. [Google Scholar] [CrossRef]
- Kumari, S.; Mehta, S.L.; Li, P.A. Glutamate induces mitochondrial dynamic imbalance and autophagy activation: Preventive effects of selenium. PLoS ONE 2012, 7, e39382. [Google Scholar] [CrossRef]
- Fukui, M.; Song, J.H.; Choi, J.; Choi, H.J.; Zhu, B.T. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur. J. Pharmacol. 2009, 617, 1–11. [Google Scholar] [CrossRef]
- Park, S.Y.; Jung, W.J.; Kang, J.S.; Kim, C.M.; Park, G.; Choi, Y.W. Neuroprotective effects of α-iso-cubebene against glutamate-induced damage in the HT22 hippocampal neuronal cell line. Int. J. Mol. Med. 2015, 35, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yuan, B.; Chu, Q.; Wang, C.; Bi, H. Protective roles of isoastilbin against Alzheimer’s disease via Nrf2-mediated antioxidation and anti-apoptosis. Int. J. Mol. Med. 2019, 43, 1406–1416. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Choi, H.G.; Hwang, J.H.; Shim, S.H.; Kang, K.S. Neuroprotective Effect of Tricyclic Pyridine Alkaloids from Fusarium lateritium SSF2, against Glutamate-Induced Oxidative Stress and Apoptosis in the HT22 Hippocampal Neuronal Cell Line. Antioxidants 2020, 9, 1115. https://doi.org/10.3390/antiox9111115
Lee D, Choi HG, Hwang JH, Shim SH, Kang KS. Neuroprotective Effect of Tricyclic Pyridine Alkaloids from Fusarium lateritium SSF2, against Glutamate-Induced Oxidative Stress and Apoptosis in the HT22 Hippocampal Neuronal Cell Line. Antioxidants. 2020; 9(11):1115. https://doi.org/10.3390/antiox9111115
Chicago/Turabian StyleLee, Dahae, Hyun Gyu Choi, Ji Hye Hwang, Sang Hee Shim, and Ki Sung Kang. 2020. "Neuroprotective Effect of Tricyclic Pyridine Alkaloids from Fusarium lateritium SSF2, against Glutamate-Induced Oxidative Stress and Apoptosis in the HT22 Hippocampal Neuronal Cell Line" Antioxidants 9, no. 11: 1115. https://doi.org/10.3390/antiox9111115
APA StyleLee, D., Choi, H. G., Hwang, J. H., Shim, S. H., & Kang, K. S. (2020). Neuroprotective Effect of Tricyclic Pyridine Alkaloids from Fusarium lateritium SSF2, against Glutamate-Induced Oxidative Stress and Apoptosis in the HT22 Hippocampal Neuronal Cell Line. Antioxidants, 9(11), 1115. https://doi.org/10.3390/antiox9111115







