Serum Selenium Status and Its Interrelationship with Serum Biomarkers of Thyroid Function and Antioxidant Defense in Hashimoto’s Thyroiditis
Abstract
1. Introduction
2. Materials and Methods
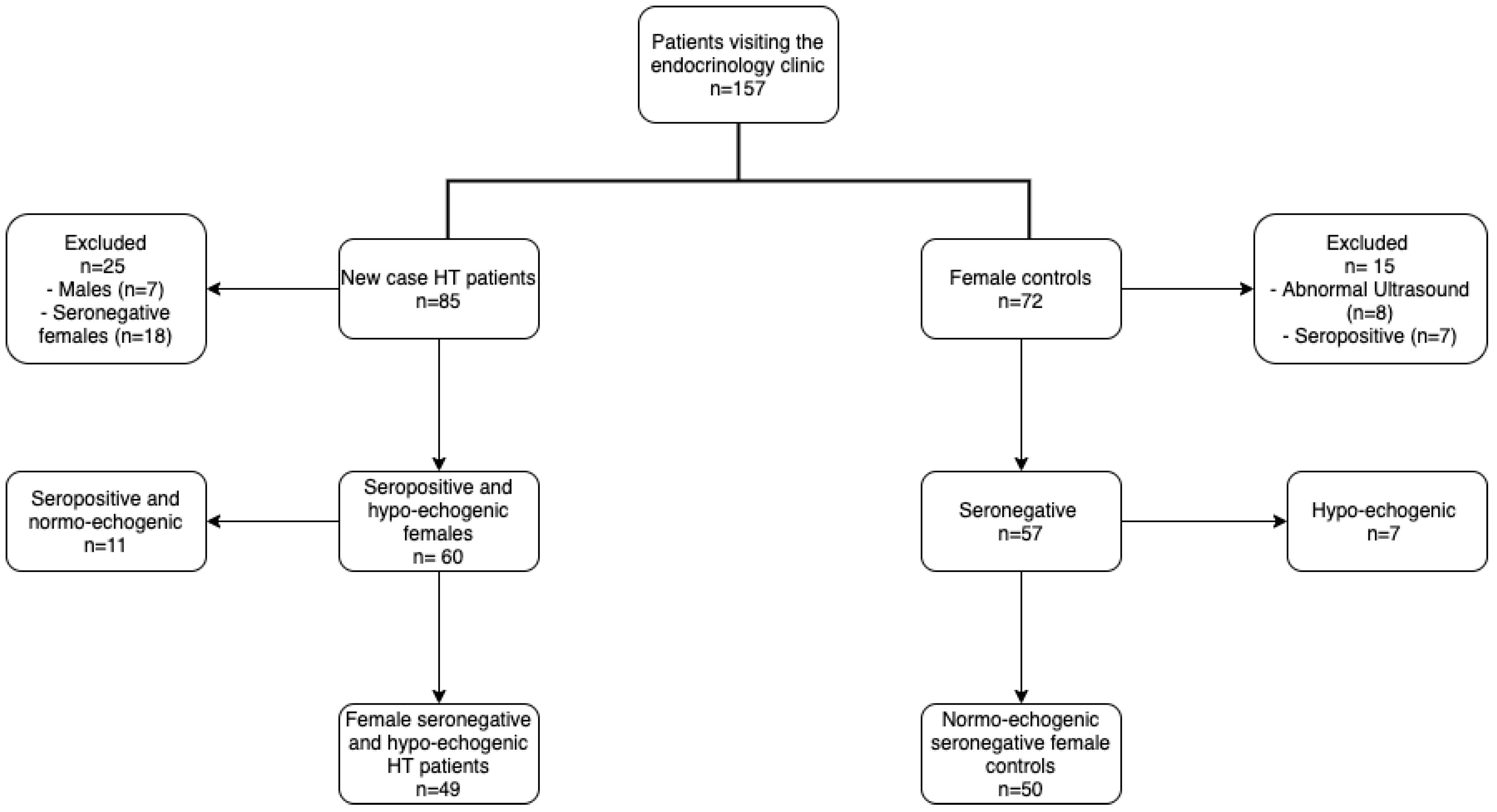
2.1. Studied Population
2.2. Blood Collection
2.3. Urine Collection
2.4. Assessment of Thyroid Volume and Thyroid Echogenicity
2.5. Thyroid Function and Antibody Tests
2.6. Selenium Assay
2.7. Assessment of Urinary Iodine Excretion (UIC)
2.8. Measurement of Urinary Creatinine (UCr)
2.9. Measurement of Serum Glutathione Peroxidase (GPx3) Activity
2.10. Glutathione Determination
2.11. Statistical Analysis
3. Results
3.1. Population Characteristics and Hypothyroidism
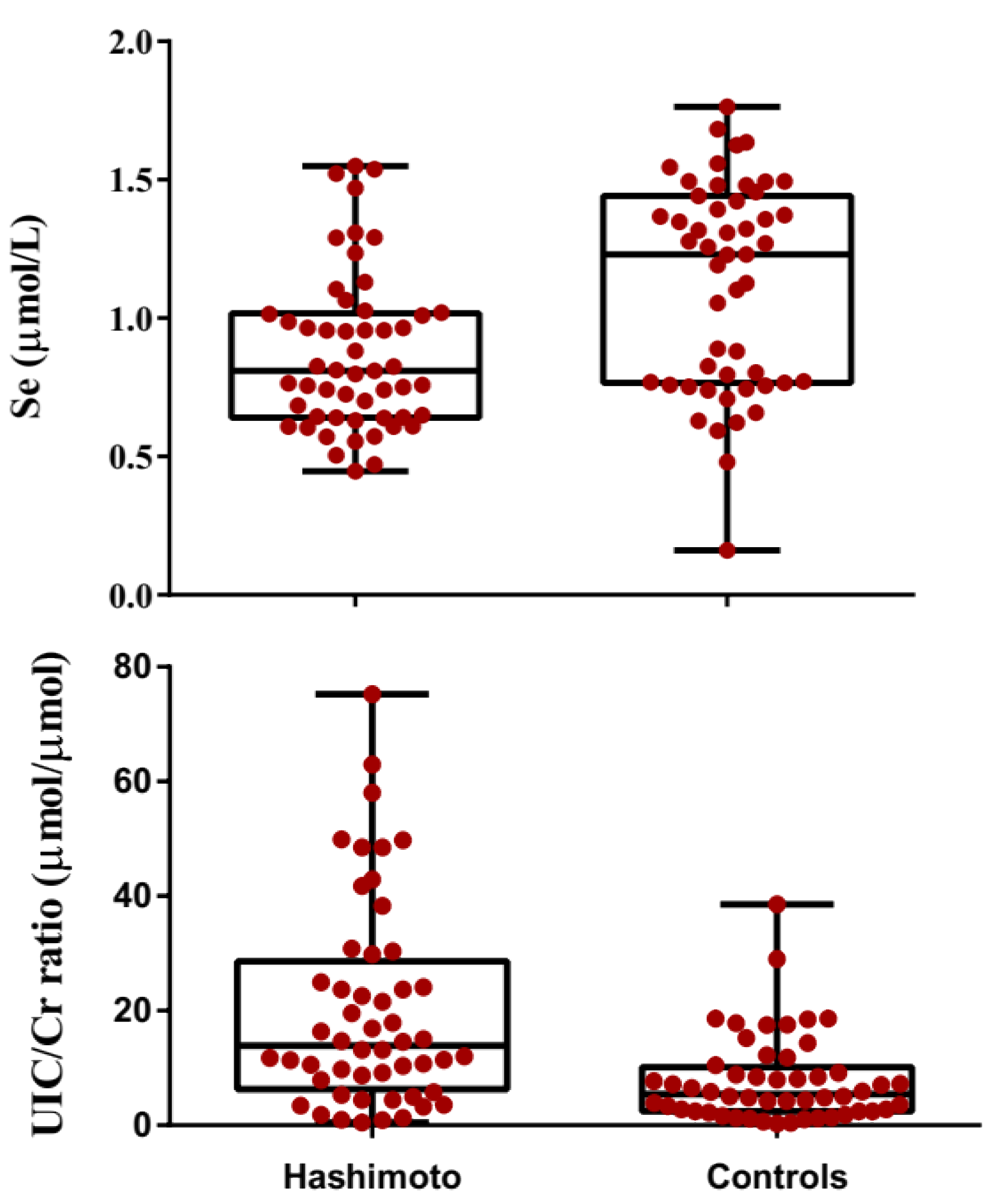
3.2. Comparison of Biochemical Paraameters in Hashimato Thyroiditis Patients and Controls
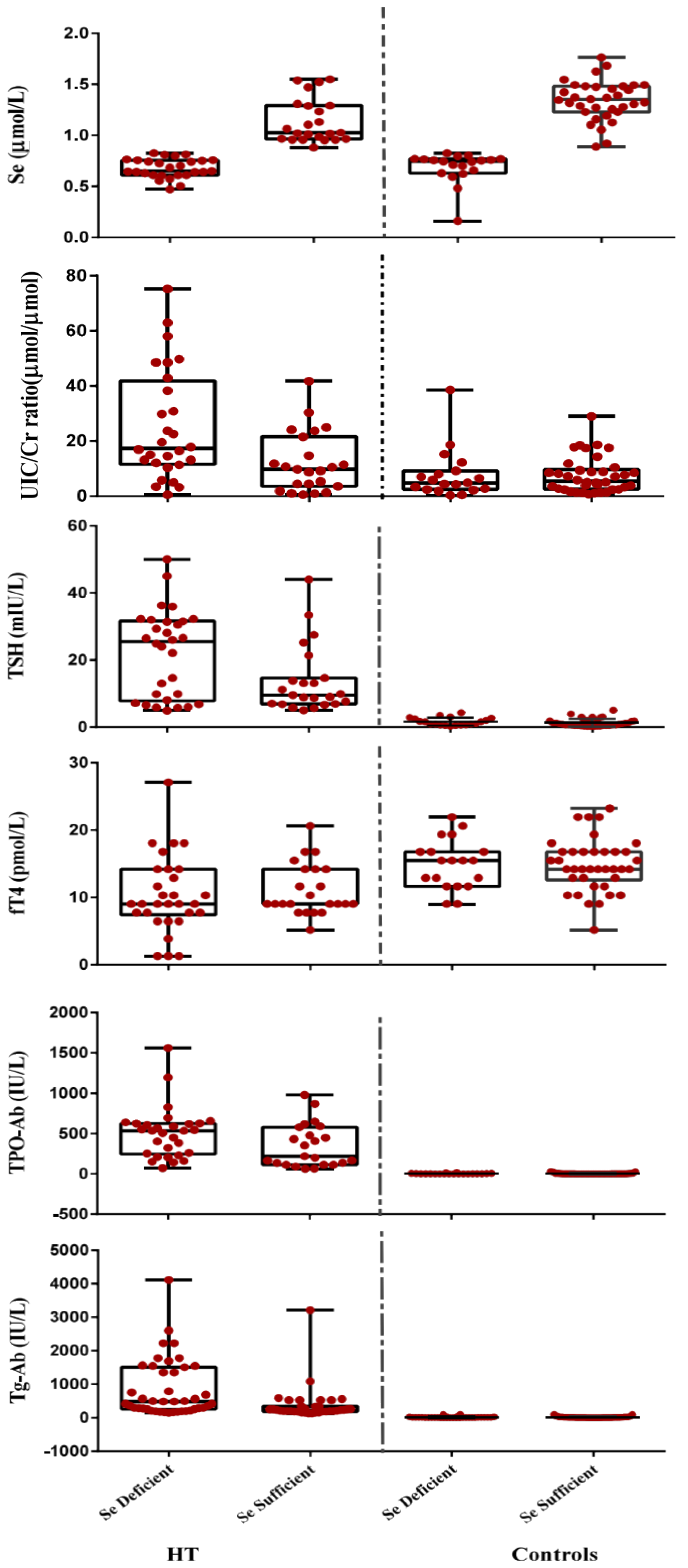
3.3. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saranac, L.; Zivanovic, S.; Bjelakovic, B.; Stamenkovic, H.; Novak, M.; Kamenov, B. Why is the thyroid so prone to autoimmune disease? Horm. Res. Paediatr. 2011, 75, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Caturegli, P.; Kimura, H.; Rocchi, R.; Rose, N.R. Autoimmune thyroid diseases. Curr. Opin. Rheumatol. 2007, 19, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Mazokopakis, E.E.; Chatzipavlidou, V. Hashimoto’s thyroiditis and the role of selenium. Current concepts. Hell. J. Nucl. Med. 2007, 10, 6–8. [Google Scholar]
- Antonelli, A.; Ferrari, S.M.; Corrado, A.; Di Domenicantonio, A.; Fallahi, P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015, 14, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H. Environmental factors and thyroid autoimmunity. Ann. d’endocrinologie 2011, 73, 108–113. [Google Scholar] [CrossRef]
- Rasic-Milutinovic, Z.; Jovanovic, D.; Bogdanovic, G.; Trifunovic, J.; Mutic, J. Potential influence of selenium, copper, zinc and cadmium on L-thyroxine substitution in patients with Hashimoto thyroiditis and hypothyroidism. Exp. Clin. Endocrinol. Diabetes. 2017, 125, 79–85. [Google Scholar] [CrossRef]
- Duntas, L.H. Environmental factors and autoimmune thyroiditis. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 454–460. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Köhrle, J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: Biochemistry and relevance to public health. Thyroid 2002, 12, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Mustacich, D.; Powis, G. Thioredoxin reductase. Biochem. J. 2000, 346, 1–8. [Google Scholar] [CrossRef]
- Duntas, L.H. The role of selenium in thyroid autoimmunity and cancer. Thyroid. 2006, 16, 455–460. [Google Scholar] [CrossRef]
- Chun, O.K.; Floegel, A.; Chung, S.J.; Chung, C.E.; Song, W.O.; Koo, S.I. Estimation of antioxidant intakes from diet and supplements in US adults. J. Nutr. 2010, 140, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Shils, M.E.; Shike, M. Modern Nutrition in Health and Disease; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Sunde, R.A. Selenium. In Present Knowledge in Nutrition, 9th ed.; Bowman, B.A., Russell, R.M., Eds.; ILSI Press: Washington, DC, USA, 2006; pp. 480–497. [Google Scholar]
- Socha, K.; Dziemianowicz, M.; Omeljaniuk, W.J.; Soroczyńska, J.; Borawska, M.H. Nawyki żywieniowe a stężenie selenu w surowicy u pacjentów z chorobą Hashimoto. Probl. Hig. Epidemiol. 2012, 93, 824–827. [Google Scholar]
- Institute of Medicine. Food and Nutrition Board, Dietary Reference Intakes: Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; The National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- Contempre, B.; Je, D.; Bebe, N.; Ch, T.; At, D.; Vanderpas, J. Effect of selenium supplementation in hypothyroid subjects of an iodine and selenium deficient area: The possible danger of indiscriminate supplementation of iodine-deficient subjects with selenium. J. Clin. Endocrinol. Metab. 1991, 73, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Rayman, M.P.; Lv, H.; Schomburg, L.; Cui, B.; Gao, C.; Chen, P.; Guihua, Z.; Zhenan, Z.; Peng, X.; et al. Low population selenium status is associated with increased prevalence of thyroid disease. J. Clin. Endocrinol. Metab. 2015, 100, 4037–4047. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef] [PubMed]
- Metere, A.; Frezzotti, F.; Graves, C.E.; Vergine, M.; De Luca, A.; Pietraforte, D.; Giacomelli, L. A possible role for selenoprotein glutathione peroxidase (GPx1) and thioredoxin reductases (TrxR1) in thyroid cancer: Our experience in thyroid surgery. Cancer Cell Int. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Przybylik-Mazurek, E.; Zagrodzki, P.; Kuźniarz-Rymarz, S.; Hubalewska-Dydejczyk, A. Thyroid disorders—Assessments of trace elements, clinical, and laboratory parameters. Biol. Trace Elem. Res. 2011, 141, 65–75. [Google Scholar] [CrossRef]
- Nourbakhsh, M.; Ahmadpour, F.; Chahardoli, B.; Malekpour-Dehkordi, Z.; Nourbakhsh, M.; Hosseini-Fard, S.R.; Doustimotlagh, A.; Golestani, A.; Razzaghy-Azar, M. Selenium and its relationship with selenoprotein P and glutathione peroxidase in children and adolescents with Hashimoto’s thyroiditis and hypothyroidism. J. Trace Elem. Med. Biol. 2016, 34, 10–14. [Google Scholar] [CrossRef]
- Safaralizadeh, R.; Kardar, G.A.; Pourpak, Z.; Moin, M.; Zare, A.; Teimourian, S. Serum concentration of selenium in healthy individuals living in Tehran. Nutr. J. 2005, 4, 1–4. [Google Scholar]
- Brunn, J.; Block, U.; Ruf, G.; Bos, I.; Kunze, W.P.; Scriba, P.C. Volumetric analysis of thyroid lobes by real-time ultrasound (author’s transl.). Dtsch. Med. Wochenschr. 1981, 106, 1338–1340. [Google Scholar] [CrossRef]
- Höfling, D.B.; Cerri, G.G.; Juliano, A.G.; Marui, S.; Chammas, M.C. Value of thyroid echogenicity in the diagnosis of chronic autoimmune thyroiditis. Radiol. Bras. 2008, 41, 409–417. [Google Scholar]
- Gunter, E.W.; Lewis, B.L.; Koncikowski, S.M. Laboratory Methods Used for the Third National Health and Nutrition Examination Survey (NHANES III); U.S. Department of Health and Human Services publisher: Washington, DC, USA, 1996; pp. 1988–1994.
- Morisi, G.; Patriarca, M.; Menotti, A. Improved determination of selenium in serum by Zeeman atomic absorption spectrometry. Clin. Chem. 1988, 34, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Rostami, R.; Beiranvend, A.; Nourooz-Zadeh, J. Nutritional iodine status in gestation and its relation to geographic features in Urmia County of northwest Iran. Food Nutr. Bull. 2012, 33, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.T.; Crutchfield, H.E.; Gutekunst, R.; Dunn, A.D. Two simple methods for measuring iodine in urine. Thyroid 1993, 3, 119–123. [Google Scholar] [CrossRef]
- Rostami, R.; Aghasi, M.R.; Mohammadi, A.; Nourooz-Zadeh, J. Enhanced oxidative stress in Hashimoto’s thyroiditis: Inter-relationships to biomarkers of thyroid function. Clin. Biochem. 2013, 46, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Naing, L.; Winn, T.; Rusli, B.N. Practical issues in calculating the sample size for prevalence studies. Arch. Orofac. Sci. 2006, 1, 9–14. [Google Scholar]
- Dröge, W.; Breitkreutz, R. Glutathione and immune function. Proc. Nutr. Soc. 2000, 59, 595–600. [Google Scholar]
- Moncayo, R.; Kroiss, A.; Oberwinkler, M.; Karakolcu, F.; Starzinger, M.; Kapelari, K.; Talasz, H.; Moncayo, H. The role of selenium, vitamin C, and zinc in benign thyroid diseases and of selenium in malignant thyroid diseases: Low selenium levels are found in subacute and silent thyroiditis and in papillary and follicular carcinoma. BMC Endocr. Disord. 2008, 8, 2. [Google Scholar] [CrossRef]
- Saiki, M.; Jaluul, O.; Sumita, N.M.; Vasconcellos, M.B.A.; Filho, W.J. Trace element contents in serum of healthy elderly population of metropolitan Sao Paulo area in Brazil. J. Trace Elem. Med. Biol. 2007, 21, 70–73. [Google Scholar] [CrossRef]
- Rayman, M.P.; Thompson, A.J.; Bekaert, B.; Catterick, J.; Galassini, R.; Hall, E.; Warren-Perry, M.; Beckett, G.J. Randomized controlled trial of the effect of selenium supplementation on thyroid function in the elderly in the United Kingdom. Am. J. Clin. Nutr. 2008, 87, 370–378. [Google Scholar] [CrossRef]
- Erdal, M.; Sahin, M.; Hasimi, A.; Uckaya, G.; Kutlu, M.; Saglam, K. Trace element levels in hashimoto thyroiditis patients with subclinical hypothyroidism. Biol. Trace Elem. Res. 2008, 123, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Colebourne, K.; Faddy, H.M.; Flower, R.; Fraser, J.F. Plasma selenium status in a group of Australian blood donors and fresh blood components. J. Trace Elem. Med. Biol. 2013, 27, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, I.; Hartmann, T.; Brustbauer, R.; Minear, G.; Dam, K. Selenium levels in patients with autoimmune thyroiditis and controls in lower Austria. Horm. Metab. Res. 2014, 46, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Maehira, F.; Luyo, G.A.; Miyagi, I.; Oshiro, M.; Yamane, N.; Kuba, M.; Nakazato, Y. Alterations of serum selenium concentrations in the acute phase of pathological conditions. Clin. Chim. Acta 2002, 316, 137–146. [Google Scholar] [CrossRef]
- Federige, M.A.F.; Romaldini, J.H.; Miklos, A.B.P.P.; Koike, M.K.; Takei, K.; Portes, E.D.S. Serum selenium and selenoprotein-P levels in autoimmune thyroid diseases patients in a select center: A transversal study. Arch. Endocrinol. Metab. 2017, 61, 600–607. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.; Zeng, J.; Sun, C. Thyroid volume, goiter prevalence, and selenium levels in an iodine-sufficient area: A cross-sectional study. BMC Public Health 2013, 13, 1153. [Google Scholar] [CrossRef] [PubMed]
- Feldt-Rasmussen, U.; Hegedüs, L.; Perrild, H.; Rasmussen, N.; Hansen, J.M. Relationship between serum thyroglobulin, thyroid volume and serum TSH in healthy non-goitrous subjects and the relationship to seasonal variations in iodine intake. Thyroidology 1989, 1, 115–118. [Google Scholar]
- Medina, D.L.; Santisteban, P. Thyrotropin-dependent proliferation of in vitro rat thyroid cell systems. Eur. J. Endocrinol. 2000, 143, 161–178. [Google Scholar] [CrossRef]
- Ozata, M.; Salk, M.; Aydin, A.; Sayin, S.; Oktenli, C.; Beyham, Z.; Isimer, A.; Ozdemir, I.C. Iodine and zinc, but not selenium and copper, deficiency exists in a male Turkish population with endemic goiter. Biol. Trace Elem. Res. 1999, 69, 211–216. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Papadakis, J.A.; Papadomanolaki, M.G.; Batistakis, A.G.; Giannakopoulos, T.G.; Protopapadakis, E.E.; Ganotakis, E.S. Effects of 12 months treatment with L-selenomethionine on serum anti-TPO Levels in Patients with Hashimoto’s thyroiditis. Thyroid 2007, 17, 609–612. [Google Scholar] [CrossRef]
- Karanikas, G.; Schuetz, M.; Kontur, S.; Duan, H.; Kommata, S.; Schoen, R.; Antoni, A.; Kletter, K.; Dudczak, R.; Willheim, M. No immunological benefit of selenium in consecutive patients with autoimmune thyroiditis. Thyroid 2008, 18, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [PubMed]
- Dominko, K.; Đikić, D. Glutationilacija–regulacijska uloga glutationa u fiziološkim procesima. Arh. Hig. Rada Toksikol. 2018, 69, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009, 91, 791–795. [Google Scholar] [CrossRef]
- Wu, D.; Meydani, S.N.; Sastre, J.; Hayek, M.; Meydani, M. In vitro glutathione supplementation enhances interleukin-2 production and mitogenic response of peripheral blood mononuclear cells from young and old subjects. J. Nutr. 1994, 124, 655–663. [Google Scholar] [CrossRef]
- Perricone, C.; De Carolis, C.; Perricone, R. Glutathione: A key player in autoimmunity. Autoimmun. Rev. 2009, 8, 697–701. [Google Scholar] [CrossRef]
- Hughes, M.M.; Mcgettrick, A.F.; O’Neill, L.A.J. Glutathione and glutathione transferase omega 1 as key posttranslational regulators in macrophages. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Peterson, J.D.; Herzenberg, L.A.; Vasquez, K.; Waltenbaugh, C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 3071–3076. [Google Scholar] [CrossRef]
- Hamilos, D.L.; Zelarney, P.; Mascali, J.J. Lymphocyte proliferation in glutathione-depleted lymphocytes: Direct relationship between glutathione availability and the proliferative response. Immunopharmacology 1989, 18, 223–235. [Google Scholar] [CrossRef]
- Morris, D.; Khurasany, M.; Nguyen, T.; Kim, J.; Guilford, F.; Mehta, R.; Gray, D.; Saviola, B.; Venketaraman, V. Glutathione and infection. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3329–3349. [Google Scholar] [CrossRef]
- Richie, J.P.; Nichenametla, S.; Neidig, W.; Calcagnotto, A.; Haley, J.S.; Schell, T.D.; Muscat, J.E. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur. J. Nutr. 2015, 54, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H. Selenium and the thyroid: A close-knit connection. J. Clin. Endocrinol. Metab. 2010, 95, 5180–5188. [Google Scholar] [CrossRef] [PubMed]
- Gamaley, I.A.; Klyubin, I.V. Roles of reactive oxygen species: Signaling and regulation of cellular functions. Int. Rev. Cytol. 1999, 188, 203–255. [Google Scholar]
- Giuliani, C.; Bucci, I.; Napolitano, G. The role of the transcription factor nuclear factor-kappa B in thyroid autoimmunity and cancer. Front. Endocrinol. (Lausanne) 2018, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Carayanniotis, G. Molecular parameters linking thyroglobulin iodination with autoimmune thyroiditis. Hormones (Athens) 2011, 10, 27–35. [Google Scholar] [CrossRef]
- Sundick, R.S.; Bagchi, N.; Brown, T.R. The role of iodine in thyroid autoimmunity: From chickens to humans: A review. Autoimmunity 1992, 13, 61–68. [Google Scholar] [CrossRef]
- Tsatsoulis, A. The role of iodine vs selenium on the rising trend of autoimmune thyroiditis in iodine sufficient countries. Endocrinol. Int. J. 2018, 6, 412–414. [Google Scholar] [CrossRef]
- Pirola, I.; Gandossi, E.; Agosti, B.; Delbarba, A.; Cappelli, C. Selenium supplementation could restore euthyroidism in subclinical hypothyroid patients with autoimmune thyroiditis. Endokrynol. Pol. 2016, 67, 567–571. [Google Scholar] [CrossRef]
- Wang, W.; Mao, J.; Zhao, J.; Lu, J.; Yan, L.; Du, J.; Lu, Z.; Wang, H.; Xu, M.; Bai, X.; et al. Decreased thyroid peroxidase antibody titer in response to selenium supplementation in autoimmune thyroiditis and the influence of a selenoprotein P gene polymorphism: A prospective, multicenter study in China. Thyroid 2018, 28, 1674–1681. [Google Scholar] [CrossRef]
- Esposito, D.; Rotondi, M.; Accardo, G.; Vallone, G.; Conzo, G.; Docimo, G.; Selvaggi, F.; Cappelli, C.; Chiovato, L.; Giugliano, D.; et al. Influence of short-term selenium supplementation on the natural course of Hashimoto’s thyroiditis: Clinical results of a blinded placebo-controlled randomized prospective trial. J. Endocrinol. Investig. 2017, 40, 83–89. [Google Scholar] [CrossRef]
| Variables. | Hashimoto’s Thyroiditis (n = 49) | Control (n = 50) | p-Value |
|---|---|---|---|
| Age (years) | 33.77 ± 10.46 | 33.32 ± 9.98 | NS |
| BMI (Kg/m2) | 28.53 ± 8.27 | 26.34 ± 5.42 | NS |
| TSH (mIU/L) | 18.57 ± 12.46 | 1.41 ± 1.17 | <0.001 |
| T4 (nmol/L) | 84.4 ± 33.3 | 127.8 ± 20.4 | <0.001 |
| T3 (nmol/L) | 1.9 ± 0.84 | 2.9 ± 0.78 | <0.001 |
| fT4 (pmol/L) | 10.4 ± 4.89 | 14.8 ± 3.86 | <0.001 |
| fT3 (pmol/L) | 4.5 ± 1.18 | 5.6 ± 1.01 | <0.001 |
| T3up (%) | 30.51 ± 2.07 | 28.71 ± 3.58 | <0.001 |
| Tg (μg/L) | 23.63 ± 19.63 | 11.85 ± 12.41 | <0.001 |
| TVol (mL) | 14.4 | 9.35 | <0.001 |
| TPO-Ab (IU/mL) | 442.65 ± 304.60 | 4.77 ± 5.75 | <0.001 |
| Tg-Ab (IU/mL) | 701.68 ± 842.25 | 16.15 ± 19.14 | <0.001 |
| Se (μmol/L) | 0.87 ± 0.29 | 1.11 ± 0.37 | <0.001 |
| GSH (μmol/L) | 2.09 ± 2.06 | 6.35 ± 3.91 | <0.001 |
| GPX3 (IU/L) | 326.29 ± 63.45 | 272.62 ± 44.33 | <0.001 |
| UIC (nmol/L) | 985 | 780 | NS |
| UIC/Cr (µmol/µmol) | 11.36 | 6.27 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostami, R.; Nourooz-Zadeh, S.; Mohammadi, A.; Khalkhali, H.R.; Ferns, G.; Nourooz-Zadeh, J. Serum Selenium Status and Its Interrelationship with Serum Biomarkers of Thyroid Function and Antioxidant Defense in Hashimoto’s Thyroiditis. Antioxidants 2020, 9, 1070. https://doi.org/10.3390/antiox9111070
Rostami R, Nourooz-Zadeh S, Mohammadi A, Khalkhali HR, Ferns G, Nourooz-Zadeh J. Serum Selenium Status and Its Interrelationship with Serum Biomarkers of Thyroid Function and Antioxidant Defense in Hashimoto’s Thyroiditis. Antioxidants. 2020; 9(11):1070. https://doi.org/10.3390/antiox9111070
Chicago/Turabian StyleRostami, Rahim, Sarmad Nourooz-Zadeh, Afshin Mohammadi, Hamid Reza Khalkhali, Gordon Ferns, and Jaffar Nourooz-Zadeh. 2020. "Serum Selenium Status and Its Interrelationship with Serum Biomarkers of Thyroid Function and Antioxidant Defense in Hashimoto’s Thyroiditis" Antioxidants 9, no. 11: 1070. https://doi.org/10.3390/antiox9111070
APA StyleRostami, R., Nourooz-Zadeh, S., Mohammadi, A., Khalkhali, H. R., Ferns, G., & Nourooz-Zadeh, J. (2020). Serum Selenium Status and Its Interrelationship with Serum Biomarkers of Thyroid Function and Antioxidant Defense in Hashimoto’s Thyroiditis. Antioxidants, 9(11), 1070. https://doi.org/10.3390/antiox9111070




