Curcumin Administration Improves Force of mdx Dystrophic Diaphragm by Acting on Fiber-Type Composition, Myosin Nitrotyrosination and SERCA1 Protein Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Antibodies and Reagents
2.3. Western Blotting
2.4. Immunoperoxidase and Routine Histology Stainings
2.5. Immunofluorescence
2.6. Serum Creatine Kinase (CK) Assay
2.7. Mechanical Recordings
2.8. Oxyblot and Tropomyosin Oxidation
2.9. Cell Cultures
2.10. Statistical Analyses
3. Results
3.1. Curcumin Treatment of mdx Mice
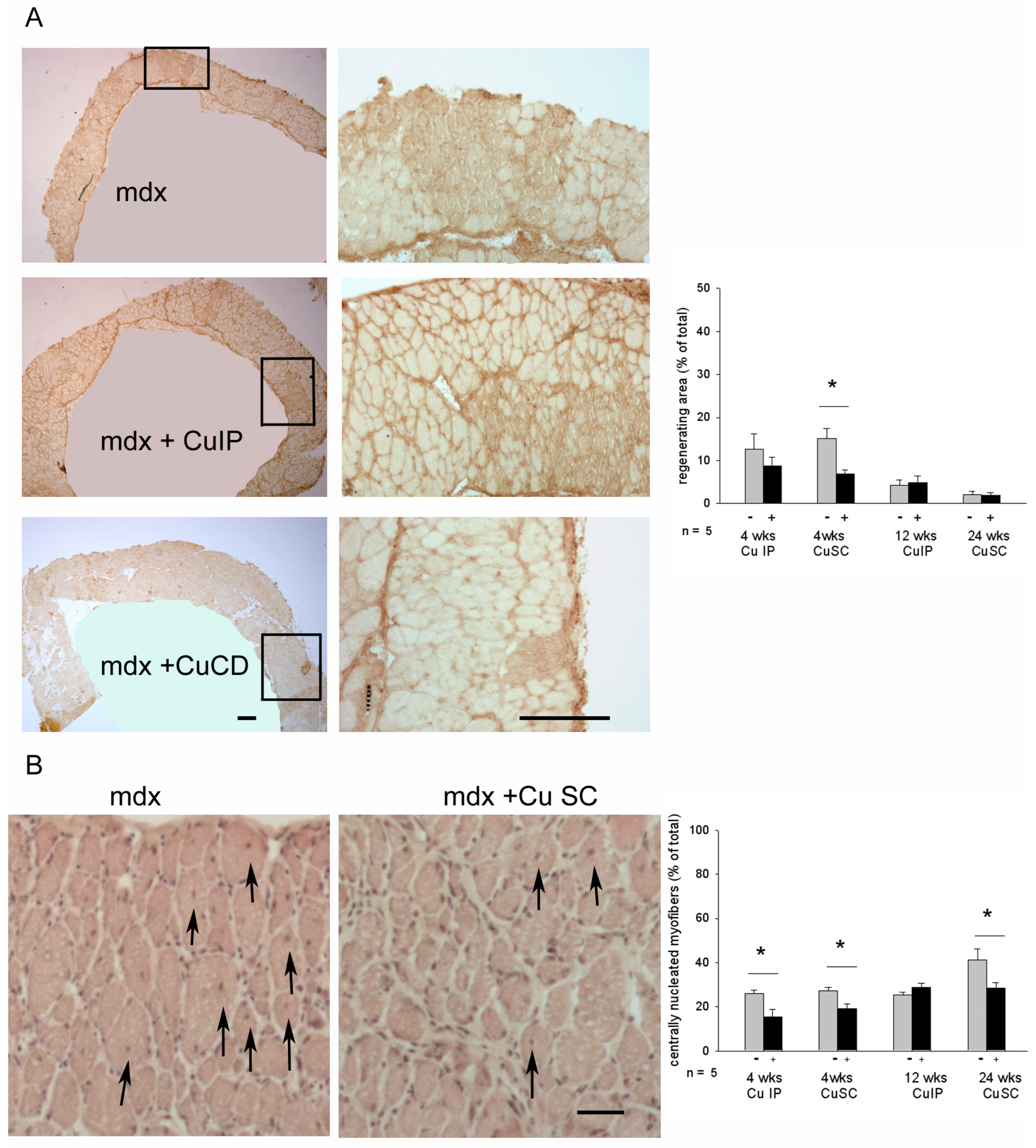
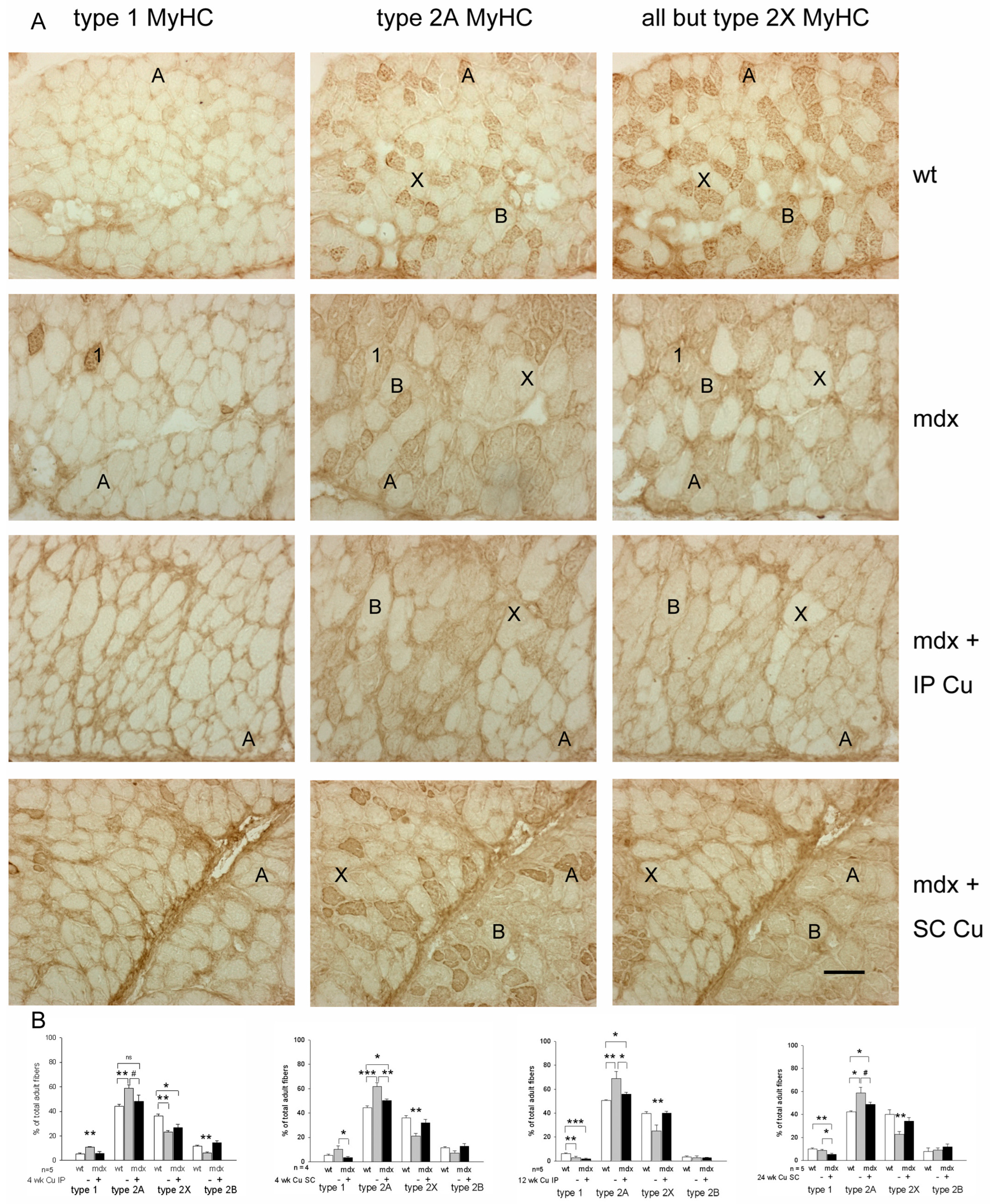
3.2. Effects of Curcumin Treatment on Diaphragm Muscle: Dystrophic Signatures and Fiber-Type Composition
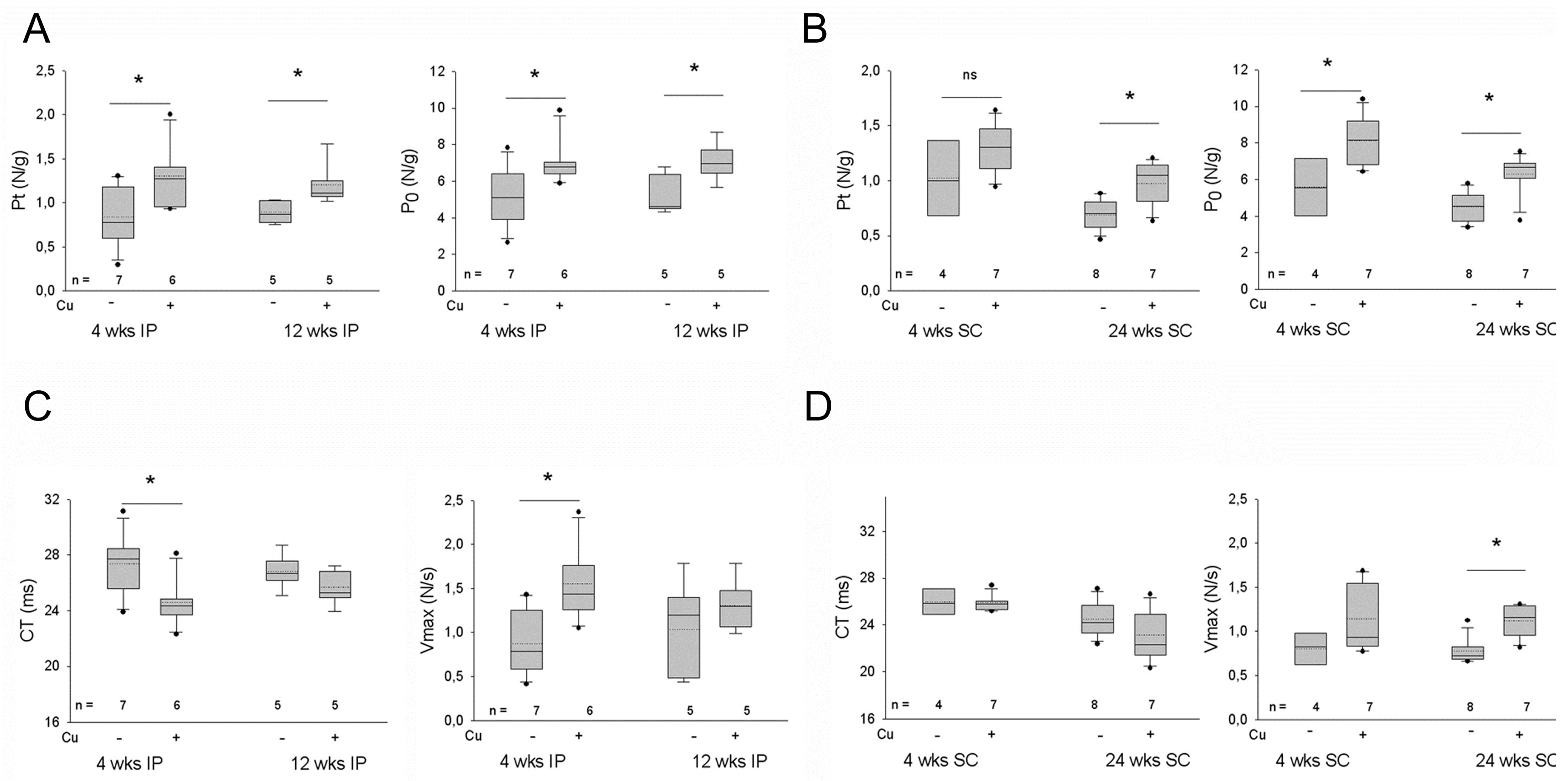
3.3. Effects of Curcumin Treatment on the Contractility of the Dystrophic Diaphragm
3.4. Curcumin Effects on Myofiber Oxidative and Nitrosative Stress
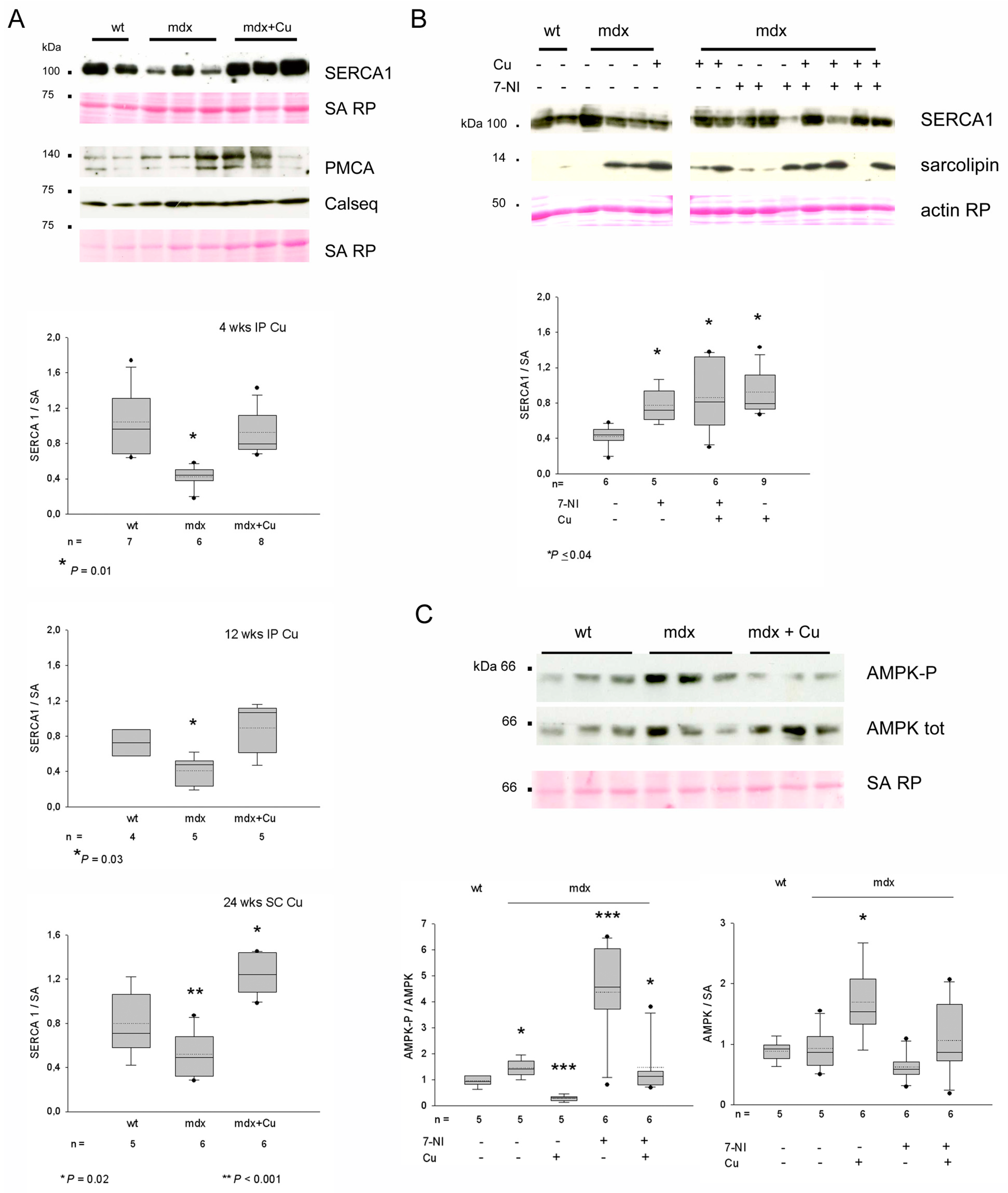
3.5. Curcumin Effects on Protein Levels of Grp94, nNOS and nNOS Regulators
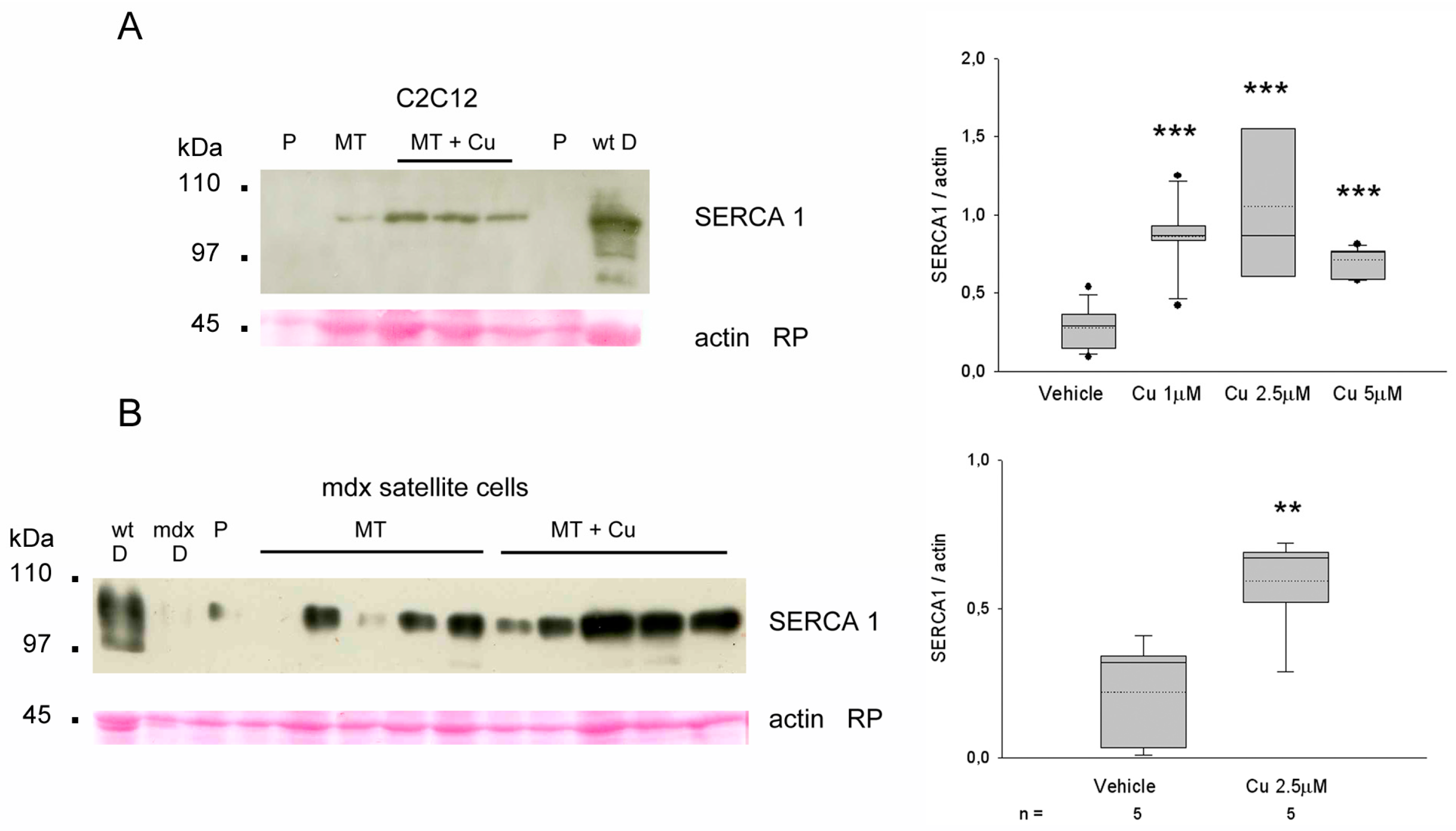
3.6. Curcumin Upregulates SERCA1 in Cultured Myotubes
4. Discussion
5. Study Limitations and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Whitehead, N.P.; Froehner, S.C. Absence of dystrophin disrupts skeletal muscle signaling: Roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol. Rev. 2016, 96, 253–305. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwak, H.B.; Thompson, L.V.; Lawler, J.M. Contribution of oxidative stress to pathology in diaphragm and limb muscles with Duchenne muscular dystrophy. J. Muscle Res. Cell Motil. 2013, 34, 1–13. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharm. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Trujillo, J.; Granados-Castro, L.F.; Zazueta, C.; Andérica-Romero, A.C.; Chirino, Y.I.; Pedraza-Chaverrí, J. Mitochondria as a target in the therapeutic properties of curcumin. Arch. Pharm. 2014, 347, 873–884. [Google Scholar] [CrossRef]
- Pietrocola, F.; Lachkar, S.; Enot, D.P.; Niso-Santano, M.; Bravo-San Pedro, J.M.; Sica, V.; Izzo, V.; Maiuri, M.C.; Madeo, F.; Mariño, G.; et al. Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ. 2015, 22, 509–516. [Google Scholar] [CrossRef]
- Franceschi, F.; Feregalli, B.; Togni, S.; Cornelli, U.; Giacomelli, L.; Eggenhoffner, R.; Belcaro, G. A novel phospholipid delivery system of curcumin (Meriva®) preserves muscular mass in healthy aging subjects. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 762–766. [Google Scholar]
- Gomes Suhett, L.; de Miranda Monteiro Santos, R.; Souza Silveira, B.K.; Gualandi Leal, A.C.; Melo de Brito, A.D.; Faries de Novaes, J.; Mattos della Lucia, C. Effects of curcumin supplementation on sport and physical exercise: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 946–958. [Google Scholar]
- Gorza, L.; Germinario, E.; Tibaudo, L.; Vitadello, M.; Tusa, C.; Guerra, I.; Bondì, M.; Salmaso, S.; Caliceti, P.; Vitiello, L.; et al. Chronic Systemic Curcumin Administration Antagonizes Murine Sarcopenia and Presarcopenia. Int. J. Mol. Sci. 2021, 22, 11789. [Google Scholar] [CrossRef] [PubMed]
- Receno, C.N.; Liang, C.; Korol, D.L.; Atalay, M.; Heffernan, K.S.; Brutsaert, T.D.; DeRuisseau, K.C. Effects of prolonged dietary curcumin exposure on skeletal muscle biochemical and functional responses of aged male rats. Int. J. Mol. Sci. 2019, 20, 1178. [Google Scholar] [CrossRef] [PubMed]
- Vitadello, M.; Germinario, E.; Ravara, B.; Dalla Libera, L.; Danieli Betto, D.; Gorza, L. Curcumin counteracts loss of force and atrophy of unloaded hindlimb rat soleus by hampering neuronal nitric oxide synthase untethering from sarcolemma. J. Physiol. 2014, 592, 2637–2652. [Google Scholar] [CrossRef]
- Lawler, J.M.; Garcia-Villatoro, E.L.; Guzzoni, V.; Hord, J.M.; Botchlett, R.; Holly, D.; Lawler, M.S.; Janini Gomes, M.; Ryan, P.; Rodriguez, D.; et al. Effect of combined fish oil & Curcumin on murine skeletal muscle morphology and stress response proteins during mechanical unloading. Nutr. Res. 2019, 65, 17–28. [Google Scholar]
- Durham, W.J.; Arbogast, S.; Gerken, E.; Li, Y.P.; Reid, M.B. Progressive nuclear factor-kappaB activation resistant to inhibition by contraction and curcumin in mdx mice. Muscle Nerve. 2006, 34, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, C.; Shen, Y.; Zhu, C.H.; Wang, G.; Wang, X.C.; Chen, H.Q.; Zhu, M.S. Curcumin alleviates dystrophic muscle pathology in mdx mice. Mol. Cells 2008, 25, 531–537. [Google Scholar]
- Vitadello, M.; Doria, A.; Tarricone, E.; Ghirardello, A.; Gorza, L. Myofiber stress-response in myositis: Parallel investigations on patients and experimental animal models of muscle regeneration and systemic inflammation. Arthritis Res. Ther. 2010, 12, R52. [Google Scholar] [CrossRef]
- Gorza, L. Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J. Histochem. Cytochem. 1990, 38, 257–265. [Google Scholar] [CrossRef]
- Lechado i Terradas, A.; Vitadello, M.; Traini, L.; Namuduri, A.V.; Gastaldello, S.; Gorza, L. Sarcolemmal loss of active nNOS (Nos1) is an oxidative stress-dependent, early event driving disuse atrophy. J. Pathol. 2018, 246, 433–446. [Google Scholar] [CrossRef]
- Coulton, G.R.; Morgan, J.E.; Partridge, G.A.; Sloper, J.C. The mdx mouse skeletal muscle myopathy: I. A histological, morphometric and biochemical investigation. Neuropathol. Appl. Neurobiol. 1988, 14, 53–70. [Google Scholar] [CrossRef]
- Pizzo, P.; Scapin, C.; Vitadello, M.; Florean, C.; Gorza, L. Grp94 acts as a mediator of curcumin-induced anti-oxidant defence in myogenic cells. J. Cell. Mol. Med. 2010, 14, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Pauly, M.; Daussin, F.; Burelle, Y.; Li, T.; Godin, R.; Fauconnier, J.; Koechlin-Ramonatxo, C.; Hugon, G.; Lacampagne, A.; Coisy-Quivy, M.; et al. AMPK activation stimulates autophagy and ameliorates muscular dystrophy in the mdx mouse diaphragm. Am. J. Pathol. 2012, 181, 583–592. [Google Scholar] [CrossRef]
- Barros Maranhão, J.; de Oliveira Moreira, D.; Maurício, A.F.; de Carvalho, S.C.; Ferretti, R.; Pereira, J.A.; Santo Neto, H.; Marques, M.J. Changes in calsequestrin, TNF-α, TGF-β and MyoD levels during the progression of skeletal muscle dystrophy in mdx mice: A comparative analysis of the quadriceps, diaphragm and intrinsic laryngeal muscles. Int. J. Exp. Pathol. 2015, 96, 285–293. [Google Scholar] [CrossRef]
- Briguet, A.; Courdier-Fruh, I.; Foster, M.; Meier, T.; Magyar, J.P. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul. Disord. 2004, 14, 675–682. [Google Scholar] [CrossRef]
- Petrof, B.J.; Stedman, H.H.; Shrager, J.B.; Eby, J.; Sweeney, H.L.; Kelly, A.M. Adaptations in myosin heavy chain expression and contractile function in dystrophic mouse diaphragm. Am. J. Physiol. 1993, 265, C834–C841. [Google Scholar] [CrossRef]
- Bates, G.; Sigurdardottir, S.; Kachmar, L.; Zitouni, N.B.; Benedetti, A.; Petrof, B.J.; Rassier, D.; Lauzon, A.M. Molecular, cellular, and muscle strip mechanics of the mdx mouse diaphragm. Am. J. Physiol. Cell Physiol. 2013, 304, C873–C880. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Mojumdar, K.; Liang, F.; Lemaire, C.; Li, T.; Richardson, J.; Divangahi, M.; Qureshi, S.; Petrof, B.J. Toll-like receptor 4 ablation in mdx mice reveals innate immunity as a therapeutic target in Duchenne muscular dystrophy. Hum. Mol. Gen. 2015, 24, 2147–2162. [Google Scholar] [CrossRef]
- Menazza, S.; Blaauw, B.; Tiepolo, T.; Toniolo, L.; Braghetta, P.; Spolaore, B.; Reggiani, C.; Di Lisa, F.; Bonaldo, P.; Canton, M. Oxidative stress by monoamine oxidases is causally involved in myofiber damage in muscular dystrophy. Hum. Mol. Gen. 2010, 19, 4207–4215. [Google Scholar] [CrossRef]
- Li, D.; Yue, Y.; Lai, Y.; Hakim, C.H.; Duan, D. Nitrosative stress elicited by nNOSµ delocalization inhibits muscle force in dystrophin-null mice. J. Pathol. 2011, 223, 88–98. [Google Scholar] [CrossRef]
- Timpani, C.A.; Trewin, A.J.; Stojanovska, V.; Robinson, A.; Goodman, C.A.; Nurgali, K.; Betik, A.C.; Stepto, N.; Hayes, A.; McConell, G.K.; et al. Attempting to Compensate for Reduced Neuronal Nitric Oxide Synthase Protein with Nitrate Supplementation Cannot Overcome Metabolic Dysfunction but Rather Has Detrimental Effects in Dystrophin-Deficient mdx Muscle. Neurotherapeutics 2017, 14, 429–446. [Google Scholar] [CrossRef]
- Hoffman, E.P.; Morgan, J.E.; Watkins, S.C.; Partridge, T.A. Somatic reversion/suppression of the mouse mdx phenotype in vivo. J. Neurol. Sci. 1990, 99, 9–25. [Google Scholar] [CrossRef]
- Suhr, F.; Gehlert, S.; Grau, M.; Bloch, W. Skeletal muscle function during exercise-fine-tuning of diverse subsystems by nitric oxide. Int. J. Mol. Sci. 2013, 14, 7109–7139. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.Y.; Turner, P.R.; Denetclaw, W.F.; Steinhardt, R.A. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science 1990, 250, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Tupling, A.R.; Bombardier, E.; Gupta, S.C.; Hussain, D.; Vigna, C.; Bloemberg, D.; Quadrilatero, J.; Trivieri, M.G.; Babu, G.J.; Backx, P.H.; et al. Enhanced Ca2+ transport and muscle relaxation in skeletal muscle from sarcolipin-null mice. Am. J. Physiol. Cell Physiol. 2011, 301, C841–C849. [Google Scholar] [CrossRef]
- Zhao, X.; Moloughney, J.G.; Zhang, S.; Komazaki, S.; Weisleder, N. Orai1 Mediates Exacerbated Ca2+ Entry in Dystrophic Skeletal Muscle. PLoS ONE 2012, 7, e49862. [Google Scholar] [CrossRef]
- Schneider, J.S.; Shanmugam, M.; Gonzalez, J.P.; Lopez, H.; Gordan, R.; Fraidenraich, D.; Babu, G.J. Increased sarcolipin expression and decreased sarco(endo)plasmic reticulum Ca2+ uptake in skeletal muscles of mouse models of Duchenne muscular dystrophy. J. Muscle Res. Cell Motil. 2013, 34, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Chambers, P.J.; Juracic, E.S.; Fajardo, V.A.; Tupling, A.R. Role of SERCA and sarcolipin in adaptive muscle remodeling. Am. J. Physiol. Cell Physiol. 2022, 322, C382–C394. [Google Scholar] [CrossRef]
- Garbincius, J.F.; Michele, D.E. Dystrophin-glycoprotein complex regulates muscle nitric oxide production through mechanoregulation of AMPK signaling. Proc. Natl. Acad. Sci. USA 2015, 112, 13663–13668. [Google Scholar] [CrossRef]
- Kellogg, D.L., 3rd; McCammon, K.M.; Hinchee-Rodriguez, K.S.; Adamo, M.L.; Roman, L.J. Neuronal nitric oxide synthase mediates insulin- and oxidative stress-induced glucose uptake in skeletal muscle myotubes. Free Radic. Biol. Med. 2017, 110, 261–269. [Google Scholar] [CrossRef]
- Lakshmanan, A.P.; Watanabe, K.; Thandavarayan, R.A.; Sari, F.R.; Meilei, H.; Soetikno, V.; Arumugam, S.; Giridharan, V.V.; Suzuki, K.; Kodama, M. Curcumin attenuates hyperglycaemia-mediated AMPK activation and oxidative stress in cerebrum of streptozotocin-induced diabetic rat. Free Radic. Res. 2011, 45, 788–795. [Google Scholar] [CrossRef]
- Kar, R.; Kellogg, D.L., 3rd; Roman, L.J. Oxidative stress induces phosphorylation of neuronal NOS in cardiomyocytes through AMP-activated protein kinase (AMPK). Biochem. Biophys. Res. Comm. 2015, 459, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Lee-Young, R.S.; Griffee, S.R.; Lynes, S.E.; Bracy, D.P.; Ayala, J.E.; McGuinness, O.P.; Wasserman, D.H. Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J. Biol. Chem. 2009, 284, 23925–23934. [Google Scholar] [CrossRef] [PubMed]
- Goonasekera, S.A.; Lam, C.K.; Millay, D.P.; Sargent, M.A.; Hajjar, R.J.; Kranias, E.G.; Molkentin, J.D. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J. Clin. Investig. 2011, 121, 1044–1052. [Google Scholar] [CrossRef]
- Mázala, D.A.; Pratt, S.; Chen, D.; Molkentin, J.D.; Lovering, R.M.; Chin, E.R. SERCA1 overexpression minimizes skeletal muscle damage in dystrophic mouse models. Am. J. Physiol. Cell Physiol. 2015, 308, C699–C709. [Google Scholar] [CrossRef]
- Kurosawa, T.; Minato, K.; Ikemoto-Uezumi, M.; Hino, J.; Tsuchida, K.; Uezumi, A. Transgenic Expression of Bmp3b in Mesenchymal Progenitors Mitigates Age-Related Muscle Mass Loss and Neuromuscular Junction Degeneration. Int. J. Mol. Sci. 2021, 22, 10246. [Google Scholar] [CrossRef] [PubMed]
- Thaloor, D.; Miller, K.J.; Gephart, J.; Mitchell, P.O.; Pavlath, G.K. Systemic administration of the NF-kappaB inhibitor curcumin stimulates muscle regeneration after traumatic injury. Am. J. Physiol. 1999, 277, C320–C329. [Google Scholar] [CrossRef]
- Hong, S.J.; Gokulrangan, G.; Schöneich, C. Proteomic analysis of age dependent nitration of rat cardiac proteins by solution isoelectric focusing coupled to nanoHPLC tandem mass spectrometry. Exp. Gerontol. 2007, 42, 639–651. [Google Scholar] [CrossRef]
- Corpeno, R.; Dworkin, B.; Cacciani, N.; Salah, H.; Bergman, H.M.; Ravara, B.; Vitadello, M.; Gorza, L.; Gustafson, A.M.; Hedström, Y.; et al. Time course analysis of mechanical ventilation-induced diaphragm contractile muscle dysfunction in the rat. J. Physiol. 2014, 592, 3859–3880. [Google Scholar] [CrossRef]
- Snook, J.H.; Li, J.; Helmke, B.P.; Guilford, W.H. Peroxynitrite inhibits myofibrillar protein function in an in vitro assay of motility. Free Radic. Biol. Med. 2008, 44, 14–23. [Google Scholar] [CrossRef]
- Altamirano, F.; López, J.R.; Henríquez, C.; Molinski, T.; Allen, P.D.; Jaimovich, E. Increased resting intracellular calcium modulates NF-κB-dependent inducible nitric-oxide synthase gene expression in dystrophic mdx skeletal myotubes. J. Biol. Chem. 2012, 287, 20876–20887. [Google Scholar] [CrossRef]
- Li, D.; Shin, J.H.; Duan, D. iNOS ablation does not improve specific force of the extensor digitorum longus muscle in dystrophin-deficient mdx4cv mice. PLoS ONE 2011, 6, e21618. [Google Scholar] [CrossRef]
- Momken, I.; Lechene, P.; Ventura-Clapier, R.; Veksler, V. Voluntary physical activity alterations in endothelial nitric oxide synthase knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H914–H920. [Google Scholar] [CrossRef]
- Gorza, L.; Sorge, M.; Seclì, L.; Brancaccio, M. Master Regulators of Muscle Atrophy: Role of Costamere Components. Cells 2021, 10, 61. [Google Scholar] [CrossRef]
- Bilmen, J.G.; Khan, S.Z.; Javed, M.H.; Michelangeli, F. Inhibition of the SERCA Ca2+ pumps by curcumin. Curcumin putatively stabilizes the interaction between the nucleotide-binding and phosphorylation domains in the absence of ATP. Eur. J. Biochem. 2001, 268, 6318–6327. [Google Scholar] [CrossRef] [PubMed]
- Summermatter, S.; Thurnheer, R.; Santos, G.; Mosca, B.; Baum, O.; Treves, S.; Hoppeler, H.; Zorzato, F.; Handschin, C. Remodelingof calcium handling in skeletal muscle through PGC-1: Impact on force, fatigability, and fiber type. Am. J. Physiol. Cell Physiol. 2012, 302, C88–C99. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Dienes, B.; Benedetti, A.; Tuluc, P.; Szentesi, P.; Sztretye, M.; Rainer, J.; Hess, M.W.; Schwarzer, C.; Obermair, G.J.; et al. Restricting calcium currents is required for correct fiber type specification in skeletal muscle. Development 2016, 143, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Smerdu, V.; Karsch-Mizrachi, I.; Campione, M.; Leinwand, L.; Schiaffino, S. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am. J. Physiol. 1994, 267, C1723–C1728. [Google Scholar] [CrossRef]
- Webster, C.; Silberstein, L.; Hays, A.P.; Blau, H.M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell 1988, 52, 503–513. [Google Scholar] [CrossRef]
- Kiviluoto, S.; Decuypere, J.P.; De Smedt, H.; Missiaen, L.; Parys, J.B.; Bultynck, G. STIM1 as a key regulator for Ca2+ homeostasis in skeletal-muscle development and function. Skelet. Muscle 2011, 1, 16. [Google Scholar] [CrossRef]
- Gayan-Ramirez, G.; Vanzeir, L.; Wuytack, F.; Decramer, M. Corticosteroids decrease mRNA levels of SERCA pumps, whereas they increase sarcolipin mRNA in the rat diaphragm. J. Physiol. 2000, 524, 387–397. [Google Scholar] [CrossRef]
- Timpani, C.A.; Mamchaoui, K.; Butler-Browne, G.; Rybalka, E. Nitric Oxide (NO) and Duchenne Muscular Dystrophy: NO Way to Go? Antioxidants 2020, 9, 1268. [Google Scholar] [CrossRef] [PubMed]



| Age + Treatment (Mice Number) | Type of Treatment | Inflammation (Area, %) | M1 Macrophages (iNOS+ CD206−, %) | M2 Macrophages (CD206+ F4/80+, %) | Fibrosis (Area, %) |
|---|---|---|---|---|---|
| 5 + 4 wks (n = 8) | IP vehicle | 6.19 ± 1.17 | 58.50 ± 0.64 | 51.33 ± 3.34 | 28.19 ± 3.54 |
| 5 + 4 wks (n = 6) | IP Cu | 7.55 ± 1.67 | 53.50 ± 0.28 | 40.00 ± 5.45 | 30.02 ± 3.72 |
| 5 + 12 wks (n = 5) | IP vehicle | 10.45 ± 1.53 | 27.50 ± 6.00 | 50.20 ± 4.49 | 41.80 ± 7.12 |
| 5 + 12 wks (n = 9) | IP Cu | 9.35 ± 0.80 | 31.25 ± 5.93 | 51.16 ± 2.77 | 40.69 ± 3.67 |
| 5 + 24 wks (n = 4) | SC vehicle | 2.37 ± 0.50 | 35.87 ± 2.47 | 42.52 ± 7.94 | 43.47 ± 1.57 |
| 5 + 24 wks (n = 6) | SC Cu | 1.97 ± 0.30 | 33.28 ± 3.45 | 30.00 ± 3.10 | 46.85 ± 1.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorza, L.; Germinario, E.; Vitadello, M.; Guerra, I.; De Majo, F.; Gasparella, F.; Caliceti, P.; Vitiello, L.; Danieli-Betto, D. Curcumin Administration Improves Force of mdx Dystrophic Diaphragm by Acting on Fiber-Type Composition, Myosin Nitrotyrosination and SERCA1 Protein Levels. Antioxidants 2023, 12, 1181. https://doi.org/10.3390/antiox12061181
Gorza L, Germinario E, Vitadello M, Guerra I, De Majo F, Gasparella F, Caliceti P, Vitiello L, Danieli-Betto D. Curcumin Administration Improves Force of mdx Dystrophic Diaphragm by Acting on Fiber-Type Composition, Myosin Nitrotyrosination and SERCA1 Protein Levels. Antioxidants. 2023; 12(6):1181. https://doi.org/10.3390/antiox12061181
Chicago/Turabian StyleGorza, Luisa, Elena Germinario, Maurizio Vitadello, Irene Guerra, Federica De Majo, Francesca Gasparella, Paolo Caliceti, Libero Vitiello, and Daniela Danieli-Betto. 2023. "Curcumin Administration Improves Force of mdx Dystrophic Diaphragm by Acting on Fiber-Type Composition, Myosin Nitrotyrosination and SERCA1 Protein Levels" Antioxidants 12, no. 6: 1181. https://doi.org/10.3390/antiox12061181
APA StyleGorza, L., Germinario, E., Vitadello, M., Guerra, I., De Majo, F., Gasparella, F., Caliceti, P., Vitiello, L., & Danieli-Betto, D. (2023). Curcumin Administration Improves Force of mdx Dystrophic Diaphragm by Acting on Fiber-Type Composition, Myosin Nitrotyrosination and SERCA1 Protein Levels. Antioxidants, 12(6), 1181. https://doi.org/10.3390/antiox12061181









