Natural Carotenoids: Recent Advances on Separation from Microbial Biomass and Methods of Analysis
Abstract
1. Introduction
2. Natural Carotenoids
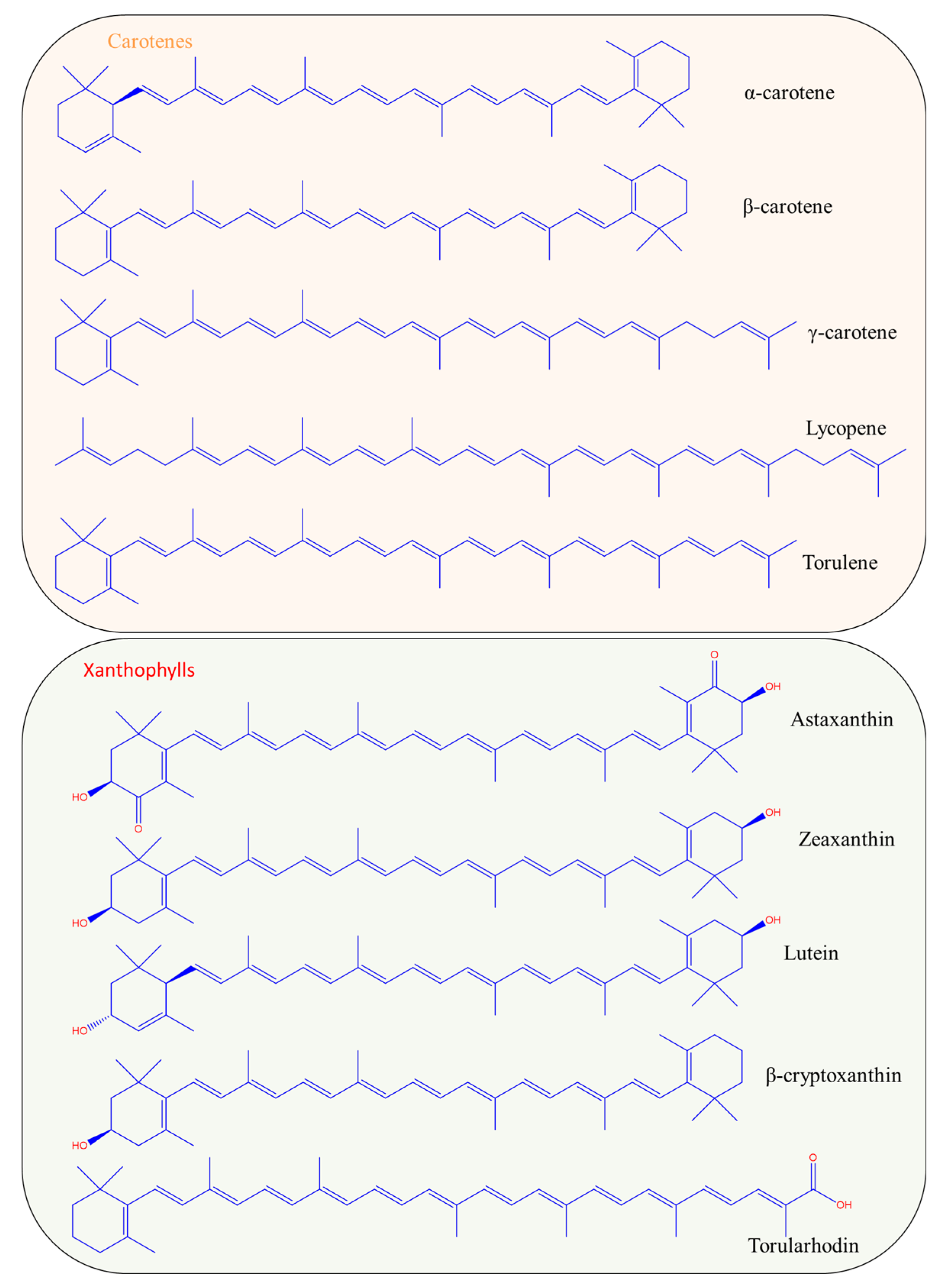
2.1. Types and Chemistry of Carotenoids
2.2. Sources of Natural Carotenoids
2.2.1. Plants
2.2.2. Microorganisms
2.3. Biotechnological Production of Carotenoids
| Main Carotenoid Produced | Microbial Strain | Ref |
|---|---|---|
| α-carotene | Rhodotorula mucilaginosa | [44] |
| β-carotene | Rhodotorula glutinis CCT-2186 | [45] |
| Xanthophyllomyces dendrorhous | [46] | |
| Phaffia rhodozyma | [47] | |
| Rhodotorula mucilaginosa | [44] | |
| Blakeslea trispora | [48] | |
| Dunaliella salina CCAP 19/41 | [49] | |
| Rhodosporidium kratochvilovae Y-42 and Y-43 | [28] | |
| γ-carotene | Rhodotorula mucilaginosa Blakeslea trispora | [44] [48] |
| Lycopene | Blakeslea trispora | [48] |
| Torulene | Rhodotorula glutinis CCT-2186 | [45] |
| Rhodotorula mucilaginosa | [44] | |
| Astaxanthin | Xanthophyllomyces dendrorhous | [46,50,51] |
| Phaffia rhodozyma | [47] | |
| Zeaxanthin | Flavobacterium sp. P8 | [52] |
| Synechococcus sp. PCC7002, Synechocystis sp. PCC6803 and Rhodosorus sp. | [53] | |
| Lutein | Asterarcys quadricellulare PUMCC 5.1.1 | [54] |
| Auxenochlorella spp. LEU27 | [55] | |
| Chlorella minutissima | [56] | |
| Chlorella pyrenoidosa | [57] | |
| Chlorella sorokiniana AK-1 | [58] | |
| Chlorella sorokiniana FZU60 | [59,60] | |
| Chlorella sorokiniana MB-1-M12 | [61,62,63] | |
| Chlorella sorokiniana MUM002 | [64] | |
| Chlorella saccharophila UTEX247 | [65] | |
| Chlorella sp. GY-H4 | [66] | |
| Chlorella vulgaris | [67] | |
| Tetraselmis sp. CTP4 | [68] | |
| Scenedesmus sp. | [69] | |
| Torularhodin | Sporobolomyces ruberrimus | [70] |
| Rhodotorula glutinis CCT-2186 | [45] | |
| Rhodotorula mucilaginosa | [44] |
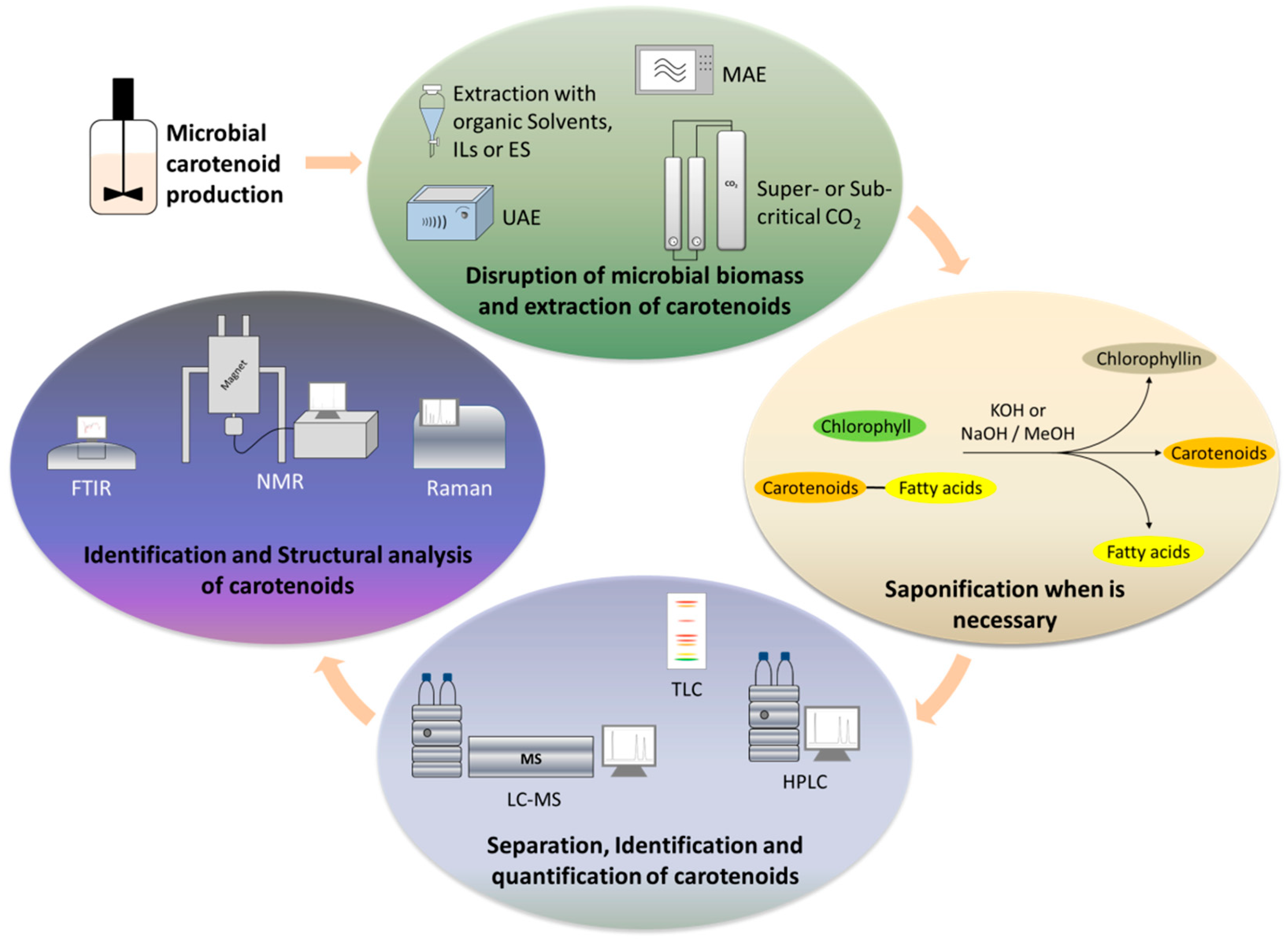
3. Extraction of Microbial Carotenoids
3.1. Solvent Extraction
3.2. Green Technologies for the Separation of Carotenoids
3.2.1. Ultrasound-Assisted Extraction (UAE)
3.2.2. Microwave-Assisted Extraction (MAE)
3.2.3. Enzyme-Assisted Extraction (EAE)
3.2.4. Supercritical Fluid Extraction (SFE)
3.2.5. Ionic Liquids and Deep Eutectic Solvents
4. Analysis of Carotenoids
4.1. Sample Pretreatment before Analysis—Saponification
4.2. Thin-Layer Chromatography
4.3. Liquid Chromatography
4.4. Spectroscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anonymous. Carotenoids Market by Type (Astaxanthin, Beta-Carotene, Lutein, Lycopene, Canthaxanthin, and Zeaxanthin), Application (Feed, Food & Beverages, Dietary Supplements, Cosmetics, and Pharmaceuticals), Source, Formulation, and Region—Global Forecast to 2026; Research and Markets: Dublin, Ireland, 2020. [Google Scholar]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef] [PubMed]
- Obana, A.; Asaoka, R.; Miura, A.; Nozue, M.; Takayanagi, Y.; Nakamura, M. Improving Skin Carotenoid Levels in Young Students through Brief Dietary Education Using the Veggie Meter. Antioxidants 2022, 11, 1570. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jaiswal, V.; Lee, H.J. Dietary Intake of Flavonoids and Carotenoids Is Associated with Anti-Depressive Symptoms: Epidemiological Study and In Silico—Mechanism Analysis. Antioxidants 2022, 11, 53. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green Natural Colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef]
- Alexandri, M.; Kachrimanidou, V.; Papapostolou, H.; Papadaki, A.; Kopsahelis, N. Sustainable Food Systems: The Case of Functional Compounds towards the Development of Clean Label Food Products. Foods 2022, 11, 2796. [Google Scholar] [CrossRef]
- Mussagy, C.; Winterburn, J.; Santos-Ebinuma, V.; Pereira, J. Production and Extraction of Carotenoids Produced by Microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 1095–1114. [Google Scholar] [CrossRef] [PubMed]
- Pfander, H. Carotenoids: An Overview. In Methods in Enzymology; Packer, L., Ed.; Academic Press: Cambridge, MA, USA, 1992; Volume 213, pp. 3–13. [Google Scholar]
- Ruiz-Sola, M.Á.; Rodríguez-Concepción, M. Carotenoid Biosynthesis in Arabidopsis: A Colorful Pathway. In The Arabidopsis Book; The American Society of Plant Biologists: Rockville, MD, USA, 2012; Volume 10, p. e0158. [Google Scholar]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological Production of Carotenoids by Yeasts: An Overview. Microb. Cell Fact. 2014, 13, 12. [Google Scholar] [CrossRef]
- Zhang, C. Biosynthesis of Carotenoids and Apocarotenoids by Microorganisms and Their Industrial Potential. In Progress in Carotenoid Research; Queiroz Zepka, L., Jacob-Lopes, E., Vera De Rosso, V., Eds.; IntechOpen: Rijeka, Croatia, 2018; pp. 85–105. [Google Scholar]
- Rodriguez-Amaya, D.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysis; HarvestPlus: Washington, DC, USA, 2004; ISBN 978-953-307-683-6. [Google Scholar]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Hornero-Méndez, D.; Vicario, I.M. Chapter 1 Structures, Nomenclature and General Chemistry of Carotenoids and Their Esters. In Carotenoid Esters in Foods: Physical, Chemical and Biological Properties; The Royal Society of Chemistry: London, UK, 2019; pp. 1–50. ISBN 978-1-78801-242-3. [Google Scholar]
- Honda, M.; Kageyama, H.; Hibino, T.; Ichihashi, K.; Takada, W.; Goto, M. Isomerization of Commercially Important Carotenoids (Lycopene, β-Carotene, and Astaxanthin) by Natural Catalysts: Isothiocyanates and Polysulfides. J. Agric. Food Chem. 2020, 68, 3228–3237. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, L.; Tsao, R. Chemistry and Biochemistry of Dietary Carotenoids: Bioaccessibility, Bioavailability and Bioactivities Cheng. J. Food Bioact. 2020, 10, 32–46. [Google Scholar] [CrossRef]
- Honda, M.; Takasu, S.; Nakagawa, K.; Tsuda, T. Differences in Bioavailability and Tissue Accumulation Efficiency of (All-E)- and (Z)-Carotenoids: A Comparative Study. Food Chem. 2021, 361, 130119. [Google Scholar] [CrossRef]
- Liaaen-Jensen, S. Basic Carotenoid Chemistry. In Carotenoids in Health and Disease; Krinsky, N.I., Mayne, S.T., Sies, H., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 1–30. [Google Scholar]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From Plants to Food and Feed Industries. Methods Mol. Biol. 2018, 1852, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Stephen, N.; Ravichandran, G.; Niranjana, R.; Prasad, Y. Carotenoids: Types, Sources, and Biosynthesis. In Plant Secondary Metabolites: Volume 2 Stimulation, Extraction, and Utilization; Siddiqui, M., Bansal, V., Prasad, K., Eds.; Apple Academic Press: Waretown, NJ, USA, 2016; pp. 77–106. ISBN 978-1-77188-355-9. [Google Scholar]
- Cassani, L.; Marcovich, N.E.; Gomez-Zavaglia, A. Valorization of Fruit and Vegetables Agro-Wastes for the Sustainable Production of Carotenoid-Based Colorants with Enhanced Bioavailability. Food Res. Int. 2022, 152, 110924. [Google Scholar] [CrossRef]
- Metličar, V.; Vovk, I.; Albreht, A. Japanese and Bohemian Knotweeds as Sustainable Sources of Carotenoids. Plants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Metličar, V.; Kranjc, K.; Albreht, A. Utilization of Plant-Based Wastes for a Sustainable Preparation of Xanthophyll Esters via Acid Anhydrides Using β-Pinene as a Bio-Derived Solvent. ACS Sustain. Chem. Eng. 2021, 9, 10651–10661. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Flora, G.; Sevanan, M.; Sripriya, R.; Chen, W.H.; Park, J.; Rajesh, J.; Kumar, G. Technological Advances in the Production of Carotenoids and Their Applications—A Critical Review. Bioresour. Technol. 2023, 367, 128215. [Google Scholar] [CrossRef]
- Böhm, V. (Ed.) Carotenoids; Antioxidants MDPI: Basel, Switzerland, 2019; ISBN 9783039218646. [Google Scholar]
- Demain, A.L.; Sánchez, S. Advancement of Biotechnology by Genetic Modifications. In Microbial Carotenoids: Methods and Protocols; Barreiro, C., Barredo, J.-L., Eds.; Springer: New York, NY, USA, 2018; pp. 1–43. ISBN 978-1-4939-8742-9. [Google Scholar]
- Gupta, I.; Adin, S.N.; Panda, B.P.; Mujeeb, M. β-Carotene—Production Methods, Biosynthesis from Phaffia Rhodozyma, Factors Affecting Its Production during Fermentation, Pharmacological Properties: A Review. Biotechnol. Appl. Biochem. 2022, 69, 2517–2529. [Google Scholar] [CrossRef]
- Sereti, F.; Papadaki, A.; Alexandri, M.; Kachrimanidou, V.; Kopsahelis, N. Exploring the Potential of Novel R. Kratochvilovae Red Yeasts towards the Sustainable Synthesis of Natural Carotenoids. Sustainability 2023, 31, 100927. [Google Scholar] [CrossRef]
- Siziya, I.N.; Hwang, C.Y.; Seo, M. Antioxidant Potential and Capacity of Microorganism-Sourced C30 Carotenoids—A Review. Antioxidants 2022, 11, 1963. [Google Scholar] [CrossRef]
- Kholany, M.; Coutinho, J.A.P.; Ventura, S.P.M. Carotenoid Production from Microalgae: The Portuguese Scenario. Molecules 2022, 27, 2540. [Google Scholar] [CrossRef]
- Casella, P.; Iovine, A.; Mehariya, S.; Marino, T.; Musmarra, D.; Molino, A. Smart Method for Carotenoids Characterization in Haematococcus pluvialis Red Phase and Evaluation of Astaxanthin Thermal Stability. Antioxidants 2020, 9, 422. [Google Scholar] [CrossRef]
- Srivastava, A.; Kalwani, M.; Chakdar, H.; Pabbi, S.; Shukla, P. Biosynthesis and Biotechnological Interventions for Commercial Production of Microalgal Pigments: A Review. Bioresour. Technol. 2022, 352, 127071. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Microalgal Lutein Biosynthesis: Recent Trends and Challenges to Enhance the Lutein Content in Microalgal Cell Factories. Front. Mar. Sci. 2022, 9, 1015419. [Google Scholar] [CrossRef]
- Assunção, J.; Malcata, F.X. Enclosed “Non-Conventional” Photobioreactors for Microalga Production: A Review. Algal Res. 2020, 52, 102107. [Google Scholar] [CrossRef]
- Mannazzu, I.; Landolfo, S.; da Silva, T.L.; Buzzini, P. Red Yeasts and Carotenoid Production: Outlining a Future for Non-Conventional Yeasts of Biotechnological Interest. World J. Microbiol. Biotechnol. 2015, 31, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Kopsahelis, N.; Mallouchos, A.; Mandala, I.; Koutinas, A.A. Bioprocess Development for the Production of Novel Oleogels from Soybean and Microbial Oils. Food Res. Int. 2019, 126, 108684. [Google Scholar] [CrossRef]
- Cipolatti Pereira, E.; Diaz Remedi, R.; do Santos Sá, S.; Bueno Rodrigues, A.; Markowiski Gonçalves, J.; Veiga Burkert, C.A.; Badiale Furlong, E.; Fernandes, J.; de Medeiros Burkert, J.F. Biocatalysis and Agricultural Biotechnology Use of Agroindustrial Byproducts as Substrate for Production of Carotenoids with Antioxidant Potential by Wild Yeasts. Biocatal. Agric. Biotechnol. 2019, 20, 101208. [Google Scholar] [CrossRef]
- Li, W.; Luna-Flores, C.H.; Anangi, R.; Zhou, R.; Tan, X.; Jessen, M.; Liu, L.; Zhou, R.; Zhang, T.; Gissibl, A.; et al. Oxidative Stress Induced by Plasma-Activated Water Stimulates Astaxanthin Production in Phaffia rhodozyma. Bioresour. Technol. 2023, 369, 128370. [Google Scholar] [CrossRef]
- Mantzouridou, F.; Tsimidou, M.Z. Lycopene Formation in Blakeslea Trispora. Chemical Aspects of a Bioprocess. Trends Food Sci. Technol. 2008, 19, 363–371. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, Y.; Li, X.; Wang, Z.M.; Yang, L.R.; Zhang, Y.Z.; Wu, M.; Yao, J.M. Analysis of Nicotine-Induced Metabolic Changes in Blakeslea Trispora by GC-MS. J. Zhejiang Univ. Sci. B 2020, 21, 172–177. [Google Scholar] [CrossRef]
- Giani, M.; Martínez-Espinosa, R.M. Carotenoids as a Protection Mechanism against Oxidative Stress in Haloferax Mediterranei. Antioxidants 2020, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Lizama, C.; Romero-Parra, J.; Andrade, D.; Riveros, F.; Bórquez, J.; Ahmed, S.; Venegas-Salas, L.; Cabalín, C.; Simirgiotis, M.J. Analysis of Carotenoids in Haloarchaea Species from Atacama Saline Lakes by High Resolution Uhplc-q-Orbitrap-Mass Spectrometry: Antioxidant Potential and Biological Effect on Cell Viability. Antioxidants 2021, 10, 1230. [Google Scholar] [CrossRef]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from Cyanobacteria: Biotechnological Potential and Optimization Strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef]
- Ghilardi, C.; Sanmartin Negrete, P.; Carelli, A.A.; Borroni, V. Evaluation of Olive Mill Waste as Substrate for Carotenoid Production by Rhodotorula mucilaginosa. Bioresour. Bioprocess. 2020, 7, 11. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Santos-Ebinuma, V.C.; Gonzalez-Miquel, M.; Coutinho, J.A.P.; Pereira, J.F.B. Protic Ionic Liquids as Cell-Disrupting Agents for the Recovery of Intracellular Carotenoids from Yeast Rhodotorula Glutinis CCT-2186. ACS Sustain. Chem. Eng. 2019, 7, 16765–16776. [Google Scholar] [CrossRef]
- Gervasi, T.; Pellizzeri, V.; Benameur, Q.; Gervasi, C.; Santini, A.; Cicero, N.; Dugo, G. Valorization of Raw Materials from Agricultural Industry for Astaxanthin and β-Carotene Production by Xanthophyllomyces Dendrorhous. Nat. Prod. Res. 2018, 32, 1554–1561. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Santos-Ebinuma, V.C.; Herculano, R.D.; Coutinho, J.A.P.; Pereira, J.F.B.; Pessoa, A. Ionic Liquids or Eutectic Solvents? Identifying the Best Solvents for the Extraction of Astaxanthin and β-Carotene from Phaffia rhodozyma Yeast and Preparation of Biodegradable Films. Green Chem. 2022, 24, 118–123. [Google Scholar] [CrossRef]
- Shariati, S.; Zare, D.; Mirdamadi, S. Screening of Carbon and Nitrogen Sources Using Mixture Analysis Designs for Carotenoid Production by Blakeslea trispora. Food Sci. Biotechnol. 2019, 28, 469–479. [Google Scholar] [CrossRef]
- Xu, Y.; Harvey, P.J. Carotenoid Production by Dunaliella Salina under Red Light. Antioxidants 2019, 8, 123. [Google Scholar] [CrossRef]
- Gervasi, T.; Santini, A.; Daliu, P.; Salem, A.Z.M.; Gervasi, C.; Pellizzeri, V.; Barrega, L.; De Pasquale, P.; Dugo, G.; Cicero, N. Astaxanthin Production by Xanthophyllomyces Dendrorhous Growing on a Low Cost Substrate. Agrofor. Syst. 2019, 6, 1229–1234. [Google Scholar] [CrossRef]
- Hara, K.Y.; Kageyama, Y.; Tanzawa, N.; Hirono-Hara, Y.; Kikukawa, H.; Wakabayashi, K. Development of Astaxanthin Production from Citrus Peel Extract Using Xanthophyllomyces Dendrorhous. Environ. Sci. Pollut. Res. 2021, 28, 12640–12647. [Google Scholar] [CrossRef]
- Vila, E.; Hornero-Méndez, D.; Lareo, C.; Saravia, V. Biotechnological Production of Zeaxanthin by an Antarctic Flavobacterium: Evaluation of Culture Conditions. J. Biotechnol. 2020, 319, 54–60. [Google Scholar] [CrossRef]
- Bourdon, L.; Jensen, A.A.; Kavanagh, J.M.; McClure, D.D. Microalgal Production of Zeaxanthin. Algal Res. 2021, 55, 102266. [Google Scholar] [CrossRef]
- Singh, D.P.; Khattar, J.S.; Rajput, A.; Chaudhary, R.; Singh, R. High Production of Carotenoids by the Green Microalga Asterarcys quadricellulare PUMCC. PLoS ONE 2019, 14, e0221930. [Google Scholar] [CrossRef] [PubMed]
- Asker, D.; Awad, T.S. Isolation and Characterization of a Novel Lutein-Producing Marine Microalga Using High Throughput Screening. Food Res. Int. 2019, 116, 660–667. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Sen, R.; Sarmah, A.K. Consolidated Bioprocessing of Wastewater Cocktail in an Algal Biorefinery for Enhanced Biomass, Lipid and Lutein Production Coupled with Efficient CO2 Capture: An Advanced Optimization Approach. J. Environ. Manag. 2019, 252, 109696. [Google Scholar] [CrossRef]
- Sampathkumar, S.J.; Gothandam, K.M. Sodium Bicarbonate Augmentation Enhances Lutein Biosynthesis in Green Microalgae Chlorella pyrenoidosa. Biocatal. Agric. Biotechnol. 2019, 22, 101406. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kuo, E.W.; Nagarajan, D.; Di Dong, C.; Lee, D.J.; Varjani, S.; Lam, S.S.; Chang, J.S. Semi-Batch Cultivation of Chlorella sorokiniana AK-1 with Dual Carriers for the Effective Treatment of Full Strength Piggery Wastewater Treatment. Bioresour. Technol. 2021, 326, 124773. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, Z.; Ho, S.H.; Ruan, C.; Li, J.; Xie, Y.; Shi, X.; Liu, L.; Chen, J. Two-Stage Bioprocess for Hyper-Production of Lutein from Microalga Chlorella sorokiniana FZU60: Effects of Temperature, Light Intensity, and Operation Strategies. Algal Res. 2020, 52, 102119. [Google Scholar] [CrossRef]
- Xie, Y.; Li, J.; Ho, S.H.; Ma, R.; Shi, X.; Liu, L.; Chen, J. Pilot-Scale Cultivation of Chlorella sorokiniana FZU60 with a Mixotrophy/Photoautotrophy Two-Stage Strategy for Efficient Lutein Production. Bioresour. Technol. 2020, 314, 123767. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Kato, Y.; Matsuda, M.; Chen, C.Y.; Nagarajan, D.; Hasunuma, T.; Kondo, A.; Chang, J.S. Lutein Production with Chlorella sorokiniana MB-1-M12 Using Novel Two-Stage Cultivation Strategies—Metabolic Analysis and Process Improvement. Bioresour. Technol. 2021, 334, 125200. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Chen, C.Y.; Hasunuma, T.; Kondo, A.; Chang, C.H.; Ng, I.S.; Chang, J.S. Enhancing Lutein Production with Mixotrophic Cultivation of Chlorella sorokiniana MB-1-M12 Using Different Bioprocess Operation Strategies. Bioresour. Technol. 2019, 278, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Kato, Y.; Matsuda, M.; Chen, C.Y.; Nagarajan, D.; Hasunuma, T.; Kondo, A.; Di Dong, C.; Lee, D.J.; Chang, J.S. A Novel Process for the Mixotrophic Production of Lutein with Chlorella sorokiniana MB-1-M12 Using Aquaculture Wastewater. Bioresour. Technol. 2019, 290, 121786. [Google Scholar] [CrossRef]
- Shiong, K.; Mun, Y.; Sing, W.; Min, J.; Chern, S. Permeabilization of Chlorella sorokiniana and Extraction of Lutein by Distillable CO 2 -Based Alkyl Carbamate Ionic Liquids. Sep. Purif. Technol. 2021, 256, 117471. [Google Scholar] [CrossRef]
- Paliwal, C.; Rehmanji, M.; Mohd, K.; Uz, S.; Pavan, P. Green Extraction Processing of Lutein from Chlorella saccharophila in Water-Based Ionic Liquids as a Sustainable Innovation in Algal Biorefineries. Algal Res. 2022, 66, 102809. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Sun, Z.; Liu, S.; Qin, Z.; Mou, J.; Zhou, Z.; Sze, C.; Lin, K. Sustainable Lipid and Lutein Production from Chlorella Mixotrophic Fermentation by Food Waste Hydrolysate. J. Hazard. Mater. 2020, 400, 123258. [Google Scholar] [CrossRef] [PubMed]
- McClure, D.D.; Nightingale, J.K.; Luiz, A.; Black, S.; Zhu, J.; Kavanagh, J.M. Pilot-Scale Production of Lutein Using Chlorella vulgaris. Algal Res. 2019, 44, 101707. [Google Scholar] [CrossRef]
- Schüler, L.M.; Santos, T.; Pereira, H.; Duarte, P.; Katkam, N.G.; Florindo, C.; Schulze, P.S.C.; Barreira, L.; Varela, J.C.S. Improved Production of Lutein and β-Carotene by Thermal and Light Intensity Upshifts in the Marine Microalga Tetraselmis sp. CTP4. Algal Res. 2020, 45, 101732. [Google Scholar] [CrossRef]
- Rajendran, L.; Nagarajan, N.G.; Karuppan, M. Enhanced Biomass and Lutein Production by Mixotrophic Cultivation of Scenedesmus sp. Using Crude Glycerol in an Airlift Photobioreactor. Biochem. Eng. J. 2020, 161, 107684. [Google Scholar] [CrossRef]
- Kanno, K.Y.F.; Karp, S.G.; Rodrigues, C.; de Andrade Tanobe, V.O.; Soccol, C.R.; da Costa Cardoso, L.A. Influence of Organic Solvents in the Extraction and Purification of Torularhodin from Sporobolomyces ruberrimus. Biotechnol. Lett. 2021, 43, 89–98. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid Extraction Methods: A Review of Recent Developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Fidan, O.; Zhan, J. Discovery and Engineering of an Endophytic Pseudomonas Strain from Taxus chinensis for Efficient Production of Zeaxanthin Diglucoside. J. Biol. Eng. 2019, 13, 66. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, H.; Tang, J.; Bi, C.; Li, Q.; Zhang, X. Coordinated Expression of Astaxanthin Biosynthesis Genes for Improved Astaxanthin Production in Escherichia coli. J. Agric. Food Chem. 2020, 68, 14917–14927. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Lin, Y.; He, M.; Gong, Y.; Huang, J. Induced High-Yield Production of Zeaxanthin, Lutein, and β-Carotene by a Mutant of Chlorella zofingiensis. J. Agric. Food Chem. 2018, 66, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Park, S.H.; Kim, S.; Kim, S.W.; Hahn, J.S. Efficient Production of Lycopene in Saccharomyces cerevisiae by Enzyme Engineering and Increasing Membrane Flexibility and NAPDH Production. Appl. Microbiol. Biotechnol. 2019, 103, 211–223. [Google Scholar] [CrossRef]
- Marzorati, S.; Schievano, A.; Idà, A.; Verotta, L. Carotenoids, Chlorophylls and Phycocyanin from Spirulina: Supercritical CO2 and Water Extraction Methods for Added Value Products Cascade. Green Chem. 2020, 22, 187–196. [Google Scholar] [CrossRef]
- Gkioni, M.D.; Andriopoulos, V.; Koutra, E.; Hatziantoniou, S.; Kornaros, M.; Lamari, F.N. Ultrasound-Assisted Extraction of Nannochloropsis Oculata with Ethanol and Betaine: 1,2-Propanediol Eutectic Solvent for Antioxidant Pigment-Rich Extracts Retaining Nutritious the Residual Biomass. Antioxidants 2022, 11, 1103. [Google Scholar] [CrossRef]
- Byrtusová, D.; Szotkowski, M.; Kurowska, K.; Shapaval, V.; Márová, I. Rhodotorula Kratochvilovae CCY 20-2-26—The Source of Multifunctional Metabolites. Microorganisms 2021, 9, 1280. [Google Scholar] [CrossRef]
- Boukroufa, M.; Boutekedjiret, C.; Chemat, F. Development of a Green Procedure of Citrus Fruits Waste Processing to Recover Carotenoids. Resour. Technol. 2017, 3, 252–262. [Google Scholar] [CrossRef]
- Maher, T.; Kabbashi, N.A.; Mirghani, M.E.S.; Alam, M.Z.; Daddiouaissa, D.; Abdulhafiz, F.; Reduan, M.F.H.; Omran, J.I.; Razab, M.K.A.A.; Mohammed, A. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Acacia Seyal Gum Using Response Surface Methodology and Their Chemical Content Identification by Raman, FTIR, and GC-TOFMS. Antioxidants 2021, 10, 1612. [Google Scholar] [CrossRef]
- Sarkarat, R.; Mohamadnia, S.; Tavakoli, O. Recent Advances in Non-Conventional Techniques for Extraction of Phycobiliproteins and Carotenoids from Microalgae. Braz. J. Chem. Eng. 2022, 1–22. [Google Scholar] [CrossRef]
- Sarnaik, A.; Nambissan, V.; Pandit, R.; Lali, A. Recombinant Synechococcus elongatus PCC 7942 for Improved Zeaxanthin Production under Natural Light Conditions. Algal Res. 2018, 36, 139–151. [Google Scholar] [CrossRef]
- Park, S.Y.; Binkley, R.M.; Kim, W.J.; Lee, M.H.; Lee, S.Y. Metabolic Engineering of Escherichia coli for High-Level Astaxanthin Production with High Productivity. Metab. Eng. 2018, 49, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, G.; Ye, X.; Kakuda, Y.; Meng, R. Stability of All-Trans-β-Carotene under Ultrasound Treatment in a Model System: Effects of Different Factors, Kinetics and Newly Formed Compounds. Ultrason. Sonochem. 2010, 17, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Ur, M.; Khan, F. Introduction to Natural Products; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128164556. [Google Scholar]
- Sarkar, S.; Manna, M.S.; Bhowmick, T.K.; Gayen, K. Extraction of Chlorophylls and Carotenoids from Dry and Wet Biomass of Isolated Chlorella thermophila: Optimization of Process Parameters and Modelling by Artificial Neural Network. Process Biochem. 2020, 96, 58–72. [Google Scholar] [CrossRef]
- Fabrowska, J.; Messyasz, B.; Szyling, J.; Walkowiak, J.; Łęska, B. Isolation of Chlorophylls and Carotenoids from Freshwater Algae Using Different Extraction Methods. Phycol. Res. 2018, 66, 52–57. [Google Scholar] [CrossRef]
- Sarma, M.K.; Saha, K.; Choudhury, R.; Pabbi, S.; Madamwar, D.; Subudhi, S. Development of a Microwave-Assisted Solvent Extraction Process for the Extraction of High-Value Carotenoids from Chlorella Biomass. Biofuels Bioprod. Biorefining 2022, 16, 698–709. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Vlysidis, A.; Papanikolaou, S.; Kookos, I.K.; Monje Martínez, B.; Escrig Rondán, M.C.; Koutinas, A.A. Downstream Separation of Poly(Hydroxyalkanoates) Using Crude Enzyme Consortia Produced via Solid State Fermentation Integrated in a Biorefinery Concept. Food Bioprod. Process. 2016, 100, 323–334. [Google Scholar] [CrossRef]
- Michelon, M.; de Matos de Borba, T.; da Silva Rafael, R.; Burkert, C.A.V.; de Medeiros Burkert, J.F. Extraction of Carotenoids from Phaffia rhodozyma: A Comparison between Different Techniques of Cell Disruption. Food Sci. Biotechnol. 2012, 21, 1–8. [Google Scholar] [CrossRef]
- Chandi, G.K.; Gill, B.S. Production and Characterization of Microbial Carotenoids as an Alternative to Synthetic Colors: A Review. Int. J. Food Prop. 2011, 14, 503–513. [Google Scholar] [CrossRef]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical Fluid Extraction of Seed Oils—A Short Review of Current Trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Martínez-Ávila, M.; Rodríguez-Rodríguez, J.; Gutiérrez Uribe, J.A.; Guajardo-Flores, D. Selective Supercritical Fluid Extraction of Non-Polar Phytochemicals from Black Beans (Phaseolus vulgaris L.) by-Products. J. Supercrit. Fluids 2022, 189, 105730. [Google Scholar] [CrossRef]
- Mehariya, S.; Iovine, A.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Casella, P.; Karatza, D.; Marino, T.; Musmarra, D.; et al. Supercritical Fluid Extraction of Lutein from Scenedesmus Almeriensis. Molecules 2019, 24, 1324. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Extraction of Astaxanthin and Lutein from Microalga Haematococcus pluvialis in the Red Phase Using CO₂ Supercritical Fluid Extraction Technology with Ethanol as Co-Solvent. Mar. Drugs 2018, 16, 432. [Google Scholar] [CrossRef] [PubMed]
- Morcelli, A.; Cassel, E.; Vargas, R.; Rech, R.; Marcílio, N. Supercritical Fluid (CO2+ethanol) Extraction of Chlorophylls and Carotenoids from Chlorella sorokiniana: COSMO-SAC Assisted Prediction of Properties and Experimental Approach. J. CO2 Util. 2021, 51, 101649. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Amorim-Carrilho, K.T.; Cepeda, A.; Fente, C.; Regal, P. Review of Methods for Analysis of Carotenoids. TrAC—Trends Anal. Chem. 2014, 56, 49–73. [Google Scholar] [CrossRef]
- Britton, G. Getting to Know Carotenoids. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2022; Volume 670, pp. 1–56. ISBN 9780323999755. [Google Scholar]
- Adadi, P.; Barakova, N.V.; Krivoshapkina, E.F. Selected Methods of Extracting Carotenoids, Characterization, and Health Concerns: A Review. J. Agric. Food Chem. 2018, 66, 5925–5947. [Google Scholar] [CrossRef]
- Hong, H.T.; Takagi, T.; Hare, T.J.O. An Optimal Saponification and Extraction Method to Determine Carotenoids in Avocado. Food Chem. 2022, 387, 132923. [Google Scholar] [CrossRef]
- Maswanna, T.; Maneeruttanarungroj, C. Identification of Major Carotenoids from Green Alga Tetraspora sp. CU2551: Partial Purification and Characterization of Lutein, Canthaxanthin, Neochrome, and β-Carotene. World J. Microbiol. Biotechnol. 2022, 38, 129. [Google Scholar] [CrossRef]
- Mishra, S.; Singh Chanotiya, C.; Shanker, K.; Kumar Tripathi, A. Characterization of Carotenoids and Genes Encoding Their Biosynthetic Pathways in Azospirillum brasilense. FEMS Microbiol. Lett. 2021, 368, 25. [Google Scholar] [CrossRef] [PubMed]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, Identification and Quantification of Carotenoids and Chlorophylls in Dietary Supplements Containing Chlorella vulgaris and Spirulina platensis Using High Performance Thin Layer Chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Garcia-Perez, P.; Carreira-Casais, A.; Silva, A.; Simal-Gandara, J.; Prieto, M.A. A HPLC-DAD Method for Identifying and Estimating the Pigment Content of Fucoxanthin, β-Carotene and Chlorophyll a in Brown Algae Extracts. Food Chem. Adv. 2022, 1, 100095. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Roca, M. Recent Developments in the Analysis of Carotenoids by Mass Spectrometry. In Progress in Carotenoid Research; Zepka, L.Q., Jacob-Lopes, E., De Rosso, V.V., Eds.; IntechOpen: Rijeka, Croatia, 2018; pp. 17–44. [Google Scholar]
- Soares, A.T.; da Costa, D.C.; Vieira, A.A.H.; Antoniosi Filho, N.R. Analysis of Major Carotenoids and Fatty Acid Composition of Freshwater Microalgae. Heliyon 2019, 5, e01529. [Google Scholar] [CrossRef]
- Vila, E.; Hornero-Méndez, D.; Azziz, G.; Lareo, C.; Saravia, V. Carotenoids from Heterotrophic Bacteria Isolated from Fildes Peninsula, King George Island, Antarctica. Biotechnol. Rep. 2019, 21, e00306. [Google Scholar] [CrossRef] [PubMed]
- Gurkok, S. A Novel Carotenoid from Metabacillus idriensis LipT27: Production, Extraction, Partial Characterization, Biological Activities and Use in Textile Dyeing. Arch. Microbiol. 2022, 204, 296. [Google Scholar] [CrossRef]
- Osterrothová, K.; Culka, A.; Němečková, K.; Kaftan, D.; Nedbalová, L.; Procházková, L.; Jehlička, J. Analyzing Carotenoids of Snow Algae by Raman Microspectroscopy and High-Performance Liquid Chromatography. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2019, 212, 262–271. [Google Scholar] [CrossRef]

| Microorganism | Method of Extraction | Carotenoids | Method of Analysis | Ref |
|---|---|---|---|---|
| Xanthophyllomyces dendrorhous | Cell disruption with zirconia beads and extraction with acetone | Astaxanthin | HPLC—UV detector. Solvent acetonitrile: methanol: 2-propanol (85:10:5) (isocratic) | [51] |
| Rhodosporidium kratochvilovae Y-42 and Y4-3 | Cell disruption with DMSO, followed by acetone and solvent extraction with petroleum ether | β-carotene, lycopene | HPLC—PDA detector. Solvent: acetonitrile: methanol: THF 4 (stabilized with 0.025% BHT) (40:56:4) (isocratic) | [28] |
| Chlorella saccharophila | Solvent extraction with IL (tetrabutyl phosphonium hydroxide) | Lutein | HPLC—UV detector. Solvent A: methanol, solvent B: 200 mM acetic acid and solvent C: MTBE 1 (gradient) | [65] |
| Synechococcus sp. PCC7002, Synechocystis sp. PCC6803 and Rhodosorus sp. | Cell disruption with zirconia beads and extraction with methanol | Zeaxanthin | HPLC—PDA detector. Solvent: methanol: MTBE: water 75:22:3 (isocratic) | [53] |
| Pseudomonas sp. 102515 and genetically modified strains of E. coli and Pseudomonas putida | Methanol and sonication for the extraction, redissolved in DMSO 2—methanol for the analysis | Zeaxanthin diglucoside | HPLC—MS (ESI-MS). Solvent: acetonitrile: water from 50% to 90% (gradient) and methanol: tetrahydrofuran (6:4) (isocratic) | [72] |
| Xanthophyllomyces dendrorhous | DMSO, addition of Na3PO4 and hexane/ethyl acetate 1:1 (v/v); redissolved in MTBE | Astaxanthin and β-carotene | HPLC—LC/MS. Solvent A: water with formic acid 0.01%/ammonium formate 5 mM; solvent B: acetonitrile—methanol (7:3) methanol with formic acid 0.01%/ammonium formate 5 mM (gradient) | [46,50] |
| Genetically engineered strains of E. coli | Extraction with acetone | Astaxanthin | HPLC—UV detector. Solvent A methanol: acetonitrile: DCM 3, 21:21:8 and solvent B: methanol: water, 1:9 (gradient) | [73] |
| Chlorella zofingiensis (mutant) | Extraction with acetone | Zeaxanthin, lutein and β-carotene | HPLC—DAD. Solvent A: methanol and solvent B: MTBE (gradient) | [74] |
| Sporobolomyces ruberrimus | Cell disruption with glass beads; extraction with different combinations of hexane, petroleum ether, ethyl ether and acetone | β-carotene | TLC analysis with silica gel and acetone: hexane (3:7 v/v) HPLC—DAD. Solvent A: acetone 99.8% and solvent B: water (gradient) | [70] |
| Chlorella sorokiniana | Extraction with CO2-based alkyl carbamate ILs (dipropylammonium dipropylcarbamate, diallylammonium diallylcarbamate, dibutylammonium dibutylcarbamate) | Torulene and torularhodin | HPLC—PDA detector. Solvent: methanol: water 97:3 (isocratic) | [64] |
| Genetically engineered Saccharomyces cerevisiae | Sequential boiling and cooling with 1 N HCl for cell disruption; extraction with acetone | Lutein | HPLC—PDA detector. Solvent methanol: DCM: acetonitrile (47:18:35) (isocratic) | [75] |
| Dunaliella salina rubeus D. salina salina D. salina bardawil | Extraction with MTBE–MeOH (20:80) assisted by sonication | α-carotene, β-carotene, lutein and zeaxanthin | HPLC—DAD. Solvent: 80% methanol: 20% MTBE (isocratic) | [49] |
| Spirulina platensis | Supercritical CO2 extractions (300 bar and 45 °C) | Zeaxanthin β-cryptoxanthin β-carotene | UPLC—MS (ESI) analysis Mobile phase acetonitrile: methanol (70:30) (isocratic) | [76] |
| Haloarcula sp. Halorubrum tebenquichense | Extraction with acetone: water (8:2) assisted by vortex, sonication and centrifugation | Bacterioruberin | UHPLC—MS analysis Mobile phase solvent A: 1% formic acid aqueous solution, solvent B: methanol with 1% formic acid and solvent C: acetonitrile with 1% formic acid (gradient system) | [42] |
| Nannochloropsis oculata | UAE combined with ES (ethanol of betaine: 1,2 propanediol at a molar ratio of 2:5) | Violaxanthin | LS-MS with mobile phase 0.2% formic acid in water (solvent A), 0.2% formic acid in acetonitrile (solvent B) (gradient system) HPLC—DAD with mobile phase 0.1% formic acid (solvent A), methanol (solvent B), acetonitrile (solvent C), methanol (solvent D) (gradient system) 1H-NMR | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papapostolou, H.; Kachrimanidou, V.; Alexandri, M.; Plessas, S.; Papadaki, A.; Kopsahelis, N. Natural Carotenoids: Recent Advances on Separation from Microbial Biomass and Methods of Analysis. Antioxidants 2023, 12, 1030. https://doi.org/10.3390/antiox12051030
Papapostolou H, Kachrimanidou V, Alexandri M, Plessas S, Papadaki A, Kopsahelis N. Natural Carotenoids: Recent Advances on Separation from Microbial Biomass and Methods of Analysis. Antioxidants. 2023; 12(5):1030. https://doi.org/10.3390/antiox12051030
Chicago/Turabian StylePapapostolou, Harris, Vasiliki Kachrimanidou, Maria Alexandri, Stavros Plessas, Aikaterini Papadaki, and Nikolaos Kopsahelis. 2023. "Natural Carotenoids: Recent Advances on Separation from Microbial Biomass and Methods of Analysis" Antioxidants 12, no. 5: 1030. https://doi.org/10.3390/antiox12051030
APA StylePapapostolou, H., Kachrimanidou, V., Alexandri, M., Plessas, S., Papadaki, A., & Kopsahelis, N. (2023). Natural Carotenoids: Recent Advances on Separation from Microbial Biomass and Methods of Analysis. Antioxidants, 12(5), 1030. https://doi.org/10.3390/antiox12051030













