Neuroprotective Properties of Berberine: Molecular Mechanisms and Clinical Implications
Abstract
:1. Introduction
2. An Overview of the Neuroprotective Effects of BBR
3. Molecular Mechanisms of BBR’s Neuroprotection
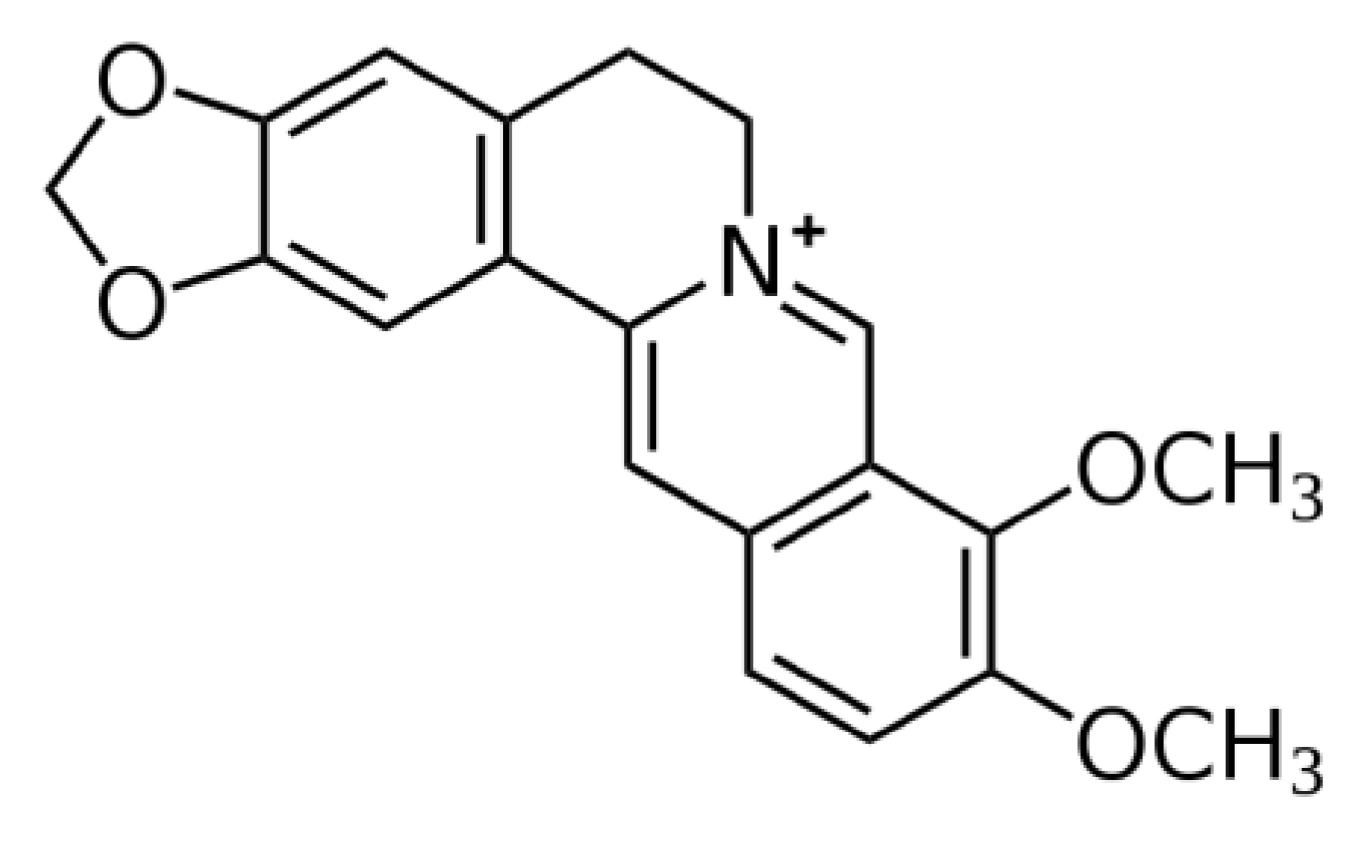
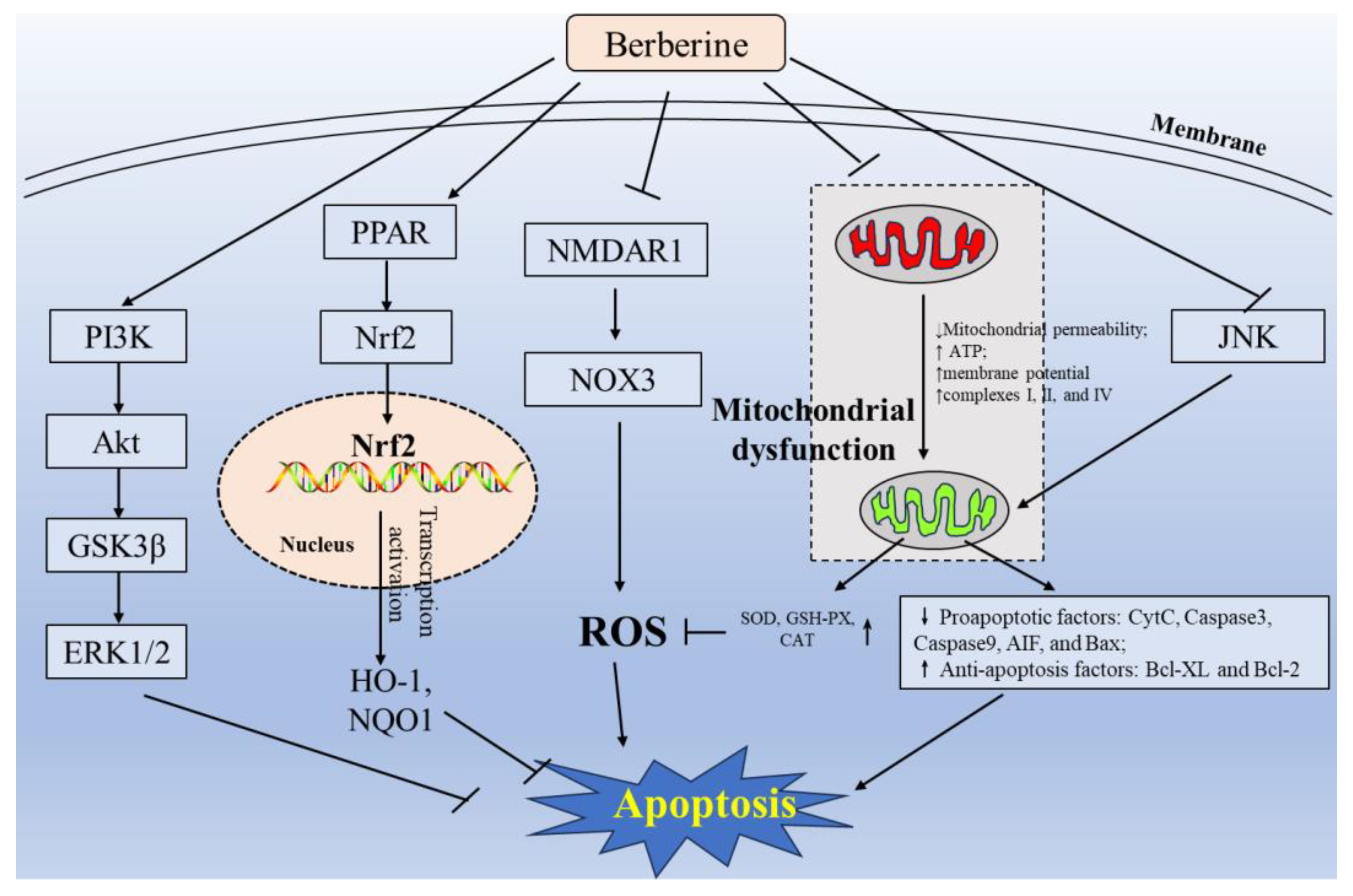
3.1. Inhibition of Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis
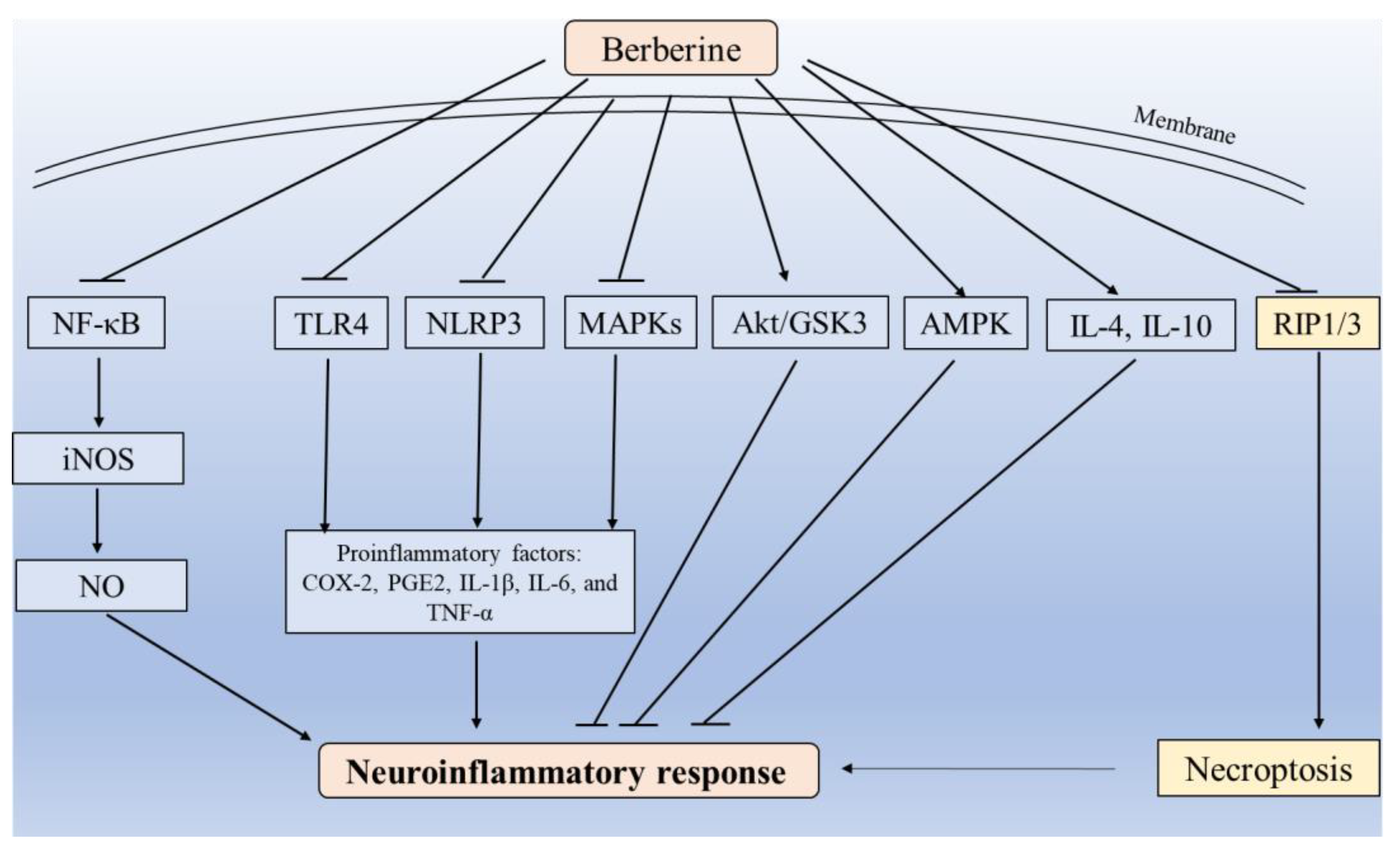
3.2. Blockade of Inflammatory Response and Necroptosis
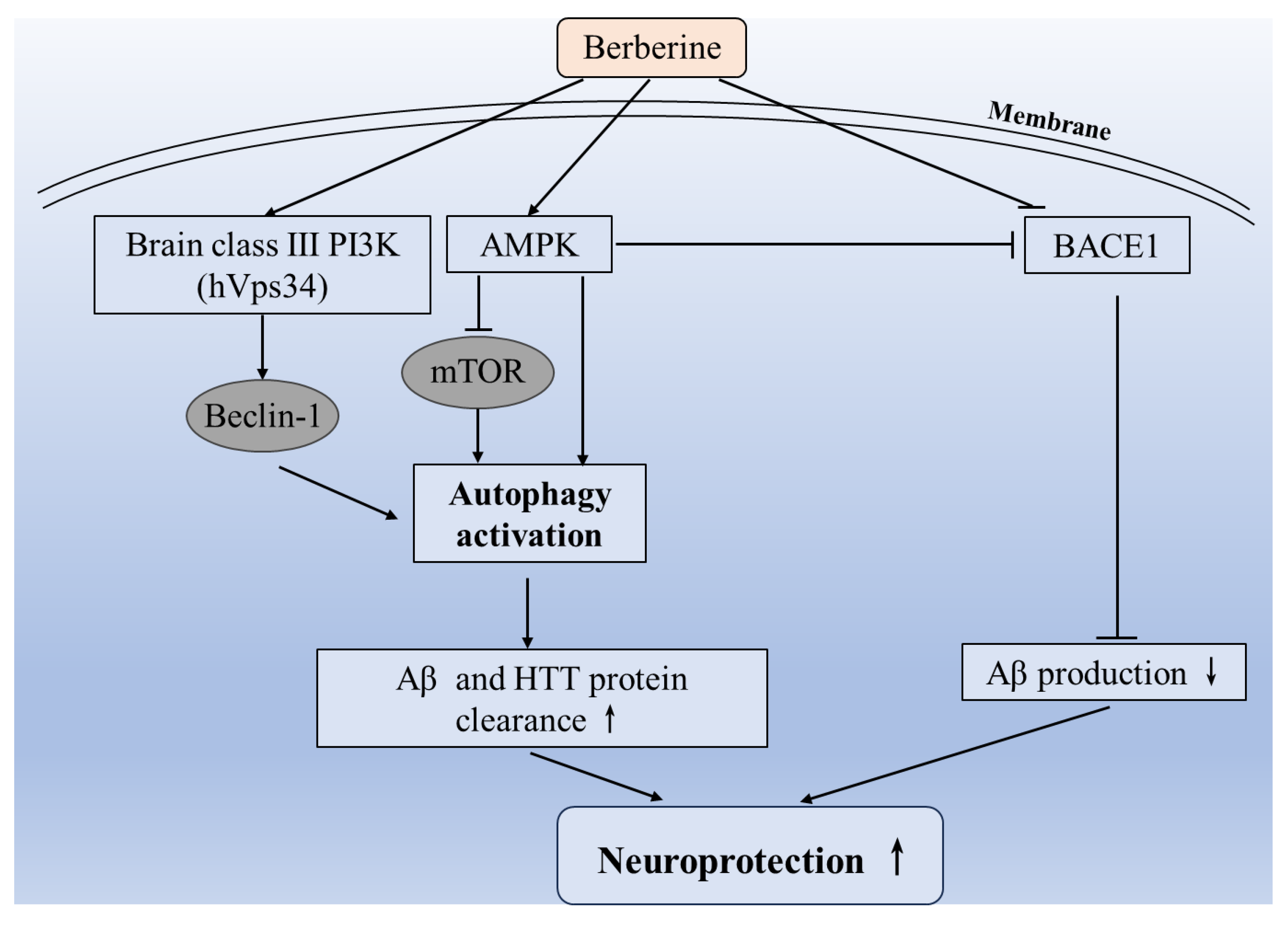
3.3. Induction of Autophagy
3.4. Modulation of Neurotransmitters
3.5. Modulation of CYP450 Enzyme Activities
3.6. Others
4. Safety and Toxic Adverse of BBR
5. Clinical Trials and Therapeutic Applications
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hesari, A.; Ghasemi, F.; Cicero, A.F.G.; Mohajeri, M.; Rezaei, O.; Hayat, S.M.G.; Sahebkar, A. Berberine: A potential adjunct for the treatment of gastrointestinal cancers? J. Cell. Biochem. 2018, 119, 9655–9663. [Google Scholar] [CrossRef] [PubMed]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.; Chintala, R.; Duddukuri, G.R. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin. Cancer Biol. 2016, 40–41, 48–81. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis 2015, 243, 449–461. [Google Scholar] [CrossRef]
- Zhou, H.; Feng, L.; Xu, F.; Sun, Y.; Ma, Y.; Zhang, X.; Liu, H.; Xu, G.; Wu, X.; Shen, Y.; et al. Berberine inhibits palmitate-induced NLRP3 inflammasome activation by triggering autophagy in macrophages: A new mechanism linking berberine to insulin resistance improvement. Biomed. Pharmacother. 2017, 89, 864–874. [Google Scholar] [CrossRef]
- Shayganfard, M. Berberine: Is a Promising Agent for Mental Disorders Treatment? Curr. Mol. Pharmacol. 2022, 16, 307–320. [Google Scholar] [CrossRef]
- Li, Z.; Geng, Y.N.; Jiang, J.D.; Kong, W.J. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid.-Based Complement. Altern. Med. 2014, 2014, 289264. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, D.; Wang, Y.; Zhang, M.; Qiang, X.; Liao, M.; Liu, X.; Wu, H.; Zhang, Y. Berberine protects liver from ethanol-induced oxidative stress and steatosis in mice. Food Chem. Toxicol. 2014, 74, 225–232. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Kang, C.; Che, S.; Su, J.; Sun, Q.; Ge, T.; Guo, Y.; Lv, J.; Sun, Z.; Yang, W.; et al. Berberine: A Promising Treatment for Neurodegenerative Diseases. Front. Pharmacol. 2022, 13, 845591. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberine and barberry (Berberis vulgaris): A clinical review. Phytother. Res. PTR 2019, 33, 504–523. [Google Scholar] [CrossRef]
- Habtemariam, S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol. Res. 2020, 155, 104722. [Google Scholar] [CrossRef] [PubMed]
- Khoshandam, A.; Imenshahidi, M.; Hosseinzadeh, H. Pharmacokinetic of berberine, the main constituent of Berberis vulgaris L.: A comprehensive review. Phytother. Res. PTR 2022, 36, 4063–4079. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.K.; Dhir, A. Berberine: A plant alkaloid with therapeutic potential for central nervous system disorders. Phytother. Res. PTR 2010, 24, 317–324. [Google Scholar] [CrossRef]
- Ahmed, T.; Gilani, A.U.; Abdollahi, M.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. Berberine and neurodegeneration: A review of literature. Pharmacol. Rep. 2015, 67, 970–979. [Google Scholar] [CrossRef]
- Zhu, J.R.; Lu, H.D.; Guo, C.; Fang, W.R.; Zhao, H.D.; Zhou, J.S.; Wang, F.; Zhao, Y.L.; Li, Y.M.; Zhang, Y.D.; et al. Berberine attenuates ischemia-reperfusion injury through inhibiting HMGB1 release and NF-κB nuclear translocation. Acta Pharmacol. Sin. 2018, 39, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.S.; Matsumoto, K.; Murakami, Y.; Hori, H.; Zhao, Q.; Obi, R. Berberine exerts neuroprotective actions against in vitro ischemia-induced neuronal cell damage in organotypic hippocampal slice cultures: Involvement of B-cell lymphoma 2 phosphorylation suppression. Biol. Pharm. Bull. 2009, 32, 79–85. [Google Scholar] [CrossRef]
- Zhu, F.; Qian, C. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer’s disease. BMC Neurosci. 2006, 7, 78. [Google Scholar] [CrossRef]
- Simões Pires, E.N.; Frozza, R.L.; Hoppe, J.B.; Menezes Bde, M.; Salbego, C.G. Berberine was neuroprotective against an in vitro model of brain ischemia: Survival and apoptosis pathways involved. Brain Res. 2014, 1557, 26–33. [Google Scholar] [CrossRef]
- Wang, L.; Sheng, W.; Tan, Z.; Ren, Q.; Wang, R.; Stoika, R.; Liu, X.; Liu, K.; Shang, X.; Jin, M. Treatment of Parkinson’s disease in Zebrafish model with a berberine derivative capable of crossing blood brain barrier, targeting mitochondria, and convenient for bioimaging experiments. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2021, 249, 109151. [Google Scholar] [CrossRef]
- Sadeghnia, H.R.; Kolangikhah, M.; Asadpour, E.; Forouzanfar, F.; Hosseinzadeh, H. Berberine protects against glutamate-induced oxidative stress and apoptosis in PC12 and N2a cells. Iran. J. Basic Med. Sci. 2017, 20, 594–603. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, N. Berberine: Pathways to protect neurons. Phytother. Res. PTR 2018, 32, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, B.Q.; Li, Y.H.; Jiang, Q.Q.; Cong, W.H.; Chen, K.J.; Wen, X.M.; Wu, Z.Z. Lactoferrin modification of berberine nanoliposomes enhances the neuroprotective effects in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2023, 18, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, X.; Yang, L.; Wang, Y. Berberine Protects against Neurological Impairments and Blood-Brain Barrier Injury in Mouse Model of Intracerebral Hemorrhage. Neuroimmunomodulation 2021, 29, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Chen, S.; Li, Z.; Jia, X.; Wang, K.; Bao, J.; Liang, Y.; Wang, X.; Chen, M.; et al. Berberine protects against 6-OHDA-induced neurotoxicity in PC12 cells and zebrafish through hormetic mechanisms involving PI3K/AKT/Bcl-2 and Nrf2/HO-1 pathways. Redox Biol. 2017, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, S.; Li, X. Therapeutic potential of berberine against neurodegenerative diseases. Sci. China Life Sci. 2015, 58, 564–569. [Google Scholar] [CrossRef]
- Shaker, F.H.; El-Derany, M.O.; Wahdan, S.A.; El-Demerdash, E.; El-Mesallamy, H.O. Berberine ameliorates doxorubicin-induced cognitive impairment (chemobrain) in rats. Life Sci. 2021, 269, 119078. [Google Scholar] [CrossRef]
- Ye, M.; Fu, S.; Pi, R.; He, F. Neuropharmacological and pharmacokinetic properties of berberine: A review of recent research. J. Pharm. Pharmacol. 2009, 61, 831–837. [Google Scholar] [CrossRef]
- Abdel Moneim, A.E. The neuroprotective effect of berberine in mercury-induced neurotoxicity in rats. Metab. Brain Dis. 2015, 30, 935–942. [Google Scholar] [CrossRef]
- Hussien, H.M.; Abd-Elmegied, A.; Ghareeb, D.A.; Hafez, H.S.; Ahmed, H.E.A.; El-Moneam, N.A. Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food Chem. Toxicol. 2018, 111, 432–444. [Google Scholar] [CrossRef]
- Saleh, S.R.; Abady, M.M.; Nofal, M.; Yassa, N.W.; Abdel-Latif, M.S.; Nounou, M.I.; Ghareeb, D.A.; Abdel-Monaem, N. Berberine Nanoencapsulation Attenuates Hallmarks of Scoplomine Induced Alzheimer’s-like Disease in Rats. Curr. Rev. Clin. Exp. Pharmacol. 2021, 16, 139–154. [Google Scholar] [CrossRef]
- Raju, M.; Kunde, S.S.; Auti, S.T.; Kulkarni, Y.A.; Wairkar, S. Berberine loaded nanostructured lipid carrier for Alzheimer’s disease: Design, statistical optimization and enhanced in vivo performance. Life Sci. 2021, 285, 119990. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Yang, Q.; Xiang, Y.; Zhang, Y.; Wan, J.; Liu, S.; Li, N.; Peng, W. Nose to brain drug delivery—A promising strategy for active components from herbal medicine for treating cerebral ischemia reperfusion. Pharmacol. Res. 2020, 159, 104795. [Google Scholar] [CrossRef]
- Tavakkoli, A.; Iranshahi, M.; Hasheminezhad, S.H.; Hayes, A.W.; Karimi, G. The neuroprotective activities of natural products through the Nrf2 upregulation. Phytother. Res. PTR 2019, 33, 2256–2273. [Google Scholar] [CrossRef] [PubMed]
- Abo El-Enin, H.A.; Elkomy, M.H.; Naguib, I.A.; Ahmed, M.F.; Alsaidan, O.A.; Alsalahat, I.; Ghoneim, M.M.; Eid, H.M. Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route. Pharmaceuticals 2022, 15, 281. [Google Scholar] [CrossRef]
- Fan, D.; Liu, L.; Wu, Z.; Cao, M. Combating Neurodegenerative Diseases with the Plant Alkaloid Berberine: Molecular Mechanisms and Therapeutic Potential. Curr. Neuropharmacol. 2019, 17, 563–579. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, T.; Lou, H.; Song, H.; Li, C.; Fan, P. Anticancer Effects of Honokiol via Mitochondrial Dysfunction Are Strongly Enhanced by the Mitochondria-Targeting Carrier Berberine. J. Med. Chem. 2020, 63, 11786–11800. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Q.; Ma, S.R.; Zhao, Z.X.; Pan, L.B.; Cong, L.; Han, P.; Peng, R.; Yu, H.; Lin, Y.; et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct. Target. Ther. 2021, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Vejux, A. Cell Death, Inflammation and Oxidative Stress in Neurodegenerative Diseases: Mechanisms and Cytoprotective Molecules. Int. J. Mol. Sci. 2021, 22, 13657. [Google Scholar] [CrossRef]
- Saleem, U.; Sabir, S.; Niazi, S.G.; Naeem, M.; Ahmad, B. Role of Oxidative Stress and Antioxidant Defense Biomarkers in Neurodegenerative Diseases. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 311–322. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Thanan, R.; Oikawa, S.; Hiraku, Y.; Ohnishi, S.; Ma, N.; Pinlaor, S.; Yongvanit, P.; Kawanishi, S.; Murata, M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2014, 16, 193–217. [Google Scholar] [CrossRef]
- Li, J.; Ao, W.; Li, W.; Jiang, Z.-G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Fernandes, C.; Oliveira, C.; Benfeito, S.; Soares, P.; Garrido, J.; Borges, F. Nanotechnology and antioxidant therapy: An emerging approach for neurodegenerative diseases. Curr. Med. Chem. 2014, 21, 4311–4327. [Google Scholar] [CrossRef]
- Danta, C.C.; Piplani, P. The discovery and development of new potential antioxidant agents for the treatment of neurodegenerative diseases. Expert Opin. Drug Discov. 2014, 9, 1205–1222. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Araiza, C.; Álvarez-Mejía, A.L.; Sánchez-Torres, S.; Farfan-García, E.; Mondragón-Lozano, R.; Pinto-Almazán, R.; Salgado-Ceballos, H. Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free Radic. Res. 2013, 47, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases. Molecules 2018, 23, 3305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Zhou, S.W. Protective effect of berberine on antioxidant enzymes and positive transcription elongation factor b expression in diabetic rat liver. Fitoterapia 2011, 82, 184–189. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Li, G.; Gao, J. Berberine-Albumin Nanoparticles: Preparation, Thermodynamic Study and Evaluation Their Protective Effects Against Oxidative Stress in Primary Neuronal Cells as a Model of Alzheimer’s Disease. J. Biomed. Nanotechnol. 2021, 17, 1088–1097. [Google Scholar] [CrossRef]
- Yaw, L.S.; Lindsei, S.; Karmin, O. Redox regulation in health and disease—Therapeutic potential of berberine. Food Res. Int. 2011, 44, 2409–2417. [Google Scholar] [CrossRef]
- Thirupurasundari, C.J.; Padmini, R.; Devaraj, S.N. Effect of berberine on the antioxidant status, ultrastructural modifications and protein bound carbohydrates in azoxymethane-induced colon cancer in rats. Chem. Biol. Interact. 2009, 177, 190–195. [Google Scholar] [CrossRef]
- Tan, Y.; Tang, Q.; Hu, B.R.; Xiang, J.Z. Antioxidant properties of berberine on cultured rabbit corpus cavernosum smooth muscle cells injured by hydrogen peroxide. Acta Pharmacol. Sin. 2007, 28, 1914–1918. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.K.; Nandi, M.K.; Mishra, G.; Maurya, A.; Rai, A.; Rai, G.K.; Awasthi, R.; Sharma, B.; Kulkarni, G.T. Berberine: A Plant-derived Alkaloid with Therapeutic Potential to Combat Alzheimer’s disease. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 154–170. [Google Scholar] [CrossRef]
- Shirwaikar, A.; Shirwaikar, A.; Rajendran, K.; Punitha, I.S. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol. Pharm. Bull. 2006, 29, 1906–1910. [Google Scholar] [CrossRef]
- Pirmoradi, Z.; Yadegari, M.; Moradi, A.; Khojasteh, F.; Zare Mehrjerdi, F. Effect of berberine chloride on caspase-3 dependent apoptosis and antioxidant capacity in the hippocampus of the chronic cerebral hypoperfusion rat model. Iran. J. Basic Med. Sci. 2019, 22, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Hei, Z.Q.; Nie, H.; Tang, F.T.; Huang, H.Q.; Li, X.J.; Deng, Y.H.; Chen, S.R.; Guo, F.F.; Huang, W.G.; et al. Berberine ameliorates renal injury in streptozotocin-induced diabetic rats by suppression of both oxidative stress and aldose reductase. Chin. Med. J. 2008, 121, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Kazaz, I.O.; Mentese, A.; Demir, S.; Kerimoglu, G.; Colak, F.; Bodur, A.; Alver, A.; Kutlu, O.; Turedi, S. Berberine inhibits the ischemia-reperfusion induced testicular injury through decreasing oxidative stress. Am. J. Emerg. Med. 2020, 38, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Kuo, W.H.; Lin, T.W.; Chang, H.R.; Lin, T.H.; Chen, P.N.; Chu, S.C. Protective effects of berberine against low-density lipoprotein (LDL) oxidation and oxidized LDL-induced cytotoxicity on endothelial cells. J. Agric. Food Chem. 2007, 55, 10437–10445. [Google Scholar] [CrossRef]
- Germoush, M.O.; Mahmoud, A.M. Berberine mitigates cyclophosphamide-induced hepatotoxicity by modulating antioxidant status and inflammatory cytokines. J. Cancer Res. Clin. Oncol. 2014, 140, 1103–1109. [Google Scholar] [CrossRef]
- Deng, H.; Jia, Y.; Pan, D.; Ma, Z. Berberine alleviates rotenone-induced cytotoxicity by antioxidation and activation of PI3K/Akt signaling pathway in SH-SY5Y cells. Neuroreport 2020, 31, 41–47. [Google Scholar] [CrossRef]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Tawari, S.; Patil, S.; Dixit, P.; Umathe, S.; Mundhada, D. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav. Brain Res. 2011, 220, 30–41. [Google Scholar] [CrossRef]
- Yokozawa, T.; Ishida, A.; Kashiwada, Y.; Cho, E.J.; Kim, H.Y.; Ikeshiro, Y. Coptidis Rhizoma: Protective effects against peroxynitrite-induced oxidative damage and elucidation of its active components. J. Pharm. Pharmacol. 2004, 56, 547–556. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oboh, G.; Okeke, B.M. Comparative effects of berberine and piperine on the neuroprotective potential of neostigmine. J. Complement. Integr. Med. 2021, 18, 491–497. [Google Scholar] [CrossRef]
- Sarna, L.K.; Wu, N.; Hwang, S.Y.; Siow, Y.L.; Karmin, O. Berberine inhibits NADPH oxidase mediated superoxide anion production in macrophages. Can. J. Physiol. Pharmacol. 2010, 88, 369–378. [Google Scholar] [CrossRef]
- Zhuang, W.; Li, T.; Wang, C.; Shi, X.; Li, Y.; Zhang, S.; Zhao, Z.; Dong, H.; Qiao, Y. Berberine exerts antioxidant effects via protection of spiral ganglion cells against cytomegalovirus-induced apoptosis. Free Radic. Biol. Med. 2018, 121, 127–135. [Google Scholar] [CrossRef]
- Dai, C.; Tian, E.; Hao, Z.; Tang, S.; Wang, Z.; Sharma, G.; Jiang, H.; Shen, J. Aflatoxin B1 Toxicity and Protective Effects of Curcumin: Molecular Mechanisms and Clinical Implications. Antioxidants 2022, 11, 2031. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Kang, Y.M.; Gao, H.L.; Shi, X.L.; Fu, L.Y.; Li, Y.; Jia, X.Y.; Liu, K.L.; Qi, J.; Li, H.B.; et al. Chronic infusion of berberine into the hypothalamic paraventricular nucleus attenuates hypertension and sympathoexcitation via the ROS/Erk1/2/iNOS pathway. Phytomed. Int. J. Phytother. Phytopharm. 2019, 52, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.W.; Li, X.X.; Tang, Y.S.; Lim-Ho Kong, B.; Wu, H.Y.; Xiao, M.J.; Cheung, C.K.; Shaw, P.C. Novel mechanistic insight on the neuroprotective effect of berberine: The role of PPARδ for antioxidant action. Free Radic. Biol. Med. 2022, 181, 62–71. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; León, R. Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol. Ther. 2016, 157, 84–104. [Google Scholar] [CrossRef]
- Li, D.; Dai, C.; Yang, X.; Wang, F.; Yu, X.; Xiao, X.; Tang, S. Critical role of p21 on olaquindox-induced mitochondrial apoptosis and S-phase arrest involves activation of PI3K/AKT and inhibition of Nrf2/HO-1pathway. Food Chem. Toxicol. 2017, 108, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Tang, S.; Zhang, S.; Zhang, C.; Wang, C.; Zhou, Y.; Dai, C.; Xiao, X. Furazolidone induces apoptosis through activating reactive oxygen species-dependent mitochondrial signaling pathway and suppressing PI3K/Akt signaling pathway in HepG2 cells. Food Chem. Toxicol. 2015, 75, 173–186. [Google Scholar] [CrossRef]
- Hsu, Y.Y.; Tseng, Y.T.; Lo, Y.C. Berberine, a natural antidiabetes drug, attenuates glucose neurotoxicity and promotes Nrf2-related neurite outgrowth. Toxicol. Appl. Pharmacol. 2013, 272, 787–796. [Google Scholar] [CrossRef]
- Hsu, Y.Y.; Chen, C.S.; Wu, S.N.; Jong, Y.J.; Lo, Y.C. Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent mechanism in NSC34 motor neuron-like cells. Eur. J. Pharm. Sci. 2012, 46, 415–425. [Google Scholar] [CrossRef]
- Phadwal, K.; Vrahnas, C.; Ganley, I.G.; MacRae, V.E. Mitochondrial Dysfunction: Cause or Consequence of Vascular Calcification? Front. Cell Dev. Biol. 2021, 9, 611922. [Google Scholar] [CrossRef]
- Bonora, M.; Giorgi, C.; Pinton, P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat. Rev. Mol. Cell Biol. 2022, 23, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Su, P.; Lv, C.; Guo, L.; Cao, G.; Qin, C.; Zhang, W. Berberine Alleviates Amyloid β-Induced Mitochondrial Dysfunction and Synaptic Loss. Oxidative Med. Cell. Longev. 2019, 2019, 7593608. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Q.; Zeng, X.N.; Kong, H.; Sun, X.L. Neuroprotective effects of berberine on stroke models in vitro and in vivo. Neurosci. Lett. 2008, 447, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Chopra, K. Verapamil augments the neuroprotectant action of berberine in rat model of transient global cerebral ischemia. Eur. J. Pharmacol. 2013, 720, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qian, Z.; Pan, L.; Li, H.; Zhu, H. Hypoxia-inducible factor 1 mediates the anti-apoptosis of berberine in neurons during hypoxia/ischemia. Acta Physiol. Hung. 2012, 99, 311–323. [Google Scholar] [CrossRef]
- Klegeris, A.; McGeer, P.L. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr. Alzheimer Res. 2005, 2, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Di Matteo, V.; Benigno, A.; Pierucci, M.; Crescimanno, G.; Di Giovanni, G. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp. Neurol. 2007, 205, 295–312. [Google Scholar] [CrossRef]
- Apetz, N.; Munch, G.; Govindaraghavan, S.; Gyengesi, E. Natural compounds and plant extracts as therapeutics against chronic inflammation in Alzheimer’s disease--a translational perspective. CNS Neurol. Disord. Drug Targets 2014, 13, 1175–1191. [Google Scholar] [CrossRef]
- Lu, D.Y.; Tang, C.H.; Chen, Y.H.; Wei, I.H. Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J. Cell. Biochem. 2010, 110, 697–705. [Google Scholar] [CrossRef]
- Gabet, B.; Kuo, P.C.; Fuentes, S.; Patel, Y.; Adow, A.; Alsakka, M.; Avila, P.; Beam, T.; Yen, J.H.; Brown, D.A. Identification of N-benzyltetrahydroisoquinolines as novel anti-neuroinflammatory agents. Bioorg. Med. Chem. 2018, 26, 5711–5717. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Hung, T.H.; Lee, C.Y.; Wang, L.F.; Wu, C.H.; Ke, C.H.; Chen, S.F. Berberine protects against neuronal damage via suppression of glia-mediated inflammation in traumatic brain injury. PLoS ONE 2014, 9, e115694. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Shi, D.D.; Li, W.; Cheng, D.; Zhang, Y.D.; Zhang, S.; Tsoi, B.; Zhao, J.; Wang, Z.; Zhang, Z.J. Berberine ameliorates depression-like behaviors in mice via inhibiting NLRP3 inflammasome-mediated neuroinflammation and preventing neuroplasticity disruption. J. Neuroinflamm. 2023, 20, 54. [Google Scholar] [CrossRef]
- Wong, L.R.; Tan, E.A.; Lim, M.E.J.; Shen, W.; Lian, X.L.; Wang, Y.; Chen, L.; Ho, P.C. Functional effects of berberine in modulating mitochondrial dysfunction and inflammatory response in the respective amyloidogenic cells and activated microglial cells—In vitro models simulating Alzheimer’s disease pathology. Life Sci. 2021, 282, 119824. [Google Scholar] [CrossRef]
- Sadraie, S.; Kiasalari, Z.; Razavian, M.; Azimi, S.; Sedighnejad, L.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Roghani, M. Berberine ameliorates lipopolysaccharide-induced learning and memory deficit in the rat: Insights into underlying molecular mechanisms. Metab. Brain Dis. 2019, 34, 245–255. [Google Scholar] [CrossRef]
- Jia, L.; Liu, J.; Song, Z.; Pan, X.; Chen, L.; Cui, X.; Wang, M. Berberine suppresses amyloid-beta-induced inflammatory response in microglia by inhibiting nuclear factor-kappaB and mitogen-activated protein kinase signalling pathways. J. Pharm. Pharmacol. 2012, 64, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Wang, C.; Li, Y.; Dong, L.; Cui, L.; Wang, L.; Liu, Z.; Qiao, H.; Zhu, C.; et al. Neuroprotection of early and short-time applying berberine in the acute phase of cerebral ischemia: Up-regulated pAkt, pGSK and pCREB, down-regulated NF-κB expression, ameliorated BBB permeability. Brain Res. 2012, 1459, 61–70. [Google Scholar] [CrossRef]
- Nam, K.N.; Kim, J.H.; Jung, H.J.; Park, J.-M.; Moon, S.K.; Kim, Y.; Kim, S.Y.; Lee, E.H. Berberine inhibits inflammatory activation of rat brain microglia. Neural Regen. Res. 2010, 5, 1384–1390. [Google Scholar] [CrossRef]
- Liu, Y.M.; Niu, L.; Wang, L.L.; Bai, L.; Fang, X.Y.; Li, Y.C.; Yi, L.T. Berberine attenuates depressive-like behaviors by suppressing neuro-inflammation in stressed mice. Brain Res. Bull. 2017, 134, 220–227. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Li, F.; An, L. Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int. Immunopharmacol. 2016, 38, 426–433. [Google Scholar] [CrossRef]
- Tian, Y.; Zheng, Y.; Wang, Q.; Yan, F.; Tao, Z.; Zhao, F.; Wang, Y.; Huang, Y.; Li, F.; Du, Y.; et al. Berberine Ameliorates Cognitive Impairment by Regulating Microglial Polarization and Increasing Expression of Anti-inflammatory Factors following Permanent Bilateral Common Carotid Artery Occlusion in Rats. CNS Neurol. Disord. Drug Targets 2022, 21, 869–879. [Google Scholar] [CrossRef]
- Yoo, K.Y.; Hwang, I.K.; Kim, J.D.; Kang, I.J.; Park, J.; Yi, J.S.; Kim, J.K.; Bae, Y.S.; Won, M.H. Antiinflammatory effect of the ethanol extract of Berberis koreana in a gerbil model of cerebral ischemia/reperfusion. Phytother. Res. PTR 2008, 22, 1527–1532. [Google Scholar] [CrossRef]
- Lomphithak, T.; Akara-Amornthum, P.; Murakami, K.; Hashimoto, M.; Usubuchi, H.; Iwabuchi, E.; Unno, M.; Cai, Z.; Sasano, H.; Jitkaew, S. Tumor necroptosis is correlated with a favorable immune cell signature and programmed death-ligand 1 expression in cholangiocarcinoma. Sci. Rep. 2021, 11, 11743. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, L.; Fei, X.C.; Jiang, X.F.; Zheng, Z.; Zhao, Y.; Wang, C.F.; Li, B.; Chen, S.J.; Janin, A.; et al. MYC is a positive regulator of choline metabolism and impedes mitophagy-dependent necroptosis in diffuse large B-cell lymphoma. Blood Cancer J. 2017, 7, 8503511. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, J.; Ai, G.; Liu, J.; Luo, N.; Li, C.; Cheng, Z. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol. Res. 2019, 52, 37. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Chowdhury, K.D.; Chatterjee, S.; Sarkar, A.; Paul, S.; Sur, P.K.; Sadhukhan, G.C. Modulation of adenylate cyclase signaling in association with MKK3/6 stabilization under combination of SAC and berberine to reduce HepG2 cell survivability. Apoptosis 2017, 22, 1362–1379. [Google Scholar] [CrossRef]
- Ou, X.; Hua, Y.; Liao, X.; Gong, C.; Kang, Y. Cognitive impairments induced by severe acute pancreatitis are attenuated by berberine treatment in rats. Mol. Med. Rep. 2018, 18, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Lee, Y.C.; Lee, K.H.; Lin, H.C.; Chen, C.L.; Shen, C.J.; Huang, C.C. Therapeutic effect of berberine on TDP-43-related pathogenesis in FTLD and ALS. J. Biomed. Sci. 2016, 23, 72. [Google Scholar] [CrossRef]
- Jiang, W.; Wei, W.; Gaertig, M.A.; Li, S.; Li, X.J. Therapeutic Effect of Berberine on Huntington’s Disease Transgenic Mouse Model. PLoS ONE 2015, 10, e0134142. [Google Scholar] [CrossRef]
- Huang, M.; Jiang, X.; Liang, Y.; Liu, Q.; Chen, S.; Guo, Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp. Gerontol. 2017, 91, 25–33. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Xing, X.; Lin, R.; Li, Q.; Zhou, W.; Qiu, W.; Zheng, W. Protective Mechanism of Berberine on Human Retinal Pigment Epithelial Cells against Apoptosis Induced by Hydrogen Peroxide via the Stimulation of Autophagy. Oxidative Med. Cell. Longev. 2021, 2021, 7654143. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ji, Y.; Yan, X.; Su, G.; Chen, L.; Xiao, J. Berberine attenuates apoptosis in rat retinal Müller cells stimulated with high glucose via enhancing autophagy and the AMPK/mTOR signaling. Biomed. Pharmacother. 2018, 108, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, P.; Xu, T.; Chen, Z.; Kong, H.; Chu, W.; Wang, Y.; Liu, Y. Berberine Induces Autophagic Cell Death in Acute Lymphoblastic Leukemia by Inactivating AKT/mTORC1 Signaling. Drug Des. Dev. Ther. 2020, 14, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Cao, X.; Hao, P.; Zhang, Y.; Chen, Y.; Zhang, J.; Li, J.; Gao, C.; Li, L. Berberine attenuates mitochondrial dysfunction by inducing autophagic flux in myocardial hypoxia/reoxygenation injury. Cell Stress Chaperones 2020, 25, 417–426. [Google Scholar] [CrossRef]
- Li, M.H.; Zhang, Y.J.; Yu, Y.H.; Yang, S.H.; Iqbal, J.; Mi, Q.Y.; Li, B.; Wang, Z.M.; Mao, W.X.; Xie, H.G.; et al. Berberine improves pressure overload-induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur. J. Pharmacol. 2014, 728, 67–76. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yang, S.H.; Li, M.H.; Iqbal, J.; Bourantas, C.V.; Mi, Q.Y.; Yu, Y.H.; Li, J.J.; Zhao, S.L.; Tian, N.L.; et al. Berberine attenuates adverse left ventricular remodeling and cardiac dysfunction after acute myocardial infarction in rats: Role of autophagy. Clin. Exp. Pharmacol. Physiol. 2014, 41, 995–1002. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y.; Zhu, M.; Tsang, C.M.; Man, K.; Tong, Y.; Tsao, S.W. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: The cellular mechanism. J. Cell. Biochem. 2010, 111, 1426–1436. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, M.; Yan, H.; Han, Y.; Zhang, F.; Hu, Z.; Cui, A.; Ma, F.; Liu, Z.; Gong, Q.; et al. Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21. Br. J. Pharmacol. 2018, 175, 374–387. [Google Scholar] [CrossRef]
- Lin, Y.; Sheng, M.; Weng, Y.; Xu, R.; Lu, N.; Du, H.; Yu, W. Berberine protects against ischemia/reperfusion injury after orthotopic liver transplantation via activating Sirt1/FoxO3α induced autophagy. Biochem. Biophys. Res. Commun. 2017, 483, 885–891. [Google Scholar] [CrossRef]
- Hou, Q.; Tang, X.; Liu, H.; Tang, J.; Yang, Y.; Jing, X.; Xiao, Q.; Wang, W.; Gou, X.; Wang, Z. Berberine induces cell death in human hepatoma cells in vitro by downregulating CD147. Cancer Sci. 2011, 102, 1287–1292. [Google Scholar] [CrossRef]
- He, Q.; Mei, D.; Sha, S.; Fan, S.; Wang, L.; Dong, M. ERK-dependent mTOR pathway is involved in berberine-induced autophagy in hepatic steatosis. J. Mol. Endocrinol. 2016, 57, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.L.; Kuo, W.H.; Tseng, H.C.; Chou, F.P. Synergistic tumor-killing effect of radiation and berberine combined treatment in lung cancer: The contribution of autophagic cell death. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Dai, C.H.; Shi, Z.H.; Wang, Y.; Wu, J.N.; Chen, K.; Su, J.Y.; Li, J. Synergistic inhibitory effect of berberine and icotinib on non-small cell lung cancer cells via inducing autophagic cell death and apoptosis. Apoptosis 2021, 26, 639–656. [Google Scholar] [CrossRef]
- Chitra, P.; Saiprasad, G.; Manikandan, R.; Sudhandiran, G. Berberine inhibits Smad and non-Smad signaling cascades and enhances autophagy against pulmonary fibrosis. J. Mol. Med. 2015, 93, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.Z.; de Moraes, F.R.; Tedesco, A.C.; Arni, R.K.; Rahal, P.; Calmon, M.F. Berberine associated photodynamic therapy promotes autophagy and apoptosis via ROS generation in renal carcinoma cells. Biomed. Pharmacother. 2020, 123, 109794. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Cao, S.; Sun, Y.; He, X.; Jiang, B.; Yu, Y.; Duan, J.; Qiu, F.; Kang, N. Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomed. Pharmacother. 2020, 128, 110245. [Google Scholar] [CrossRef]
- Han, B.; Wang, K.; Tu, Y.; Tan, L.; He, C. Low-Dose Berberine Attenuates the Anti-Breast Cancer Activity of Chemotherapeutic Agents via Induction of Autophagy and Antioxidation. Dose-Response 2020, 18, 1559325820939751. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, C.; Cao, G.; Guo, L.; Zhang, S.; Liang, Y.; Qin, C.; Su, P.; Li, H.; Zhang, W. Berberine modulates amyloid-β peptide generation by activating AMP-activated protein kinase. Neuropharmacology 2017, 125, 408–417. [Google Scholar] [CrossRef]
- Fan, X.; Wang, J.; Hou, J.; Lin, C.; Bensoussan, A.; Chang, D.; Liu, J.; Wang, B. Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway. J. Transl. Med. 2015, 13, 92. [Google Scholar] [CrossRef]
- Kline, A.E.; Leary, J.B.; Radabaugh, H.L.; Cheng, J.P.; Bondi, C.O. Combination therapies for neurobehavioral and cognitive recovery after experimental traumatic brain injury: Is more better? Prog. Neurobiol. 2016, 142, 45–67. [Google Scholar] [CrossRef]
- Peng, W.H.; Lo, K.L.; Lee, Y.H.; Hung, T.H.; Lin, Y.C. Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sci. 2007, 81, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, L.P.; Sochilina, E.E.; Faddeeva, M.D.; Iagodina, O.V. Effect of some isoquinoline alkaloids on enzymatic activity of acetylcholinesterase and monoamine oxidase. Ukr. Biokhim. Zh. 2005, 77, 147–153. [Google Scholar]
- Ji, H.F.; Shen, L. Molecular basis of inhibitory activities of berberine against pathogenic enzymes in Alzheimer’s disease. Sci. World J. 2012, 2012, 823201. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.Y.; Qu, S.Y.; Ni, S.J.; Yao, Z.Y.; Qi, Y.Y.; Zhao, X.; Guo, R.; Yang, N.Y.; Zhang, Q.C.; Zhu, H.X. Berberine ameliorates depression-like behavior in CUMS mice by activating TPH1 and inhibiting IDO1-associated with tryptophan metabolism. Phytother. Res. PTR 2023, 37, 342–357. [Google Scholar] [CrossRef]
- Lee, T.; Heo, H.; Kim Kwon, Y. Effect of Berberine on Cell Survival in the Developing Rat Brain Damaged by MK-801. Exp. Neurobiol. 2010, 19, 140–145. [Google Scholar] [CrossRef]
- Lin, T.Y.; Lin, Y.W.; Lu, C.W.; Huang, S.K.; Wang, S.J. Berberine Inhibits the Release of Glutamate in Nerve Terminals from Rat Cerebral Cortex. PLoS ONE 2013, 8, e67215. [Google Scholar] [CrossRef]
- Liu, M.; Hurn, P.D.; Alkayed, N.J. Cytochrome P450 in neurological disease. Curr. Drug Metab. 2004, 5, 225–234. [Google Scholar] [CrossRef]
- Zverinsky, I.V.; Zverinskaya, H.G.; Sutsko, I.P.; Telegin, P.G.; Shlyahtun, A.G. Effects of berberine on the recovery of rat liver xenobiotic-metabolizing enzymes after partial hepatectomy. Biomed. Khim. 2015, 61, 381–383. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.J.; Wang, X.; Bu, X.Y.; Lou, Y.Q.; Zhang, G.L. Effect of berberine on hepatocyte proliferation, inducible nitric oxide synthase expression, cytochrome P450 2E1 and 1A2 activities in diethylnitrosamine- and phenobarbital-treated rats. Biomed. Pharmacother. 2008, 62, 567–572. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jang, H.; Lee, J.Y.; Ma, J.Y.; Oh, S.J.; Kim, S.K. Inhibitory effects of Hwang-Ryun-Hae-Dok-Tang on cytochrome P450 in human liver microsomes. Xenobiotica 2015, 45, 131–138. [Google Scholar] [CrossRef]
- Guo, Y.; Pope, C.; Cheng, X.; Zhou, H.; Klaassen, C.D. Dose-response of berberine on hepatic cytochromes P450 mRNA expression and activities in mice. J. Ethnopharmacol. 2011, 138, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, Y.; Tan, Z.R.; Klaassen, C.D.; Zhou, H.H. Repeated administration of berberine inhibits cytochromes P450 in humans. Eur. J. Clin. Pharmacol. 2012, 68, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.M.; Zhang, Q.Y.; Wang, J.L.; Chen, J.L.; Zhang, Y.L.; Tong, X.L. In vitro studies of berberine metabolism and its effect of enzyme induction on HepG2 cells. J. Ethnopharmacol. 2014, 158 Pt A, 388–396. [Google Scholar] [CrossRef]
- Chatuphonprasert, W.; Nemoto, N.; Sakuma, T.; Jarukamjorn, K. Modulations of cytochrome P450 expression in diabetic mice by berberine. Chem. Biol. Interact. 2012, 196, 23–29. [Google Scholar] [CrossRef]
- McDonald, M.G.; Tian, D.D.; Thummel, K.E.; Paine, M.F.; Rettie, A.E. Modulation of Major Human Liver Microsomal Cytochromes P450 by Component Alkaloids of Goldenseal: Time-Dependent Inhibition and Allosteric Effects. Drug Metab. Dispos. Biol. Fate Chem. 2020, 48, 1018–1027. [Google Scholar] [CrossRef]
- Wen, C.J.; Wu, L.X.; Fu, L.J.; Shen, D.Y.; Zhang, X.; Zhang, Y.W.; Yu, J.; Zhou, H.H. Preferential induction of CYP1A1 over CYP1B1 in human breast cancer MCF-7 cells after exposure to berberine. Asian Pac. J. Cancer Prev. 2014, 15, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.N.; Wang, C.W.; Chen, Y.S.; Huang, C.C.; Wu, T.S.; Li, L.A.; Lee, I.J.; Ueng, Y.F. Berberine Activates Aryl Hydrocarbon Receptor but Suppresses CYP1A1 Induction through miR-21-3p Stimulation in MCF-7 Breast Cancer Cells. Molecules 2017, 22, 1847. [Google Scholar] [CrossRef]
- Lo, S.N.; Chang, Y.P.; Tsai, K.C.; Chang, C.Y.; Wu, T.S.; Ueng, Y.F. Inhibition of CYP1 by berberine, palmatine, and jatrorrhizine: Selectivity, kinetic characterization, and molecular modeling. Toxicol. Appl. Pharmacol. 2013, 272, 671–680. [Google Scholar] [CrossRef]
- Falero-Perez, J.; Song, Y.S.; Sorenson, C.M.; Sheibani, N. CYP1B1: A key regulator of redox homeostasis. Trends Cell Mol. Biol. 2018, 13, 27–45. [Google Scholar]
- Falero-Perez, J.; Sorenson, C.M.; Sheibani, N. Cyp1b1-deficient retinal astrocytes are more proliferative and migratory and are protected from oxidative stress and inflammation. Am. J. Physiol. Cell Physiol. 2019, 316, C767–C781. [Google Scholar] [CrossRef]
- Qiu, W.; Jiang, X.H.; Liu, C.X.; Ju, Y.; Jin, J.X. Effect of berberine on the pharmacokinetics of substrates of CYP3A and P-gp. Phytother. Res. PTR 2009, 23, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, J.; Zhao, H.; Wan, D.; Jiang, Z. Berberine combined with cyclosporine A alleviates acute graft-versus-host disease in murine models. Int. Immunopharmacol. 2020, 81, 106205. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ekavali, M.J.; Chopra, K.; Dhull, D.K. Possible role of P-glycoprotein in the neuroprotective mechanism of berberine in intracerebroventricular streptozotocin-induced cognitive dysfunction. Psychopharmacology 2016, 233, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.J.; Yao, Z.Y.; Wei, X.; Heng, X.; Qu, S.Y.; Zhao, X.; Qi, Y.Y.; Ge, P.Y.; Xu, C.P.; Yang, N.Y.; et al. Vagus nerve stimulated by microbiota-derived hydrogen sulfide mediates the regulation of berberine on microglia in transient middle cerebral artery occlusion rats. Phytother. Res. PTR 2022, 36, 2964–2981. [Google Scholar] [CrossRef]
- Hong, J.S.; Chu, Y.K.; Lee, H.; Ahn, B.H.; Park, J.H.; Kim, M.J.; Lee, S.; Ryoo, H.S.; Jang, J.H.; Lee, S.R.; et al. Effects of berberine on hippocampal neuronal damage and matrix metalloproteinase-9 activity following transient global cerebral ischemia. J. Neurosci. Res. 2012, 90, 489–497. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, Y.; Wu, A.; Chen, X.; Pi, R.; Liu, M.; Liu, Y. Berberine attenuates experimental autoimmune encephalomyelitis in C57 BL/6 mice. PLoS ONE 2010, 5, e13489. [Google Scholar] [CrossRef]
- Wu, C.; Yang, K.; Liu, Q.; Wakui, M.; Jin, G.Z.; Zhen, X.; Wu, J. Tetrahydroberberine blocks ATP-sensitive potassium channels in dopamine neurons acutely-dissociated from rat substantia nigra pars compacta. Neuropharmacology 2010, 59, 567–572. [Google Scholar] [CrossRef]
- Shigeta, K.; Ootaki, K.; Tatemoto, H.; Nakanishi, T.; Inada, A.; Muto, N. Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by a Coptidis Rhizoma extract and protoberberine alkaloids. Biosci. Biotechnol. Biochem. 2002, 66, 2491–2494. [Google Scholar] [CrossRef]
- Han, A.M.; Heo, H.; Kwon, Y.K. Berberine promotes axonal regeneration in injured nerves of the peripheral nervous system. J. Med. Food 2012, 15, 413–417. [Google Scholar] [CrossRef]
- Kheir, M.M.; Wang, Y.; Hua, L.; Hu, J.; Li, L.; Lei, F.; Du, L. Acute toxicity of berberine and its correlation with the blood concentration in mice. Food Chem. Toxicol. 2010, 48, 1105–1110. [Google Scholar] [CrossRef]
- Rad, S.Z.K.; Rameshrad, M.; Hosseinzadeh, H. Toxicology effects of Berberis vulgaris (barberry) and its active constituent, berberine: A review. Iran. J. Basic Med. Sci. 2017, 20, 516–529. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Zamani Taghizadeh Rabe, S.; Balali-Mood, M.; Karimi, G.; Memar, B.; Rahnama, M.; Tabasi, N.; Khazaee, M.; Riahi-Zanjani, B. Immunotoxicity induced in mice by subacute exposure to berberine. J. Immunotoxicol. 2016, 13, 255–262. [Google Scholar] [CrossRef]
- Küpeli, E.; Koşar, M.; Yeşilada, E.; Hüsnü, K.; Başer, C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002, 72, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Terpstra, A.H. Differences between humans and mice in efficacy of the body fat lowering effect of conjugated linoleic acid: Role of metabolic rate. J. Nutr. 2001, 131, 2067–2068. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hua, W.; Li, Y.; Xian, X.; Zhao, Z.; Liu, C.; Zou, J.; Li, J.; Fang, X.; Zhu, Y. Berberine suppresses colon cancer cell proliferation by inhibiting the SCAP/SREBP-1 signaling pathway-mediated lipogenesis. Biochem. Pharmacol. 2020, 174, 113776. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, K.; Kumar, G.S. Therapeutic potential of nucleic acid-binding isoquinoline alkaloids: Binding aspects and implications for drug design. Med. Res. Rev. 2011, 31, 821–862. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.C.; Kumar, G.S.; Bhadra, K.; Giri, P.; Sinha, R.; Pal, S.; Maiti, M. Berberine, a strong polyriboadenylic acid binding plant alkaloid: Spectroscopic, viscometric, and thermodynamic study. Bioorg. Med. Chem. 2005, 13, 165–174. [Google Scholar] [CrossRef]
- Rauf, A.; Abu-Izneid, T.; Khalil, A.A.; Imran, M.; Shah, Z.A.; Emran, T.B.; Mitra, S.; Khan, Z.; Alhumaydhi, F.A.; Aljohani, A.S.M.; et al. Berberine as a Potential Anticancer Agent: A Comprehensive Review. Molecules 2021, 26, 7368. [Google Scholar] [CrossRef]
- Jahnke, G.D.; Price, C.J.; Marr, M.C.; Myers, C.B.; George, J.D. Developmental toxicity evaluation of berberine in rats and mice. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2006, 77, 195–206. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, B. Toxicological Effects of Berberine and Sanguinarine. Front. Mol. Biosci. 2018, 5, 21. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metab. Clin. Exp. 2008, 57, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-q.; Chen, L.; Zhu, J.-h. Influence of berberine combining with atorvastatin on serum high-sensitivity C-reactive protein and adipocyte fatty acid-binding protein in patients with acute ischemic stroke. Chin. J. Contemp. Neurol. Neurosurg. 2015, 15, 43–47. [Google Scholar] [CrossRef]
- Ming, J.; Yu, X.; Xu, X.; Wang, L.; Ding, C.; Wang, Z.; Xie, X.; Li, S.; Yang, W.; Luo, S.; et al. Effectiveness and safety of Bifidobacterium and berberine in human hyperglycemia and their regulatory effect on the gut microbiota: A multi-center, double-blind, randomized, parallel-controlled study. Genome Med. 2021, 13, 125. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Chai, M.J.; Yang, F.; Li, H.S.; Zhao, J.; Wang, H.; Lu, D.D. Effects of berberine on serum inflammatory factors and carotid atherosclerotic plaques in patients with acute cerebral ischemic stroke. Zhongguo Zhong Yao Za Zhi 2016, 41, 4066–4071. [Google Scholar] [CrossRef]
- Liu, C.S.; Zheng, Y.R.; Zhang, Y.F.; Long, X.Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.S.; Ma, J.Y.; Feng, R.; Ma, C.; Chen, W.J.; Sun, Y.P.; Fu, J.; Huang, M.; He, C.Y.; Shou, J.W.; et al. Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS ONE 2013, 8, e77969. [Google Scholar] [CrossRef]
- Wang, Q.S.; Li, K.; Gao, L.N.; Zhang, Y.; Lin, K.M.; Cui, Y.L. Intranasal delivery of berberine via in situ thermoresponsive hydrogels with non-invasive therapy exhibits better antidepressant-like effects. Biomater. Sci. 2020, 8, 2853–2865. [Google Scholar] [CrossRef]
- Xu, D.; Qiu, C.; Wang, Y.; Qiao, T.; Cui, Y.L. Intranasal co-delivery of berberine and evodiamine by self-assembled thermosensitive in-situ hydrogels for improving depressive disorder. Int. J. Pharm. 2021, 603, 120667. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, E.; Sharma, G.; Dai, C. Neuroprotective Properties of Berberine: Molecular Mechanisms and Clinical Implications. Antioxidants 2023, 12, 1883. https://doi.org/10.3390/antiox12101883
Tian E, Sharma G, Dai C. Neuroprotective Properties of Berberine: Molecular Mechanisms and Clinical Implications. Antioxidants. 2023; 12(10):1883. https://doi.org/10.3390/antiox12101883
Chicago/Turabian StyleTian, Erjie, Gaurav Sharma, and Chongshan Dai. 2023. "Neuroprotective Properties of Berberine: Molecular Mechanisms and Clinical Implications" Antioxidants 12, no. 10: 1883. https://doi.org/10.3390/antiox12101883
APA StyleTian, E., Sharma, G., & Dai, C. (2023). Neuroprotective Properties of Berberine: Molecular Mechanisms and Clinical Implications. Antioxidants, 12(10), 1883. https://doi.org/10.3390/antiox12101883







