Red Blood Cell Antioxidant State in Fanconi Anemia: The Highlighted Roles of Pi-Class Glutathione S-Transferase and Glutathione Peroxidase
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Cells and Cell Cultures
2.3. Cytogenetic Analysis
2.4. GSH Content
2.5. Enzymatic Activities
2.5.1. Catalase
2.5.2. Superoxide Dismutase (SOD)
2.5.3. Glutathione Peroxidase (GPx)
2.5.4. Pi-Class Glutathione S-Transferase (GSTP1)
2.6. Statistical Analysis
3. Results
3.1. Evaluation of Chromosome Instability in Study Groups
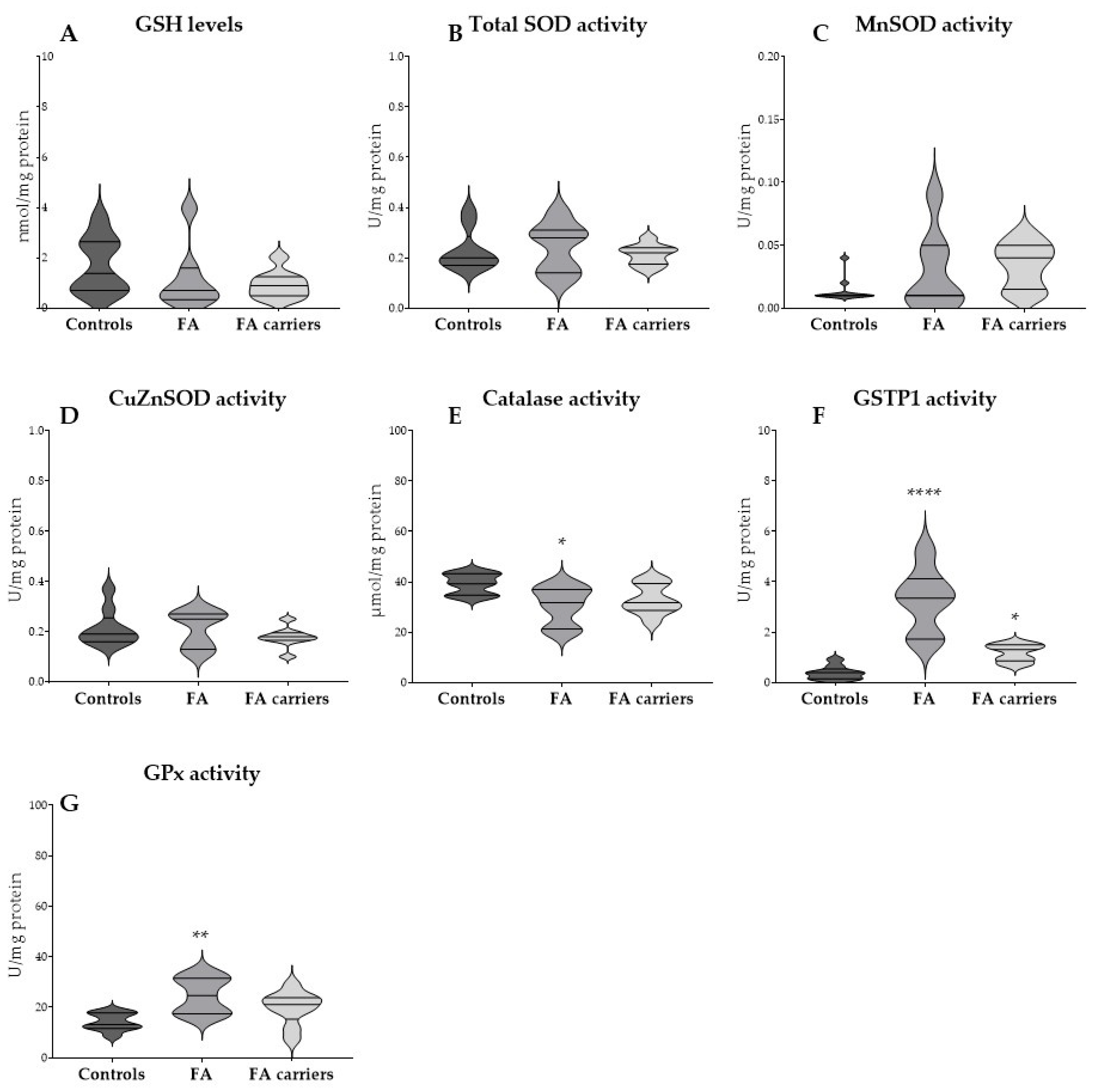
3.2. Basal GSH Levels and Antioxidant Enzyme Activities in RBCs
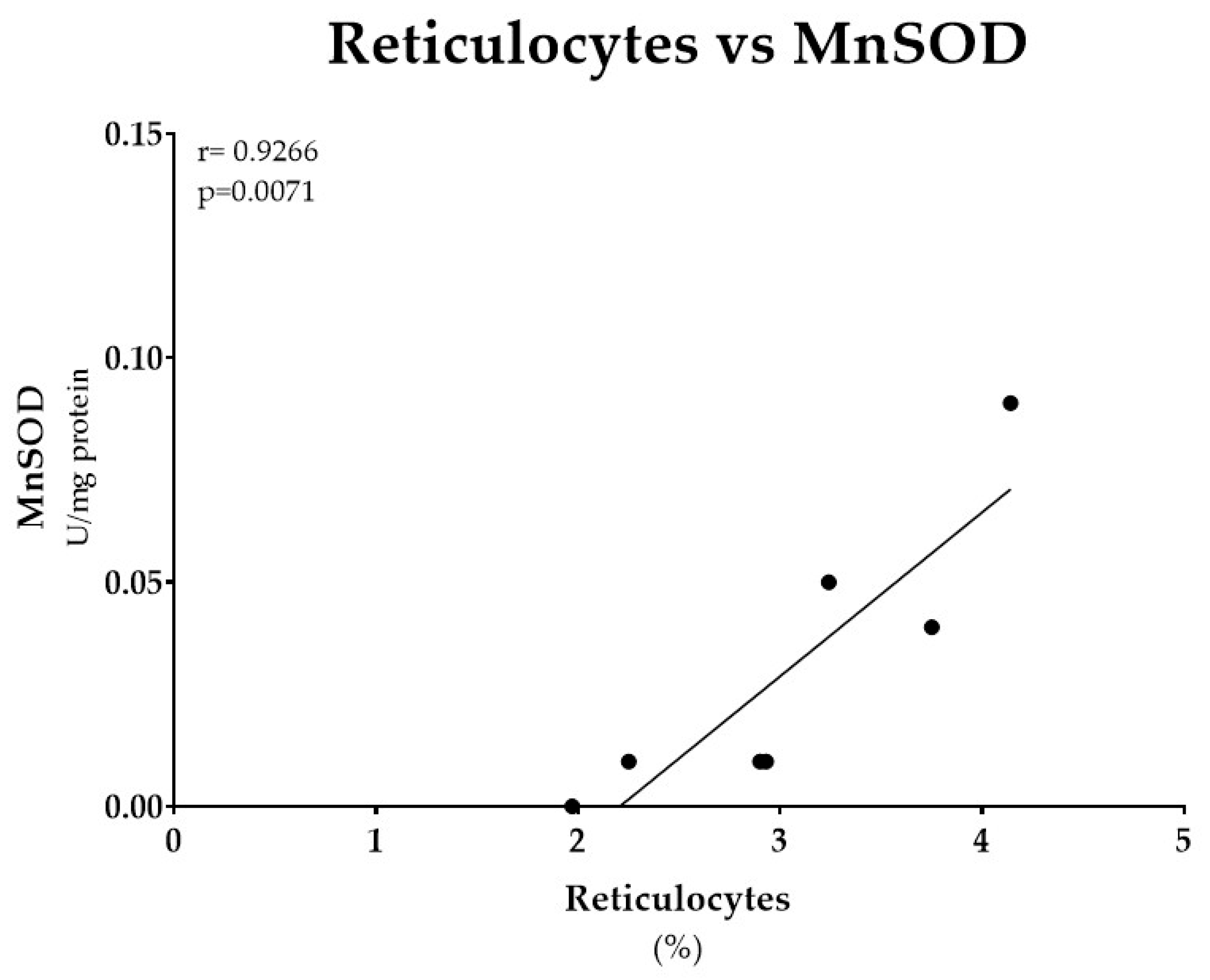
3.3. Erythroid Parameters and MnSOD Activity from FA Patients
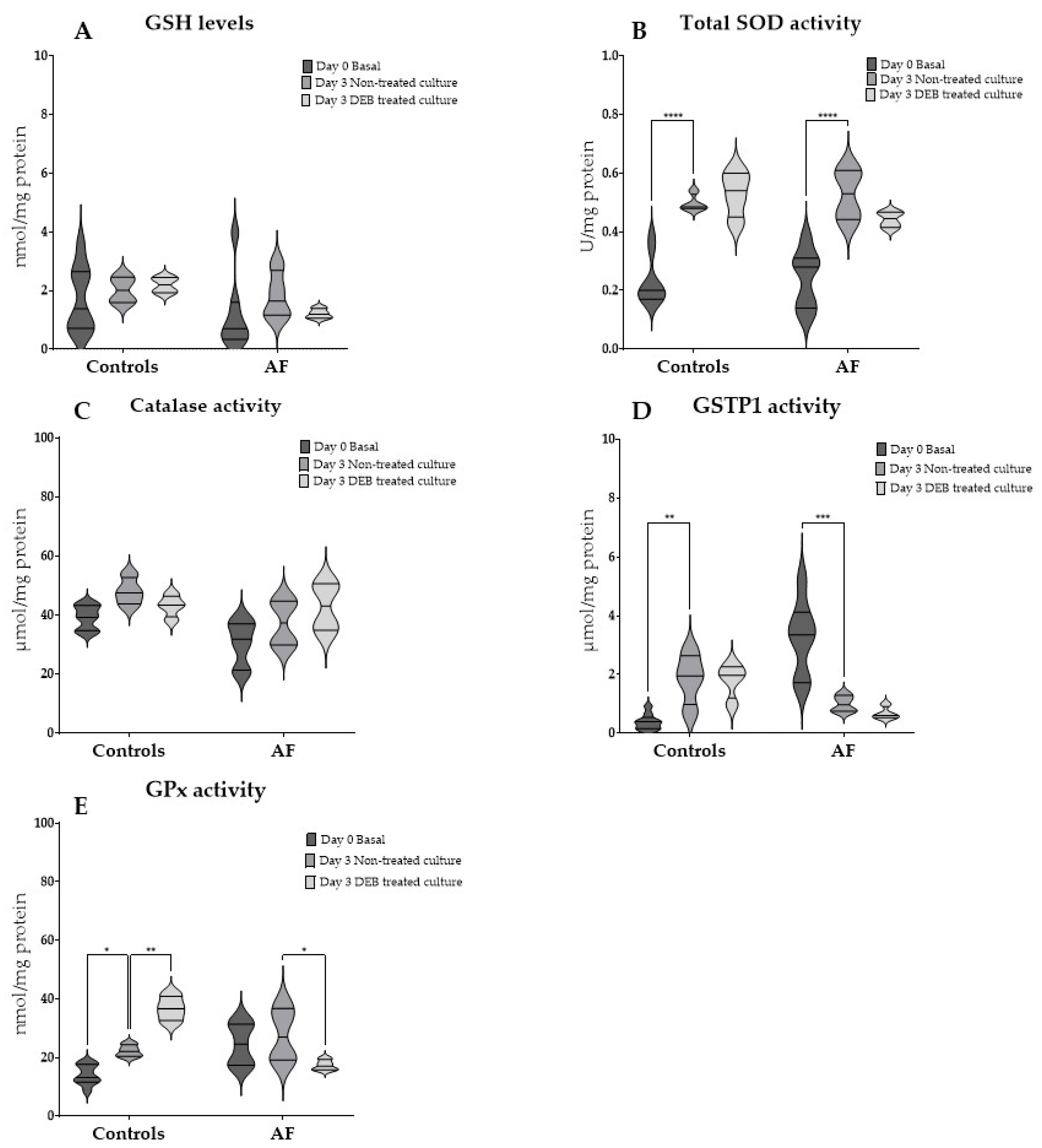
3.4. GSH Levels and Antioxidant Enzyme Activities in RBCs After Culture and the Effect of DEB-Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALA | α-lipoic acid |
| BMF | Bone marrow failure |
| CDNB | 1-chloro-2,4-dinitrobenzene |
| CI | Chromosome instability |
| CuZnSOD | Copper/zinc superoxide dismutase |
| DEB | 1,2,3,4-diepoxybutane |
| FA | Fanconi anemia |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| GST | Glutathione S-transferase |
| GSTP1 | Pi-class glutathione S-transferase |
| Hb | Hemoglobin |
| HbF | Fetal hemoglobin |
| MnSOD | Manganese superoxide dismutase |
| NAC | N-acetylcysteine |
| NADH | Nicotinamide Adenine Dinucleotide Phosphate |
| NBT | Nitroblue tetrazolium |
| OS | Oxidative stress |
| RBC | Red blood cell count |
| RBCs | Red blood cells |
| RDW | Red cell distribution width |
| RPMI | Roswell Park Memorial Institute |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
References
- Knies, K.; Inano, S.; Ramírez, M.J.; Ishiai, M.; Surrallés, J.; Takata, M.; Schindler, D. Biallelic mutations in the ubiquitin ligase RFWD3 cause Fanconi anemia. J. Clin. Investig. 2017, 127, 3013–3027. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhu, Z.; Cao, J.; Huang, J.; Xu, Y. Comprehensive review on Fanconi anemia: Insights into DNA interstrand cross-links, repair pathways, and associated tumors. Orphanet J. Rare Dis. 2025, 20, 389. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, A.; Alter, B.P. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 2010, 24, 101–122. [Google Scholar] [CrossRef]
- Altintas, B.; Giri, N.; McReynolds, L.J.; Best, A.; Alter, B.P. Genotype-phenotype and outcome associations in patients with Fanconi anemia: The National Cancer Institute cohort. Haematologica 2023, 108, 69–82. [Google Scholar] [CrossRef]
- Dufour, C.; Pierri, F. Modern management of Fanconi anemia. Hematol. Am. Soc. Hematol. Educ. Program. 2022, 2022, 649–657. [Google Scholar] [CrossRef]
- Auerbach, A.D. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr. Protoc. Hum. Genet. 2015, 85, 8.7.1–8.7.17. [Google Scholar] [CrossRef]
- Du, W.; Adam, Z.; Rani, R.; Zhang, X. Oxidative stress in Fanconi anemia hematopoiesis and disease progression. Antioxid. Redox Signal. 2008, 10, 1909–1921. [Google Scholar] [CrossRef]
- Bagby, G. Recent advances in understanding hematopoiesis in Fanconi Anemia. F1000Res 2018, 7, 105. [Google Scholar] [CrossRef]
- Pagano, G.; Tiano, L.; Pallardó, F.V.; Lyakhovich, A.; Mukhopadhyay, S.S.; Di Bartolomeo, P.; Zatterale, A.; Trifuoggi, M. Re-definition and supporting evidence toward Fanconi Anemia as a mitochondrial disease: Prospects for new design in clinical management. Redox Biol. 2021, 40, 101860. [Google Scholar] [CrossRef]
- Solanki, A.; Rajendran, A.; Mohan, S.; Raj, R.; Vundinti, B.R. Mitochondrial DNA variations and mitochondrial dysfunction in Fanconi anemia. PLoS ONE 2020, 15, e0227603. [Google Scholar] [CrossRef] [PubMed]
- Siems, W.G.; Sommerburg, O.; Grune, T. Erythrocyte free radical and energy metabolism. Clin. Nephrol. 2000, 53, S9–S17. [Google Scholar]
- Pagano, G.; Talamanca, A.A.; Castello, G.; d’Ischia, M.; Pallardó, F.V.; Petrović, S.; Porto, B.; Tiano, L.; Zatterale, A. From clinical description, to in vitro and animal studies, and backward to patients: Oxidative stress and mitochondrial dysfunction in Fanconi anemia. Free Radic. Biol. Med. 2013, 58, 118–125. [Google Scholar] [CrossRef]
- Möller, M.N.; Orrico, F.; Villar, S.F.; López, A.C.; Silva, N.; Donzé, M.; Thomson, L.; Denicola, A. Oxidants and Antioxidants in the Redox Biochemistry of Human Red Blood Cells. ACS Omega 2023, 8, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Arbos, K.A.; Claro, L.M.; Borges, L.; Santos, C.A.; Weffort-Santos, A.M. Human erythrocytes as a system for evaluating the antioxidant capacity of vegetable extracts. Nutr. Res. 2008, 28, 457–463. [Google Scholar] [CrossRef]
- Orrico, F.; Laurance, S.; Lopez, A.C.; Lefevre, S.D.; Thomson, L.; Möller, M.N.; Ostuni, M.A. Oxidative Stress in Healthy and Pathological Red Blood Cells. Biomolecules 2023, 13, 1262. [Google Scholar] [CrossRef]
- Spinelli, S.; Marino, A.; Remigante, A.; Morabito, R. Redox Homeostasis in Red Blood Cells: From Molecular Mechanisms to Antioxidant Strategies. Curr. Issues Mol. Biol. 2025, 47, 655. [Google Scholar] [CrossRef]
- Bocedi, A.; Noce, A.; Marrone, G.; Noce, G.; Cattani, G.; Gambardella, G.; Di Lauro, M.; Di Daniele, N.; Ricci, G. Glutathione Transferase P1-1 an Enzyme Useful in Biomedicine and as Biomarker in Clinical Practice and in Environmental Pollution. Nutrients 2019, 11, 1741. [Google Scholar] [CrossRef]
- Alnasser, S.M. The role of glutathione S-transferases in human disease pathogenesis and their current inhibitors. Genes. Dis. 2025, 12, 101482. [Google Scholar] [CrossRef] [PubMed]
- Cumming, R.C.; Lightfoot, J.; Beard, K.; Youssoufian, H.; O’Brien, P.J.; Buchwald, M. Fanconi anemia group C protein prevents apoptosis in hematopoietic cells through redox regulation of GSTP1. Nat. Med. 2001, 7, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Kruyt, F.A.; Hoshino, T.; Liu, J.M.; Joseph, P.; Jaiswal, A.K.; Youssoufian, H. Abnormal microsomal detoxification implicated in Fanconi anemia group C by interaction of the FAC protein with NADPH cytochrome P450 reductase. Blood 1998, 92, 3050–3056. [Google Scholar] [CrossRef]
- Mavelli, I.; Ciriolo, M.R.; Rotilio, G.; De Sole, P.; Castorino, M.; Stabile, A. Superoxide dismutase, glutathione peroxidase and catalase in oxidative hemolysis. A study of Fanconi’s anemia erythrocytes. Biochem. Biophys. Res. Commun. 1982, 106, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Yoshimitsu, K.; Kobayashi, Y.; Usui, T. Decreased superoxide dismutase activity of erythrocytes and leukocytes in Fanconi’s anemia. Acta Haematol. 1984, 72, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Malorni, W.; Straface, E.; Pagano, G.; Monti, D.; Zatterale, A.; Del Principe, D.; Deeva, I.B.; Franceschi, C.; Masella, R.; Korkina, L.G. Cytoskeleton alterations of erythrocytes from patients with Fanconi’s anemia. FEBS Lett. 2000, 468, 125–128. [Google Scholar] [CrossRef]
- Sousa, R.; Gonçalves, C.; Guerra, I.C. Increased red cell distribution width in Fanconi anemia: A novel marker of stress erythropoiesis. Orphanet J. Rare Dis. 2016, 11, 102. [Google Scholar] [CrossRef]
- Pagano, G.; Talamanca, A.A.; Castello, G.; Pallardó, F.V.; Zatterale, A.; Degan, P. Oxidative stress in Fanconi anaemia: From cells and molecules towards prospects in clinical management. Biol. Chem. 2012, 393, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ponte, F.; Carvalho, F.; Porto, B. Protective effect of acetyl-l-carnitine and α-lipoic acid against the acute toxicity of diepoxybutane to human lymphocytes. Toxicology 2011, 289, 52–58. [Google Scholar] [CrossRef]
- Ponte, F.; Sousa, R.; Fernandes, A.P.; Gonçalves, C.; Barbot, J.; Carvalho, F.; Porto, B. Improvement of genetic stability in lymphocytes from Fanconi anemia patients through the combined effect of α-lipoic acid and N-acetylcysteine. Orphanet J. Rare Dis. 2012, 7, 28. [Google Scholar] [CrossRef]
- Mehta, P.A.; Nelson, A.; Loveless, S.; Lane, A.; Fukuda, T.; Teusink-Cross, A.; Elder, D.; Lagory, D.; Miller, E.; Cancelas, J.A.; et al. Phase 1 study of quercetin, a natural antioxidant for children and young adults with Fanconi anemia. Blood Adv. 2025, 9, 1927–1939. [Google Scholar] [CrossRef]
- Carvalho, M.; Milhazes, N.; Remião, F.; Borges, F.; Fernandes, E.; Amado, F.; Monks, T.J.; Carvalho, F.; Bastos, M.L. Hepatotoxicity of 3,4-methylenedioxyamphetamine and alpha-methyldopamine in isolated rat hepatocytes: Formation of glutathione conjugates. Arch. Toxicol. 2004, 78, 16–24. [Google Scholar] [CrossRef]
- Flohé, L.; Otting, F. Superoxide dismutase assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Du, W.; Rani, R.; Sipple, J.; Schick, J.; Pang, Q. The FA pathway counteracts oxidative stress through selective protection of antioxidant defense gene promoters. Blood 2012, 119, 4142–4151. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Kobayashi, S.; Warang, P.; Madkaikar, M.; Mukherjee, M.B. Erythrocytes as a preferential target of oxidative stress in blood. Free Radic. Res. 2021, 55, 562–580. [Google Scholar] [CrossRef]
- Moras, M.; Lefevre, S.D.; Ostuni, M.A. From Erythroblasts to Mature Red Blood Cells: Organelle Clearance in Mammals. Front. Physiol. 2017, 8, 1076. [Google Scholar] [CrossRef]
- Moriconi, C.; Dzieciatkowska, M.; Roy, M.; D’Alessandro, A.; Roingeard, P.; Lee, J.Y.; Gibb, D.R.; Tredicine, M.; McGill, M.A.; Qiu, A.; et al. Retention of functional mitochondria in mature red blood cells from patients with sickle cell disease. Br. J. Haematol. 2022, 198, 574–586. [Google Scholar] [CrossRef]
- Esperti, S.; Nader, E.; Stier, A.; Boisson, C.; Carin, R.; Marano, M.; Robert, M.; Martin, M.; Horand, F.; Cibiel, A.; et al. Increased retention of functional mitochondria in mature sickle red blood cells is associated with increased sickling tendency, hemolysis and oxidative stress. Haematologica 2023, 108, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Morimoto, K. Increased formation of 8-hydroxydeoxyguanosine, an oxidative DNA damage, in lymphoblasts from Fanconi’s anemia patients due to possible catalase deficiency. Carcinogenesis 1993, 14, 1115–1120. [Google Scholar] [CrossRef]
- Nordenson, I. Effect of superoxide dismutase and catalase on spontaneously occurring chromosome breaks in patients with Fanconi’s anemia. Hereditas 1977, 86, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, S.; Leskovac, A.; Kotur-Stevuljevic, J.; Joksic, J.; Guc-Scekic, M.; Vujic, D.; Joksic, G. Gender-related differences in the oxidant state of cells in Fanconi anemia heterozygotes. Biol. Chem. 2011, 392, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Porto, B.; Sousa, R.; Malheiro, I.; Gaspar, J.; Rueff, J.; Gonçalves, C.; Barbot, J. Protective effect of red blood cells against diepoxybutane-induced chromosome instability in Fanconi anaemia lymphocytes. Cell Prolif. 2010, 43, 573–578. [Google Scholar] [CrossRef]
- Porto, B.; Chiecchio, L.; Gaspar, J.; Faber, A.; Pinho, L.; Rueff, J.; Malheiro, I. Role of haemoglobin in the protection of cultured lymphocytes against diepoxybutane (DEB), assessed by in vitro induced chromosome breakage. Mutat. Res. 2003, 536, 61–67. [Google Scholar]
- Porto, B.; Oliveira, R.D.; Sousa, C.; Gaspar, J.; Rueff, J.; Carvalho, F.; Malheiro, I. The role of foetal red blood cells in protecting cultured lymphocytes against diepoxybutane-induced chromosome breaks. Mutat. Res. 2006, 603, 41–47. [Google Scholar] [PubMed]
- Auerbach, A.D. Fanconi anemia and its diagnosis. Mutat. Res. 2009, 668, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Suda, T. Microenvironmental dynamics in steady-state and stress erythropoiesis. Blood Sci. 2025, 7, e00219. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Agarwal, S.; Tewari, S. Biomarker of Oxidative Stress in Erythrocytes: Clinical Significance. The Power of Antioxidants—Unleashing Nature’s Defense Against Oxidative Stress; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Bertola, N.; Regis, S.; Bruno, S.; Mazzarello, A.N.; Serra, M.; Lupia, M.; Sabatini, F.; Corsolini, F.; Ravera, S.; Cappelli, E. Effects of Deacetylase Inhibition on the Activation of the Antioxidant Response and Aerobic Metabolism in Cellular Models of Fanconi Anemia. Antioxidants 2023, 12, 1100. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Deater, M.; Schubert, K.; Marquez-Loza, L.; Pelz, C.; Sinclair, D.A.; Grompe, M. The Sirt1 activator SRT3025 expands hematopoietic stem and progenitor cells and improves hematopoiesis in Fanconi anemia mice. Stem Cell Res. 2015, 15, 130–140. [Google Scholar] [CrossRef]
| Controls | FA | FA Carriers | ||||
|---|---|---|---|---|---|---|
| % Aberrant Cells | Mean No Breaks/Cell | % Aberrant Cells | Mean No Breaks/Cell | % Aberrant Cells | Mean No Breaks/Cell | |
| 1.00 | 0.01 | 85.70 | 3.14 | 2.10 | 0.02 | |
| 18.20 | 0.20 | 98.00 | 14.36 | 1.00 | 0.01 | |
| 1.00 | 0.01 | 83.30 | 2.70 | 4.00 | 0.05 | |
| 13.8 | 0.19 | 55.00 | 4.20 | 5.00 | 0.07 | |
| 6.00 | 0.06 | 96.00 | 9.91 | 2.00 | 0.03 | |
| 6.00 | 0.06 | 100.00 | 9.74 | 4.00 | 0.04 | |
| 3.80 | 0.05 | 86.70 | 3.18 | 3.00 | 0.03 | |
| 1.00 | 0.01 | 10.00 | 0.11 | |||
| 1.00 | 0.01 | 3.00 | 0.04 | |||
| Mean ± SD | 5.76 ± 5.91 | 0.01 ± 0.70 | 86.39 ± 14.18 | 6.75 ± 4.24 | 3.79 ± 2.48 | 0.04 ± 0.003 |
| RBC (×106/µL) | Hb (g/dL) | HbF (%) | MCV (fL) | RDW (%) | Reticulocytes (%) | |
|---|---|---|---|---|---|---|
| [4.00–5.20] | [11.10–14.10] | [<2.00%] | [77.00–95.00] | [11.60–14.00] | [1.00–2.00%] | |
| FA1 | 3.74 | 9.50 | 12.70 | 104.10 | 19.20 | 3.24 |
| FA2 | 3.00 | 10.60 | -- | 103.80 | 17.30 | 4.14 |
| FA3 | 4.60 | 12.20 | 1.20 | 105.20 | 14.70 | 2.25 |
| FA4 | 4.17 | 13.80 | -- | 96.60 | 15.40 | 2.90 |
| FA5 | 2.10 | 7.70 | 12.60 | 117.80 | 18.90 | 3.75 |
| FA6 | 1.90 | 7.10 | 8.10 | 109.00 | 21.10 | 2.93 |
| FA7 | 2.67 | 8.60 | -- | 97.00 | 15.10 | 1.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, C.; Dinis-Oliveira, R.J.; Carvalho, F.; Jorge, P.; Porto, B. Red Blood Cell Antioxidant State in Fanconi Anemia: The Highlighted Roles of Pi-Class Glutathione S-Transferase and Glutathione Peroxidase. Antioxidants 2025, 14, 1150. https://doi.org/10.3390/antiox14101150
Oliveira C, Dinis-Oliveira RJ, Carvalho F, Jorge P, Porto B. Red Blood Cell Antioxidant State in Fanconi Anemia: The Highlighted Roles of Pi-Class Glutathione S-Transferase and Glutathione Peroxidase. Antioxidants. 2025; 14(10):1150. https://doi.org/10.3390/antiox14101150
Chicago/Turabian StyleOliveira, Cláudia, Ricardo Jorge Dinis-Oliveira, Félix Carvalho, Paula Jorge, and Beatriz Porto. 2025. "Red Blood Cell Antioxidant State in Fanconi Anemia: The Highlighted Roles of Pi-Class Glutathione S-Transferase and Glutathione Peroxidase" Antioxidants 14, no. 10: 1150. https://doi.org/10.3390/antiox14101150
APA StyleOliveira, C., Dinis-Oliveira, R. J., Carvalho, F., Jorge, P., & Porto, B. (2025). Red Blood Cell Antioxidant State in Fanconi Anemia: The Highlighted Roles of Pi-Class Glutathione S-Transferase and Glutathione Peroxidase. Antioxidants, 14(10), 1150. https://doi.org/10.3390/antiox14101150









