Abstract
Peripheral artery disease (PAD) is caused by narrowing of arteries in the limbs, normally occurring in the lower extremities, with severe cases resulting in amputation of the foot or leg. A potential approach for treatment is to stimulate the formation of new blood vessels to restore blood flow to limb tissues. This is a process called angiogenesis and involves the proliferation, migration, and differentiation of endothelial cells. Angiogenesis can be stimulated by reactive oxygen species (ROS), with NADPH oxidases (NOX) being a major source of ROS in endothelial cells. This review summarizes the recent evidence implicating NOX isoforms in their ability to regulate angiogenesis in vascular endothelial cells in vitro, and in PAD in vivo. Increasing our understanding of the involvement of the NOX isoforms in promoting therapeutic angiogenesis may lead to new treatment options to slow or reverse PAD.
1. Introduction
Peripheral artery disease (PAD) is a condition where narrowed arteries reduce blood flow to peripheral arteries, most commonly in the lower extremities. This is a major risk factor for lower-limb amputations [1] and a major risk factor for heart attack [2,3]. Current interventions are insufficient in many PAD patients because extensive disease precludes effective revascularization. This has prompted alternate treatment strategies including angiogenesis, or the stimulation of new blood vessel growth, to restore blood flow and preserve tissue survival.
Angiogenesis is the growth of capillary networks consisting of endothelial cell (EC) tubes, driven by hypoxia-induced mediators; the most characterized being vascular endothelial growth factor (VEGF) [4]. Remodeling, maturation, and stabilization of existing vessels by perivascular cells (pericytes in capillaries) or vascular smooth muscle cells (VSMCs; in larger vessels) are essential for generating functional collateral vessel networks in ischemia. Excessive reactive oxygen species (ROS) can promote oxidative stress, exacerbate atherosclerosis and cardiovascular disease (CVD), however, physiological levels are essential for cell signaling and maintaining homeostasis by their ability to regulate proliferation, migration, and differentiation of cells. Indeed, ROS have been implicated in angiogenesis and stability of the newly formed vessels [5,6,7,8,9,10], and NADPH oxidases (NOXs) are a major source of ROS in ECs. In this review, we summarize the recent progress in understanding ROS-derived NOX, and the role NOXs play in angiogenesis in vitro, and in PAD in vivo.
2. NOX Structure and Function
The nicotinamide adenine dinucleotide phosphate (NADPH) system is responsible for generating free radicals in immune and other cells in the body. In humans, 7 isoforms exist, including NOX-1 to -5 and two dual oxidases, DUOX-1 and DUOX-2. Except for NOX-5 and DUOX-1 and -2, NOX are phagocytic oxidases, whose main task is to generate ROS to kill foreign pathogens at homeostasis. They are found in various parts of the body, and each implicated in multiple functions including defense mechanisms, signal transduction, and hormone biosynthesis [11]. Of note, the major source of EC ROS comes from NOX-1, -2, -4 and -5 [12,13]; these isoforms will be the focus of this review.
NOX are transmembrane proteins that transport electrons across the cell membrane to reduce oxygen and produce superoxide (O2•–). While each NOX contains six transmembrane domains along with a cytoplasmic domain that binds NADPH and flavin adenine dinucleotide, each isoform is differentiated by specific cytosolic and membrane-bound subunits with the prototypic and most characterized being NOX-2 (also known as gp91phox). For the prototypic NOX isoform, O2•– generation is induced upon assembly of membrane-bound subunits e.g., NOX-2 and p22phox, together with cytosolic subunits p67phox, p47phox, p40phox, and a small G-protein, namely Rac. Like NOX-2, NOX-1, and -4 also assemble with p22phox, however NOX-1 oxidase activity requires NOX organizer 1 (NOXO1) and NOX activator 1 (NOXA1); isoforms of p67phox and p47phox. NOX-4 differs from the prototypic NOX as it is constitutively active, but can be activated by polymerase-δ-interacting protein 2 (POLDIP2). On the other hand, NOX-5 is calcium-dependent and can be regulated by calmodulin [14,15]. NOX-1, -2, -3, and -5 produce O2•–. NOX-4 also produces O2•–, but primarily emits H2O2 [16,17], potentially due to an extended extracytosolic loop that only occurs in the NOX-4 isoform [18]. Structures of NOX-1, -2, -4, and -5 are depicted in Figure 1.
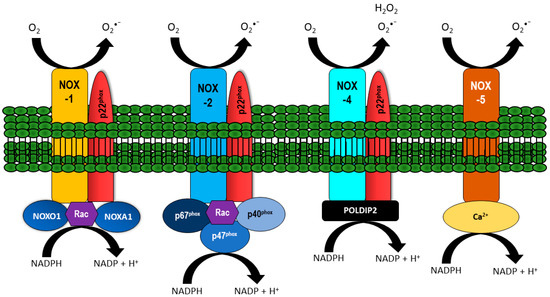
Figure 1.
Vascular NADPH oxidase (NOX) and their regulatory subunits. NOX-1, -2, and -4 are localized at the membrane together with p22phox. The activity of NOX-1 is regulated by NOXO1, NOXA1, and Rac. NOX-2 activity is dependent on binding p67phox, p40phox, p47phox, and Rac, whilst NOX-4 is constitutively active and can be regulated by polymerase-δ-interacting protein 2 (POLDIP2). NOX-5 activity is influenced by calcium. All NOX isoforms generate O2•−, with NOX-4 preferentially producing H2O2.
3. NOX: Role in Angiogenesis
Angiogenesis is a normal, important, and carefully controlled process where capillaries form from pre-existing blood vessels. It is driven by pro- and anti-angiogenic factors acting on ECs, and proceeds through several defined stages. Initially, quiescent ECs are activated, stimulating expression of growth factor receptors, and the release of proteases that breakdown the basement membrane. ECs then proliferate and begin sprouting, followed by migration in the direction of the angiogenic stimulus, and form tubules (Figure 2). These events ultimately result in the formation of a lumen.
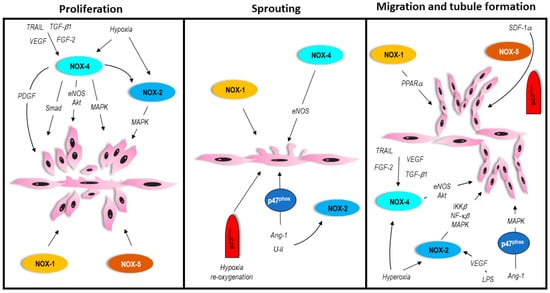
Figure 2.
In vitro process of angiogenesis involving vascular NOX isoforms. Please refer to text for details.
eNOS-derived nitric oxide (NO) activity produced by eNOS (which catalyzes the conversion of l-arginine into NO and l-citrulline), is essential in EC homeostasis. Any imbalance or impairment in eNOS-catalyzed NO production/bioactivity leads to a dysfunctional endothelium. Importantly, both eNOS and NO are important mediators of angiogenesis with NO being the effector molecule [19,20]. Though the exact mechanism(s) are not fully understood, NO increases EC proliferation and migration by increasing growth factor levels and expression; growth factors including VEGF, fibroblast growth factor-2 (FGF-2), TNF-related apoptosis-inducing ligand (TRAIL), and urokinase type plasminogen activator (uPA) [19,21,22,23]. Importantly, EC-derived NOX is activated by VEGF, FGF-2, platelet derived growth factor (PDGF), Angiopoietin 1 or -2 (Ang1, Ang2), and TRAIL. NOX are also activated by conditions which induce angiogenesis, namely hypoxia and ischemia, and involve multiple cytokines and enzymes. Here, we will describe the recent advances in NOX isoforms in regulating angiogenic processes in vitro, but also in PAD in vivo.
3.1. In Vitro Process of Angiogenesis
While excessive or supraphysiological levels of ROS can damage and kill ECs [24,25,26,27,28], ROS at physiological levels are essential in mediating regular cellular functions including proliferation, migration, and differentiation [29,30,31]; in vitro processes of angiogenesis. Importantly, these cellular functions have been shown to require NOX isoforms and are described in detail below.
3.2. Proliferation
NOX can produce ROS to inhibit EC proliferation and promote cell senescence in vitro [32,33], however, NOX can also stimulate proliferation and cell survival. The first evidence demonstrating NOX-1’s involvement in cell growth comes from Suh et al. (1999). In this study, NOX-1 overexpression stimulated proliferation of multiple cells, including VSMCs [34], but it wasn’t until recently, that EC proliferation was shown to be regulated by NOX-1, where silencing NOX-1 impaired hypoxia-induced human pulmonary artery EC proliferation [35]. NOX-5 overexpression also promoted human microvascular EC cell growth [36]. While these studies suggest a positive role for NOX-1 and -5 in EC proliferation, mechanism(s) for this are currently lacking and require further elucidation. The majority of the evidence linking NOX’s to EC proliferation comes from work involving NOX-2 and NOX-4. For example, NOX-2 and NOX-4 over expression increased human hybridoma EC proliferation and ROS production [37], but silencing them inhibited ROS and EC proliferation [37]. NOX-4 binding proteins have also been implicated in EC growth; compared to control, siRNA to POLDIP2 inhibited serum-inducible human umbilical vein EC proliferation, suggesting that POLDIP2 directly influences cell cycle processes [38]. Induction of NOX-4 either by NOX-4 overexpression, VEGF [39], FGF-2, TRAIL [40], or transforming-growth-factor-β1 (TGFβ1) exposure [41,42] can stimulate human microvascular EC growth. Importantly, NOX-2 or NOX-4-induced human microvascular EC proliferation appears to involve mitogen activated protein kinase (MAPK), Akt, or Smad pathways [40,43,44]. These pathways can facilitate eNOS activation, nitric oxide or H2O2 production to stimulate proliferation [40,42,45,46,47]. NOX-4 is also known to interact with phosphorylated VEGF receptor 2 and stimulate VEGF-induced human retinal microvascular EC proliferation [39]. Interestingly, NOX-4 derived H2O2 can stimulate NOX-2, resulting in VEGF receptor activation, and increased human and mouse EC proliferation [33,42,48]. These findings imply cross-talk between NOX isoforms. Interestingly, both NOX-2 and NOX-4 can increase PDGF receptor protein expression, implying that NOX isoforms may stimulate other growth factors to promote EC proliferation in vitro [49]. Collectively, these studies suggest that NOX-2 and -4 are major contributors to EC growth.
3.3. Sprouting
The majority of studies linking NOX isoforms to EC sprouting come from tumor angiogenesis [50,51,52,53,54]. In this review, we have focused on NOX isoforms that mediate vascular EC sprouting, which predominantly include NOX-2 and NOX-4. A study by Craig et al. (2011) [45] demonstrated that aortas from NOX-4 transgenic (NOX-4 TG) mice cultured in Matrigel for 7 days ex vivo, demonstrated ~25% enhanced sprouting compared to wildtype aortas [45]. Importantly, this effect was eNOS dependent, since eNOS−/− NOX-4 TG mice did not display enhanced sprouting ability compared to eNOS−/− mice alone [45]. Two hours of hypoxia followed by 4 h re-oxygenation also resulted in growth of sprouts in a model of pig coronary artery endothelial spheroids [55]. These effects however, were diminished when p47phox knockout spheroids were exposed to hypoxia/re-oxygenation, implicating NOX cytosolic proteins in sprouting [55]. In a second model, aortas of p47phox−/− mice had impaired sprouting ability compared to wildtype in response to hypoxia and re-oxygenation [55]. p47phox has also been implicated in Ang-1 [56] and urotensin II (U-II) [57] stimulated sprouting. Ang-1 is an important regulator of vascular angiogenesis [58]. Importantly, in response to Ang-1, pig coronary artery endothelial spheroid sprouting was inhibited by broad-spectrum NOX inhibitors (apocynin or diphenylene iodonium), with p47phox−/− EC spheroids and aortic ring vessel outgrowths showing modest sprouting compared to wildtype [56]. U-II is a peptide ligand and a potent vasoconstrictor [59]. U-II-induced sprouting in cultured mouse vena cava explants was inhibited by treatment with urantide, a U-II antagonist [57]. Importantly, vascular sprouting of the explants in Nox2−/− mice was considerably reduced [57]. Furthermore, Nox2−/− mice had reduced U-II-induced invasion of new vessels into Matrigel plugs [57]. Collectively, these studies suggest that NOX-2 and p47phox are important in EC sprouting processes.
3.4. Migration and Tubule Formation
The role of NOX-1 in regulating migration and tubule formation has been described [53]. In NOX-1 deficient mouse lung ECs, VEGF, and FGF-2-stimulated intracellular ROS was severely compromised compared to wildtype ECs. Importantly, NOX-1 deficient ECs were unable to migrate in Matrigel, nor could they form tubules on fibrin gel [53]. Interestingly, these EC displayed increased peroxisome proliferator-activated receptor-α (PPARα) expression, and exposure of these cells to GW6471 (PPARα antagonist), restored their ability to migrate and form tubules [53]. While this is suggestive that PPARα may play an antagonistic role in NOX-1-induced angiogenesis, the exact mechanism(s) for NOX-1 mediated tubule formation requires further study.
Migration of ECs is often initiated upon detection of a hypoxic environment [4,60], but can also be initiated in an environment of excess oxygen. Pendyala et al. (2009) found that hyperoxia-stimulated production of ROS and EC migration, was in part, due to NOX-2 in human lung microvascular endothelial cells [61]. Silencing NOX-2 by adenovirus also inhibited Ang-1-inducible ROS production and tubule formation of human umbilical vein EC’s [62]. Moreover, Ang-1-induced migration was impaired in ECs isolated from p47phox−/− mice, associating with reduced intracellular ROS and reduced activation of Akt and p42/44 MAP kinase [55]. The role of Ang-2 in angiogenesis has also been described. Here, lipopolysaccharide (LPS)-induced VEGF and Ang-2 expression resulted in increased human pulmonary microvascular EC ROS production, however, only inhibition of NOX-2 by siRNA, but not NOX-1 and -4, reduced O2•– levels [63]. LPS also stimulated the formation of EC tubules, which was attenuated by NOX-2, involving IκB kinase-β (IKKβ)/NF-κB and MAPK/AP-1 pathways [63].
Like NOX-2, NOX-4 can promote EC migration and tubule formation. A role for NOX-4 in hyperoxia has been identified [61]. Here, the authors found that hyperoxia-induced migration and tubule formation of human lung ECs in vitro, was significantly attenuated when NOX-2 and NOX-4 were silenced [61]. In addition to hyperoxia, the role of NOX-4 has been further examined in response to other factors. For example, H2O2 production and TGFβ1-induced capillary formation was abolished when NOX-4 was silenced in ECs [41]. Silencing NOX-4 also inhibited intracellular ROS production, attenuated eNOS phosphorylation, as well as impaired TRAIL-inducible human microvascular EC migration and tubule formation [40]. Stromal cell-derived factor-1 (SDF-1α) is a potent angiogenic chemokine and induces migration of human microvascular EC [64]. When p22phox or NOX-5 were silenced by siRNA, migration induced by SDF-1α was inhibited [65]. Silencing of p22phox and NOX-5 subunits also significantly reduced SDF-1α-induced tubule formation after 72 h. This suggests that multiple NOXs with p22phox subunits and NOX-5, are involved in SDF-1α migration [65].
3.5. In Vivo Processes of Angiogenesis in PAD
Hindlimb ischemia is a common model of PAD. In most cases, this model involves ligation and excision of the femoral artery and all side branches, with the ischemic process naturally promoting angiogenesis in normal mice [66]. However, variations to this model do exist, and can affect angiogenic outcomes [67]. For example, cutting or partial/complete resection of the femoral artery, iliac artery, or femoral vein, independently, or collectively, or injury via coagulation, can alter the degree of ischaemic damage, affecting degree of vascularity and limb perfusion [67,68]. As described above, NOX isoforms are upregulated in response to hypoxic conditions and implicated in angiogenic processes in vitro. These suggest that NOX may play a role in angiogenesis in response to ischemic injury in vivo. However, to date only NOX-2 and NOX-4 have been proven to control the angiogenic process in PAD following hindlimb ischemia. For example, NOX-2 protein expression was significantly increased in newly formed capillaries and leukocytes in the ischemic limbs of wildtype mice [69]. Hindlimb ischemia also increased NOX-2, -4, and p47phox mRNA expression in collateral arteries of rats at day 1, 3, and 7 post-surgery, whereas NOX-1 expression was undetected [70]. The importance of NOX-2 in stimulating angiogenesis in PAD was confirmed in Nox2−/− mice; blood perfusion was significantly impaired 7 days after ischemic injury when compared to wildtype, associating with reduced capillary density and reduced O2•– production, but not inflammation [69]. Reduced collateral vessel growth was also observed in rats where apocynin treatment or NOX-2 inhibition suppressed angiogenesis, and inhibition of p47phox by siRNA in rats, or in p47phox−/− mice, led to reduced collateral vessel growth in response to ischemia [70]. The bone marrow has also been implicated in NOX-2-induced angiogenesis in PAD. ROS production, hypoxia, and HIF-1α expression were increased in bone marrow after ischemic injury; a finding not observed in Nox2−/− mice [71]. Importantly, reduced ROS in bone marrow of Nox2−/− mice associated with impaired vascularity and blood flow, as well as reduced endothelial progenitor cell numbers in peripheral blood [71]. The authors concluded that NOX-2 dependent hypoxia in bone marrow stimulates EC progenitor expansion to facilitate mobilization of these cells to areas of ischemic injury for tissue repair [71]. In support, more recent studies suggest that EC-specific lineage and angiogenic potency of progenitor stem cells in mice require NOX-2 [72]. While these studies imply that NOX-2 is crucial in stimulating angiogenesis in PAD, other studies contrastingly demonstrate no change in blood perfusion or vascularity in Nox2−/− mice [45,73]. It is unclear as to why there are discrepancies, however, variations in the way ischemia was initiated is different between studies, confirming that variations to the model can result in differences in vascularization [67,68].
Like NOX-2, NOX-4 can promote angiogenesis after hindlimb ischemia. This has been demonstrated using multiple models in vivo including NOX-4 adenoviral gene therapy, and using transgenic and Nox4−/− mice [45,74]. For example, local adenoviral NOX-4 injection 3 days prior to ischemia improved blood flow recovery and stimulated capillary density in mice 28 days after injury [45]. A similar finding was observed in EC-specific NOX-4 transgenic mice [45]. Not only did vascular tissue from these mice display enhanced capillary sprouting ex vivo, in response to hindlimb ischemia, these mice had enhanced capillary density in skeletal muscle, and marked improvement in blood perfusion compared to littermate control mice [45]. Importantly the authors showed that eNOS activation was critical for NOX-4-induced angiogenesis in PAD [45]. In contrast, global Nox4−/− or tamoxifen-inducible deletion of NOX-4 in mice significantly attenuated blood flow recovery and vascularity in response to hindlimb ischemia [74]. Furthermore, a reduction in NOX-4 mRNA expression in skeletal muscle 3 days after hindlimb ischemia was observed in Trail−/− mice associating with impaired blood flow and capillary density [40]. Importantly, NOX-4 deletion in mice also resulted in reduced eNOS and nitric oxide production [74]. Poldip2−/− mice are embryonic lethal [75], however, Poldip2+/− mice exhibited reduced blood perfusion to the lower hindlimb, associating with reduced capillary density, macrophage infiltration, and ~44% reduction in H2O2 production in gastrocnemius muscle after ischaemic injury [38]. The authors proposed that new collaterals and maintenance of new vascular networks were dependent on POLDIP2, since Poldip2+/− mice displayed regressed vasculature [38]. Using the porcine small intestine submucosa implant model of angiogenesis, Poldip2+/− mice also had reduced endothelial invasion by approximately 50%. Collectively, these demonstrate the importance NOX-2 and -4 and their subunits in angiogenic responses in vivo.
3.6. NOX-Mediated Perivascular Cell Processes Contributing to Vessel Stability
Perivascular cells including pericytes and VSMCs, play important roles in the stability of new blood vessels. Stabilization and maturation of the newly-formed vessels is achieved by the activation, proliferation, and recruitment of these cells, which wrap around the EC-tubes, providing support and maintenance [76,77,78]. Pericytes closely associate and are in direct contact with small vessels such as capillaries or microvasculature, whereas VSMCs wrap around larger arteries and veins, however, they are separated by an extracellular matrix and are not in direct contact with the endothelium [79].
NOX-4 was identified as the main source of ROS in human brain pericytes, since NOX-4 RNAi, but not rotenone, l-Name, or oxypurinol inhibited ROS production [80]. Importantly, silencing NOX-4 also blocked basal proliferation of these cells [80]. While this study implicates NOX-4-mediated ROS on pericyte proliferation, the majority of work involving NOX isoforms in regulating proliferation and migration in perivascular cells comes from VSMCs. For example, NOX-1 expression was increased within 3 d after carotid artery ligation in Sprague Dawley rats, associating with elevated O2•– in vessels derived from intimal and medial VSMCs [81]. The role of NOX-1-induced ROS in VSMC proliferation and migration in vivo was further confirmed using mice with global NOX-1 deletion (Nox1−/y), or mice expressing NOX-1 specifically in VSMCs (TgSMCnox1) [82]. Nox1−/y, but not TgSMCnox1 mice had reduced intimal thickening in response to femoral artery wire injury [82]. The role of NOX-1 was further characterized in VSMCs isolated from aortas of wildtype, Nox1−/y and TgSMCnox1 mice. Importantly, TgSMCnox1 VSMCs exhibited increased PDGF-induced O2•– production, associating with increased growth rate and migration [82]. Multiple factors have been shown to regulate VSMC processes via NOX. These include epidermal growth factor receptor [83], FGF-2 [84], AngII [85], and the plasminogen/plasmin system; important in blood coagulation and known to participate in vascular remodeling [86]. Of note, uPA-inducible ROS ranged from 20 to 80 nmol/L in VSMCs; and using pharmacological or molecular approaches, inhibition of NOX-1 significantly reduced ROS generation and aortic VSMC proliferation [86]. Reports suggest NOX-2 is expressed in VSMCs [87,88], however, its role in processes relating to angiogenesis is not fully defined, whereas, NOX-4 has been implicated. For example, silencing NOX-4 reduced human VSMC proliferation and increased senescence [89], implicating a role for NOX-4 in VSMC growth. NOX-4 also stimulated VSMC differentiation [90], and affected VSMC integrity and migration, in part via POLDIP2 [91]. Here, silencing POLDIP2 reduced intracellular O2•– and H2O2, as well as POLDIP2, NOX-4, and p22phox protein expression, with marked reduction in the localization of these proteins to focal adhesions in rat thoracic aortic VSMCs [92]. Of note, silencing NOX-4 had a similar effect on cytoskeletal integrity [92]. Consistent with these, Poldip2+/− mice had reduced VSMC content in ischemic skeletal muscle, suggesting impaired VSMC remodeling in response to hindlimb ischemia [38]. Finally, reports linking NOX-5 to VSMC proliferation have been described [93]. SiRNA to NOX-5 significantly reduced PDGF-induced O2•– production, from ~800 to 500 pmol/mg protein (as assessed by 2-hydroethidium and HPLC) and VSMC proliferation via the janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway [93]; however, the precise mechanisms for this are unknown. Collectively, these studies provide insight into NOX signaling in perivascular cell processes related to angiogenesis. More studies are required to understand the role of NOX in differentiation, proliferation, and migration of perivascular cells, and how they may contribute to vessel stability in angiogenesis.
4. NOX—Lessons from the Clinic
PAD is often a consequence of atherosclerosis, and NOX-derived oxidative stress has been linked to lesion progression [94,95,96,97]. Of note, pharmacological inhibition of NOX can block ROS production, thereby reducing atherosclerotic activity and disease progression in preclinical models [98,99]. These suggest that drugs targeting NOX may be beneficial in atherosclerosis and in people with PAD. Importantly, NOX subunit p22phox expression was increased in atherosclerotic vs. non-atherosclerotic arteries [100]. NOX-2 and NOX-4, but not NOX-1 were found to be abundant in diseased arteries, correlating with atherosclerosis severity [101]. The first evidence of NOX as a major source of O2•– in atherosclerotic blood vessels was described by Guzic et al. (2000) [102]. This has since been confirmed by others, where O2•– was localized to coronary arteries and increased in atherosclerotic plaque shoulders, associating with NOX-2, p22phox, and to some extent, NOX-4 expression [101]. Notably, NOX-2 and p22phox mRNA was increased with the severity of disease [101]. Differential expression of NOX isoforms dependent on the vessel bed have also been described, with veins expressing more NOX-2 and p22phox, and arteries expressing more NOX-4 [103] Importantly, a strong correlation between O2•– production with p22phox and NOX-4; NOX activity and endothelial dysfunction was observed [103]. Very little has been described in relation to PAD, with one study showing increased plasma NOX-2 associating with impaired endothelial function [104]. Interestingly, dark chocolate, reduced markers of oxidative stress including circulating NOX-2, and improved walking autonomy in patients with PAD diagnosed with intermittent claudication [105]. While these studies suggest that NOX isoforms may be important mediators of ROS in artery dysfunction and cardiovascular diseases, evidence that NOX-mediated ROS increases atherosclerotic risk in people is still lacking or controversial e.g., in chronic granulomatous disease, an X-linked immune-deficiency disease caused by mutation in one or all NOX-2 components. While, these patients have profound bacterial and fungal infection due to the inability to eliminate foreign pathogens [106,107], they have reduced carotid atherosclerosis [108], raising questions of the in vivo role of NOX-2 in CVD. The role of NOX isoforms in angiogenesis in vascular diseases are also unclear. As such, more studies are needed to fully elucidate the role of NOX-mediated ROS in vascular disease.
5. Conclusions
PAD can lead to gangrene and potential limb amputation. While current treatments include lifestyle changes and stent placement, a new approach is to increase vascularization in the affected limb by targeting ECs and stimulating angiogenesis. At physiological levels EC-derived ROS is pro-angiogenic. In vitro studies have implicated NOX in important stages of angiogenesis; proliferation, sprouting, migration, and tubule formation. Furthermore, pre-clinical in vivo studies have confirmed the importance of NOX family members in reperfusion after hind limb ischemia. Crucially, clinical investigations have shown changes in NOX expression in the vessels of patients diagnosed with PAD, suggesting these isoforms may be beneficial as therapeutic targets. Increasing our understanding of the mechanism(s) regulating NOX-induced angiogenesis will be highly beneficial in the development of treatments to increase both lifespan and life quality for patients with PAD.
Author Contributions
Pradeep Manuneedhi Cholan, Siân P. Cartland, and Mary M. Kavurma contributed in writing this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Swaminathan, A.; Vemulapalli, S.; Patel, M.R.; Jones, W.S. Lower extremity amputation in peripheral artery disease: Improving patient outcomes. Vasc. Health Risk Manag. 2014, 10, 417–424. [Google Scholar] [PubMed]
- Heald, C.L.; Fowkes, F.G.; Murray, G.D.; Price, J.F.; Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis 2006, 189, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.A.; Jaff, M.R.; Creager, M.A. The United States preventive services task force recommendation statement on screening for peripheral arterial disease: More harm than benefit? Circulation 2006, 114, 861–866. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer 2011, 2, 1117–1133. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Byzova, T.V. Oxidative stress in angiogenesis and vascular disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, T.; Chen, Y.; Ahokas, R.A.; Sun, Y. Reactive oxygen species promote angiogenesis in the infarcted rat heart. Int. J. Exp. Pathol. 2009, 90, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Alexander, R.W. Reactive oxygen species as mediators of angiogenesis signaling: Role of NAD(P)H oxidase. Mol. Cell. Biochem. 2004, 264, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Meng, Q.; Liu, L.Z.; Rojanasakul, Y.; Wang, X.R.; Jiang, B.H. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007, 67, 10823–10830. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Quintans, N.; Sanchez-Ramos, C.; Tierrez, A.; Olmo, Y.; Luque, A.; Arza, E.; Alfranca, A.; Miguel Redondo, J.; Monsalve, M. Control of endothelial function and angiogenesis by PGC-1α relies on ROS control of vascular stability. Free Rad. Biol. Med. 2014, 75, S5. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Quintans, N.; Sanchez-Ramos, C.; Prieto, I.; Tierrez, A.; Arza, E.; Alfranca, A.; Redondo, J.M.; Monsalve, M. Oxidative stress induces loss of pericyte coverage and vascular instability in PGC-1α-deficient mice. Angiogenesis 2016, 19, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Overmyer, K.; Brosche, M.; Kangasjarvi, J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003, 8, 335–342. [Google Scholar] [CrossRef]
- Pendyala, S.; Usatyuk, P.V.; Gorshkova, I.A.; Garcia, J.G.; Natarajan, V. Regulation of NADPH oxidase in vascular endothelium: The role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid. Redox Signal. 2009, 11, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.R.; Sobey, C.G. Endothelial NADPH oxidases: Which NOX to target in vascular disease? Trends Endocrinol. Metab. 2014, 25, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Gratton, J.P.; Rafikov, R.; Black, S.M.; Fulton, D.J. Calcium/calmodulin-dependent kinase II mediates the phosphorylation and activation of NADPH oxidase 5. Mol. Pharmacol. 2011, 80, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Burger, D.; Paravicini, T.M.; Chignalia, A.Z.; Yusuf, H.; Almasri, M.; He, Y.; Callera, G.E.; He, G.; Krause, K.H.; et al. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin ii and endothelin-1 is mediated via calcium/calmodulin-dependent, Rac-1-independent pathways in human endothelial cells. Circ. Res. 2010, 106, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Nakagawa, K.; Yamasaki, T.; Nakamura, K.; Takeya, R.; Kuribayashi, F.; Imajoh-Ohmi, S.; Igarashi, K.; Shibata, Y.; Sueishi, K.; et al. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells 2005, 10, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Nisimoto, Y.; Diebold, B.A.; Cosentino-Gomes, D.; Lambeth, J.D. Nox4: A hydrogen peroxide-generating oxygen sensor. Biochemistry 2014, 53, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Takac, I.; Schroder, K.; Zhang, L.; Lardy, B.; Anilkumar, N.; Lambeth, J.D.; Shah, A.M.; Morel, F.; Brandes, R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011, 286, 13304–13313. [Google Scholar] [CrossRef] [PubMed]
- Namba, T.; Koike, H.; Murakami, K.; Aoki, M.; Makino, H.; Hashiya, N.; Ogihara, T.; Kaneda, Y.; Kohno, M.; Morishita, R. Angiogenesis induced by endothelial nitric oxide synthase gene through vascular endothelial growth factor expression in a rat hindlimb ischemia model. Circulation 2003, 108, 2250–2257. [Google Scholar] [CrossRef] [PubMed]
- Morbidelli, L.; Donnini, S.; Ziche, M. Role of nitric oxide in the modulation of angiogenesis. Curr. Pharm. Des. 2003, 9, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J.; Jozkowicz, A.; Dembinska-Kiec, A.; Guevara, I.; Zdzienicka, A.; Zmudzinska-Grochot, D.; Florek, I.; Wojtowicz, A.; Szuba, A.; Cooke, J.P. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arterioscler. Thromb. Vas. Biol. 2000, 20, 659–666. [Google Scholar] [CrossRef]
- Ziche, M.; Parenti, A.; Ledda, F.; Dell’Era, P.; Granger, H.J.; Maggi, C.A.; Presta, M. Nitric oxide promotes proliferation and plasminogen activator production by coronary venular endothelium through endogenous bFGF. Circ. Res. 1997, 80, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Kavurma, M.M.; Bennett, M.R. Expression, regulation and function of trail in atherosclerosis. Biochem. Pharmacol. 2008, 75, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.S.; Angkeow, P.; Huang, J.; Ozaki, M.; Irani, K. Rac1 inhibits TNF-α-induced endothelial cell apoptosis: Dual regulation by reactive oxygen species. FASEB J. 2000, 14, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; DeLano, F.A.; Schmid-Schonbein, G.W. Oxidative stress promotes endothelial cell apoptosis and loss of microvessels in the spontaneously hypertensive rats. Arterioscler. Thromb. Vas. Biol. 2005, 25, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; McCrae, K.R. Endothelial-cell apoptosis induced by cleaved high-molecular-weight kininogen (HKa) is matrix dependent and requires the generation of reactive oxygen species. Blood 2006, 107, 4714–4720. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.A.; Choi, S.; Suh, S.H. Superoxide is a potential culprit of caspase-3 dependent endothelial cell death induced by lysophosphatidylcholine. J. Physiol. Pharmacol. 2010, 61, 375–381. [Google Scholar] [PubMed]
- Peng, C.; Ma, J.; Gao, X.; Tian, P.; Li, W.; Zhang, L. High glucose induced oxidative stress and apoptosis in cardiac microvascular endothelial cells are regulated by FoxO3a. PLoS ONE 2013, 8, e79739. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Frey, R.S.; Ushio-Fukai, M.; Malik, A.B. NADPH oxidase-dependent signaling in endothelial cells: Role in physiology and pathophysiology. Antioxid. Redox Signal. 2009, 11, 791–810. [Google Scholar] [CrossRef] [PubMed]
- Schilder, Y.D.; Heiss, E.H.; Schachner, D.; Ziegler, J.; Reznicek, G.; Sorescu, D.; Dirsch, V.M. NADPH oxidases 1 and 4 mediate cellular senescence induced by resveratrol in human endothelial cells. Free Rad. Biol. Med. 2009, 46, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Shafique, E.; Torina, A.; Reichert, K.; Colantuono, B.; Nur, N.; Zeeshan, K.; Ravichandran, V.; Liu, Y.; Feng, J.; Zeeshan, K.; et al. Mitochondrial redox plays a critical role in the paradoxical effects of NAPDH oxidase-derived ROS on coronary endothelium. Cardiovasc. Res. 2017, 113, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.A.; Arnold, R.S.; Lassegue, B.; Shi, J.; Xu, X.; Sorescu, D.; Chung, A.B.; Griendling, K.K.; Lambeth, J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999, 401, 79–82. [Google Scholar] [PubMed]
- Al Ghouleh, I.; Sahoo, S.; Meijles, D.N.; Amaral, J.H.; de Jesus, D.S.; Sembrat, J.; Rojas, M.; Goncharov, D.A.; Goncharova, E.A.; Pagano, P.J. Endothelial Nox1 oxidase assembly in human pulmonary arterial hypertension; driver of Gremlin1-mediated proliferation. Clin. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- BelAiba, R.S.; Djordjevic, T.; Petry, A.; Diemer, K.; Bonello, S.; Banfi, B.; Hess, J.; Pogrebniak, A.; Bickel, C.; Gorlach, A. Nox5 variants are functionally active in endothelial cells. Free Rad. Biol. Med. 2007, 42, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Petry, A.; Djordjevic, T.; Weitnauer, M.; Kietzmann, T.; Hess, J.; Gorlach, A. Nox2 and Nox4 mediate proliferative response in endothelial cells. Antioxid. Redox Signal. 2006, 8, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Amanso, A.M.; Lassegue, B.; Joseph, G.; Landazuri, N.; Long, J.S.; Weiss, D.; Taylor, W.R.; Griendling, K.K. Polymerase δ-interacting protein 2 promotes postischemic neovascularization of the mouse hindlimb. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Z.; Jiang, Y.; Hartnett, M.E. Endothelial NADPH oxidase 4 mediates vascular endothelial growth factor receptor 2-induced intravitreal neovascularization in a rat model of retinopathy of prematurity. Mol. Vis. 2014, 20, 231–241. [Google Scholar] [PubMed]
- Di Bartolo, B.A.; Cartland, S.P.; Prado-Lourenco, L.; Griffith, T.S.; Gentile, C.; Ravindran, J.; Azahri, N.S.; Thai, T.; Yeung, A.W.; Thomas, S.R.; et al. Tumor necrosis factor-related apoptosis-inducing ligand (trail) promotes angiogenesis and ischemia-induced neovascularization via NADPH oxidase 4 (NOX4) and nitric oxide-dependent mechanisms. J. Am. Heart Assoc. 2015, 4, e002527. [Google Scholar] [CrossRef] [PubMed]
- Peshavariya, H.M.; Chan, E.C.; Liu, G.S.; Jiang, F.; Dusting, G.J. Transforming growth factor-β1 requires NADPH oxidase 4 for angiogenesis in vitro and in vivo. J. Cell. Mol. Med. 2014, 18, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiao, J.; Kuroda, J.; Ago, T.; Sadoshima, J.; Cohen, R.A.; Tong, X. Both hydrogen peroxide and transforming growth factor β 1 contribute to endothelial Nox4 mediated angiogenesis in endothelial Nox4 transgenic mouse lines. Biochim. Biophys. Acta 2014, 1842, 2489–2499. [Google Scholar] [CrossRef] [PubMed]
- Peshavariya, H.; Dusting, G.J.; Jiang, F.; Halmos, L.R.; Sobey, C.G.; Drummond, G.R.; Selemidis, S. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009, 380, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Hakami, N.Y.; Wong, H.; Shah, M.H.; Dusting, G.J.; Jiang, F.; Peshavariya, H.M. Smad-independent pathway involved in transforming growth factor β1-induced Nox4 expression and proliferation of endothelial cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Craige, S.M.; Chen, K.; Pei, Y.; Li, C.; Huang, X.; Chen, C.; Shibata, R.; Sato, K.; Walsh, K.; Keaney, J.F., Jr. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 2011, 124, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Rouaud, F.; Romero-Perez, M.; Wang, H.; Lobysheva, I.; Ramassamy, B.; Henry, E.; Tauc, P.; Giacchero, D.; Boucher, J.L.; Deprez, E.; et al. Regulation of NADPH-dependent nitric oxide and reactive oxygen species signalling in endothelial and melanoma cells by a photoactive NADPH analogue. Oncotarget 2014, 5, 10650–10664. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.L.; Gao, L.; Cai, H. Differential roles of protein complexes NOX1-NOXO1 and NOX2-p47phox in mediating endothelial redox responses to oscillatory and unidirectional laminar shear stress. J. Biol. Chem. 2016, 291, 8653–8662. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.M.; Thompson, M.D.; Bolotina, V.M.; Tong, X.; Cohen, R.A. Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 s-glutathiolation and endothelial cell migration. Free Rad. Biol. Med. 2012, 53, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Datla, S.R.; Peshavariya, H.; Dusting, G.J.; Mahadev, K.; Goldstein, B.J.; Jiang, F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Block, K.; Gorin, Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat. Rev. Cancer 2012, 12, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Helfinger, V.; Henke, N.; Harenkamp, S.; Walter, M.; Epah, J.; Penski, C.; Mittelbronn, M.; Schroder, K. The NADPH oxidase NOX4 mediates tumour angiogenesis. Acta Physiol. 2016, 216, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Kamata, T. Roles of Nox1 and other Nox isoforms in cancer development. Cancer Sci. 2009, 100, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Urbani, S.; Jemelin, S.; Deffert, C.; Carnesecchi, S.; Basset, O.; Szyndralewiez, C.; Heitz, F.; Page, P.; Montet, X.; Michalik, L.; et al. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARα mediated mechanism. PLoS ONE 2011, 6, e14665. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Nakamura, Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008, 266, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Zeng, H.; Tuo, Q.H.; Yu, H.; Meyrick, B.; Aschner, J.L. NADPH oxidase modulates myocardial Akt, ERK1/2 activation, and angiogenesis after hypoxia-reoxygenation. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1664–H1674. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Zeng, H.; Lawrence, M.L.; Blackwell, T.S.; Meyrick, B. Angiopoietin-1-induced angiogenesis is modulated by endothelial NADPH oxidase. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Diebold, I.; Petry, A.; Sabrane, K.; Djordjevic, T.; Hess, J.; Gorlach, A. The HIF1 target gene NOX2 promotes angiogenesis through urotensin-II. J. Cell Sci. 2012, 125, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Augustin, H.G.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Ames, R.S.; Sarau, H.M.; Chambers, J.K.; Willette, R.N.; Aiyar, N.V.; Romanic, A.M.; Louden, C.S.; Foley, J.J.; Sauermelch, C.F.; Coatney, R.W.; et al. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature 1999, 401, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Pendyala, S.; Gorshkova, I.A.; Usatyuk, P.V.; He, D.; Pennathur, A.; Lambeth, J.D.; Thannickal, V.J.; Natarajan, V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid. Redox Signal. 2009, 11, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Harel, S.; Mayaki, D.; Sanchez, V.; Hussain, S.N.A. Nox2, Nox4, and mitochondrial-derived reactive oxygen species contribute to angiopoietin-1 signaling and angiogenic responses in endothelial cells. Vasc. Pharmacol. 2017, 92, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Menden, H.; Welak, S.; Cossette, S.; Ramchandran, R.; Sampath, V. Lipopolysaccharide (LPS)-mediated angiopoietin-2-dependent autocrine angiogenesis is regulated by NADPH oxidase 2 (Nox2) in human pulmonary microvascular endothelial cells. J. Biol. Chem. 2015, 290, 5449–5461. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.K.; Tsui, J.; Xu, S.; Leoni, P.; Abraham, D.J.; Baker, D.M. Angiogenic effects of stromal cell-derived factor-1 (SDF-1/CXCL12) variants in vitro and the in vivo expressions of CXCL12 variants and CXCR4 in human critical leg ischemia. J. Vasc. Surg. 2010, 51, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Xie, L.; Portbury, A.L.; Kumar, S.; Lockyer, P.; Li, X.; Patterson, C. NADPH oxidase-generated reactive oxygen species are required for stromal cell-derived factor-1α-stimulated angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Couffinhal, T.; Silver, M.; Zheng, L.P.; Kearney, M.; Witzenbichler, B.; Isner, J.M. Mouse model of angiogenesis. Am. J. Pathol. 1998, 152, 1667–1679. [Google Scholar] [PubMed]
- Hellingman, A.A.; Bastiaansen, A.J.; de Vries, M.R.; Seghers, L.; Lijkwan, M.A.; Lowik, C.W.; Hamming, J.F.; Quax, P.H. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Fukuyama, N.; Aki, A.; Kanabuchi, K.; Kimura, K.; Taira, H.; Tanaka, E.; Wakana, N.; Mori, H.; Inoue, H. Search for appropriate experimental methods to create stable hind-limb ischemia in mouse. Tokai J. Exp. Clin. Med. 2006, 31, 128–132. [Google Scholar] [PubMed]
- Tojo, T.; Ushio-Fukai, M.; Yamaoka-Tojo, M.; Ikeda, S.; Patrushev, N.; Alexander, R.W. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 2005, 111, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- DiStasi, M.R.; Unthank, J.L.; Miller, S.J. Nox2 and p47phox modulate compensatory growth of primary collateral arteries. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1435–H1443. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Urao, N.; McKinney, R.D.; Fukai, T.; Ushio-Fukai, M. NADPH oxidase 2 regulates bone marrow microenvironment following hindlimb ischemia: Role in reparative mobilization of progenitor cells. Stem Cells 2012, 30, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wei, X.; Wang, X.; Jiang, L.; Niu, C.; Zhang, J.; Chen, S.; Meng, D. Nox2 contributes to the arterial endothelial specification of mouse induced pluripotent stem cells by upregulating notch signaling. Sci. Rep. 2016, 6, 33737. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, T.G.; Heymes, C.; You, D.; Blanc-Brude, O.; Mees, B.; Waeckel, L.; Duriez, M.; Vilar, J.; Brandes, R.P.; Levy, B.I.; et al. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am. J. Pathol. 2006, 169, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.I.; Lassegue, B.; Lee, M.; Zafari, R.; Long, J.S.; Saavedra, H.I.; Griendling, K.K. Poldip2 knockout results in perinatal lethality, reduced cellular growth and increased autophagy of mouse embryonic fibroblasts. PLoS ONE 2014, 9, e96657. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncology 2005, 7, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Wanjare, M.; Kusuma, S.; Gerecht, S. Perivascular cells in blood vessel regeneration. Biotechnol. J. 2013, 8, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, Z.; Bayraktutan, U. NADPH oxidase mediates TNF-α-evoked in vitro brain barrier dysfunction: Roles of apoptosis and time. Mol. Cell. Neurosci. 2014, 61, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Crivellato, E. The role of pericytes in angiogenesis. Int. J. Dev. Biol. 2011, 55, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Ago, T.; Nishimura, A.; Nakamura, K.; Matsuo, R.; Wakisaka, Y.; Kamouchi, M.; Kitazono, T. Nox4 is a major source of superoxide production in human brain pericytes. J. Vasc. Res. 2014, 51, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Szocs, K.; Lassegue, B.; Sorescu, D.; Hilenski, L.L.; Valppu, L.; Couse, T.L.; Wilcox, J.N.; Quinn, M.T.; Lambeth, J.D.; Griendling, K.K. Upregulation of NOX-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; San Martin, A.; Mehta, P.K.; Dikalova, A.E.; Garrido, A.M.; Datla, S.R.; Lyons, E.; Krause, K.H.; Banfi, B.; Lambeth, J.D.; et al. Mechanisms of vascular smooth muscle NADPH oxidase 1 (NOX1) contribution to injury-induced neointimal formation. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesha, D.K.; Takapoo, M.; Banfi, B.; Bhalla, R.C.; Miller, F.J., Jr. Nox1 transactivation of epidermal growth factor receptor promotes N-cadherin shedding and smooth muscle cell migration. Cardiovasc. Res. 2012, 93, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Helmcke, I.; Palfi, K.; Krause, K.H.; Busse, R.; Brandes, R.P. Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Lassegue, B.; Sorescu, D.; Szocs, K.; Yin, Q.; Akers, M.; Zhang, Y.; Grant, S.L.; Lambeth, J.D.; Griendling, K.K. Novel gp91phox homologues in vascular smooth muscle cells: Nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ. Res. 2001, 88, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Menshikov, M.; Plekhanova, O.; Cai, H.; Chalupsky, K.; Parfyonova, Y.; Bashtrikov, P.; Tkachuk, V.; Berk, B.C. Urokinase plasminogen activator stimulates vascular smooth muscle cell proliferation via redox-dependent pathways. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Chen, X.; Tabet, F.; Yao, G.; He, G.; Quinn, M.T.; Pagano, P.J.; Schiffrin, E.L. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: Regulation by angiotensin II. Circ. Res. 2002, 90, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Tabet, F.; Callera, G.E.; Montezano, A.C.; Yogi, A.; He, Y.; Quinn, M.T.; Salaices, M.; Touyz, R.M. Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR. J. Am. Soc. Hypertens. 2011, 5, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Przybylska, D.; Janiszewska, D.; Gozdzik, A.; Bielak-Zmijewska, A.; Sunderland, P.; Sikora, E.; Mosieniak, G. Nox4 downregulation leads to senescence of human vascular smooth muscle cells. Oncotarget 2016, 7, 66429–66443. [Google Scholar] [CrossRef] [PubMed]
- Deliri, H.; McNamara, C.A. Nox 4 regulation of vascular smooth muscle cell differentiation marker gene expression. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 12–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Datla, S.R.; McGrail, D.J.; Vukelic, S.; Huff, L.P.; Lyle, A.N.; Pounkova, L.; Lee, M.; Seidel-Rogol, B.; Khalil, M.K.; Hilenski, L.L.; et al. Poldip2 controls vascular smooth muscle cell migration by regulating focal adhesion turnover and force polarization. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H945–H957. [Google Scholar] [CrossRef] [PubMed]
- Lyle, A.N.; Deshpande, N.N.; Taniyama, Y.; Seidel-Rogol, B.; Pounkova, L.; Du, P.; Papaharalambus, C.; Lassegue, B.; Griendling, K.K. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ. Res. 2009, 105, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Jay, D.B.; Papaharalambus, C.A.; Seidel-Rogol, B.; Dikalova, A.E.; Lassegue, B.; Griendling, K.K. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Rad. Biol. Med. 2008, 45, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Jialal, I. Oxidative stress and atherosclerosis. Pathophysiology 2006, 13, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Horke, S.; Forstermann, U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 2014, 237, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, E.; Gray, S.P.; Chew, P.; Koulis, C.; Ziegler, A.; Szyndralewiez, C.; Touyz, R.M.; Schmidt, H.H.; Cooper, M.E.; Slattery, R.; et al. Pharmacological inhibition of NOX reduces atherosclerotic lesions, vascular ROS and immune-inflammatory responses in diabetic Apoe−/− mice. Diabetologia 2014, 57, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.P.; Di Marco, E.; Okabe, J.; Szyndralewiez, C.; Heitz, F.; Montezano, A.C.; de Haan, J.B.; Koulis, C.; El-Osta, A.; Andrews, K.L.; et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 2013, 127, 1888–1902. [Google Scholar] [CrossRef] [PubMed]
- Azumi, H.; Inoue, N.; Takeshita, S.; Rikitake, Y.; Kawashima, S.; Hayashi, Y.; Itoh, H.; Yokoyama, M. Expression of NADH/NADPH oxidase p22phox in human coronary arteries. Circulation 1999, 100, 1494–1498. [Google Scholar] [CrossRef] [PubMed]
- Sorescu, D.; Weiss, D.; Lassegue, B.; Clempus, R.E.; Szocs, K.; Sorescu, G.P.; Valppu, L.; Quinn, M.T.; Lambeth, J.D.; Vega, J.D.; et al. Superoxide production and expression of NOX family proteins in human atherosclerosis. Circulation 2002, 105, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; West, N.E.; Black, E.; McDonald, D.; Ratnatunga, C.; Pillai, R.; Channon, K.M. Vascular superoxide production by NAD(P)H oxidase: Association with endothelial dysfunction and clinical risk factors. Circ. Res. 2000, 86, E85–E90. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Sadowski, J.; Kapelak, B.; Jopek, A.; Rudzinski, P.; Pillai, R.; Korbut, R.; Channon, K.M. Systemic regulation of vascular NAD(P)H oxidase activity and Nox isoform expression in human arteries and veins. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Carnevale, R.; Cangemi, R.; Angelico, F.; Augelletti, T.; Di Santo, S.; Calabrese, C.M.; Della Volpe, L.; Pignatelli, P.; Perri, L.; et al. Nox2 up-regulation is associated with artery dysfunction in patients with peripheral artery disease. Int. J. Cardiol. 2013, 165, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Perri, L.; Catasca, E.; Pignatelli, P.; Brancorsini, M.; Nocella, C.; De Falco, E.; Bartimoccia, S.; Frati, G.; Carnevale, R.; et al. Dark chocolate acutely improves walking autonomy in patients with peripheral artery disease. J. Am. Heart Assoc. 2014, 3, e001072. [Google Scholar] [CrossRef] [PubMed]
- Jurkowska, M.; Bernatowska, E.; Bal, J. Genetic and biochemical background of chronic granulomatous disease. Arch. Immunol. Ther. Exp. 2004, 52, 113–120. [Google Scholar]
- Roos, D.; de Boer, M. Molecular diagnosis of chronic granulomatous disease. Clin. Exp. Immunol. 2014, 175, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.T.; Estwick, T.; Zavodni, A.; Huang, C.Y.; Kwan, A.C.; Soule, B.P.; Long Priel, D.A.; Remaley, A.T.; Rudman Spergel, A.K.; Turkbey, E.B.; et al. Assessment of atherosclerosis in chronic granulomatous disease. Circulation 2014, 130, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).