Anti-Inflammatory and Antioxidant Properties of β-Sitosterol in Copper Sulfate-Induced Inflammation in Zebrafish (Danio rerio)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Experimental Conditions
2.2. Main Reagents
2.3. Experimental Design
2.3.1. Preparation of Test Samples
2.3.2. Chelating Ability of β-Sitosterol for Cu2+
2.3.3. Antioxidant Effect of β-Sitosterol In Vitro
2.3.4. In Vivo Antioxidant Test
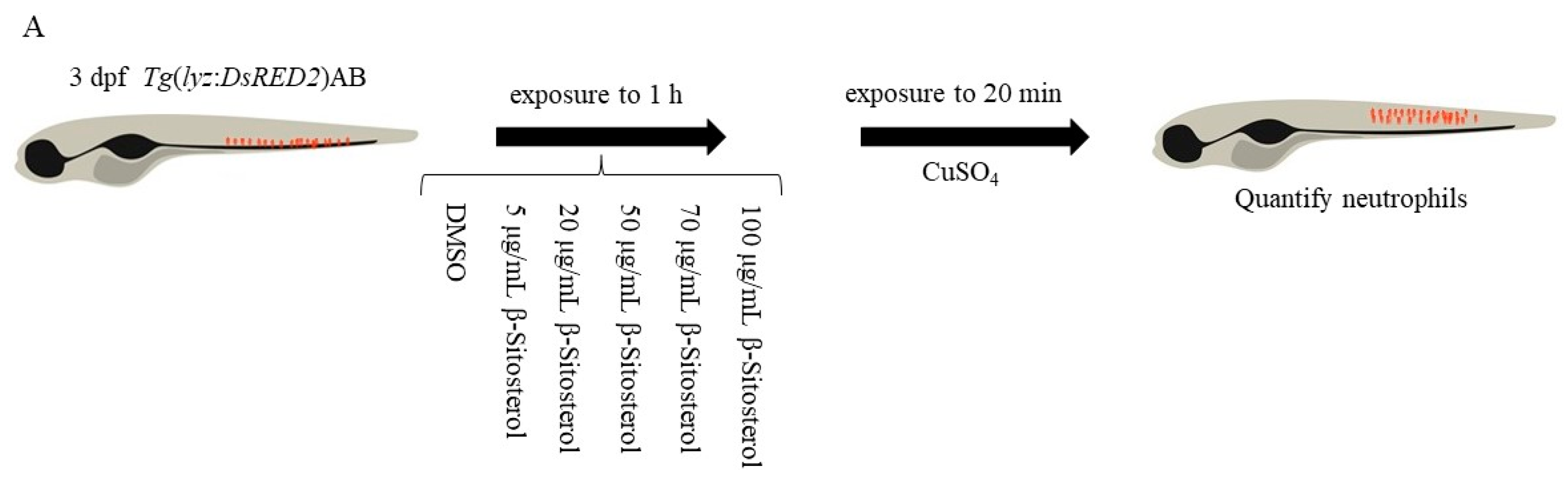
2.3.5. Neutrophil Migration Assay
2.3.6. Fluorescence Assay Quantification Method
2.3.7. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.3.8. Data Presentation and Statistical Analyses
3. Results
3.1. Chelating Ability of β-Sitosterol for Cu2+
3.2. Antioxidant Effect of β-Sitosterol
3.2.1. In Vitro Antioxidant Effect of β-Sitosterol
3.2.2. In Vivo Antioxidant Effect of β-Sitosterol
3.2.3. Effect of β-Sitosterol on the Expressions of the Antioxidant Genes sod and gpx4b
3.3. Anti-Inflammatory Effect of β-Sitosterol in Zebrafish
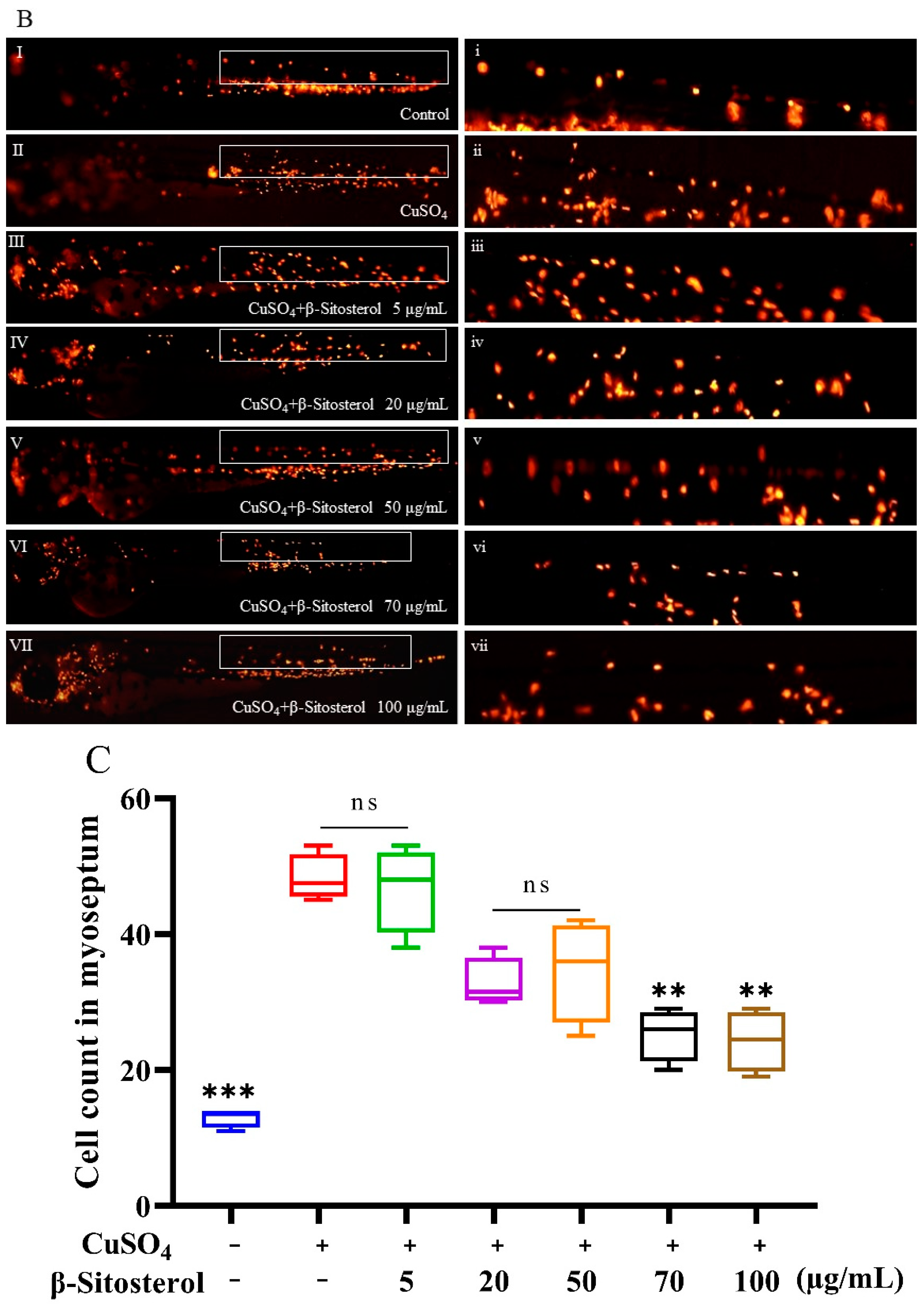
3.3.1. Effect of β-Sitosterol on Neutrophil Migration
3.3.2. Effects of β-Sitosterol on the Expressions of il-8 and myd88
4. Discussion
4.1. In Vivo Antioxidant Effect of β-Sitosterol
4.2. Anti-Inflammatory Effect of β-Sitosterol in Zebrafish
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef]
- Li, F.; Xiaoyu, L.; Weiliang, Z.; Fujiang, G.; Yingchun, W.; Rui, W.; Kaixian, C.; Cheng, H.; Yiming, L. Inhibition of human neutrophil elastase by pentacyclic triterpenes. PLoS ONE 2013, 8, e82794. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Jun, L.; Yixin, W.; John, H.W.; Yu, C.; Zhaoting, L.; Quanyin, H. Mucoadhesive probiotic backpacks with ROS nanoscavengers enhance the bacteriotherapy for inflammatory bowel diseases. Sci. Adv. 2022, 8, eabp8798. [Google Scholar] [CrossRef]
- Alessandro, D.; Jessica, K.; Mona, S.; Federica, M.; Bruno, C.; Fowler, C.J.; Valentina, O. Exploring the fatty acid amide hydrolase and cyclooxygenase inhibitory properties of novel amide derivatives of ibuprofen. J. Enzym. Inhib. Med. Chem. 2020, 35, 815–823. [Google Scholar] [CrossRef]
- Christian, C.; Emmanuel, M. Non-steroidal anti-inflammatory drugs in the pharmacological management of osteoarthritis in the very old: Prescribe or proscribe? Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211022149. [Google Scholar] [CrossRef]
- Liang, M.; Tzu-Wen, L.; Wallig, M.A.; Dobrucki, I.T.; Dobrucki, L.W.; Nelson, E.R.; Swanson, K.S.; Smith, A.M. Efficient Targeting of Adipose Tissue Macrophages in Obesity with Polysaccharide Nanocarriers. ACS Nano 2016, 10, 6952–6962. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Kim, H.-S.; Kim, S.-Y.; Lee, S.-H.; Lee, W.W.; Jeon, Y.-J. Identification of sterols from the soft coral Dendronephthya gigantea and their anti-inflammatory potential. Environ. Toxicol. Pharmacol. 2017, 55, 37–43. [Google Scholar] [CrossRef]
- Hussain, A.; AlAjmi, M.F.; Rehman, M.T.; Amir, S.; Husain, F.M.; Alsalme, A.; Siddiqui, M.A.; AlKhedhairy, A.A.; Khan, R.A. Copper(II) complexes as potential anticancer and Nonsteroidal anti-inflammatory agents: In vitro and in vivo studies. Sci. Rep. 2019, 9, 5237. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.J.; Medici, V.; Heffern, M.C. Correction: A caged imidazopyrazinone for selective bioluminescence detection of labile extracellular copper(ii). Chem. Sci. 2022, 13, 5774. [Google Scholar] [CrossRef]
- Brandão, P.T.C.; Martha, C.M.; Reis, B.M. Copper toxicology, oxidative stress and inflammation using zebrafish as experimental model. J. Appl. Toxicol. 2016, 36, 876–885. [Google Scholar] [CrossRef]
- Di Paola, D.; Capparucci, F.; Lanteri, G.; Crupi, R.; Marino, Y.; Franco, G.A.; Cuzzocrea, S.; Spano, N.; Gugliandolo, E.; Peritore, A.F. Environmental Toxicity Assessment of Sodium Fluoride and Platinum-Derived Drugs Co-Exposure on Aquatic Organisms. Toxics 2022, 10, 272. [Google Scholar] [CrossRef]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef]
- Valerio, M.; Awad, A.B. β-Sitosterol down-regulates some pro-inflammatory signal transduction pathways by increasing the activity of tyrosine phosphatase SHP-1 in J774A.1 murine macrophages. Int. Immunopharmacol. 2011, 11, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Tan, Y.Y.; Ding, Y.; Fang, Y.; Yang, X.; Fang, J.; Xu, D.C.; Zhang, H.; Lu, W.; Li, M.; et al. β-Sitosterol improves experimental colitis in mice with a target against pathogenic bacteria. J. Cell. Biochem. 2019, 120, 5687–5694. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Pérez, R.; Madrigal-Bujaida, E.; Reyes-Cadena, S.; Álvarez-González, I.; Sánchez-Chapul, L.; Pérez-Gallaga, L.; Hernández, N.; Flores-Mondragón, G.; Velasco, O. Cell protection induced by beta-sitosterol: Inhibition of genotoxic damage, stimulation of lymphocyte production, and determination of its antioxidant capacity. Arch. Toxicol. 2008, 82, 615–622. [Google Scholar] [CrossRef]
- Di Paola, D.; Abbate, J.M.; Iaria, C.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Environmental Risk Assessment of Dexamethasone Sodium Phosphate and Tocilizumab Mixture in Zebrafish Early Life Stage (Danio rerio). Toxics 2022, 10, 279. [Google Scholar] [CrossRef]
- Santos, J.S.; Alvarenga Brizola, V.R.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Yuan, H.H.; Bao, X.L.; Lan, M.B. In vitro antioxidant and cytotoxic properties of ethanol extract of Alpinia oxyphylla fruits. Pharm. Biol. 2013, 51, 1419–1425. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Le, H.D.; Kim, T.N.T.; The, H.P.; Nguyen, T.M.; Cornet, V.; Lambert, J.; Kestemont, P. Anti–Inflammatory and Antioxidant Properties of the Ethanol Extract of Clerodendrum Cyrtophyllum Turcz in Copper Sulfate-Induced Inflammation in Zebrafish. Antioxidants 2020, 9, 192. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Jia, X.; Wang, K.; Tu, Y.; Wang, R.; Liu, K.; Lu, T.; He, C. In Vitro and In Vivo Anti-inflammatory Effects of Polyphyllin Vii Through Downregulating Mapk and Nf-kappa B Pathways. Chem. Chem. 2019, 24, 875. [Google Scholar] [CrossRef]
- Marc Sultan, V.A.; Risch, T.; Schuette, M.; Dökel, S.; Ralser, M.; Balzereit, D.; Lehrach, H.; Yaspo, M.-L. Influence of RNA extraction methods and library selection schemes on RNA-seq data. BMC Genom. 2014, 15, 675. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Leite, C.E.; Maboni, L.d.O.; Cruz, F.F.; Rosemberg, D.B.; Zimmermann, F.F.; Pereira, T.C.B.; Bogo, M.R.; Bonan, C.D.; Campos, M.M.; Morrone, F.B.; et al. Involvement of purinergic system in inflammation and toxicity induced by copper in zebrafish larvae. Toxicol. Appl. Pharmacol. 2013, 272, 681–689. [Google Scholar] [CrossRef]
- Hernandez, P.P.; Undurraga, C.; Gallardo, V.E.; Mackenzie, N.; Allende, M.L.; Reyes, A.E. Sublethal concentrations of waterborne copper induce cellular stress and cell death in zebrafish embryos and larvae. Biol. Res. 2011, 44, 7–15. [Google Scholar] [CrossRef]
- Olivari, F.A.; Hernández, P.P.; Allende, M.L. Acute copper exposure induces oxidative stress and cell death in lateral line hair cells of zebrafish larvae. Brain Res. 2008, 1244, 1–12. [Google Scholar] [CrossRef]
- Francesco, S.; Gianluca, P.; Domenico, C.; Domenico, C.; Tonino, C.; Ermanno, V. Monitoring antioxidants by coulometry: Quantitative assessment of the strikingly high antioxidant capacity of bergamot (Citrus bergamia R.) by-products. Talanta 2023, 251, 123765. [Google Scholar] [CrossRef]
- Jesús, R.M. Anti-Inflammatory and Antioxidant Properties of Plant Extracts. Antioxidants 2021, 10, 921. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Wang, M.-H. Antioxidant and nitric oxide release inhibition activities of methanolic extract from Clerodendrum cyrtophyllum Turcz. Hortic. Environ. Biotechnol. 2011, 52, 309–314. [Google Scholar] [CrossRef]
- Wang, Y.T.; Chen, G.C. Regulation of oxidative stress-induced autophagy by ATG9A ubiquitination. Autophagy 2022, 18, 2008–2010. [Google Scholar] [CrossRef] [PubMed]
- Lirong, H.; Kun, J.; Haibin, X.; Guiyou, T.; Jiaxin, X.; Wei, Y.; Chen, L.; Xiaoping, X.; Huiqiang, L. Oxyfluorfen exposure can cause acute kidney injury by promoting ROS-induced oxidative stress and inflammation in zebrafish. J. Hazard. Mater. 2022, 440, 129823. [Google Scholar] [CrossRef]
- Ying, W.; Junhua, L.; Huiming, Q.; Chunjing, C.; Hui, L.; Jie, C. β-Carotene extracted from Blakeslea trispora attenuates oxidative stress, inflammatory, hepatic injury and immune damage induced by copper sulfate in zebrafish (Danio rerio). Comp. Biochem. Physiology. Toxicol. Pharmacol. 2022, 258, 109366. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ko, C.-I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.-S.; Jeon, Y.-J. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar] [CrossRef]
- Shibo, S.; Yici, Z.; Weiping, X.; Yue, Z.; Rui, Y.; Jianli, G.; Shui, G.; Qiang, M.; Kun, M.; Jianqiang, X. Chlorophyllin Inhibits Mammalian Thioredoxin Reductase 1 and Triggers Cancer Cell Death. Antioxidants 2021, 10, 1733. [Google Scholar] [CrossRef]
- Devi, R.R.; Arumughan, C. Antiradical efficacy of phytochemical extracts from defatted rice bran. Food Chem. Toxicol. 2007, 45, 2014–2021. [Google Scholar] [CrossRef]
- Mohanan, P.V.; Devi, K.S. Toxicological evaluation of sobatum. Cancer Lett. 1998, 127, 135–140. [Google Scholar] [CrossRef]
- Ambavade, S.D.; Misar, A.V.; Ambavade, P.D. Pharmacological, nutritional, and analytical aspects of β-sitosterol: A review. Orient. Pharm. Exp. Med. 2014, 14, 193–211. [Google Scholar] [CrossRef]
- Maharajan, K.; Muthulakshmi, S.; Nataraj, B.; Ramesh, M.; Kadirvelu, K. Toxicity assessment of pyriproxyfen in vertebrate model zebrafish embryos (Danio rerio): A multi biomarker study. Aquat. Toxicol. 2018, 196, 132–145. [Google Scholar] [CrossRef]
- Wang, C.; Harwood, J.D.; Zhang, Q. Oxidative stress and DNA damage in common carp (Cyprinus carpio) exposed to the herbicide mesotrione. Chemosphere 2018, 193, 1080–1086. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Park, S.Y.; Choi, J. Expression of catalase and glutathione S-transferase genes in Chironomus riparius on exposure to cadmium and nonylphenol. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 399–408. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Huang, H.; Ma, Y.; Wang, R.; Hu, Y.; Zheng, X.; Chen, C.; Tang, H. Squid Ink Polysaccharides Protect Human Fibroblast Against Oxidative Stress by Regulating NADPH Oxidase and Connexin43. Front. Pharmacol. 2019, 10, 1574. [Google Scholar] [CrossRef]
- Chaudhary, V.; Katyal, P.; Panwar, H.; Kaur, J.; Aluko, R.E.; Puniya, A.K.; Poonia, A.K. Antioxidative, anti-inflammatory, and anticancer properties of the red biopigment extract from Monascus purpureus (MTCC 369). J. Food Biochem. 2022, 46, e14249. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. β-Sitosterol Alleviates Inflammatory Response via Inhibiting the Activation of ERK/p38 and NF-κB Pathways in LPS-Exposed BV2 Cells. Biomed. Res. Int. 2020, 2020, 7532306. [Google Scholar] [CrossRef]
- Christine, W.; Markus, R.; Shah, A.H.; Ralf, M.; Urban, L.; Clemens, G. Facilitating drug discovery: An automated high-content inflammation assay in zebrafish. J. Vis. Exp. 2012, 16, e4203. [Google Scholar] [CrossRef]
- d’Alençon, C.A.; Oscar, P.; Christine, W.; Viviana, G.; Rebecca, J.; Felix, L.; Urban, L.; Clemens, G.; Miguel, A. A high-throughput chemically induced inflammation assay in zebrafish. BMC Biol. 2010, 8, 151. [Google Scholar] [CrossRef]
- Mara, C.; Keat, L.W.; Adina, G.; Nandula, S.V.; Manisha, B.; Qiong, S.; Francesco, B.; Maurilio, P.; Marta, S.; Andrea, C.; et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature 2009, 459, 717–721. [Google Scholar] [CrossRef]
- Jacques, D.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef]
- Delitto, D.; Delitto, A.E.; DiVita, B.E.; Pham, K.; Han, S.; Hartlage, E.R.; Newby, B.N.; Gerber, M.H.; Behrns, K.E.; Moldawer, L.L.; et al. Human Pancreatic Cancer Cells Induce a MyD88-Dependent Stromal Response to Promote a Tumor-Tolerant Immune Microenvironment. Cancer Res. 2017, 77, 672–683. [Google Scholar] [CrossRef]
- Zhu, X.; Burfeind, K.G.; Michaelis, K.A.; Braun, T.P.; Olson, B.; Pelz, K.R.; Morgan, T.K.; Marks, D.L. MyD88 signalling is critical in the development of pancreatic cancer cachexia. J. Cachexia Sarcopenia Muscle 2019, 10, 378–390. [Google Scholar] [CrossRef]
- Ruslan, M. The spectrum of inflammatory responses. Science 2021, 734, 1070–1075. [Google Scholar] [CrossRef]
- Holmes, W.; Lee, J.; Kuang, W.; Rice, G.; Wood, W. Structure and functional expression of a human interleukin-8 receptor. Science 1991, 253, 1278–1280. [Google Scholar] [CrossRef]
- de Oliveira, S.; Reyes-Aldasoro, C.C.; Candel, S.; Renshaw, S.A.; Mulero, V.; Calado, Â. Cxcl8 (IL-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J. Immunol. 2013, 190, 4349–4359. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, D.; Zhou, X.Q.; Yin, L.; Feng, L.; Jiang, W.D.; Liu, Y.; Tang, L.; Wu, P.; Zhao, Y. Vitamin D inhibits lipopolysaccharide-induced inflammatory response potentially through the Toll-like receptor 4 signalling pathway in the intestine and enterocytes of juvenile Jian carp (Cyprinus carpio var. Jian). Br. J. Nutr. 2015, 114, 1560–1568. [Google Scholar] [CrossRef]





| Gene Name | GenBank Accession No. | Forward and Reverse Primer Sequences (5′-3′) |
|---|---|---|
| β-actin [20] | AF057040 | F: CCCCATTGAGCACGGTATTG R: ATACATGGCAGGGGTGTTGA |
| il-8 | XM_009306855.3 | F: GAGAGGTCTGGCTGTAGATC R: AGTTGTCATCAAGGTGGCAAT |
| myd88 | NM_212814.2 | F: GTGATGCCTGTGATTTTCAGACTAA R: CGGCCTCTTCATGGATTTGT |
| sod [20] | NM_131294.1 | F: ATGGTGAACAAGGCCGTTTG R: AAAGCATGGACGTGGAAACC |
| gpx4b [20] | BC095133.1 | F: TGAGAAGGGTTTACGCATCCTG R: TGTTGTTCCCCAGTGTTCCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Liu, N.; Xue, M.; Zhang, M.; Liu, W.; Xu, C.; Fan, Y.; Meng, Y.; Zhang, Q.; Zhou, Y. Anti-Inflammatory and Antioxidant Properties of β-Sitosterol in Copper Sulfate-Induced Inflammation in Zebrafish (Danio rerio). Antioxidants 2023, 12, 391. https://doi.org/10.3390/antiox12020391
Zhang P, Liu N, Xue M, Zhang M, Liu W, Xu C, Fan Y, Meng Y, Zhang Q, Zhou Y. Anti-Inflammatory and Antioxidant Properties of β-Sitosterol in Copper Sulfate-Induced Inflammation in Zebrafish (Danio rerio). Antioxidants. 2023; 12(2):391. https://doi.org/10.3390/antiox12020391
Chicago/Turabian StyleZhang, Peng, Naicheng Liu, Mingyang Xue, Mengjie Zhang, Wei Liu, Chen Xu, Yuding Fan, Yan Meng, Qinghua Zhang, and Yong Zhou. 2023. "Anti-Inflammatory and Antioxidant Properties of β-Sitosterol in Copper Sulfate-Induced Inflammation in Zebrafish (Danio rerio)" Antioxidants 12, no. 2: 391. https://doi.org/10.3390/antiox12020391
APA StyleZhang, P., Liu, N., Xue, M., Zhang, M., Liu, W., Xu, C., Fan, Y., Meng, Y., Zhang, Q., & Zhou, Y. (2023). Anti-Inflammatory and Antioxidant Properties of β-Sitosterol in Copper Sulfate-Induced Inflammation in Zebrafish (Danio rerio). Antioxidants, 12(2), 391. https://doi.org/10.3390/antiox12020391










