Abstract
BRCA1 mutations predispose women to breast and ovarian cancer. The anticancer effect of zinc is typically linked to its antioxidant abilities and protecting cells against oxidative stress. Zinc regulates key processes in cancer development, including DNA repair, gene expression, and apoptosis. We took a blood sample from 989 female BRCA1 mutation carriers who were initially unaffected by cancer and followed them for a mean of 7.5 years thereafter. There were 172 incident cases of cancer, including 121 cases of breast cancer, 29 cases of ovarian cancers, and 22 cancers at other sites. A zinc level in the lowest tertile was associated with a modestly higher risk of ovarian cancer compared to women with zinc levels in the upper two tertiles (HR = 1.65; 95% CI 0.80 to 3.44; p = 0.18), but this was not significant. Among those women with zinc levels in the lowest tertile, the 10-year cumulative risk of ovarian cancer was 6.1%. Among those in the top two tertiles of zinc level, the ten-year cumulative risk of ovarian cancer was 4.7%. There was no significant association between zinc level and breast cancer risk. Our preliminary study does not support an association between serum zinc level and cancer risk in BRCA1 mutation carriers.
1. Introduction
In 2023, it was predicted that there would be 297,790 new cases of breast cancer in women and 19,710 ovarian cancers [1]. About 3% of breast cancers (about 7500–8500 women per year) and 10% of ovarian cancers (about 2000 women per year) are cases with BRCA1 mutations.
Approximately 13% of women in the general population will develop breast cancer during their lifetime [2]. However, in women who have inherited a deleterious BRCA1 variant, the mutation in the BRCA1 gene, the lifetime risks are 70% and 40%, respectively [2,3]. In addition to prophylactic surgery, modifiers of risks include age; hormone treatment; reproductive history; and diet, including micronutrients. Because of their extremely high risk of developing breast and ovarian cancer, we aim to find possible ways to reduce this risk.
Zinc is classified as an essential trace element and plays a crucial role in numerous cancer-suppressive mechanisms, including DNA replication, damage repair, oxidative stress response, cell cycle progression, and apoptosis [4].
Zinc functions as a cofactor for over 900 transcription factors and 300 enzymes, influencing DNA regulation, gene expression, nucleic acid synthesis, and genome stability [5]. As part of the CuZnSOD enzyme and the metallothionein protein, zinc acts as a key defender against ROS attacks [6,7,8,9]. Zinc deficiency is linked to the generation of single-strand breaks of DNA and affects repair ability, impacting processes such as repair, chromatin structure, replication, transcription, and counteracting oxidative DNA damage [10,11,12]. Moreover, zinc deficiency compromises immune responses, potentially contributing to cancer development [13,14].
There have been 18 published prospective studies on the correlation between zinc and cancer risk [5,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Additionally, numerous retrospective publications demonstrate a correlation between zinc and cancer risk [32,33,34,35,36,37,38,39,40,41]. To date, the role of zinc in tumorigenesis in women with BRCA1 mutations has not been studied, and for this reason, this was the purpose of our work.
2. Materials and Methods
The study subjects included 989 adult women, who received genetic counselling and testing between 2011 and 2017 at the Clinical Hospitals of Pomeranian Medical University in Szczecin, Poland, or at an affiliated hospital or outpatient clinic. At the first study visit, a fasting blood sample was collected from each study participant to be used for genetic testing for BRCA1 mutations. For analysis, 10 mL of peripheral blood was collected into a vacutainer tube containing ethylenediaminetetraacetic acid (EDTA) from all study participants. All blood samples were collected between 8 a.m. and 2 p.m. and stored at −80 °C until analysis. Participants were included in the study if a deleterious BRCA1 variant was detected.
Typically, these patients are offered the opportunity to participate in other clinical research studies. Medical charts were reviewed for date of diagnosis, age at enrollment (<50/≥50), preventive salpingo-oophorectomy (yes/no), smoking status (ever/never), oral contraceptive use (ever/never), diabetes (yes/no), dietary supplements (ever/never), hormonal therapy (ever/never), and BMI (low/normal/fat/obesity).
The study was conducted in accordance with the Helsinki Declaration and with the consent of the Ethics Committee of Pomeranian Medical University in Szczecin under the number KB-0012/73/10 of 21 June 2010. All participants provided written informed consent.
2.1. Measurement of Blood Zinc Level
Collected blood samples were thawed from −80 °C to room temperature on the day of analysis. Each sample was thoroughly mixed using a shaker or vortex to make the material as homogeneous as possible. This process was repeated immediately prior to taking blood volumes for dilutions due to the phenomenon of blood stratification. Using the simplest possible technique, the blood samples were diluted at a ratio of 1:30 (50 µL blood: 1450 µL buffer).
In order to achieve the specificity of the measurement, tetramethylammonium hydroxide (TMAH) solution was used for dilutions. The alkaline pH ensures good solubility of blood components, thus not causing precipitation of any of the fractions.
In addition, in order to better disperse the dissolved blood components, a non-ionic surfactant in the form of Triton X-100 was added. The use of this compound not only facilitates the dissolution of proteins, among others but also contributes to the faster flushing of the sample from the spectrometer introduction system. An internal standard in the form of rhodium (105Rh) was used to correct the matrix effect and camera drift. To achieve the stability of metal ions dissolved in solution, EDTA was used. In addition, due to the content of carbon-containing compounds, butanol was used.
The inductively coupled plasma excitation mass spectrometry (ICP-MS) technique was used to determine the content of Pb. An ELAN DRC-e mass spectrometer (PerkinElmer, Norfolk, VA, USA) and a NexION 350D mass spectrometer (PerkinElmer) were applied. Oxygen was used as a reaction gas. The ICP-MS allows for detection limits of <0.1 µg/L.
The following reference materials were used to validate the measurements: ClinCheck (Recipe, Munich, Germany), NIST 955c (National Institute of Standards and Technology, Gaithersburg, MD, USA), and BCR 634/BCR635 (European Commission, Community Bureau of Reference, Brussels, Belgium). These are reference standards commonly used in spectrometry to confirm the precision, sensitivity, and specificity of the measurement.
2.2. Statistical Analysis
All study participants were assigned to one of three categories (tertiles) depending on their blood zinc level. The cumulative risks of breast and ovarian cancer were calculated from the age at blood draw to the age of diagnosis of breast or ovarian cancer, death from another cause, or last follow-up. For estimating the risk of ovarian cancer, women with oophorectomy prior to blood draw were excluded, and subjects with oophorectomy in the follow-up period were censored at the time of oophorectomy. To estimate the ten-year cumulative risk of ovarian cancer, patients were followed from blood draw to date of preventive oophorectomy, ovarian cancer, ten years of follow-up, last follow-up, or death from another cause. For the analysis of breast cancer risk, oophorectomy was included as a time-dependent variable. In order to estimate the hazard ratios (HRs) for cancer risk, univariable and multivariable Cox proportional hazards regression analyses were performed. In multivariable models, the following variables were taken into analysis: zinc level (tertile), year of birth, age at blood draw (<40 years, 40–49.9 years ≥50 years), oral contraceptive use (yes/no), hormone replacement therapy use (yes/no), smoking history (current, former never), and BMI (<23.0 versus >23.0). All statistical analyses were performed using SAS, version 9.4.
3. Results
The study group consisted of 989 women diagnosed with a BRCA1 mutation. The patients were followed up for an average of 6.75 years, during which time 174 new cancers were reported (121 cases of breast cancer, 29 cases of ovarian cancer, and 22 cancers at other sites). The characteristics of the study group are presented in Table 1.

Table 1.
Group characteristics.
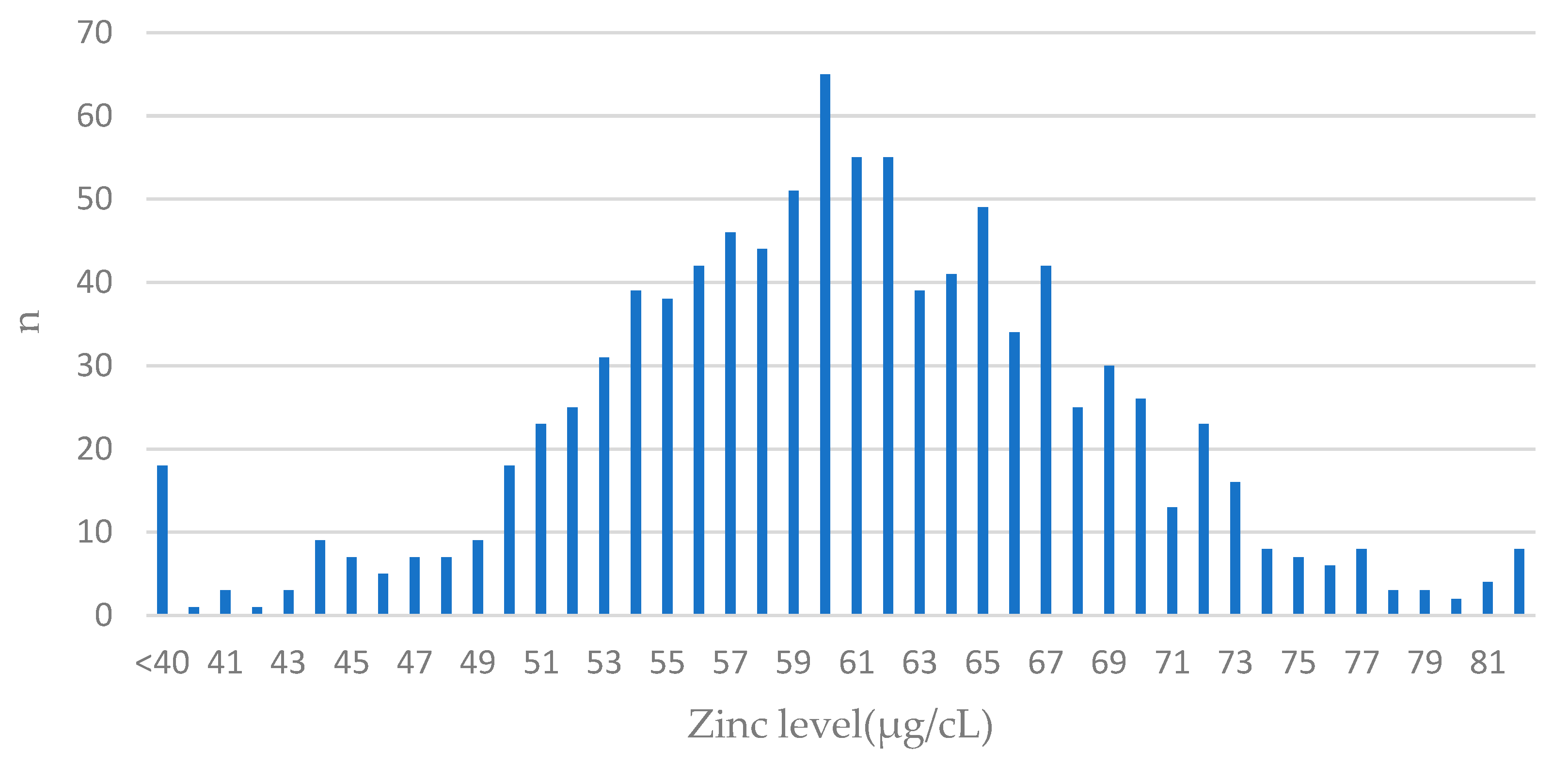
The distribution of zinc levels in the cohort is presented in Figure 1.
Figure 1.
The distribution of values of zinc levels in blood among BRCA1 carriers. Features of normal distribution can be seen. The largest number of patients had blood levels close to the mean value (61 µg/cL) in the entire group; n—number of patients.
3.1. Breast Cancer
There was no statistically significant correlation between blood zinc levels and breast cancer risk in BRCA1 carriers (Table 2). For women with zinc levels in the lowest tertile, the hazard ratio was 0.88 (95% CI 0.60 to 1.29; p = 0.51) compared to those with zinc levels in the top two tertiles.

Table 2.
The hazard ratio for breast cancer according to zinc level (tertiles).
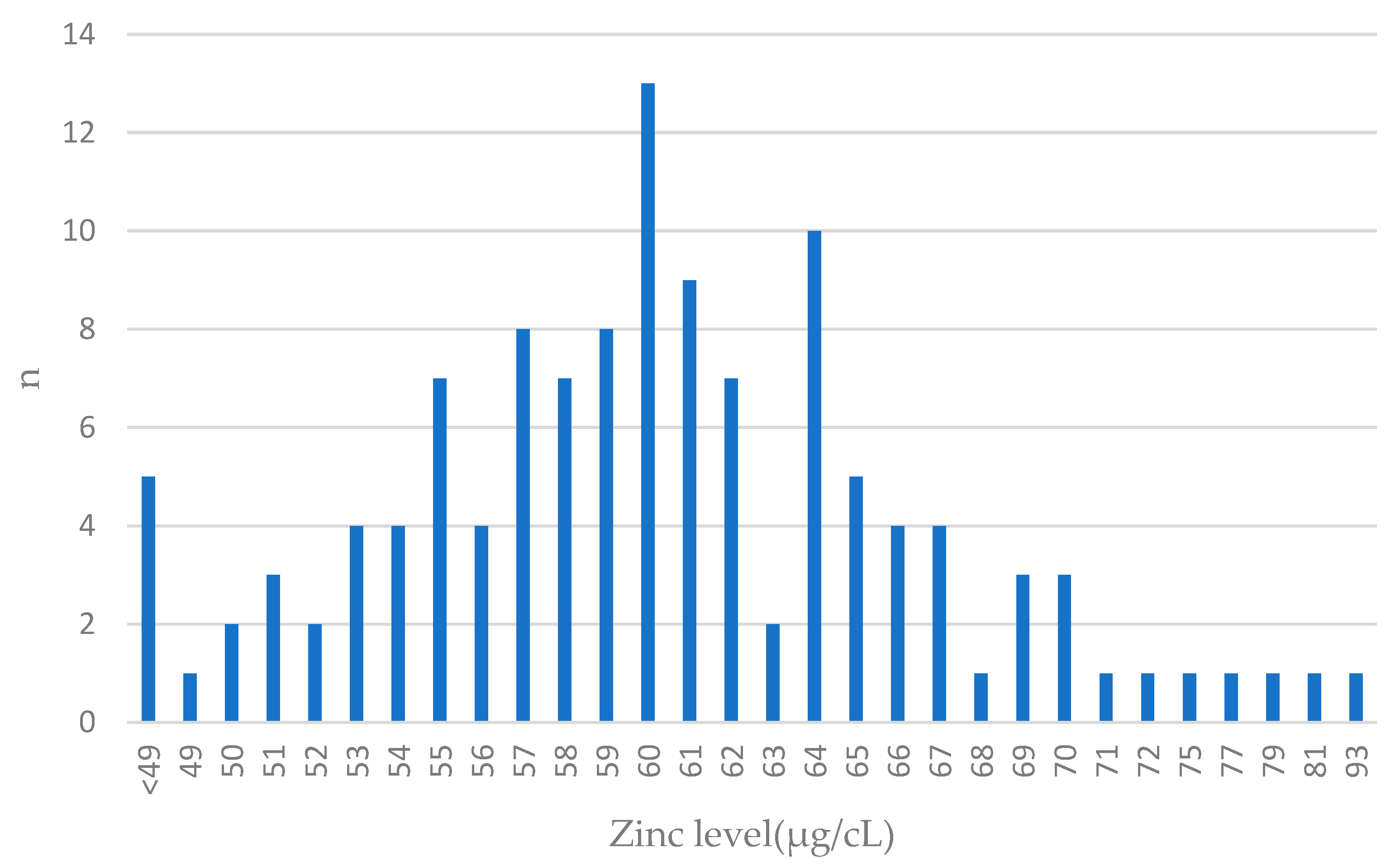
The distribution of zinc levels in breast cancer cases is presented in Figure 2.
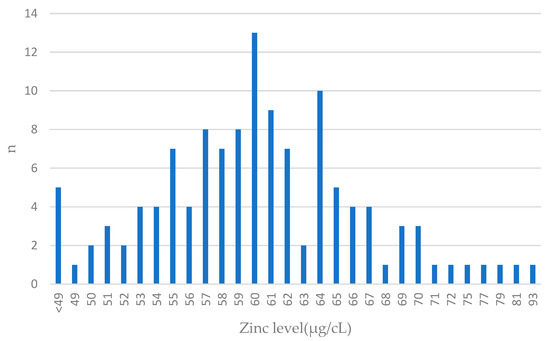
Figure 2.
Zinc levels in blood among breast cancer cases. Features close to normal distribution can be seen. The largest number of patients had blood levels close to the mean value (61 µg/cL) in the entire group; n—number of patients.
3.2. Ovarian Cancer
Initially, unaffected women with a blood zinc level below 5797 µg/L had an increased risk of ovarian cancer, compared to women with a blood zinc level greater than 5797 (tertile 1 versus tertiles 2/3; adjusted HR = 1.95 95% CI 0.92 to 4.14), but this was not significant (p = 0.08). Among those women with zinc levels in the lowest tertile, the 10-year cumulative risk of ovarian cancer was 6.1%. Among those with zinc levels in the top two tertiles, the 10-year cumulative risk of ovarian cancer was 4.7% (Table 3).

Table 3.
Hazard ratios (HRs) for ovarian cancer by zinc level (tertiles).
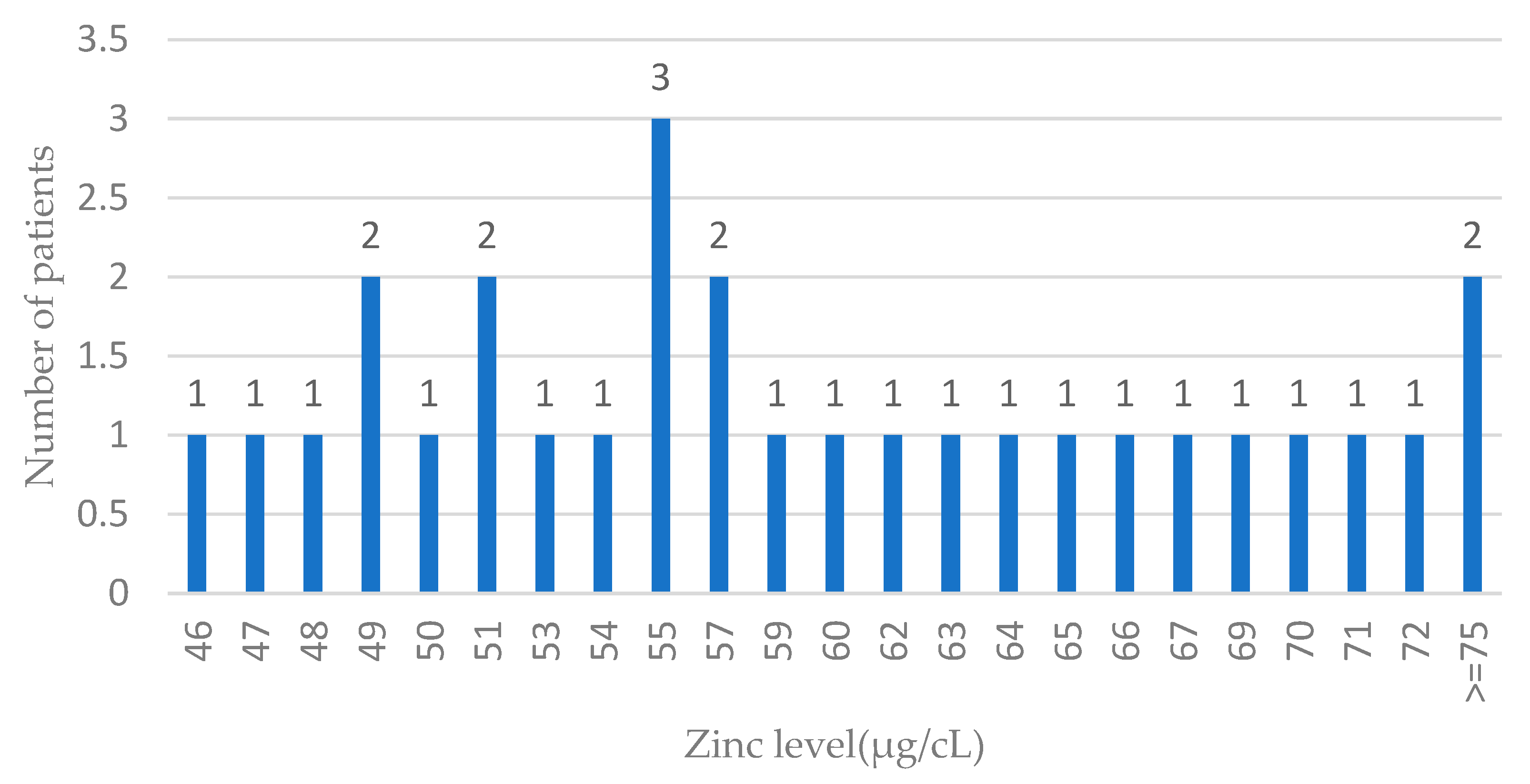
The distribution of zinc levels in ovarian cases is presented in Figure 3.
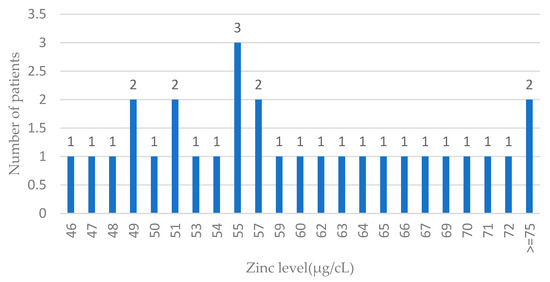
Figure 3.
Zinc levels in blood among ovarian cancer cases. Features of the normal distribution cannot be seen (probably due to the low number of ovarian cases); n—number of patients.
3.3. All Cancers
Among all the 989 women, 174 developed cancers in the follow-up period. Overall, those women with zinc levels in the bottom tertile had a modestly increased risk of any cancer, compared to those in the top two tertiles (HR = 1.10; 95% CI 0.80 to 1.52). If we exclude breast or ovarian cancer, women with zinc levels in the bottom tertile had a similar risk of cancer, compared to those in the top two tertiles (HR = 1.00; 95% CI 0.70 to 1.42). There were too few cancers at other sites to provide site-specific hazard ratios for these.
4. Discussion
Our results demonstrated a modest and non-significant association between a low blood zinc level and an increased risk of ovarian cancer in unaffected BRCA1 carriers. Initially, unaffected women with blood zinc levels > 5797 µg/L exhibited a twofold reduction in the risk of ovarian cancer compared to women with blood zinc levels ≤ 5797 (HR = 0.51 95% CI: 0.24–1.09), although this did not reach statistical significance (p = 0.08). There was no association between zinc levels and breast cancer or other cancers.
Zinc serves as a critical cofactor for enzymatic activities such as dehydrogenases, peptidases, and zinc finger domains. Zinc is involved in a number of reactions necessary for the proper functioning of the human body (Table 4).

Table 4.
The effect of zinc on carcinogenesis.
The recommended daily value of zinc is 11 mg for men and 8 mg per day for women. Thus far, there has been no suggested blood zinc level; however, the recommended concentration of zinc in serum or plasma typically ranges from 800 to 1200 mcg/L.
Zinc can be absorbed through several pathways, including passive diffusion and absorption in the digestive tract, regulated by transporters [48]. The bioavailability of zinc in the digestive tract increases in the presence of citric acid and decreases in the presence of iron, calcium, phosphorus, fiber, and phytate [49]. Individuals with a vegetable-rich diet may exhibit lower zinc absorption rates. For example, legumes contain a relatively high amount of zinc (Table 5), but the presence of phytate, which inhibits the absorption of zinc, results in less of this element being supplied to the body than in the case of providing the same amount from animal foods [50].

Table 5.
The average content of zinc and DV in selected foods with favorable bioabsorption.
There have been 18 published prospective studies on the correlation between cancer risk and zinc [5,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. There are numerous publications that demonstrate a correlation between zinc and cancer risk [32,33,34,35,36,37,38,39,40,41]. However, these are retrospective papers, and for this reason, they were not analyzed further in our publication.
We found 18 prospective studies about zinc and cancer risk (Table 6). Of these, there were eight papers on colorectal, five on prostate, two on breast, two on pancreatic, one on hepatocellular, one on lung, and one on kidney cancer. Among them, 13 showed a positive correlation between low zinc levels in the blood and cancer risk, but the remaining 7 did not show a statistically significant result. In most studies, the exposure data were based on questionnaire information about intake. The exception is one prospective study [15], in which zinc levels were measured in serum and urine.

Table 6.
Prospective studies on cancer risk.
In another study [15], in addition to the association with zinc and copper levels, the strongest correlation was shown between the highest quartile Cu/Zn ratio in serum and urine (OR, 2.37; 95% CI, 1.32–4.25). Even for serum alone, the ratio was better than for each micronutrient separately (OR—1.75; 95% CI: 1.21–2.54). Elevated copper and low zinc levels are the most common trace metal imbalances encountered in the human body [51].
Zinc interacts with the human body through a variety of mechanisms, which are crucial for its proper functioning. This is, for example, evidenced by the fact that metalloprotease activity mediates every stage from (ovarian) tumor formation to metastatic implantation [52].
The results of this study have several potential clinical implications. If confirmed, the evaluation of zinc levels and the levels of other microelements in the blood of BRCA1 carriers may be used as a marker of the presence of early cancers and as a risk factor for later cancer development. This information is potentially relevant for BRCA1 mutation carriers who are considering preventive oophorectomies. Notably, our study revealed that around 33% of women demonstrated low zinc values and would be candidates for supplementation. In the future, blood testing and dietary advice and/or supplement use might be used to optimize zinc levels among BRCA1 carriers.
In summary, our study did not prove that blood zinc levels are associated with the risk of cancers among BRCA1 carriers. However, there was a suggestive association between low zinc levels and a higher risk of ovarian cancers. It is important to perform further investigations and observations on a larger number of carriers and with longer follow-ups.
5. Conclusions
In summary, our preliminary study does not confirm an association between serum zinc levels and cancer risk in BRCA1 mutation carriers. We hypothesize that zinc levels may predict lower risks of ovarian cancer, but the correlation was not statistically significant. Further studies are needed. Additionally, there is a need to generate results with women with other genetic mutations.
Author Contributions
Conceptualization, M.M., A.K., R.D., S.A.N. and J.L.; Methodology, W.M.; Software, W.M., P.B. and A.C.; Validation, R.D. and S.A.N.; Formal analysis, W.M., R.D., P.B. and M.B.; Investigation, M.M.; Resources, K.S., C.C., T.D., J.G., T.H., M.S. (Marek Szwiec), M.S.-N., D.G., A.P., A.J. (Andrzej Jasiewicz), T.K., J.T.-S., E.K.-K., M.S. (Monika Siołek), R.W., R.P. and J.J.-T.; Data curation, W.M., R.D., K.S. and P.S.; Writing—original draft, M.M. and A.K.; Writing—review & editing, M.M., A.K., S.A.N. and J.L.; Visualization, J.L.; Supervision, R.J.S., S.A.N. and J.L.; Funding acquisition, M.R.L., A.J. (Anna Jakubowska) and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the program of Minister of Science and Higher Education “Regional Initiative of Excellence” in years 2019–2022, Grant No. 002/RID/2018/19.
Institutional Review Board Statement
This research adhered to the principles of the Declaration of Helsinki and received approval from the Ethics Committee of the Pomeranian Medical University in Szczecin (Poland) under the reference number KB-0012/73/10 (26 June 2010).
Informed Consent Statement
Informed consent was obtained from all study participants.
Data Availability Statement
Data supporting the results presented are available from the authors upon request from any interested researchers.
Conflicts of Interest
Authors W.M., R.D., C.C., J.G., T.H. and J.L. were employed by the company Read-Gene. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.-A.; Mooij, T.M.; Roos-Blom, M.-J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Silvestri, V.; Leslie, G.; Rebbeck, T.R.; Neuhausen, S.L.; Hopper, J.L.; Nielsen, H.R.; Lee, A.; Yang, X.; McGuffog, L.; et al. Cancer Risks Associated with BRCA1 and BRCA2 Pathogenic Variants. J. Clin. Oncol. 2022, 40, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, D.K.; Chadha, V.D. Zinc: A Promising Agent in Dietary Chemoprevention of Cancer. Indian J. Med. Res. 2010, 132, 676–682. [Google Scholar] [PubMed]
- Stepien, M.; Jenab, M.; Freisling, H.; Becker, N.-P.; Czuban, M.; Tjønneland, A.; Olsen, A.; Overvad, K.; Boutron-Ruault, M.-C.; Mancini, F.R.; et al. Pre-Diagnostic Copper and Zinc Biomarkers and Colorectal Cancer Risk in the European Prospective Investigation into Cancer and Nutrition Cohort. Carcinogenesis 2017, 38, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Bray, T.M.; Bettger, W.J. The Physiological Role of Zinc as an Antioxidant. Free Radic. Biol. Med. 1990, 8, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc Deficiency. BMJ 2003, 326, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Kucuk, O. Zinc in Cancer Prevention. Cancer Metastasis Rev. 2002, 21, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.R. The Antioxidant Properties of Zinc. J. Nutr. 2000, 130, 1447S–1454S. [Google Scholar] [CrossRef]
- Falchuk, K.H. The Molecular Basis for the Role of Zinc in Developmental Biology. Mol. Cell. Biochem. 1998, 188, 41–48. [Google Scholar] [CrossRef]
- Ho, E.; Courtemanche, C.; Ames, B.N. Zinc Deficiency Induces Oxidative DNA Damage and Increases P53 Expression in Human Lung Fibroblasts. J. Nutr. 2003, 133, 2543–2548. [Google Scholar] [CrossRef]
- Erkekoglu, P.; Giray, B.; Rachidi, W.; Hininger-Favier, I.; Roussel, A.; Favier, A.; Hincal, F. Effects of Di(2-ethylhexyl)Phthalate on Testicular Oxidant/Antioxidant Status in Selenium-deficient and Selenium-supplemented Rats. Environ. Toxicol. 2014, 29, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Soghoian, D.Z.; Streeck, H. Cytolytic CD4+ T Cells in Viral Immunity. Expert Rev. Vaccines 2010, 9, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and Anti-Inflammatory Effects of Zinc. Zinc-Dependent NF-ΚB Signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Pala, V.; Agnoli, C.; Cavalleri, A.; Rinaldi, S.; Orlandi, R.; Segrado, F.; Venturelli, E.; Vinceti, M.; Krogh, V.; Sieri, S. Prediagnostic Levels of Copper and Zinc and Breast Cancer Risk in the ORDET Cohort. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Wilkens, L.R.; Morris, J.S.; Henderson, B.E.; Kolonel, L.N. Serum Zinc and Prostate Cancer Risk in a Nested Case–Control Study: The Multiethnic Cohort. Prostate 2013, 73, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; Hughes, D.J.; Hybsier, S.; Bamia, C.; Tjønneland, A.; Overvad, K.; Affret, A.; His, M.; Boutron-Ruault, M.-C.; Katzke, V.; et al. Circulating Copper and Zinc Levels and Risk of Hepatobiliary Cancers in Europeans. Br. J. Cancer 2017, 116, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, Y.; Li, H.; Zheng, W.; Gao, J.; Zhang, W.; Yang, G.; Shu, X.; Xiang, Y. Dietary Trace Element Intake and Liver Cancer Risk: Results from Two Population-based Cohorts in China. Int. J. Cancer 2017, 140, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, Y.; Sandsveden, M.; Borgquist, S.; Manjer, J. Serum Zinc and Dietary Intake of Zinc in Relation to Risk of Different Breast Cancer Subgroups and Serum Levels as a Marker of Intake: A Prospective Nested Case-Control Study. Breast Cancer Res. Treat. 2021, 189, 571–583. [Google Scholar] [CrossRef]
- Banim, P.J.R.; Luben, R.; McTaggart, A.; Welch, A.; Wareham, N.; Khaw, K.-T.; Hart, A.R. Dietary Antioxidants and the Aetiology of Pancreatic Cancer: A Cohort Study Using Data from Food Diaries and Biomarkers. Gut 2013, 62, 1489–1496. [Google Scholar] [CrossRef]
- Han, X.; Li, J.; Brasky, T.M.; Xun, P.; Stevens, J.; White, E.; Gammon, M.D.; He, K. Antioxidant Intake and Pancreatic Cancer Risk. Cancer 2013, 119, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, G.; Fu, W.; Lu, Y.; Wei, W.; Chen, W.; Wu, X.; Meng, H.; Feng, Y.; Liu, Y.; et al. Circulating Essential Metals and Lung Cancer: Risk Assessment and Potential Molecular Effects. Environ. Int. 2019, 127, 685–693. [Google Scholar] [CrossRef]
- Hara, A.; Sasazuki, S.; Inoue, M.; Iwasaki, M.; Shimazu, T.; Sawada, N.; Yamaji, T.; Takachi, R.; Tsugane, S. Zinc and Heme Iron Intakes and Risk of Colorectal Cancer: A Population-Based Prospective Cohort Study in Japan. Am. J. Clin. Nutr. 2012, 96, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Anderson, K.E.; Harnack, L.J.; Folsom, A.R.; Jacobs, D.R. Heme Iron, Zinc, Alcohol Consumption, and Colon Cancer: Iowa Women’s Health Study. JNCI J. Natl. Cancer Inst. 2004, 96, 403–407. [Google Scholar] [CrossRef]
- Zhang, X.; Giovannucci, E.L.; Smith-Warner, S.A.; Wu, K.; Fuchs, C.S.; Pollak, M.; Willett, W.C.; Ma, J. A Prospective Study of Intakes of Zinc and Heme Iron and Colorectal Cancer Risk in Men and Women. Cancer Causes Control 2011, 22, 1627–1637. [Google Scholar] [CrossRef]
- Hansen, R.D.; Albieri, V.; Tjønneland, A.; Overvad, K.; Andersen, K.K.; Raaschou–Nielsen, O. Effects of Smoking and Antioxidant Micronutrients on Risk of Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2013, 11, 406–415.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, M.; Mucci, L.A.; Giovannucci, E.L. Zinc Supplement Use and Risk of Aggressive Prostate Cancer: A 30-Year Follow-up Study. Eur. J. Epidemiol. 2022, 37, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Coogan, P.; Palmer, J.R.; Strom, B.L.; Rosenberg, L. Vitamin and Mineral Use and Risk of Prostate Cancer: The Case–Control Surveillance Study. Cancer Causes Control 2009, 20, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc Supplement Use and Risk of Prostate Cancer. JNCI J. Natl. Cancer Inst. 2003, 95, 1004–1007. [Google Scholar] [CrossRef]
- Gonzalez, A.; Peters, U.; Lampe, J.W.; White, E. Zinc Intake from Supplements and Diet and Prostate Cancer. Nutr. Cancer 2009, 61, 206–215. [Google Scholar] [CrossRef]
- Wang, Y.; Jafar, T.H.; Jin, A.; Yuan, J.-M.; Koh, W.-P. Dietary Intakes of Trace Elements and the Risk of Kidney Cancer: The Singapore Chinese Health Study. Nutr. Cancer 2021, 73, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.; Liu, K.; Wang, Y.; Jiang, Y.; Zhang, C. Association of Dietary Intake of Zinc and Selenium with Breast Cancer Risk: A Case-Control Study in Chinese Women. Nutrients 2023, 15, 3253. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gai, X. The Association between Dietary Zinc Intake and Risk of Pancreatic Cancer: A Meta-Analysis. Biosci. Rep. 2017, 37, BSR20170155. [Google Scholar] [CrossRef] [PubMed]
- Białkowska, K.; Marciniak, W.; Muszyńska, M.; Baszuk, P.; Gupta, S.; Jaworska-Bieniek, K.; Sukiennicki, G.; Durda, K.; Gromowski, T.; Prajzendanc, K.; et al. Association of Zinc Level and Polymorphism in MMP-7 Gene with Prostate Cancer in Polish Population. PLoS ONE 2018, 13, e0201065. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Ponvilawan, B.; Ungprasert, P. Higher Zinc Intake Is Associated with Decreased Risk of Lung Cancer. J. Evid. Based Med. 2021, 14, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Mahabir, S.; Spitz, M.R.; Barrera, S.L.; Beaver, S.H.; Etzel, C.; Forman, M.R. Dietary Zinc, Copper and Selenium, and Risk of Lung Cancer. Int. J. Cancer 2007, 120, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, J.; Chen, J.; Yan, L.; Hu, Z.; Wu, J.; Bao, X.; Lin, L.; Wang, R.; Cai, L.; et al. Serum Copper and Zinc Levels and the Risk of Oral Cancer: A New Insight Based on Large-scale Case–Control Study. Oral Dis. 2019, 25, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Cunzhi, H.; Jiexian, J.; Xianwen, Z.; Jingang, G.; Shumin, Z.; Lili, D. Serum and Tissue Levels of Six Trace Elements and Copper/Zinc Ratio in Patients with Cervical Cancer and Uterine Myoma. Biol. Trace Elem. Res. 2003, 94, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Gallus, S.; Foschi, R.; Negri, E.; Talamini, R.; Franceschi, S.; Montella, M.; Ramazzotti, V.; Tavani, A.; Dal Maso, L.; La Vecchia, C. Dietary Zinc and Prostate Cancer Risk: A Case-Control Study from Italy. Eur. Urol. 2007, 52, 1052–1057. [Google Scholar] [CrossRef]
- Zhou, W.; Park, S.; Liu, G.; Miller, D.P.; Wang, L.I.; Pothier, L.; Wain, J.C.; Lynch, T.J.; Giovannucci, E.; Christiani, D.C. Dietary Iron, Zinc, and Calcium and the Risk of Lung Cancer. Epidemiology 2005, 16, 772–779. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Li, A.; Zhang, Y. Association between Serum Zinc Levels and Lung Cancer: A Meta-Analysis of Observational Studies. World J. Surg. Oncol. 2019, 17, 78. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc Deficiency in Humans: A Neglected Problem. J. Am. Coll. Nutr. 1998, 17, 542–543. [Google Scholar] [CrossRef]
- Ho, E. Zinc Deficiency, DNA Damage and Cancer Risk. J. Nutr. Biochem. 2004, 15, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhen, J.; Leng, J.; Cai, L.; Ji, H.; Keller, B.B. Zinc as a Countermeasure for Cadmium Toxicity. Acta Pharmacol. Sin. 2021, 42, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. The Function of Zinc Metallothionein: A Link between Cellular Zinc and Redox State. J. Nutr. 2000, 130, 1455S–1458S. [Google Scholar] [CrossRef]
- Ho, E.; Ames, B.N. Low Intracellular Zinc Induces Oxidative DNA Damage, Disrupts P53, NFκB, and AP1 DNA Binding, and Affects DNA Repair in a Rat Glioma Cell Line. Proc. Natl. Acad. Sci. USA 2002, 99, 16770–16775. [Google Scholar] [CrossRef] [PubMed]
- Hollstein, M.; Sidransky, D.; Vogelstein, B.; Harris, C.C. P53 Mutations in Human Cancers. Science 1991, 253, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Roney, N.; Osier, M.; Paikoff, S.J.; Smith, C.V.; Williams, M.; De Rosa, C.T. ATSDR Evaluation of the Health Effects of Zinc and Relevance to Public Health. Toxicol. Ind. Health 2006, 22, 423–493. [Google Scholar] [CrossRef] [PubMed]
- Cousins, R.J. Absorption, Transport, and Hepatic Metabolism of Copper and Zinc: Special Reference to Metallothionein and Ceruloplasmin. Physiol. Rev. 1985, 65, 238–309. [Google Scholar] [CrossRef]
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef]
- Khan-Mayberry, N. (Ed.) Heavy Metal Toxicity; The International Open Acces Journal of Clinical Toxicology: Houston, TX, USA, 2011. [Google Scholar]
- Carey, P.; Low, E.; Harper, E.; Stack, M.S. Metalloproteinases in Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 3403. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).