Hydrogen Sulfide Interacting with Cannabinoid 2 Receptors during Sciatic Nerve Injury-Induced Neuropathic Pain
Abstract
1. Introduction
2. Materials and Method
2.1. Animals
2.2. Neuropathic Pain Induction
2.3. Mechanical Allodynia
2.4. Thermal Hyperalgesia
2.5. Cold Allodynia
2.6. Anxiety-like Behaviors
2.7. Depressive-like Behaviors
2.8. Western Blot
2.9. Experiments
2.10. Drugs
2.11. Statistical Analyses
3. Results
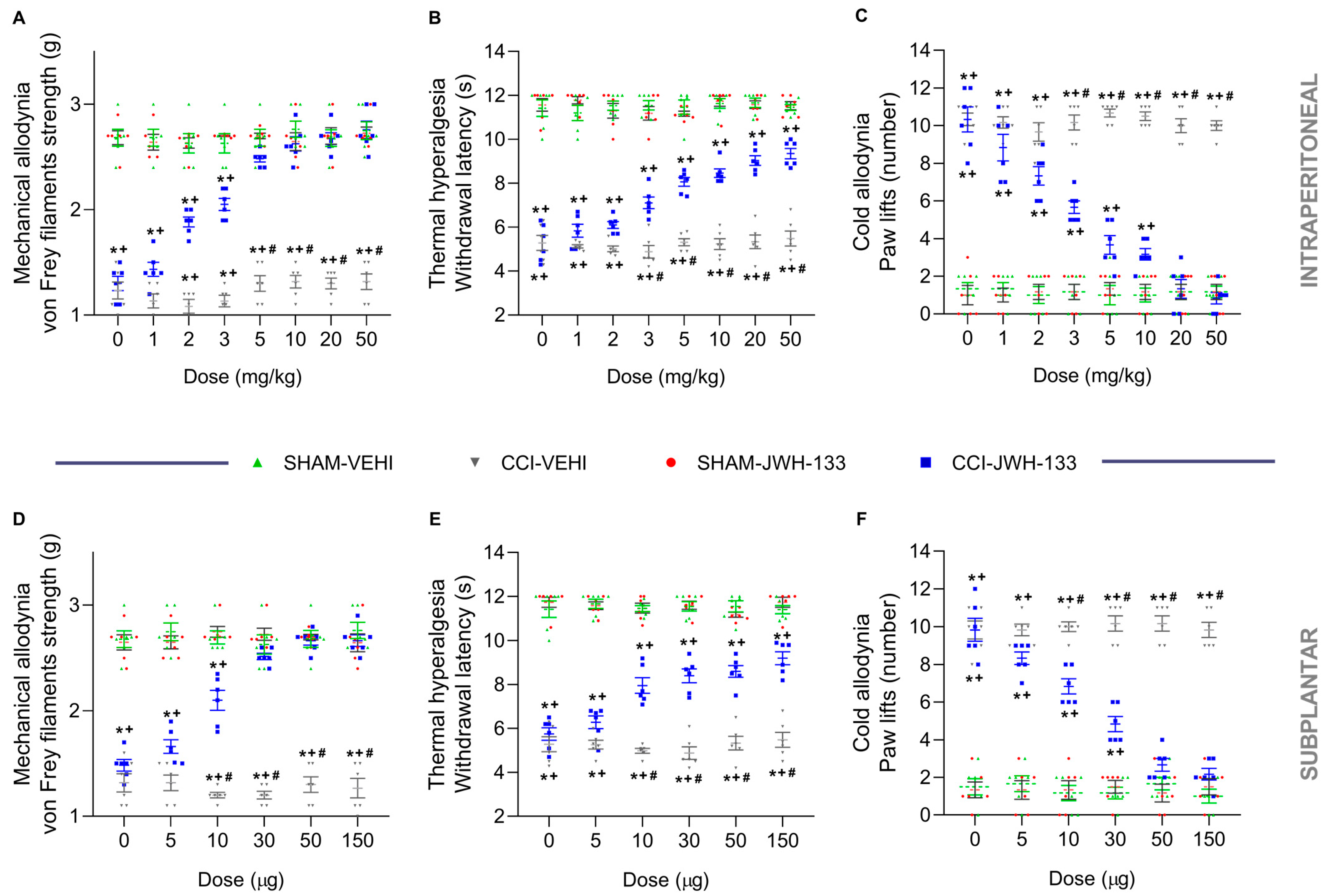
3.1. The Antinociceptive Effects of JWH-133 during Neuropathic Pain
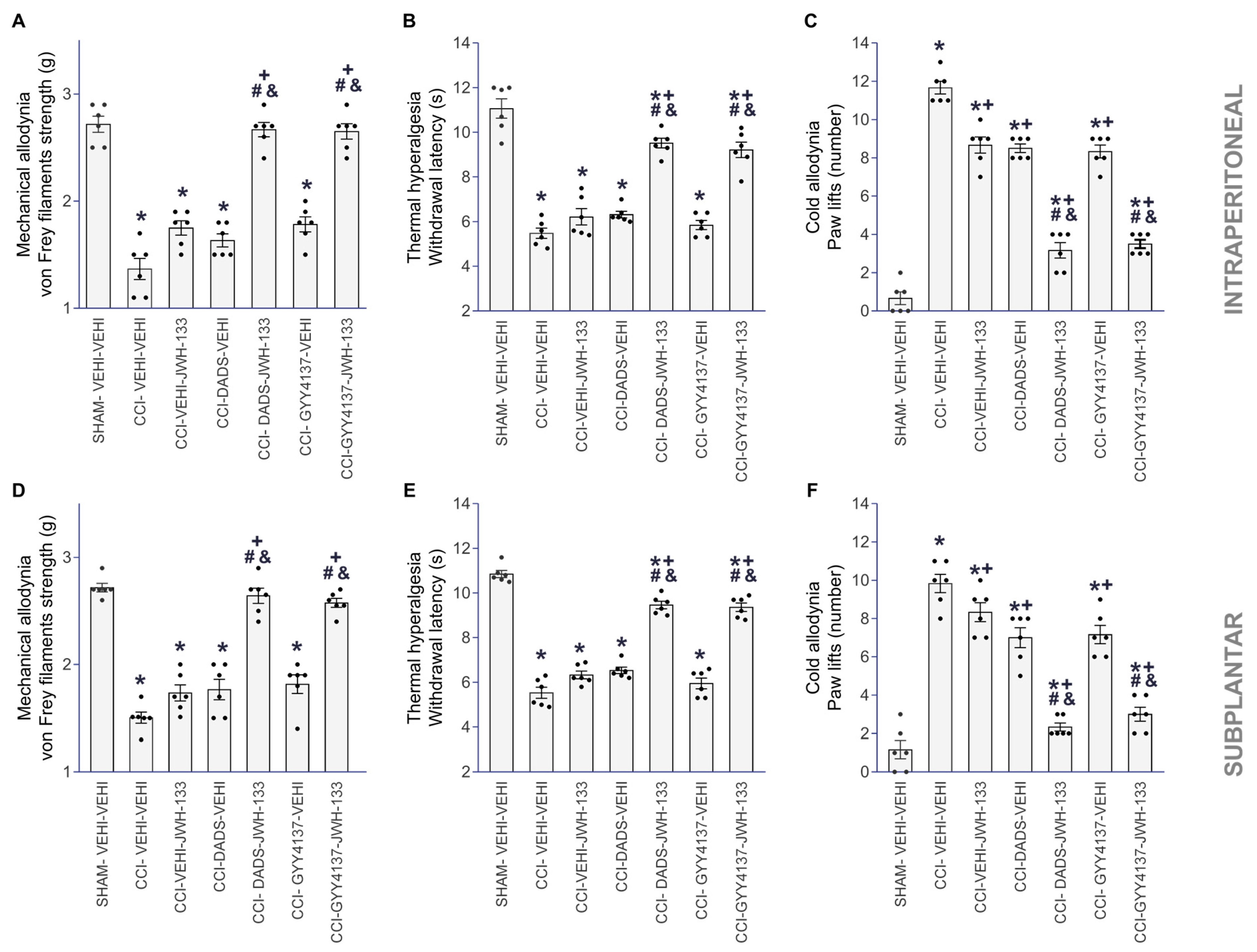
3.2. The Analgesic Effects of JWH-133 in Animals with Neuropathic Pain Co-Treated with DADS or GYY4137
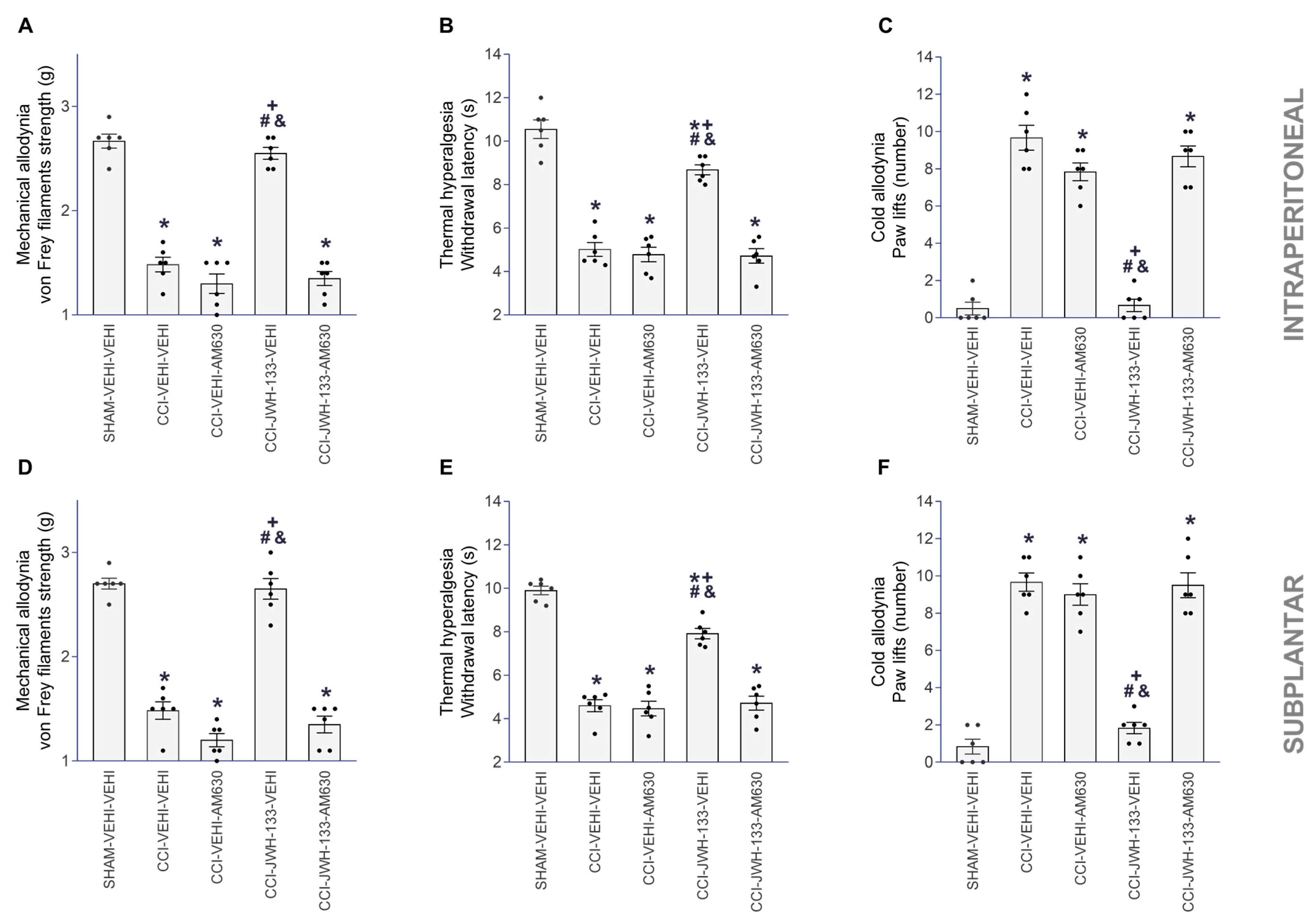
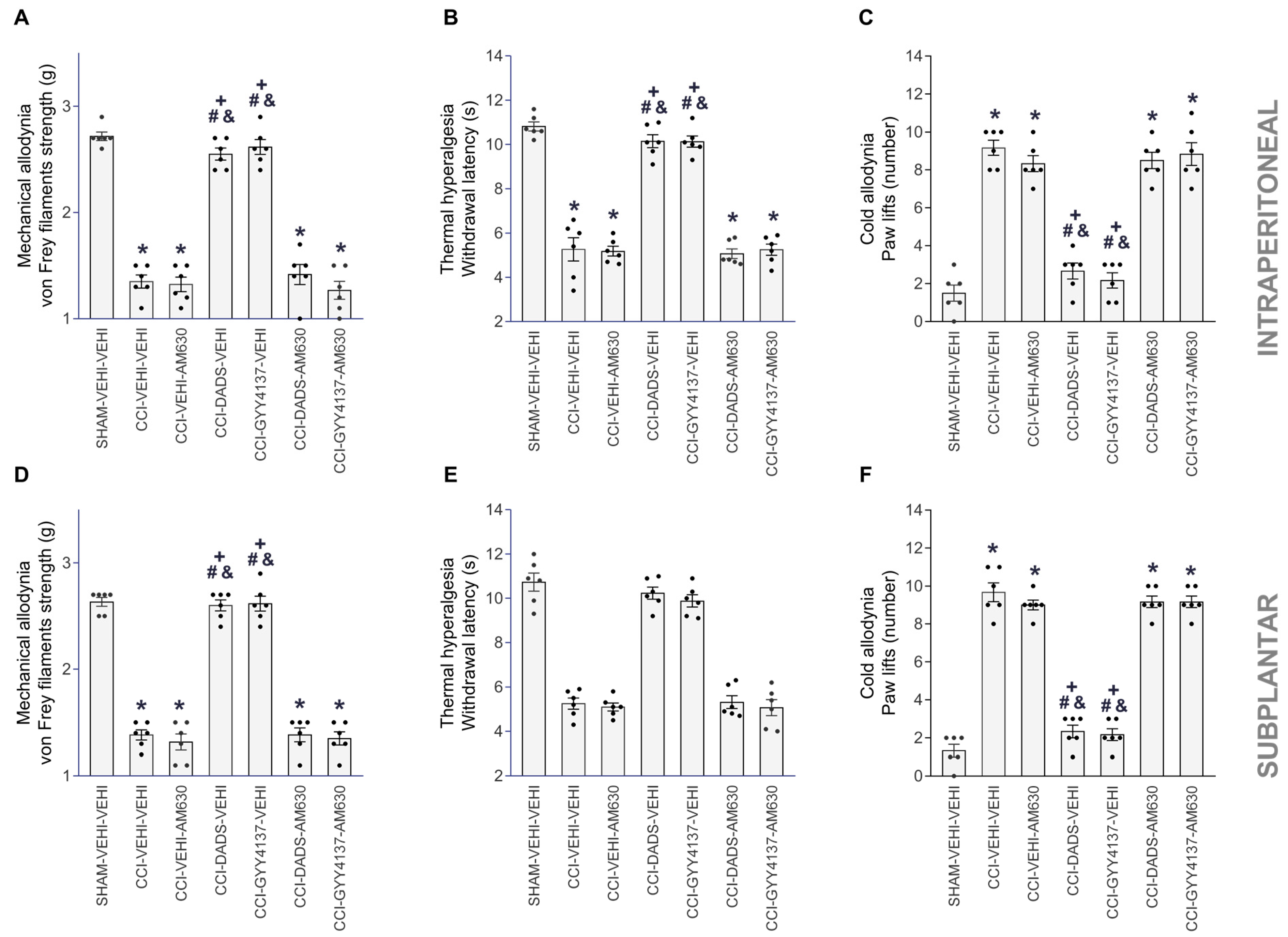
3.3. Reversion of the Antinociceptive Actions of JWH-133, DADS and GYY4137 with AM630
3.4. Effects of JWH-133 Alone or Combined with GYY4137 on the Neuropathic-Pain-Associated Anxiodepressive-liked Behaviors
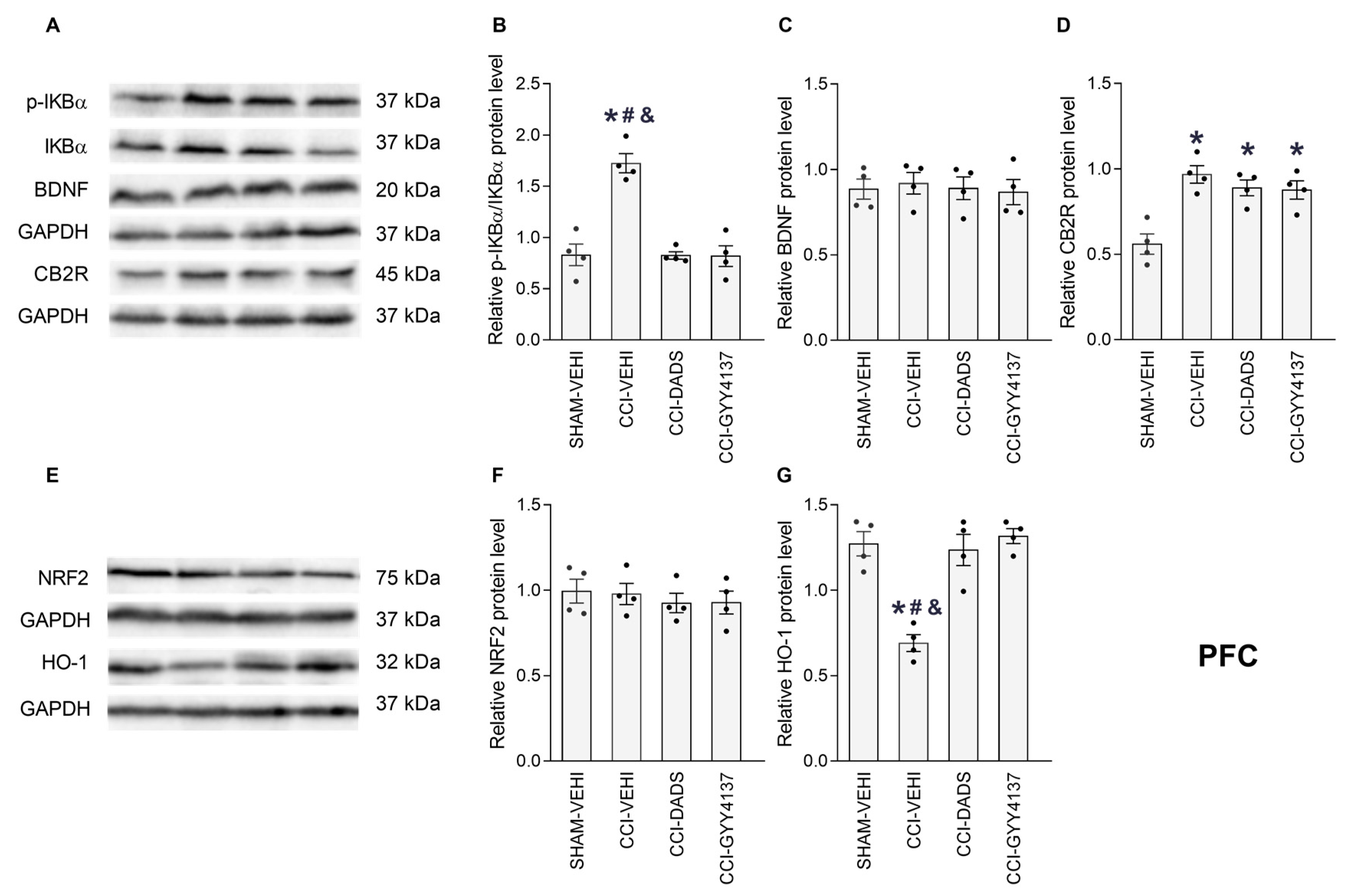
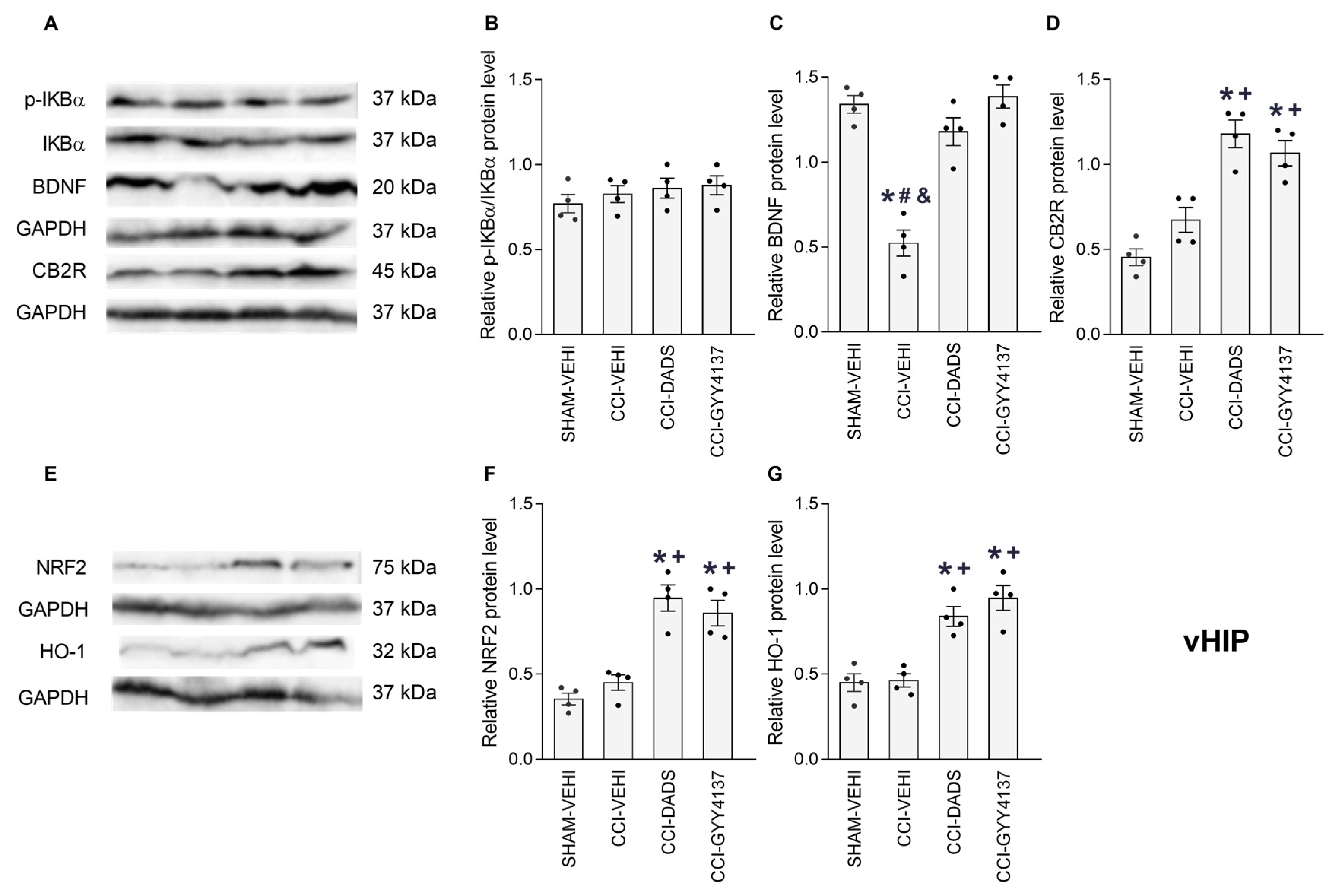
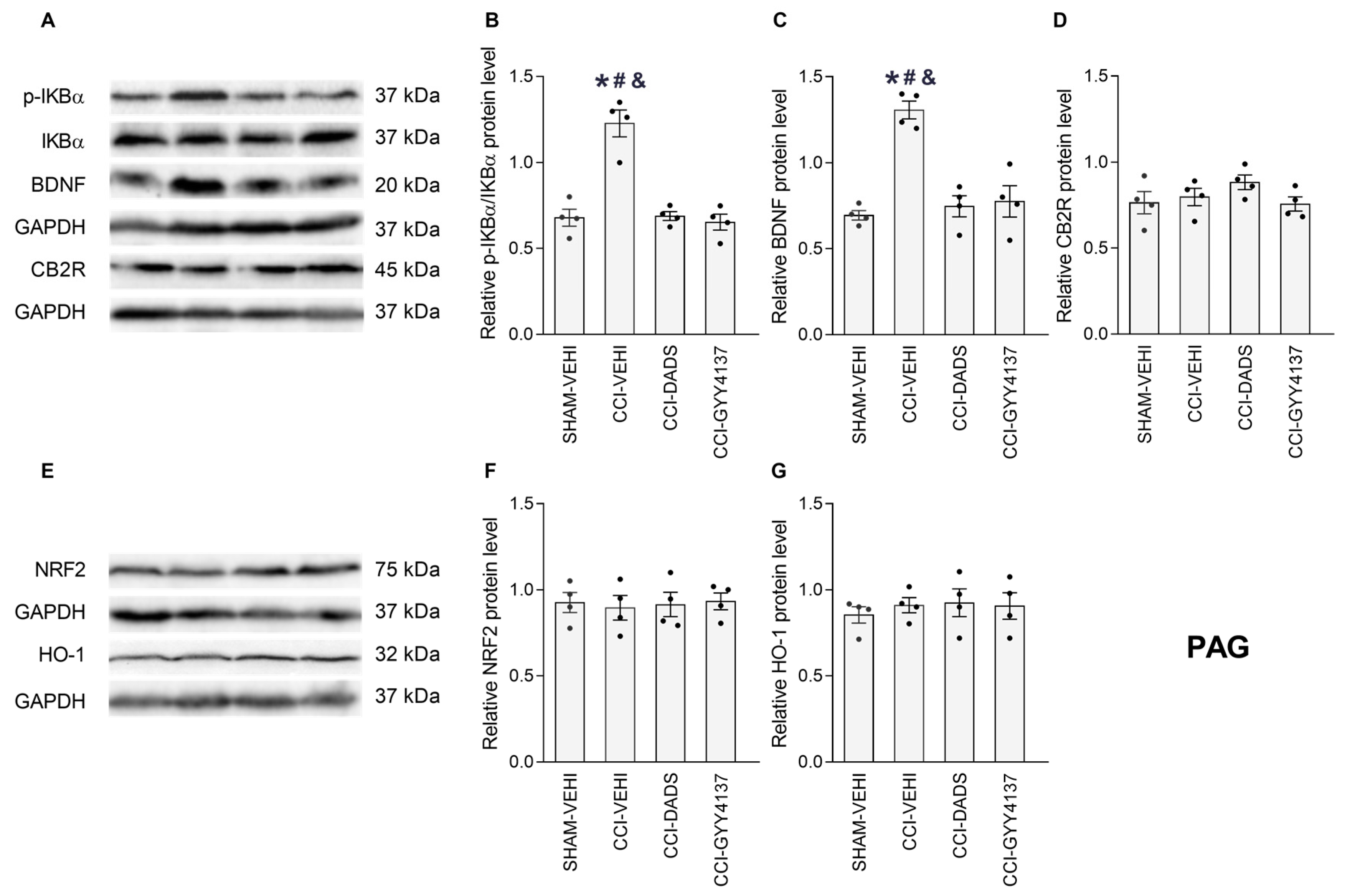
3.5. Effects of DADS and GYY4137 on the p-IKBα, BDNF, CB2R, NRF2 and HO-1 Levels in the PFC, vHIP and PAG of Mice with Neuropathic Pain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uher, T.; Bob, P. Neuropathic pain, depressive symptoms, and C-reactive protein in sciatica patients. Int. J. Neurosci. 2013, 123, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Hu, H.Y.; Xiong, Y.C.; Peng, C.; Hu, L.; Kong, Y.Z.; Wang, Y.L.; Guo, J.B.; Bi, S.; Li, T.S.; et al. Exercise for Neuropathic Pain: A Systematic Review and Expert Consensus. Front. Med. 2021, 8, 756940. [Google Scholar] [CrossRef] [PubMed]
- Reddington, M.; Baxter, S.; Walters, S.J. A qualitative exploration of patient experiences of medication for sciatica. Musculoskelet. Sci. Pract. 2021, 55, 102419. [Google Scholar] [CrossRef]
- Mathieson, S.; Maher, C.G.; McLachlan, A.J.; Latimer, J.; Koes, B.W.; Hancock, M.J.; Harris, I.; Day, R.O.; Billot, L.; Pik, J.; et al. Trial of Pregabalin for Acute and Chronic Sciatica. N. Engl. J. Med. 2017, 376, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.; Marshman, L.A.G.; Plummer, D.; Downs, E. Effect of Gabapentin vs Pregabalin on Pain Intensity in Adults With Chronic Sciatica: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 28–34. [Google Scholar] [CrossRef]
- Hashiesh, H.M.; Jha, N.K.; Sharma, C.; Gupta, P.K.; Jha, S.K.; Patil, C.R.; Goyal, S.N.; Ojha, S.K. Pharmacological potential of JWH133, a cannabinoid type 2 receptor agonist in neurodegenerative, neurodevelopmental and neuropsychiatric diseases. Eur. J. Pharmacol. 2021, 909, 174398. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Mackie, K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb. Exp. Pharmacol. 2005, 168, 299–325. [Google Scholar]
- Beltramo, M.; Bernardini, N.; Bertorelli, R.; Campanella, M.; Nicolussi, E.; Fredduzzi, S.; Reggiani, A. CB2 receptor-mediated antihyperalgesia: Possible direct involvement of neural mechanisms. Eur. J. Neurosci. 2006, 23, 1530–1538. [Google Scholar] [CrossRef]
- Atwood, B.K.; Mackie, K. CB2: A cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 2010, 160, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Valenzano, K.J.; Tafesse, L.; Lee, G.; Harrison, J.E.; Boulet, J.M.; Gottshall, S.L.; Mark, L.; Pearson, M.S.; Miller, W.; Shan, S.; et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology 2005, 48, 658–672. [Google Scholar] [CrossRef] [PubMed]
- La Porta, C.; Bura, S.A.; Llorente-Onaindia, J.; Pastor, A.; Navarrete, F.; García-Gutiérrez, M.S.; De la Torre, R.; Manzanares, J.; Monfort, J.; Maldonado, R. Role of the endocannabinoid system in the emotional manifestations of osteoarthritis pain. Pain 2015, 156, 2001–2012. [Google Scholar] [CrossRef]
- Cabañero, D.; Ramírez-López, A.; Drews, E. Protective role of neuronal and lymphoid cannabinoid CB2 receptors in neuropathic pain. elife 2020, 9, e55582. [Google Scholar] [CrossRef] [PubMed]
- Viscomi, M.T.; Oddi, S.; Latini, L.; Pasquariello, N.; Florenzano, F.; Bernardi, G.; Molinari, M.; Maccarrone, M. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J. Neurosci. 2009, 29, 4564–4570. [Google Scholar] [CrossRef]
- Jiang, F.; Xia, M.; Zhang, Y.; Chang, J.; Cao, J.; Zhang, Z.; Qian, Z.; Yang, L. Cannabinoid receptor-2 attenuates neuroinflammation by promoting autophagy-mediated degradation of the NLRP3 inflammasome post spinal cord injury. Front. Immunol. 2022, 13, 993168. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; García-Bueno, B.; Zoppi, S.; Leza, J.C.; Manzanares, J. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABA(A) receptors. Br. J. Pharmacol. 2012, 165, 951–964. [Google Scholar] [CrossRef]
- Kibret, B.G.; Ishiguro, H.; Horiuchi, Y.; Onaivi, E.S. New Insights and Potential Therapeutic Targeting of CB2 Cannabinoid Receptors in CNS Disorders. Int. J. Mol. Sci. 2022, 23, 975. [Google Scholar] [CrossRef]
- Aguilar-Ávila, D.S.; Flores-Soto, M.E.; Tapia-Vázquez, C.; Pastor-Zarandona, O.A.; López-Roa, R.I.; Viveros-Paredes, J.M. β-Caryophyllene, a Natural Sesquiterpene, Attenuates Neuropathic Pain and Depressive-Like Behavior in Experimental Diabetic Mice. J. Med. Food 2019, 22, 460–468. [Google Scholar] [CrossRef]
- Wang, G.; Yang, Y.; Wang, C.; Huang, J.; Wang, X.; Liu, Y.; Wang, H. Exploring the role and mechanisms of diallyl trisulfide and diallyl disulfide in chronic constriction-induced neuropathic pain in rats. Korean J. Pain 2020, 33, 216–225. [Google Scholar] [CrossRef]
- Batallé, G.; Bai, X.; Pouso-Vázquez, E.; Roch, G.; Rodríguez, L.; Pol, O. The Recovery of Cognitive and Affective Deficiencies Linked with Chronic Osteoarthritis Pain and Implicated Pathways by Slow-Releasing Hydrogen Sulfide Treatment. Antioxidants 2021, 10, 1632. [Google Scholar] [CrossRef] [PubMed]
- Qabazard, B.; Masocha, W.; Khajah, M.; Phillips, O.A. H2S donor GYY4137 ameliorates paclitaxel-induced neuropathic pain in mice. Biomed. Pharmacother. 2020, 127, 110210. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Batallé, G.; Balboni, G.; Pol, O. Hydrogen Sulfide Increases the Analgesic Effects of µ- and δ-Opioid Receptors during Neuropathic Pain: Pathways Implicated. Antioxidants 2022, 11, 1321. [Google Scholar] [CrossRef] [PubMed]
- Batallé, G.; Bai, X.; Pol, O. The Interaction between Carbon Monoxide and Hydrogen Sulfide during Chronic Joint Pain in Young Female Mice. Antioxidants 2022, 11, 1271. [Google Scholar] [CrossRef]
- Visavadiya, N.P.; Patel, S.P.; VanRooyen, J.L.; Sullivan, P.G.; Rabchevsky, A.G. Cellular and subcellular oxidative stress parameters following severe spinal cord injury. Redox Biol. 2016, 8, 59–67. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Hartung, J.; Eskew, O.; Wong, T.; Tchivileva, I.E.; Oladosu, F.A.; O’Buckley, S.C.; Nackley, A.G. Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation. Brain Behav. Immun. 2015, 50, 196–202. [Google Scholar] [CrossRef]
- Zhai, G.; Liang, W.; Xu, Y. High Expression of Lysophosphatidic Acid Induces Nerve Injury in LSS Patients via AKT Mediated NF-κB p65 Pathway. Front. Pharmacol. 2021, 12, 641435. [Google Scholar] [CrossRef]
- Mokhtari, T.; Yue, L.P.; Hu, L. Exogenous melatonin alleviates neuropathic pain-induced affective disorders by suppressing NF-κB/ NLRP3 pathway and apoptosis. Sci. Rep. 2023, 13, 2111. [Google Scholar] [CrossRef]
- Jiang, X.; Yan, Q.; Lao, W.; Lin, Q.; Cao, H.; Chen, L.; Chen, J.; Yu, X.; Liu, F. Irisin attenuates ethanol-induced behavioral deficits in mice through activation of Nrf2 and inhibition of NF-κB pathways. Metab. Brain Dis. 2023, 38, 1643–1656. [Google Scholar] [CrossRef]
- Huang, L.; Jin, J.; Chen, K.; You, S.; Zhang, H.; Sideris, A.; Norcini, M.; Recio-Pinto, E.; Wang, J.; Gan, W.B.; et al. BDNF produced by cerebral microglia promotes cortical plasticity and pain hypersensitivity after peripheral nerve injury. PLoS Biol. 2021, 19, e3001337. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.D.; Ghelardini, C.; Galeotti, N. HuD-mediated distinct BDNF regulatory pathways promote regeneration after nerve injury. Brain Res. 2017, 1659, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Noguchi, K. BDNF in sensory neurons and chronic pain. Neurosci. Res. 2006, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Giardini, A.C.; Dos Santos, F.M.; da Silva, J.T.; de Oliveira, M.E.; Martins, D.O.; Chacur, M. Neural Mobilization Treatment Decreases Glial Cells and Brain-Derived Neurotrophic Factor Expression in the Central Nervous System in Rats with Neuropathic Pain Induced by CCI in Rats. Pain Res. Manag. 2017, 2017, 7429761. [Google Scholar] [CrossRef]
- Fang, X.; Yang, C.; Li, S.; Zhan, G.; Zhang, J.; Huang, N.; Du, X.; Xu, H.; Hashimoto, K.; Luo, A. Brain-derived neurotrophic factor-TrkB signaling in the medial prefrontal cortex plays a role in the anhedonia-like phenotype after spared nerve injury. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 195–205. [Google Scholar] [CrossRef]
- Klein, J.; Winter, C.; Coquery, N.; Heinz, A.; Morgenstern, R.; Kupsch, A.; Juckel, G. Lesion of the medial prefrontal cortex and the subthalamic nucleus selectively affect depression-like behavior in rats. Behav. Brain Res. 2010, 213, 73–81. [Google Scholar] [CrossRef]
- Jaggi, A.S.; Singh, N. Role of different brain areas in peripheral nerve injury-induced neuropathic pain. Brain Res. 2011, 1381, 187–201. [Google Scholar] [CrossRef]
- Parfitt, G.M.; Nguyen, R.; Bang, J.Y.; Aqrabawi, A.J.; Tran, M.M.; Seo, D.K.; Richards, B.A.; Kim, J.C. Bidirectional Control of Anxiety-Related Behaviors in Mice: Role of Inputs Arising from the Ventral Hippocampus to the Lateral Septum and Medial Prefrontal Cortex. Neuropsychopharmacology 2017, 42, 1715–1728. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Liang, S.H.; Ge, J.; Lu, Y.C.; Li, J.N.; Chen, Y.B.; Luo, D.S.; Li, H.; Li, Y.Q. Involvement of the Ventrolateral Periaqueductal Gray Matter-Central Medial Thalamic Nucleus-Basolateral Amygdala Pathway in Neuropathic Pain Regulation of Rats. Front. Neuroanat. 2020, 14, 32. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B. Paxinos and Franklin’s, the Mouse Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Jaszczyk, A.; Stankiewicz, A.M.; Juszczak, G.R. Dissection of Mouse Hippocampus with Its Dorsal, Intermediate and Ventral Subdivisions Combined with Molecular Validation. Brain Sci. 2022, 12, 799. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L.; Llorca-Torralba, M.; Suárez-Pereira, I.; Berrocoso, E. Pain in neuropsychiatry: Insights from animal models. Neurosci. Biobehav. Rev. 2020, 115, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Kruk-Slomka, M.; Boguszewska-Czubara, A.; Slomka, T.; Budzynska, B.; Biala, G. Correlations between the Memory-Related Behavior and the Level of Oxidative Stress Biomarkers in the Mice Brain, Provoked by an Acute Administration of CB Receptor Ligands. Neural. Plast. 2016, 2016, 9815092. [Google Scholar] [CrossRef]
- Gobira, P.H.; Oliveira, A.C.; Gomes, J.S.; da Silveira, V.T.; Asth, L.; Bastos, J.R.; Batista, E.M.; Issy, A.C.; Okine, B.N.; de Oliveira, A.C.; et al. Opposing roles of CB1 and CB2 cannabinoid receptors in the stimulant and rewarding effects of cocaine. Br. J. Pharmacol. 2019, 176, 1541–1551. [Google Scholar] [CrossRef]
- Pol, O. The role of carbon monoxide, heme oxygenase 1, and the Nrf2 transcription factor in the modulation of chronic pain and their interactions with opioids and cannabinoids. Med. Res. Rev. 2021, 41, 136–155. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, L.; Borges Filho, C.; Cattelan Souza, L.; Savegnago, L.; Alves, D.; Henrique Schneider, P.; de Salles, H.D.; Jesse, C.R. Effects of Se-phenyl thiazolidine-4-carboselenoate on mechanical and thermal hyperalgesia in brachial plexus avulsion in mice: Mediation by cannabinoid CB1 and CB2 receptors. Brain Res. 2012, 1475, 31–36. [Google Scholar] [CrossRef]
- Vera, G.; Cabezos, P.A.; Martín, M.I.; Abalo, R. Characterization of cannabinoid-induced relief of neuropathic pain in a rat model of cisplatin-induced neuropathy. Pharmacol. Biochem. Behav. 2013, 105, 205–212. [Google Scholar] [CrossRef]
- Racz, I.; Nadal, X.; Alferink, J.; Baños, J.E.; Rehnelt, J.; Martín, M.; Pintado, B.; Gutierrez-Adan, A.; Sanguino, E.; Bellora, N.; et al. Interferon-gamma is a critical modulator of CB(2) cannabinoid receptor signaling during neuropathic pain. J. Neurosci. 2008, 28, 12136–12145. [Google Scholar] [CrossRef]
- Racz, I.; Nadal, X.; Alferink, J.; Baños, J.E.; Rehnelt, J.; Martín, M.; Pintado, B.; Gutierrez-Adan, A.; Sanguino, E.; Manzanares, J.; et al. Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain. J. Neurosci. 2008, 28, 12125–12135. [Google Scholar] [CrossRef]
- Hu, B.; Doods, H.; Treede, R.D.; Ceci, A. Depression-like behaviour in rats with mononeuropathy is reduced by the CB2-selective agonist GW405833. Pain 2009, 143, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.; Bennett, D.L. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, C.; Gong, Q.; Chai, Y.; Chen, Y.; Song, C.; Wu, Y.; Wang, L. Alterations of endogenous pain-modulatory system of the cerebral cortex in the neuropathic pain. iScience 2023, 26, 106668. [Google Scholar] [CrossRef]
- Costa, S.K.P.F.; Muscara, M.N.; Allain, T.; Dallazen, J.; Gonzaga, L.; Buret, A.G.; Vaughan, D.J.; Fowler, C.J.; de Nucci, G.; Wallace, J.L. Enhanced Analgesic Effects and Gastrointestinal Safety of a Novel, Hydrogen Sulfide-Releasing Anti-Inflammatory Drug (ATB-352): A Role for Endogenous Cannabinoids. Antioxid. Redox Signal. 2020, 33, 1003–1009. [Google Scholar] [CrossRef]
- Kinsey, S.G.; Long, J.Z.; O'Neal, S.T.; Abdullah, R.A.; Poklis, J.L.; Boger, D.L.; Cravatt, B.F.; Lichtman, A.H. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J. Pharmacol. Exp. Ther. 2009, 330, 902–910. [Google Scholar] [CrossRef]
- Ignatowska-Jankowska, B.; Wilkerson, J.L.; Mustafa, M.; Abdullah, R.; Niphakis, M.; Wiley, J.L.; Cravatt, B.F.; Lichtman, A.H. Selective monoacylglycerol lipase inhibitors: Antinociceptive versus cannabimimetic effects in mice. J. Pharmacol. Exp. Ther. 2015, 353, 424–432. [Google Scholar] [CrossRef]
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron 2010, 66, 198–204. [Google Scholar] [CrossRef]
- Merighi, A.; Salio, C.; Ghirri, A.; Lossi, L.; Ferrini, F.; Betelli, C.; Bardoni, R. BDNF as a pain modulator. Prog. Neurobiol. 2008, 85, 297–317. [Google Scholar] [CrossRef]
- Dou, S.H.; Cui, Y.; Huang, S.M.; Zhang, B. The Role of Brain-Derived Neurotrophic Factor Signaling in Central Nervous System Disease Pathogenesis. Front. Hum. Neurosci. 2022, 16, 924155. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Robbins, M.T.; Wei, F.; Zou, S.; Dubner, R.; Ren, K. Supraspinal brain-derived neurotrophic factor signaling: A novel mechanism for descending pain facilitation. J. Neurosci. 2006, 26, 126–137. [Google Scholar] [CrossRef]
- Growth, R.; Aanonsen, L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain 2002, 100, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhong, X.L.; Zhou, F.H.; Li, J.Y.; Zhou, P.; Xu, J.M.; Song, B.; Li, C.Q.; Zhou, X.F.; Dai, R.P. Peripheral Brain Derived Neurotrophic Factor Precursor Regulates Pain as an Inflammatory Mediator. Sci. Rep. 2016, 6, 27171. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Y.; Luo, S.Y.; Al-Hawwas, M.; Herselman, M.F.; Zhou, X.F.; Bobrovskaya, L. The Long-Term Effects of Ethanol and Corticosterone on the Mood-Related Behaviours and the Balance Between Mature BDNF and proBDNF in Mice. J. Mol. Neurosci. 2019, 69, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Liu, L.; Wei, J.L.; Hu, Z.L.; Li, L.; Wang, S.; Xu, J.M.; Zhou, X.F.; Li, C.Q.; Yang, Z.Y.; et al. Brain-Derived Neurotrophic Factor Precursor in the Hippocampus Regulates Both Depressive and Anxiety-Like Behaviors in Rats. Front. Psychiatry 2019, 9, 776. [Google Scholar] [CrossRef]
- Wang, X.L.; Wei, X.; Yuan, J.J.; Mao, Y.Y.; Wang, Z.Y.; Xing, N.; Gu, H.W.; Lin, C.H.; Wang, W.T.; Zhang, W.; et al. Downregulation of Fat Mass and Obesity-Related Protein in the Anterior Cingulate Cortex Participates in Anxiety- and Depression-Like Behaviors Induced by Neuropathic Pain. Front. Cell Neurosci. 2022, 16, 884296. [Google Scholar] [CrossRef]
- Bai, X.; Batallé, G.; Pol, O. The Anxiolytic and Antidepressant Effects of Diallyl Disulfide and GYY4137 in Animals with Chronic Neuropathic Pain. Antioxidants 2021, 10, 1074. [Google Scholar] [CrossRef]
- Zhang, J.; Hoffert, C.; Vu, H.K.; Groblewski, T.; Ahmad, S.; O'Donnell, D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur. J. Neurosci. 2003, 17, 2750–2754. [Google Scholar] [CrossRef]
- Carrasco, C.; Naziroǧlu, M.; Rodríguez, A.B.; Pariente, J.A. Neuropathic Pain: Delving into the Oxidative Origin and the Possible Implication of Transient Receptor Potential Channels. Front. Physiol. 2018, 9, 95. [Google Scholar] [CrossRef]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Gliozzi, M.; Malafoglia, V.; Palma, E.; Tafani, M.; Russo, M.A.; Tomino, C.; Fini, M.; et al. Natural Antioxidant Control of Neuropathic Pain-Exploring the Role of Mitochondrial SIRT3 Pathway. Antioxidants 2020, 9, 1103. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, M.R.; Eftekhari, P.; Abbaszadeh, S.; Noubarani, M.; Shafaghi, B.; Pourahmad, J. Inhibition of Different Pain Pathways Attenuates Oxidative Stress in Glial Cells: A Mechanistic View on Neuroprotective Effects of Different Types of Analgesics. Iran. J. Pharm. Res. 2021, 20, 204–215. [Google Scholar] [PubMed]
- Pane, K.; Boccella, S.; Guida, F.; Franzese, M.; Maione, S.; Salvatore, M. Role of gut microbiota in neuropathy and neuropathic pain states: A systematic preclinical review. Neurobiol. Dis. 2022, 170, 105773. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Bai, F.; Yu, Y. Spinal cord injury and gut microbiota: A review. Life Sci. 2021, 266, 118865. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, C.; Ren, Y.N.; Ye, Z.J.; Jiang, C.; Wu, Z.B. Alterations in the gut microbiota and metabolite profiles in the context of neuropathic pain. Mol. Brain 2021, 14, 50. [Google Scholar] [CrossRef]
- Ding, W.; You, Z.; Chen, Q.; Yang, L.; Doheny, J.; Zhou, X.; Li, N.; Wang, S.; Hu, K.; Chen, L.; et al. Gut Microbiota Influences Neuropathic Pain Through Modulating Proinflammatory and Anti-inflammatory T Cells. Anesth. Analg. 2021, 132, 1146–1155. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O'Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Yang, C.; Fang, X.; Zhan, G.; Huang, N.; Li, S.; Bi, J.; Jiang, R.; Yang, L.; Miao, L.; Zhu, B.; et al. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl. Psychiatry 2019, 9, 57. [Google Scholar] [CrossRef]
- Huang, L.; Lv, X.; Ze, X.; Ma, Z.; Zhang, X.; He, R.; Fan, J.; Zhang, M.; Sun, B.; Wang, F.; et al. Combined probiotics attenuate chronic unpredictable mild stress-induced depressive-like and anxiety-like behaviors in rats. Front. Psychiatry 2022, 13, 990465. [Google Scholar] [CrossRef]
- Ng, Q.X.; Peters, C.; Ho, C.Y.X.; Lim, D.Y.; Yeo, W.S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 2018, 228, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Motta, J.P.; Flannigan, K.L.; Agbor, T.A.; Beatty, J.K.; Blackler, R.W.; Workentine, M.L.; Da Silva, G.J.; Wang, R.; Buret, A.G.; Wallace, J.L. Hydrogen sulfide protects from colitis and restores intestinal microbiota biofilm and mucus production. Inflamm. Bowel Dis. 2015, 21, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, G.; Siopi, E.; Guenin-Macé, L.; Pascal, M.; Laval, T.; Rifflet, A.; Boneca, I.G.; Demangel, C.; Colsch, B.; Pruvost, A.; et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat. Commun. 2020, 11, 6363. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Motta, J.P.; Buret, A.G. Hydrogen sulfide: An agent of stability at the microbiome-mucosa interface. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G143–G149. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Batallé, G.; Martínez-Martel, I.; Pol, O. Hydrogen Sulfide Interacting with Cannabinoid 2 Receptors during Sciatic Nerve Injury-Induced Neuropathic Pain. Antioxidants 2023, 12, 1179. https://doi.org/10.3390/antiox12061179
Bai X, Batallé G, Martínez-Martel I, Pol O. Hydrogen Sulfide Interacting with Cannabinoid 2 Receptors during Sciatic Nerve Injury-Induced Neuropathic Pain. Antioxidants. 2023; 12(6):1179. https://doi.org/10.3390/antiox12061179
Chicago/Turabian StyleBai, Xue, Gerard Batallé, Ignacio Martínez-Martel, and Olga Pol. 2023. "Hydrogen Sulfide Interacting with Cannabinoid 2 Receptors during Sciatic Nerve Injury-Induced Neuropathic Pain" Antioxidants 12, no. 6: 1179. https://doi.org/10.3390/antiox12061179
APA StyleBai, X., Batallé, G., Martínez-Martel, I., & Pol, O. (2023). Hydrogen Sulfide Interacting with Cannabinoid 2 Receptors during Sciatic Nerve Injury-Induced Neuropathic Pain. Antioxidants, 12(6), 1179. https://doi.org/10.3390/antiox12061179





