Applications of Perilla frutescens Extracts in Clinical Practice
Abstract
1. Introduction
2. Materials and Methods
3. Evidence from In Vitro and Animal Studies Regarding the Perilla frutescens Effects
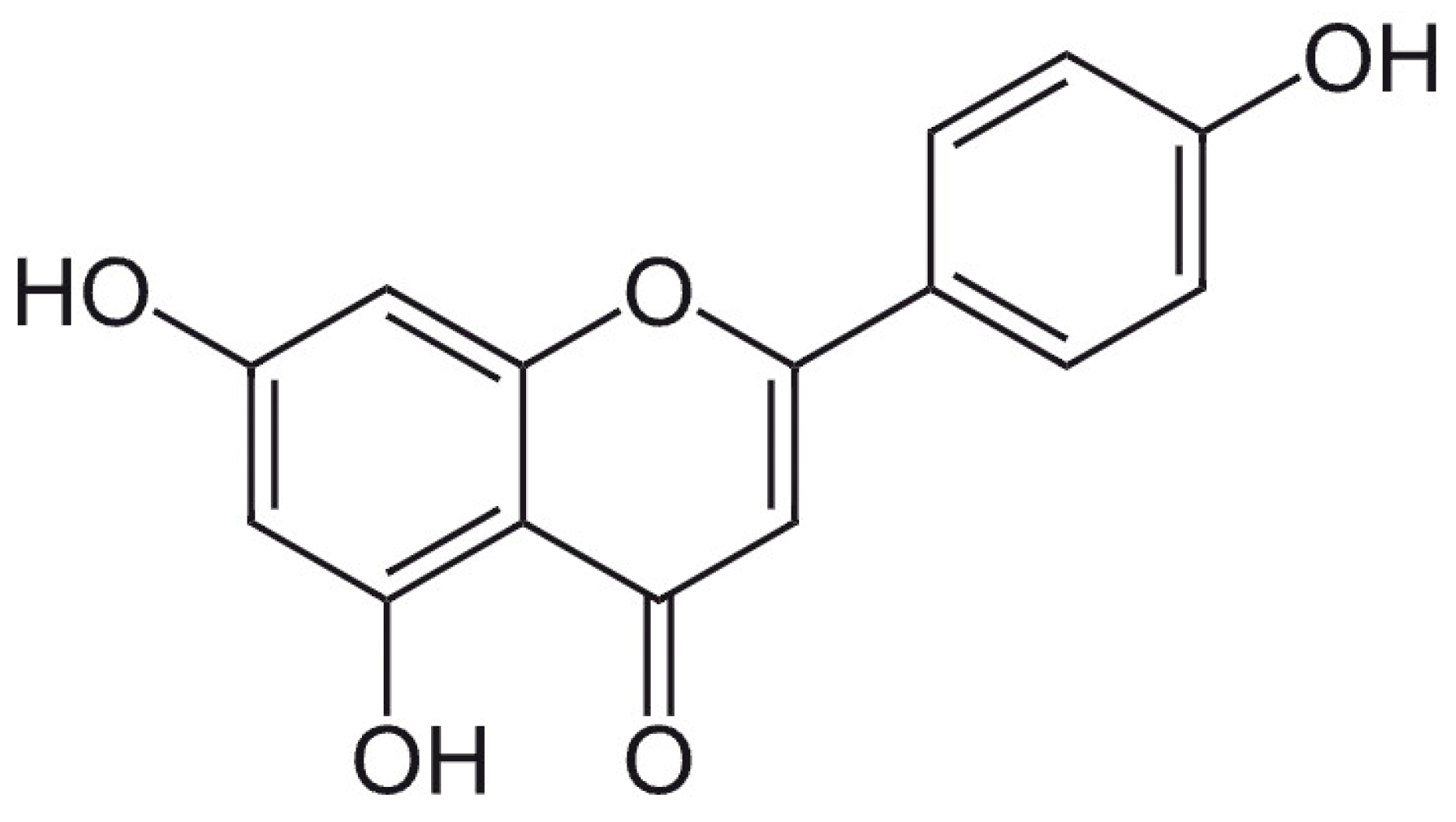
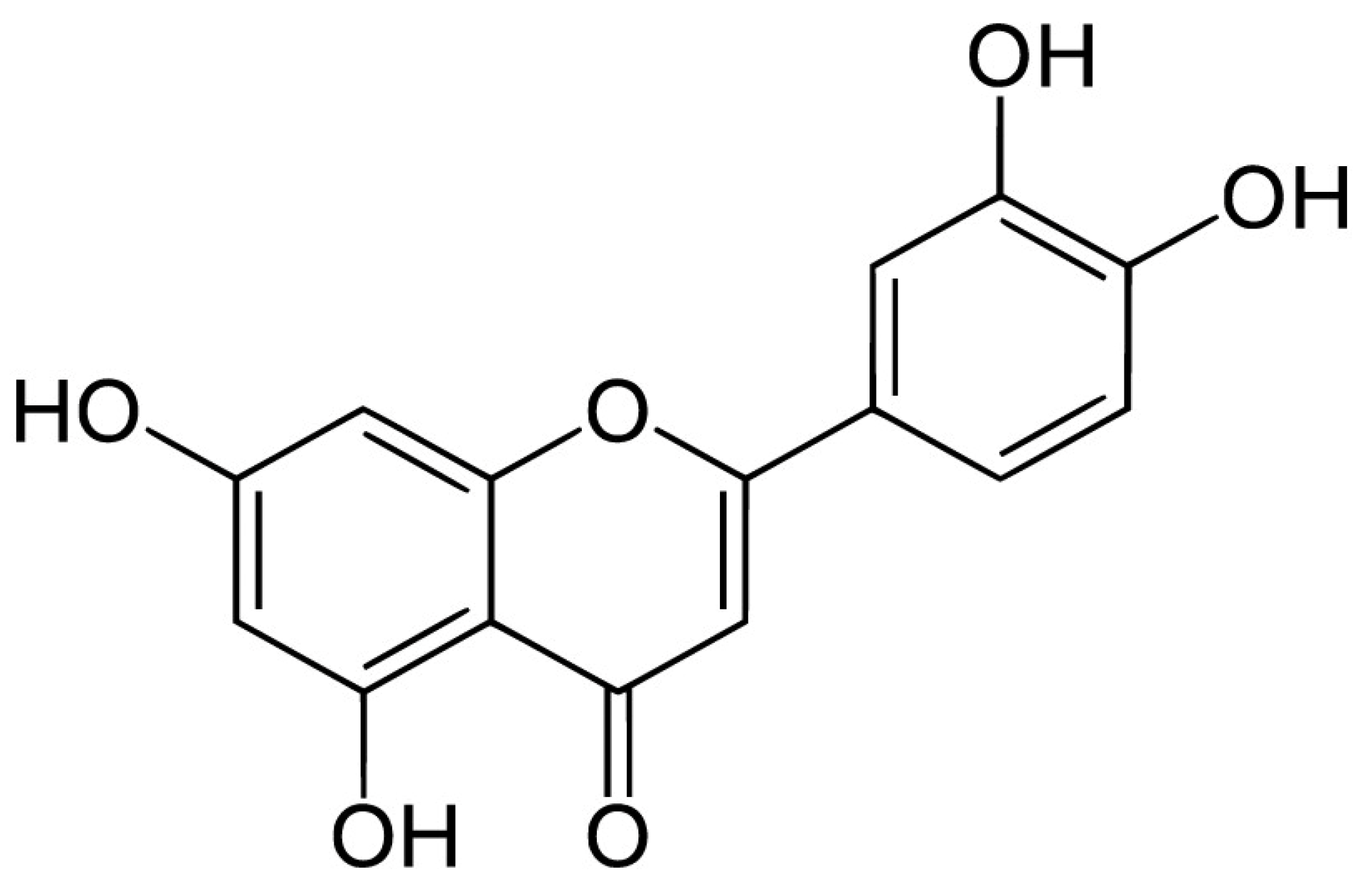
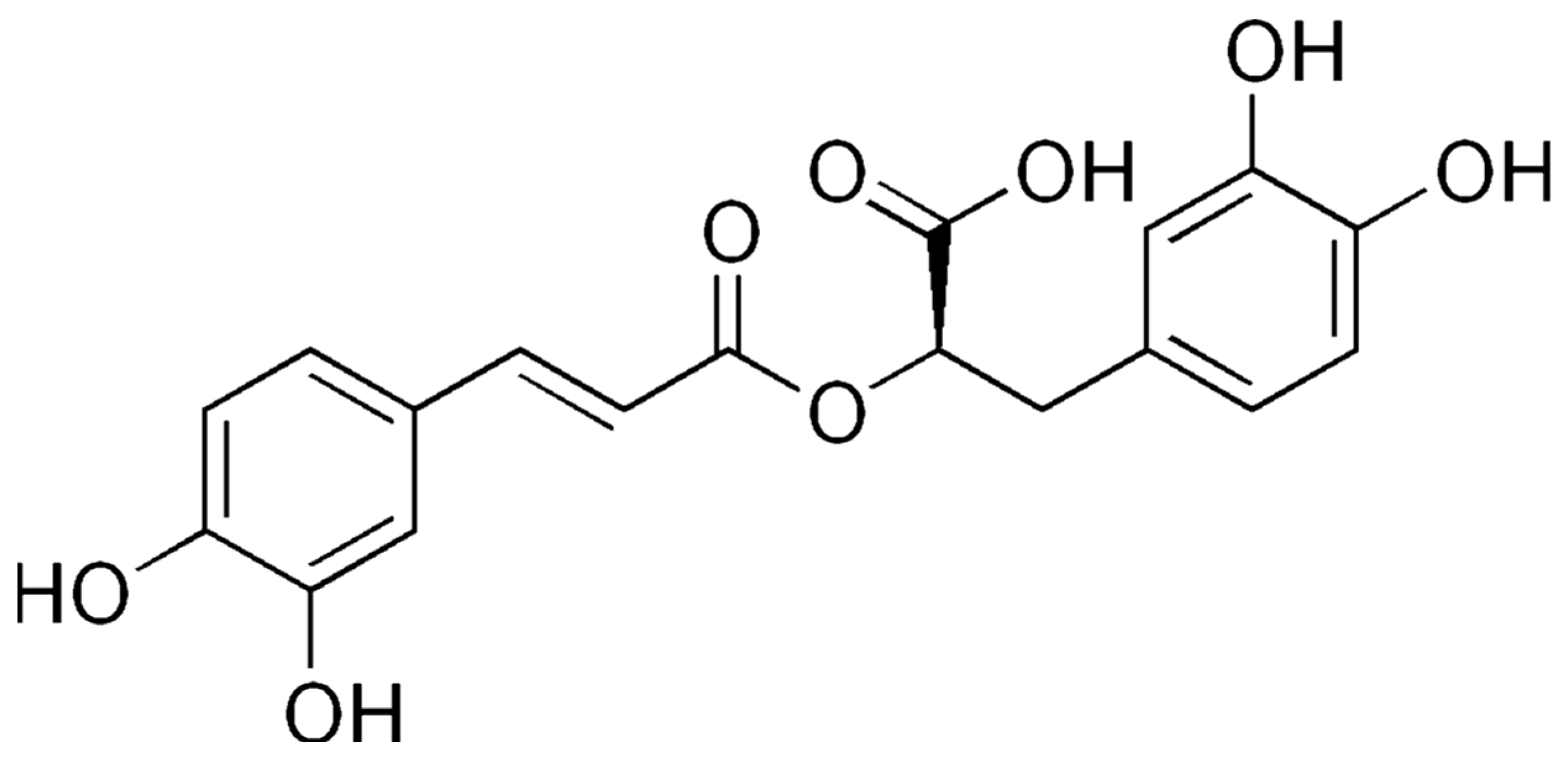
4. Apigenin, Luteolin, and Rosmarinic Acid—Three Important Constituents of Perilla frutescens
5. Anti-Inflammatory and Antiallergic Effects
6. Antioxidant and Hypolipemiant Effects
7. Miscellaneous Effects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, H.; Qiu, J.F.; Ma, L.J.; Hu, Y.J.; Li, P.; Wan, J.B. Phytochemical and phytopharmacological review of Perilla frutescens L. (Labiatae), a traditional edible-medicinal herb in China. Food Chem. Toxicol. 2017, 108 (Pt B), 375–391. [Google Scholar] [CrossRef]
- Hou, T.; Netala, V.R.; Zhang, H.; Xing, Y.; Li, H.; Zhang, Z. Perilla frutescens: A Rich Source of Pharmacological Active Compounds. Molecules 2022, 27, 3578. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.L.; Guo, B.L.; Zhang, C.W.; Zhang, F.; Tian, J.; Bai, X.L.; Zhang, S.N. Perilla resources of China and essential oil chemotypes of Perilla leaves. Zhongguo Zhong Yao Za Zhi 2016, 41, 1823–1834. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Chen, S.; Wu, L.; Zhou, J.; Jia, K.; Ju, W. Integrated Network Pharmacology and GC-MS-Based Metabolomics to Investigate the Effect of Xiang-Su Volatile Oil Against Menopausal Depression. Front. Pharmacol. 2021, 12, 765638. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Huang, Z.; Zhong, X.M.; Feng, C.R.; Pan, A.J.; Li, Z.Y.; Ip, S.P.; Che, C.T. Effects of SYJN, a Chinese herbal formula, on chronic unpredictable stress-induced changes in behavior and brain BDNF in rats. J. Ethnopharmacol. 2010, 128, 336–341. [Google Scholar] [CrossRef]
- Ragazinskiene, O.; Gailys, V.; Jankauskiene, K.; Simoniene, G.; Jurkstiene, V. Common perilla (Perilla frutescens (L.) Britton.) as a perspective immunomodulator. Medicina 2004, 40, 220–224. [Google Scholar]
- Wang, R.; Zhang, Q.; Feng, C.; Zhang, J.; Qin, Y.; Meng, L. Advances in the Pharmacological Activities and Effects of Perilla Ketone and Isoegomaketone. Evid. Based Complement. Alternat. Med. 2022, 2022, 8809792. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl. Microbiol. Biotechnol. 2018, 102, 7775–7793. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, X.; Shang, Y.; Wang, L.; Du, K.; Chen, S.; Li, J.; He, J.; Fang, S.; Chang, Y. A comprehensive review of the botany, ethnopharmacology, phytochemistry, pharmacology, toxicity and quality control of Perillae Fructus. J. Ethnopharmacol. 2023, 304, 116022. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sun, W.; Fan, Y.N.; Li, S.Y.; Yuan, J.Q.; Zhang, Z.Q.; Li, X.Y.; Lin, M.B.; Hou, Q. Perilla Leaf Extract Attenuates Asthma Airway Inflammation by Blocking the Syk Pathway. Mediators Inflamm 2021, 2021, 6611219. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.L.; Shin, Y.S.; Choi, S.H.; Oh, S.; Kim, K.; Jeong, H.S.; Mo, J.S. Extracts of Perilla frutescens var. Acuta (Odash.) Kudo Leaves Have Antitumor Effects on Breast Cancer Cells by Suppressing YAP Activity. Evid. Based Complement. Alternat. Med. 2021, 2021, 5619761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, X.; Han, J.; Zheng, W.; Ma, W. Extract of Perilla frutescens inhibits tumor proliferation of HCC via PI3K/AKT signal pathway. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hafeez, A.A.; Fujimura, T.; Kamei, R.; Hirakawa, N.; Baba, K.; Ono, K.; Kawamoto, S. Synergistic tumor suppression by a Perilla frutescens-derived methoxyflavanone and anti-cancer tyrosine kinase inhibitors in A549 human lung adenocarcinoma. Cytotechnology 2018, 70, 913–919. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, H.R.; Woo, E.R.; Hong, S.T.; Chae, H.J.; Chae, S.W. Inhibitory effects of rosmarinic acid on adriamycin-induced apoptosis in H9c2 cardiac muscle cells by inhibiting reactive oxygen species and the activations of c-Jun N-terminal kinase and extracellular signal-regulated kinase. Biochem. Pharmacol. 2005, 70, 1066–1078. [Google Scholar] [CrossRef]
- Yamasaki, K.; Nakano, M.; Kawahata, T.; Mori, H.; Otake, T.; Ueda, N.; Oishi, I.; Inami, R.; Yamane, M.; Nakamura, M.; et al. Anti-HIV-1 Activity of Herbs in Labiatae. Biol. Pharm. Bull. 1998, 21, 829–833. [Google Scholar] [CrossRef]
- Bae, J.S.; Han, M.; Shin, H.S.; Kim, M.K.; Shin, C.Y.; Lee, D.H.; Chung, J.H. Perilla frutescens leaves extract ameliorates ultraviolet radiation-induced extracellular matrix damage in human dermal fibroblasts and hairless mice skin. J. Ethnopharmacol. 2017, 195, 334–342. [Google Scholar] [CrossRef]
- Eckert, G.P.; Franke, C.; Nöldner, M.; Rau, O.; Wurglics, M.; Schubert-Zsilavecz, M.; Müller, W.E. Plant derived omega-3-fatty acids protect mitochondrial function in the brain. Pharmacol. Res. 2010, 61, 234–241. [Google Scholar] [CrossRef]
- Zhao, G.; Zang, S.Y.; Jiang, Z.H.; Chen, Y.Y.; Ji, X.H.; Lu, B.F.; Wu, J.H.; Qin, G.W.; Guo, L.H. Postischemic administration of liposome-encapsulated luteolin prevents against ischemia-reperfusion injury in a rat middle cerebral artery occlusion model. J. Nutr. Biochem. 2011, 22, 929–936. [Google Scholar] [CrossRef]
- Yang, S.Y.; Hong, C.O.; Lee, H.; Park, S.Y.; Park, B.G.; Lee, K.W. Protective effect of extracts of Perilla frutescens treated with sucrose on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in vitro and in vivo. Food Chem. 2012, 133, 337–343. [Google Scholar] [CrossRef]
- Shin, T.Y.; Kim, S.H.; Kim, S.H.; Kim, Y.K.; Park, H.J.; Chae, B.S.; Jung, H.J.; Kim, H.M. Inhibitory effect of mast cell-mediated immediate-type allergic reactions in rats by Perilla frutescens. Immunopharmacol. Immunotoxicol. 2000, 22, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Fukumori, Y.; Inoue, M.; Nakagomi, K.; Sugie, M.; Fujita, Y.; Tomizuka, N.; Yamazaki, Y.; Oka, S. Glycoprotein derived from the hot water extract of mint plant, Perilla frutescens britton. J. Agric. Food Chem. 1999, 47, 468–472. [Google Scholar] [CrossRef]
- Makino, T.; Furuta, A.; Fujii, H.; Nakagawa, T.; Wakushima, H.; Saito, K.-I.; Kano, Y. Effect of Oral Treatment of Perilla frutescens and Its Constituents on Type-I Allergy in Mice. Biol. Pharm. Bull. 2001, 24, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Takano, H.; Sanbongi, C.; Yasuda, A.; Yanagisawa, R.; Inoue, K.; Yoshikawa, T. Anti-inflammatory and anti-allergic effect of rosmarinic acid (RA); inhibition of seasonal allergic rhinoconjunctivitis (SAR) and its mechanism. Biofactors 2004, 21, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Sanbongi, C.; Takano, H.; Osakabe, N.; Sasa, N.; Natsume, M.; Yanagisawa, R.; Inoue, K.I.; Sadakane, K.; Ichinose, T.; Yoshikawa, T. Rosmarinic acid in perilla extract inhibits allergic inflammation induced by mite allergen, in a mouse model. Clin. Exp. Allergy 2004, 34, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Li, W.; Tsubouchi, R.; Haneda, M.; Murakami, K.; Takeuchi, F.; Nisimoto, Y.; Yoshino, M. Rosmarinic acid inhibits the formation of reactive oxygen and nitrogen species in RAW264.7 macrophages. Free Radic. Res. 2005, 39, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Furuta, Y.; Wakushima, H.; Fujii, H.; Saito, K.; Kano, Y. Anti-allergic effect of Perilla frutescens and its active constituents. Phytother. Res. 2003, 17, 240–243. [Google Scholar] [CrossRef]
- Yim, Y.K.; Lee, H.; Hong, K.E.; Kim, Y.I.; Ko, S.K.; Kim, J.E.; Lee, S.Y.; Park, K.S. Anti-inflammatory and Immune-regulatory Effects of Subcutaneous Perillae Fructus Extract Injections on OVA-induced Asthma in Mice. Evid. Based Complement. Alternat. Med. 2010, 7, 79–86. [Google Scholar] [CrossRef]
- Yang, E.J.; Ku, S.K.; Lee, W.; Lee, S.; Lee, T.; Song, K.S.; Bae, J.S. Barrier protective effects of rosmarinic acid on HMGB1-induced inflammatory responses in vitro and in vivo. J. Cell. Physiol. 2013, 228, 975–982. [Google Scholar] [CrossRef]
- Ueda, H.; Yamazaki, C.; Yamazaki, M. Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol. Pharm. Bull. 2002, 25, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, H.J.; Lee, M.H.; Kim, J.; Jin, C.; Ryu, J.H. Luteolin inhibits LPS-stimulated inducible nitric oxide synthase expression in BV-2 microglial cells. Planta Med. 2006, 72, 65–68. [Google Scholar] [CrossRef]
- Oh, H.A.; Park, C.S.; Ahn, H.J.; Park, Y.S.; Kim, H.M. Effect of Perilla frutescens var. acuta Kudo and rosmarinic acid on allergic inflammatory reactions. Exp. Biol. Med. 2011, 236, 99–106. [Google Scholar] [CrossRef]
- Liu, J.Y.; Chen, Y.C.; Lin, C.H.; Kao, S.H. Perilla frutescens leaf extract inhibits mite major allergen Der p 2-induced gene expression of pro-allergic and pro-inflammatory cytokines in human bronchial epithelial cell BEAS-2B. PLoS ONE 2013, 8, e77458. [Google Scholar] [CrossRef]
- Ku, S.K.; Yang, E.J.; Song, K.S.; Bae, J.S. Rosmarinic acid down-regulates endothelial protein C receptor shedding in vitro and in vivo. Food Chem. Toxicol. 2013, 59, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Banno, N.; Akihisa, T.; Tokuda, H.; Yasukawa, K.; Higashihara, H.; Ukiya, M.; Watanabe, K.; Kimura, Y.; Hasegawa, J.; Nishino, H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci. Biotechnol. Biochem. 2004, 68, 85–90. [Google Scholar] [CrossRef]
- Ueda, H.; Yamazaki, M. Anti-inflammatory and anti-allergic actions by oral administration of a perilla leaf extract in mice. Biosci. Biotechnol. Biochem. 2001, 65, 1673–1675. [Google Scholar] [CrossRef]
- Ueda, H.; Yamazaki, M. Inhibition of tumor necrosis factor-alpha production by orally administering a perilla leaf extract. Biosci. Biotechnol. Biochem. 1997, 61, 1292–1295. [Google Scholar] [CrossRef] [PubMed]
- Saita, E.; Kishimoto, Y.; Tani, M.; Iizuka, M.; Toyozaki, M.; Sugihara, N.; Kondo, K. Antioxidant activities of Perilla frutescens against low-density lipoprotein oxidation in vitro and in human subjects. J. Oleo Sci. 2012, 61, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bu, H.; Jiang, Y.; Sun, G.; Jiang, R.; Huang, X.; Duan, H.; Huang, Z.; Wu, Q. The antidepressant effects of apigenin are associated with the promotion of autophagy via the mTOR/AMPK/ULK1 pathway. Mol. Med. Rep. 2019, 20, 2867–2874. [Google Scholar] [CrossRef]
- Park, C.H.; Min, S.Y.; Yu, H.W.; Kim, K.; Kim, S.; Lee, H.J.; Kim, J.H.; Park, Y.J. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef]
- Yue, S.; Xue, N.; Li, H.; Huang, B.; Chen, Z.; Wang, X. Hepatoprotective Effect of Apigenin Against Liver Injury via the Non-canonical NF-κB Pathway In Vivo and In Vitro. Inflammation 2020, 43, 1634–1648. [Google Scholar] [CrossRef]
- Teng, C.M.; Ko, F.N.; Wang, J.P.; Lin, C.N.; Wu, T.S.; Chen, C.C.; Huang, T.F. Antihaemostatic and antithrombotic effect of some antiplatelet agents isolated from Chinese herbs. J. Pharm. Pharmacol. 1991, 43, 667–669. [Google Scholar] [CrossRef]
- Xu, L.; Zaky, M.Y.; Yousuf, W.; Ullah, A.; Abdelbaset, G.R.; Zhang, Y.; Ahmed, O.M.; Liu, S.; Liu, H. The Anticancer Potential of Apigenin Via Immunoregulation. Curr. Pharm. Des. 2021, 27, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Song, W.; Li, D.; Jin, X. Apigenin in the regulation of cholesterol metabolism and protection of blood vessels. Exp. Ther. Med. 2017, 13, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zeng, W.; Chen, M.; Huang, L.; Li, S.; Li, Z.; Pan, Q.; Lv, S.; Yang, X.; Wang, Y.; et al. Apigenin suppresses tumor angiogenesis and growth via inhibiting HIF-1α expression in non-small cell lung carcinoma. Chem. Biol. Interact. 2022, 361, 109966. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Dietary Luteolin: A Narrative Review Focusing on Its Pharmacokinetic Properties and Effects on Glycolipid Metabolism. J. Agric. Food Chem. 2021, 69, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Boeing, T.; de Souza, P.; Speca, S.; Somensi, L.B.; Mariano, L.N.B.; Cury, B.J.; Ferreira Dos Anjos, M.; Quintão, N.L.M.; Dubuqoy, L.; Desreumax, P.; et al. Luteolin prevents irinotecan-induced intestinal mucositis in mice through antioxidant and anti-inflammatory properties. Br. J. Pharmacol. 2020, 177, 2393–2408. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Alamer, M.M.; Alam, P.; Alshetaili, A.; Haq, N.; Alanazi, F.K.; Alshehri, S.; Ghoneim, M.M.; Alsarra, I.A. Hepatoprotective Effects of Bioflavonoid Luteolin Using Self-Nanoemulsifying Drug Delivery System. Molecules 2021, 26, 7497. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Kempuraj, D.D.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Chandrasekaran, P.N.; et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. Biofactors 2021, 47, 190–197. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic Acid-Human Pharmacokinetics and Health Benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Zhou, X.J.; Yan, L.L.; Yin, P.P.; Shi, L.L.; Zhang, J.H.; Liu, Y.J.; Ma, C. Structural characterisation and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var. arguta seed flour. Food Chem. 2014, 164, 150–157. [Google Scholar] [CrossRef]
- Peng, Y.; Ye, J.; Kong, J. Determination of phenolic compounds in Perilla frutescens L. by capillary electrophoresis with electrochemical detection. J. Agric. Food Chem. 2005, 53, 8141–8147. [Google Scholar] [CrossRef]
- Meng, L.; Lozano, Y.F.; Gaydou, E.M.; Li, B. Antioxidant activities of polyphenols extracted from Perilla frutescens varieties. Molecules 2008, 14, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Nakajima, J.; Yamanashi, M.; Sugiyama, M.; Makita, Y.; Springob, K.; Awazuhara, M.; Saito, K. Metabolomics and differential gene expression in anthocyanin chemo-varietal forms of Perilla frutescens. Phytochemistry 2003, 62, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wan, Y.; Zhao, Z.; Chen, H. Determination of the content of rosmarinic acid by HPLC and analytical comparison of volatile constituents by GC-MS in different parts of Perilla frutescens (L.) Britt. Chem. Cent. J. 2013, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, Y.; Ito, M. Rosmarinic acid in Perilla frutescens and perilla herb analyzed by HPLC. J. Nat. Med. 2020, 74, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.H.; Kim, H.S.; Kang, H.J.; Lee, H.S.; Jeong, S.I.; Kim, S.J.; Jang, S.I. Anti-inflammatory and antipruritic effects of luteolin from Perilla (P. frutescens L.) leaves. Molecules 2014, 19, 6941–6951. [Google Scholar] [CrossRef]
- Zhao, G.; Qin, G.W.; Wang, J.; Chu, W.J.; Guo, L.H. Functional activation of monoamine transporters by luteolin and apigenin isolated from the fruit of Perilla frutescens (L.) Britt. Neurochem. Int. 2010, 56, 168–176. [Google Scholar] [CrossRef]
- Tantipaiboonwong, P.; Chaiwangyen, W.; Suttajit, M.; Kangwan, N.; Kaowinn, S.; Khanaree, C.; Punfa, W.; Pintha, K. Molecular Mechanism of Antioxidant and Anti-Inflammatory Effects of Omega-3 Fatty Acids in Perilla Seed Oil and Rosmarinic Acid Rich Fraction Extracted from Perilla Seed Meal on TNF-α Induced A549 Lung Adenocarcinoma Cells. Molecules 2021, 26, 6757. [Google Scholar] [CrossRef]
- Osakabe, N.; Yasuda, A.; Natsume, M.; Sanbongi, C.; Kato, Y.; Osawa, T.; Yoshikawa, T. Rosmarinic acid, a major polyphenolic component of Perilla frutescens, reduces lipopolysaccharide (LPS)-induced liver injury in D-galactosamine (D-GalN)-sensitized mice. Free Radic. Biol. Med. 2002, 33, 798–806. [Google Scholar] [CrossRef]
- Urushima, H.; Nishimura, J.; Mizushima, T.; Hayashi, N.; Maeda, K.; Ito, T. Perilla frutescens extract ameliorates DSS-induced colitis by suppressing proinflammatory cytokines and inducing anti-inflammatory cytokines. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G32–G41. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.P.; Lin, C.H.; Chen, Y.C.; Kao, S.H. Anti-inflammatory effects of Perilla frutescens leaf extract on lipopolysaccharide-stimulated RAW264.7 cells. Mol. Med. Rep. 2014, 10, 1077–1083. [Google Scholar] [CrossRef]
- Jiang, T.; Guo, K.; Liu, L.; Tian, W.; Xie, X.; Wen, S.; Wen, C. Integrated transcriptomic and metabolomic data reveal the flavonoid biosynthesis metabolic pathway in Perilla frutescens (L.) leaves. Sci. Rep. 2020, 10, 16207. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. Health effects of omega-3, 6, 9 fatty acids: Perilla frutescens is a good example of plant oils. Orient. Pharm. Exp. Med. 2011, 11, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Arya, E.; Saha, S.; Saraf, S.A.; Kaithwas, G. Effect of Perilla frutescens fixed oil on experimental esophagitis in albino Wistar rats. Biomed Res. Int. 2013, 2013, 981372. [Google Scholar] [CrossRef]
- Marseglia, G.L.; Licari, A.; Ciprandi, G. A polycentric, randomized, double blind, parallel-group, placebo-controlled study on Lertal®, a multicomponent nutraceutical, as add-on treatment in children with allergic rhinoconjunctivitis: Phase I during active treatment. J. Biol. Regul. Homeost. Agents 2019, 33, 617–622. [Google Scholar]
- Shen, M.; Liu, J.; Wang, K. Effect of Traditional Chinese Medicine on Allergic Rhinitis in Children under Data Mining. Comput. Math. Methods Med. 2022, 2022, 7007370. [Google Scholar] [CrossRef]
- Marseglia, G.; Licari, A.; Leonardi, S.; Papale, M.; Zicari, A.M.; Schiavi, L.; Ciprandi, G. A polycentric, randomized, parallel-group, study on Lertal®, a multicomponent nutraceutical, as preventive treatment in children with allergic rhinoconjunctivitis: Phase II. Ital. J. Pediatr. 2019, 45, 84. [Google Scholar] [CrossRef]
- Ariano, R. Efficacy of a novel food supplement in the relief of the signs and symptoms of seasonal allergic rhinitis and in the reduction of the consumption of anti-allergic drugs. Acta Biomed. 2015, 86, 53–58. [Google Scholar]
- Lee, J.; Jung, E.; Koh, J.; Kim, Y.S.; Park, D. Effect of rosmarinic acid on atopic dermatitis. J. Dermatol. 2008, 35, 768–771. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Kim, Y.; Lee, J.; Park, J.; Hong, S.; Hyun, C.G.; Park, D.; Kim, Y.S. Rosmarinic acid as a downstream inhibitor of IKK-beta in TNF-alpha-induced upregulation of CCL11 and CCR3. Br. J. Pharmacol. 2006, 148, 366–375. [Google Scholar] [CrossRef]
- Feng, L.-J.; Yu, C.-H.; Ying, K.-J.; Hua, J.; Dai, X.-Y. Hypolipidemic and antioxidant effects of total flavonoids of Perilla Frutescens leaves in hyperlipidemia rats induced by high-fat diet. Food Res. Int. 2011, 44, 404–409. [Google Scholar] [CrossRef]
- Hashimoto, M.; Tanabe, Y.; Hossain, S.; Matsuzaki, K.; Ohno, M.; Kato, S.; Katakura, M.; Shido, O. Intake of Alpha-Linolenic Acid-Rich Perilla frutescens Leaf Powder Decreases Home Blood Pressure and Serum Oxidized Low-Density Lipoprotein in Japanese Adults. Molecules 2020, 25, 2099. [Google Scholar] [CrossRef]
- Carbonneau, M.A.; Léger, C.L.; Monnier, L.; Bonnet, C.; Michel, F.; Fouret, G.; Dedieu, F.; Descomps, B. Supplementation with wine phenolic compounds increases the antioxidant capacity of plasma and vitamin E of low-density lipoprotein without changing the lipoprotein Cu(2+)-oxidizability: Possible explanation by phenolic location. Eur. J. Clin. Nutr. 1997, 51, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Tani, M.; Kishimoto, Y.; Saita, E.; Toyozaki, M.; Kondo, K. Inhibitory effects of balsamic vinegar on LDL oxidation and lipid accumulation in THP-1 macrophages. J. Nutr. Sci. Vitaminol. 2010, 56, 421–427. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wang, Y.; Li, X.; Zhou, L.; Yang, J.; Guo, L. Metabolites and chemometric study of Perilla (Perilla frutescens) from different varieties and geographical origins. J. Food Sci. 2022, 87, 5240–5251. [Google Scholar] [CrossRef]
- Tavva, V.S.; Kim, Y.H.; Kagan, I.A.; Dinkins, R.D.; Kim, K.H.; Collins, G.B. Increased alpha-tocopherol content in soybean seed overexpressing the Perilla frutescens gamma-tocopherol methyltransferase gene. Plant Cell Rep. 2007, 26, 61–70. [Google Scholar] [CrossRef]
- Müller-Waldeck, F.; Sitzmann, J.; Schnitzler, W.H.; Grassmann, J. Determination of toxic perilla ketone, secondary plant metabolites and antioxidative capacity in five Perilla frutescens L. varieties. Food Chem. Toxicol. 2010, 48, 264–270. [Google Scholar] [CrossRef]
- Milde, J.; Elstner, E.F.; Grassmann, J. Synergistic effects of phenolics and carotenoids on human low-density lipoprotein oxidation. Mol. Nutr. Food Res. 2007, 51, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, H.; Kalinowska, M. New Polyphenol-Containing LDL Nano-Preparations in Oxidative Stress and DNA Damage: A Potential Route for Cell-Targeted PP Delivery. Materials 2020, 13, 5106. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-Based Dietary Patterns, Plant Foods, and Age-Related Cognitive Decline. Adv. Nutr. 2019, 10, S422–S436. [Google Scholar] [CrossRef]
- Phu, H.T.; Thuan, D.T.B.; Nguyen, T.H.D.; Posadino, A.M.; Eid, A.H.; Pintus, G. Herbal Medicine for Slowing Aging and Aging-associated Conditions: Efficacy, Mechanisms and Safety. Curr. Vasc. Pharmacol. 2020, 18, 369–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Matsuzaki, K.; Maruyama, K.; Sumiyoshi, E.; Hossain, S.; Wakatsuki, H.; Kato, S.; Ohno, M.; Tanabe, Y.; Kuroda, Y.; et al. Perilla frutescens seed oil combined with Anredera cordifolia leaf powder attenuates age-related cognitive decline by reducing serum triglyceride and glucose levels in healthy elderly Japanese individuals: A possible supplement for brain health. Food Funct. 2022, 13, 7226–7239. [Google Scholar] [CrossRef]
- Hashimoto, M.; Matsuzaki, K.; Maruyama, K.; Hossain, S.; Sumiyoshi, E.; Wakatsuki, H.; Kato, S.; Ohno, M.; Tanabe, Y.; Kuroda, Y.; et al. Perilla seed oil in combination with nobiletin-rich ponkan powder enhances cognitive function in healthy elderly Japanese individuals: A possible supplement for brain health in the elderly. Food Funct. 2022, 13, 2768–2781. [Google Scholar] [CrossRef]
- Hashimoto, M.; Matsuzaki, K.; Hossain, S.; Ito, T.; Wakatsuki, H.; Tanabe, Y.; Ohno, M.; Kato, S.; Yamashita, K.; Shido, O. Perilla Seed Oil Enhances Cognitive Function and Mental Health in Healthy Elderly Japanese Individuals by Enhancing the Biological Antioxidant Potential. Foods 2021, 10, 1130. [Google Scholar] [CrossRef]
- Hashimoto, M.; Matsuzaki, K.; Kato, S.; Hossain, S.; Ohno, M.; Shido, O. Twelve-month Studies on Perilla Oil Intake in Japanese Adults-Possible Supplement for Mental Health. Foods 2020, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zeng, X.; Yang, K.; Peng, H.; Chen, J. n-3 polyunsaturated fatty acids improve depression-like behavior by inhibiting hippocampal neuroinflammation in mice via reducing TLR4 expression. Immun. Inflamm. Dis. 2022, 10, e707. [Google Scholar] [CrossRef]
- Appleton, K.M.; Voyias, P.D.; Sallis, H.M.; Dawson, S.; Ness, A.R.; Churchill, R.; Perry, R. Omega-3 fatty acids for depression in adults. Cochrane Database Syst. Rev. 2021, 11, Cd004692. [Google Scholar] [CrossRef]
- Gao, X.; Su, X.; Han, X.; Wen, H.; Cheng, C.; Zhang, S.; Li, W.; Cai, J.; Zheng, L.; Ma, J.; et al. Unsaturated Fatty Acids in Mental Disorders: An Umbrella Review of Meta-Analyses. Adv. Nutr. 2022, 13, 2217–2236. [Google Scholar] [CrossRef]
- Mengelberg, A.; Leathem, J.; Podd, J.; Hill, S.; Conlon, C. The effects of docosahexaenoic acid supplementation on cognition and well-being in mild cognitive impairment: A 12-month randomised controlled trial. Int. J. Geriatr. Psychiatry 2022, 37, 1–12. [Google Scholar] [CrossRef]
- Mischoulon, D.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Lamon-Fava, S.; Rakofsky, J.J.; Nierenberg, A.A.; Clain, A.J.; Mletzko Crowe, T.; Wong, A.; et al. Omega-3 Fatty Acids for Major Depressive Disorder With High Inflammation: A Randomized Dose-Finding Clinical Trial. J. Clin. Psychiatry 2022, 83, 22cr03241. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, A.; Yang, Y.; Zhao, Y.; Wang, C.C.; Wang, Y.; Han, J.; Wang, Z.; Wen, M. DHA and EPA Prevent Seizure and Depression-Like Behavior by Inhibiting Ferroptosis and Neuroinflammation via Different Mode-of-Actions in a Pentylenetetrazole-Induced Kindling Model in Mice. Mol. Nutr. Food Res. 2022, 66, e2200275. [Google Scholar] [CrossRef]
- Lucas, M.; Mirzaei, F.; O’Reilly, E.J.; Pan, A.; Willett, W.C.; Kawachi, I.; Koenen, K.; Ascherio, A. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: A 10-y prospective follow-up study. Am. J. Clin. Nutr. 2011, 93, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Bourre, J.M. Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J. Nutr. Health Aging 2004, 8, 163–174. [Google Scholar]
- Chiu, C.C.; Frangou, S.; Chang, C.J.; Chiu, W.C.; Liu, H.C.; Sun, I.W.; Liu, S.I.; Lu, M.L.; Chen, C.H.; Huang, S.Y.; et al. Associations between n-3 PUFA concentrations and cognitive function after recovery from late-life depression. Am. J. Clin. Nutr. 2012, 95, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Egerton, S.; Donoso, F.; Fitzgerald, P.; Gite, S.; Fouhy, F.; Whooley, J.; Dinan, T.G.; Cryan, J.F.; Culloty, S.C.; Ross, R.P.; et al. Investigating the potential of fish oil as a nutraceutical in an animal model of early life stress. Nutr. Neurosci. 2022, 25, 356–378. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Able, J.; Liu, Y.; Jandacek, R.; Rider, T.; Tso, P.; Lipton, J.W. Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: Dissociation from estrogenic effects. J. Psychiatr. Res. 2009, 43, 656–663. [Google Scholar] [CrossRef]
- Verspohl, E.J.; Fujii, H.; Homma, K.; Buchwald-Werner, S. Testing of Perilla frutescens extract and Vicenin 2 for their antispasmodic effect. Phytomedicine 2013, 20, 427–431. [Google Scholar] [CrossRef]
- Buchwald-Werner, S.; Fujii, H.; Schön, C.; Doebis, C. Healthy ingredients-Investigation of a Perilla frutescens special extract. Anti-inflammatory and immune-modulatory properties. Agro. Food Industry Hi Technol. 2012, 23, 38. [Google Scholar]
- Buchwald-Werner, S.; Fujii, H.; Reule, C.; Schoen, C. Perilla extract improves gastrointestinal discomfort in a randomized placebo controlled double blind human pilot study. BMC Complement. Altern. Med. 2014, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Thumann, T.A.; Pferschy-Wenzig, E.M.; Moissl-Eichinger, C.; Bauer, R. The role of gut microbiota for the activity of medicinal plants traditionally used in the European Union for gastrointestinal disorders. J. Ethnopharmacol. 2019, 245, 112153. [Google Scholar] [CrossRef]
- Lisa, E.L.; Dragostin, O.M.; Petroaie, A.D.; Gurau, G.; Cristea, A.; Pavel, A.; Bonifate, F.; Popa, P.S.; Matei, M. The Effect of the New Imidazole Derivatives Complexation with Betacyclodextrin, on the Antifungal Activity in Oropharyngeal Infections. Processes 2022, 10, 2697. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Ogawa, T. Antimicrobial activity of perilla seed polyphenols against oral pathogenic bacteria. Biosci. Biotechnol. Biochem. 2002, 66, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Nishiyama, Y.; Hasumi, Y.; Yamaguchi, H.; Abe, S.; Uchida, K. The vapor activity of oregano, perilla, tea tree, lavender, clove, and geranium oils against a Trichophyton mentagrophytes in a closed box. J. Infect. Chemother. 2006, 12, 349–354. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, X.; Luo, M.; Li, H.; Dong, J.; Wang, J.; Leng, B.; Wang, X.; Feng, H.; Ren, W. Subinhibitory concentrations of perilla oil affect the expression of secreted virulence factor genes in Staphylococcus aureus. PLoS ONE 2011, 6, e16160. [Google Scholar] [CrossRef]
- Kanzaki, T.; Kimura, S. Occupational allergic contact dermatitis from Perilla frutescens (shiso). Contact Dermat. 1992, 26, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.Y.; Park, H.S.; Choi, J.H.; Kim, S.H.; Min, K.U. Two cases of anaphylaxis caused by perilla seed. J. Allergy Clin. Immunol. 2006, 117, 1505–1506. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, W.Y.; Yong, S.J.; Shin, K.; Kim, C.; Lee, J.-H.; Jung, Y.R.; Kim, H.; Yu, T.-S.; Kim, S.-H. Occupational asthma caused by inhaling smoke from roasting perilla seeds. Allergy Asthma Respir. Dis. 2013, 1, 90. [Google Scholar] [CrossRef]
| Activity | Part Used | Compound | Dose | Subject | Model | Reference |
|---|---|---|---|---|---|---|
| Anti-inflammatory and antiallergic effects | Leaves | AE + RA | AE—500 mg/kg RA—19 mg/kg | ddY mice | PCA response elicited by OVA | [27] |
| Herb | Water decoction | 500 mg/kg | Balb/c mice | PCA response elicited by OVA | [23] | |
| Seeds | AE | 100 mL | C57BL/6 mice | Asthma provoked by OVA | [28] | |
| Seeds | RA | 0.7 or 1.4 mg | Endothelial cell and mice | HMGB1 expression and HMGB1-mediated regulation of immune activation | [29] | |
| Leaves | AE and Luteolin | 1 mg for both | ICR mice | Induced ear edema | [30] | |
| Leaves | Luteolin | 1, 5 and 10 μM | BV-2 microglial cells | Nitric oxide production is enhanced by lipopolysaccharides | [31] | |
| Leaves | RA | 30% ethanol extract | Mice; human mast cells | Respiratory allergic manifestations | [32] | |
| Leaves | AE | 0.01 g/kg | SD rats, Rat peritoneal mast cell | Allergic response caused by anti-DNP IgE | [21] | |
| Leaves | Glycoprotein from the hot water extract | 0.5 mg/mL | Rat peritoneal mast cell | Induced histamine release | [22] | |
| Leaves | Methanol extract | 5–50 μg/mL | Human bronchial epithelial cells | Induced allergen gene expression | [33] | |
| Leaves | RA | Cell culture—0–2 μM Mice—1.4 mg/12 h | HUVECs, C57BL/6 mice | Production and interaction of inflammatory cytokines | [34] | |
| Leaves | RA | 1.5 mg/24 h | C3H/He mice | Induced allergic asthma | [25] | |
| Leaves | Nine triterpene acids from ethanol extract | Inhibitory dose—0.09–0.3 mg | ICR mice | Ear inflammation | [35] | |
| Leaves | AE | 400 μL | ICR mice | Ear edema | [36] | |
| Leaves | AE | 1 mg b.i.w. | C3H/He mice | Influence over TNF-a production | [37] | |
| Antioxidant and hypolypemiant effects | Leaves | AE | 1.7 or 4.6 mg/mL | HUVECs, healthy female volunteers | Induced lipid oxidation | [38] |
| Seeds | RA | 20 mg/mL | H9c2 cardiac muscle cells | Induced programmed cell death | [15] | |
| Seeds | RA | 10–50 μM | RAW 264.7 cells | Induced lipid production | [26] |
| Disease | Number of Patients | Compound | Dose | Time-Frame | Results | Reference |
|---|---|---|---|---|---|---|
| Allergic rhinoconjunctivitis (AR) | 128 children (Lertal Group-LG: 64 patients; Observation Group-OG: 64 patients) | Lertal: Quercetin 150 mg, Perilla frutescens 80 mg (as dry extract of the seeds containing rosmarinic acid, luteolin, apigenin and chrysoeriol), and Vitamin D3 5 mcg (200 IU). | 1 tab/day | 4–12 weeks |
| [69] |
| Allergic rhinoconjunctivitis (AR) | 146 children (LG+ standard treatment: 73 patients; OG+ standard treatment: 73 patients) | Lertal | 1 tab q.d. | Baseline, after 2 and 4 weeks |
| [67] |
| Seasonal allergic rhinoconjunctivitis (SAR) | 23 adults (16 women, and 7 men), without control group | Lertal | 1 tab b.i.d. | Baseline and after 1 month |
| [70] |
| Seasonal allergic rhinoconjunctivitis (SAR) | Rosmarinic acid 50 mg (9 patients) Rosmarinic acid 200 mg (10 patients) Placebo (n10 patients) | Rosmarinic acid | 50 or 100 mg q.d. | 21 days |
| [24] |
| Atopic dermatitis (AD) | 21 patients (14 women and 7 men) | Rosmarinic acid cream | 0.3%, topical application b.i.d | 8 weeks |
| [71] |
| Main Outcome(s) | Number of Patients | Compound | Dose | Time-Frame | Results | Reference |
|---|---|---|---|---|---|---|
| LDL oxidation and antioxidant enzyme expression | 8 healthy female volunteers | Red Perilla frutescens extract | 120 mL single dose | Plasma at baseline, 30 min, 1, 2, and 4 h |
| [38] |
| Mental function, fatty acid profile, biological antioxidant potential | 32 healthy elderly volunteers (17 women, and 15 men) | Perilla frutescens seeds oil (PO) Anredera cordifolia (AC) leaf powder | PO: 1.47 mL q.d. PO + AC: 1.47 mL of PO and 1.12 g of AC | Baseline, and at 12 months |
| [87] |
| Cognitive function | 49 healthy elderly (24 men and 25 women) | Perilla frutescens seeds oil (PO) Nobiletin-rich air-dried immature ponkan powder (PP) | PO: 1.47 mL (0.88 g of ALA) q.d. PO + PP: 1.47 mL of PO and 1.12 g ponkan powder (2.91 mg of nobiletin) | Baseline, and at 12 months |
| [88] |
| Biological Antioxidant Potential | PO group (n = 42 patients) Control group (n = 33 patients) | Perilla frutescens seeds oil (PO) | PO: 7.0 mL of PO q.d. Control: 7.0 mL of canola oil q.d. | Baseline, and at 12 months |
| [89] |
| Mental condition | PO group (n = 38 patients) Placebo group (n = 37 patients) | Perilla frutescens seeds oil (PO) | PO: 7.0 mL of PO q.d. Control: 7.0 mL of olive oil q.d. | Baseline, and at 12 months |
| [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam, G.; Robu, S.; Flutur, M.-M.; Cioanca, O.; Vasilache, I.-A.; Adam, A.-M.; Mircea, C.; Nechita, A.; Harabor, V.; Harabor, A.; et al. Applications of Perilla frutescens Extracts in Clinical Practice. Antioxidants 2023, 12, 727. https://doi.org/10.3390/antiox12030727
Adam G, Robu S, Flutur M-M, Cioanca O, Vasilache I-A, Adam A-M, Mircea C, Nechita A, Harabor V, Harabor A, et al. Applications of Perilla frutescens Extracts in Clinical Practice. Antioxidants. 2023; 12(3):727. https://doi.org/10.3390/antiox12030727
Chicago/Turabian StyleAdam, Gigi, Silvia Robu, Mihaela-Magdalena Flutur, Oana Cioanca, Ingrid-Andrada Vasilache, Ana-Maria Adam, Cornelia Mircea, Aurel Nechita, Valeriu Harabor, AnaMaria Harabor, and et al. 2023. "Applications of Perilla frutescens Extracts in Clinical Practice" Antioxidants 12, no. 3: 727. https://doi.org/10.3390/antiox12030727
APA StyleAdam, G., Robu, S., Flutur, M.-M., Cioanca, O., Vasilache, I.-A., Adam, A.-M., Mircea, C., Nechita, A., Harabor, V., Harabor, A., & Hancianu, M. (2023). Applications of Perilla frutescens Extracts in Clinical Practice. Antioxidants, 12(3), 727. https://doi.org/10.3390/antiox12030727







