Stress-Induced Premature Senescence Related to Oxidative Stress in the Developmental Programming of Nonalcoholic Fatty Liver Disease in a Rat Model of Intrauterine Growth Restriction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
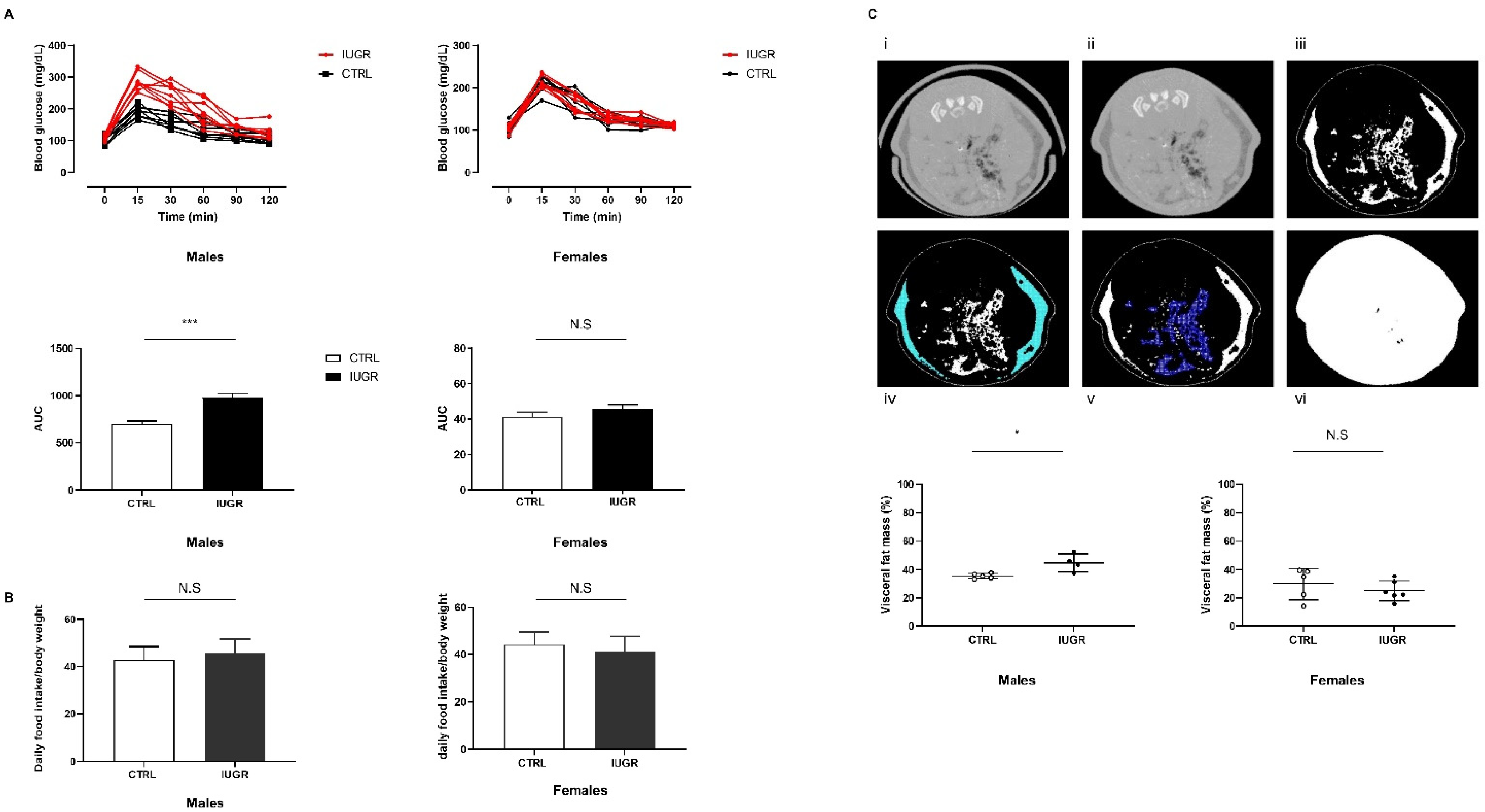
2.2. Intraperitoneal Glucose Tolerance Test (IPGTT)
2.3. Daily Food Consumption
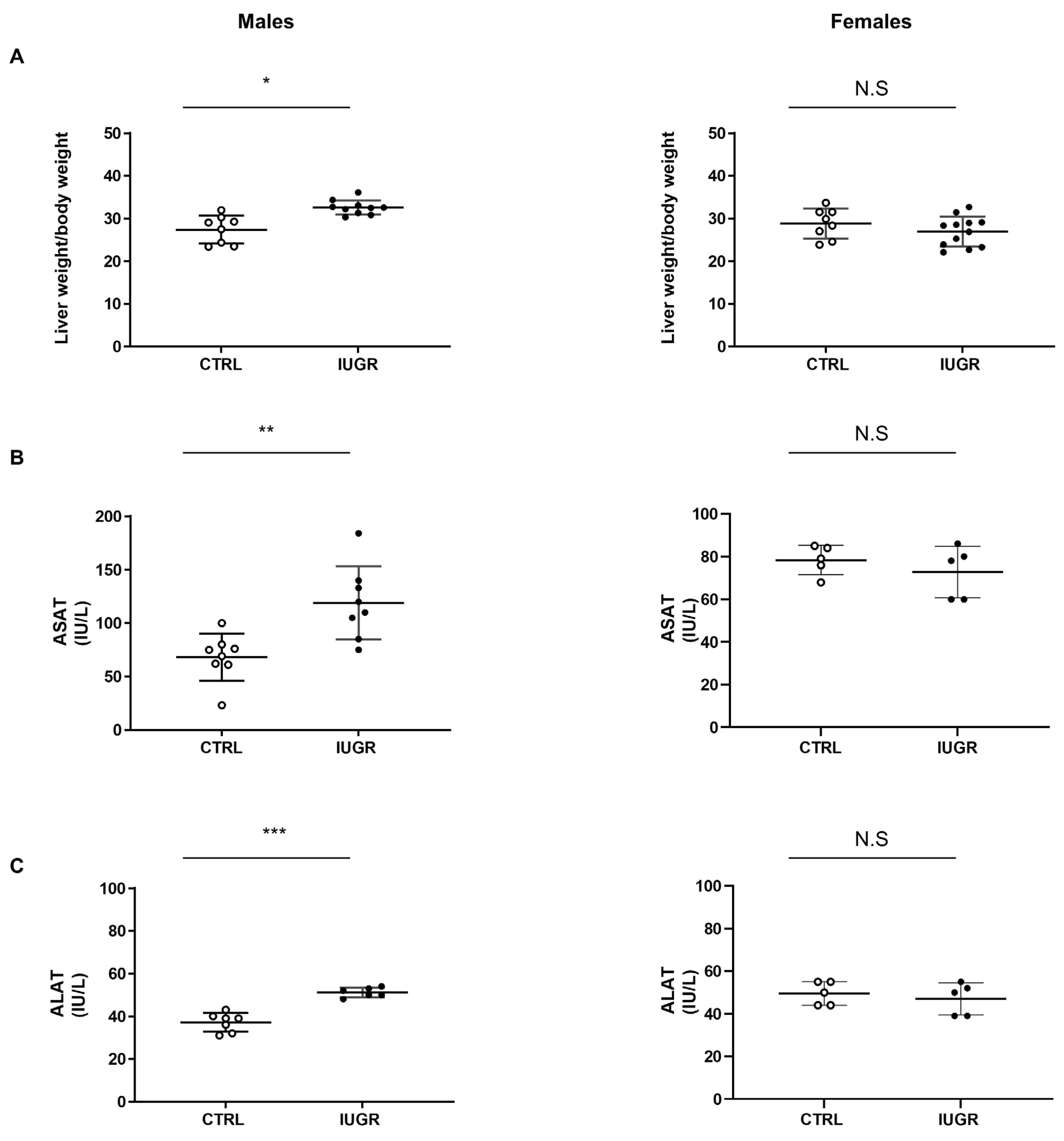
2.4. Liver Enzyme Measurement
2.5. Body Composition Measurement
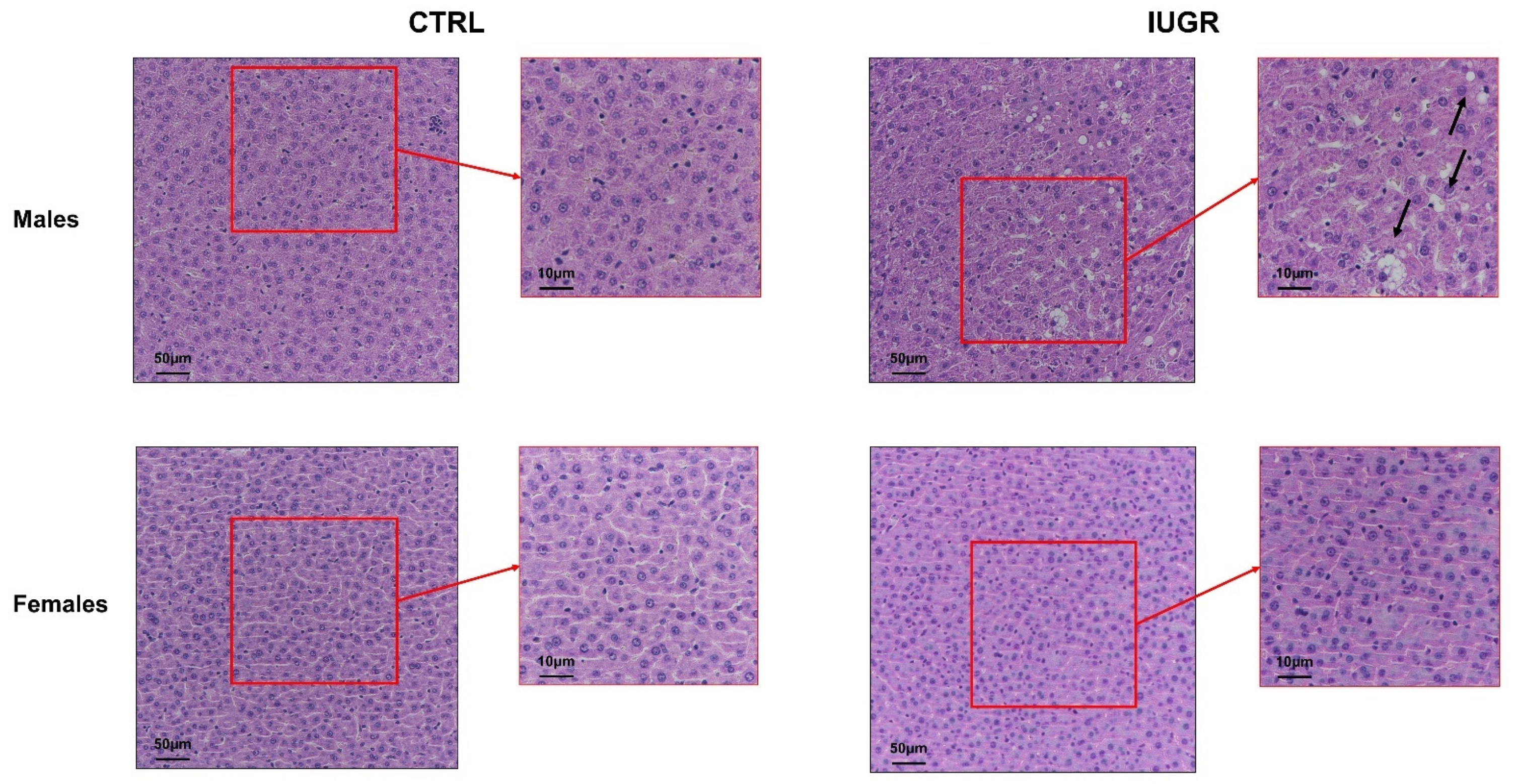
2.6. Liver Analyses
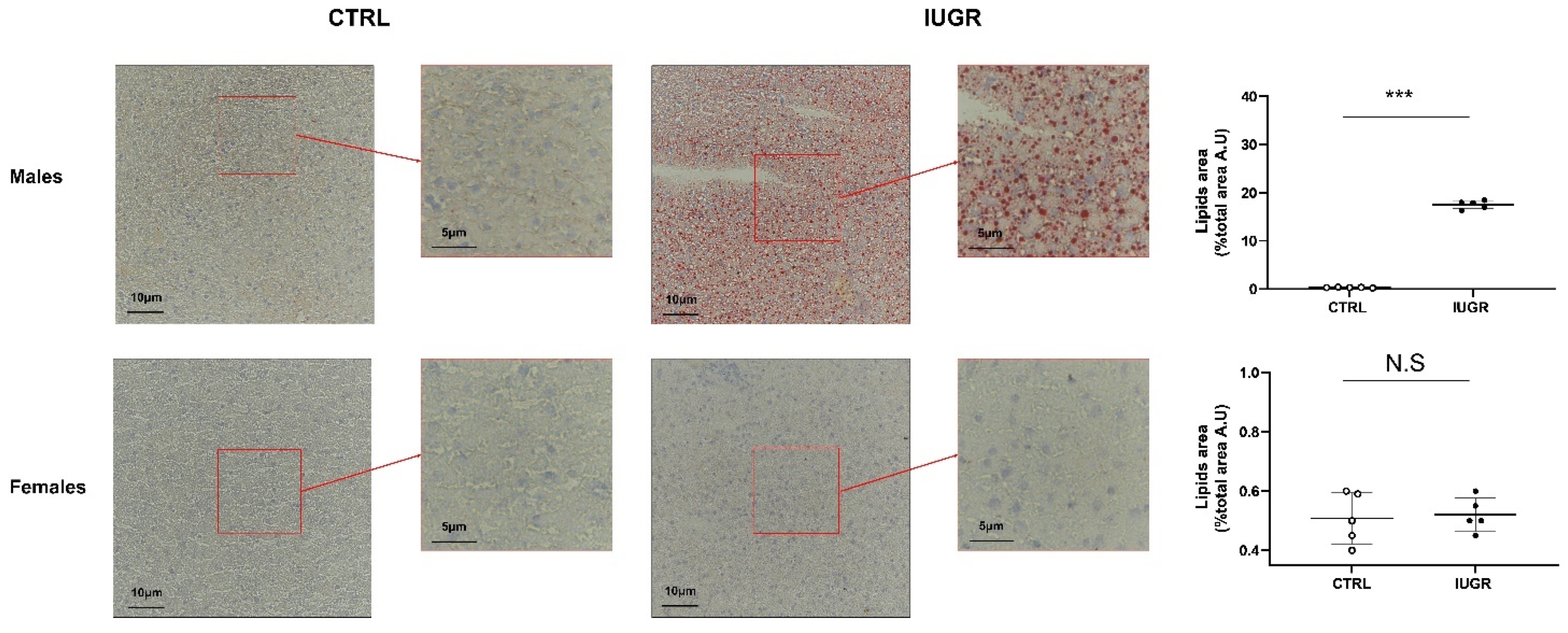
2.7. Steatosis Detection
2.8. Superoxide Anion Production Evaluation
2.9. Oxidative DNA Double-Strand Break
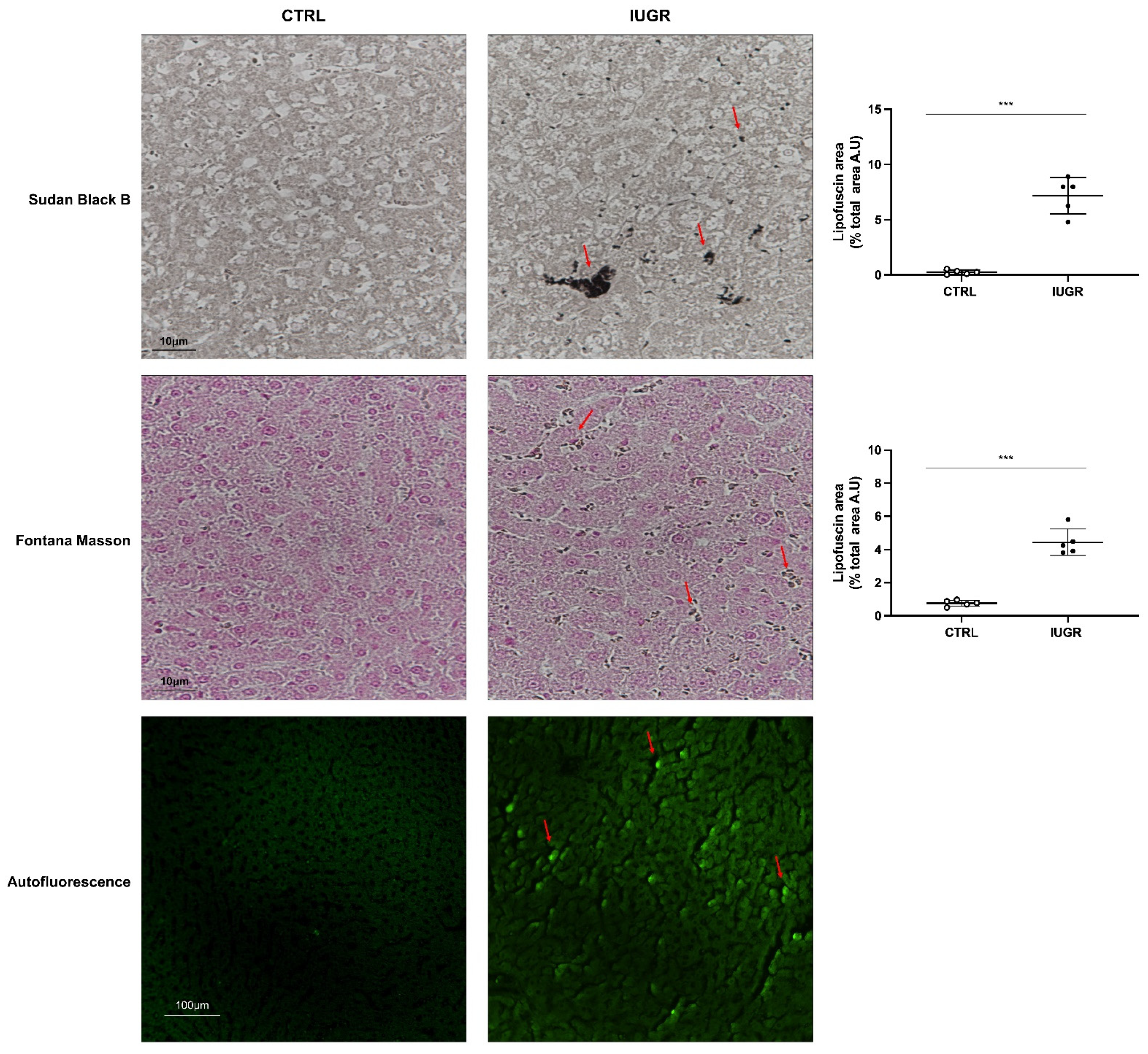
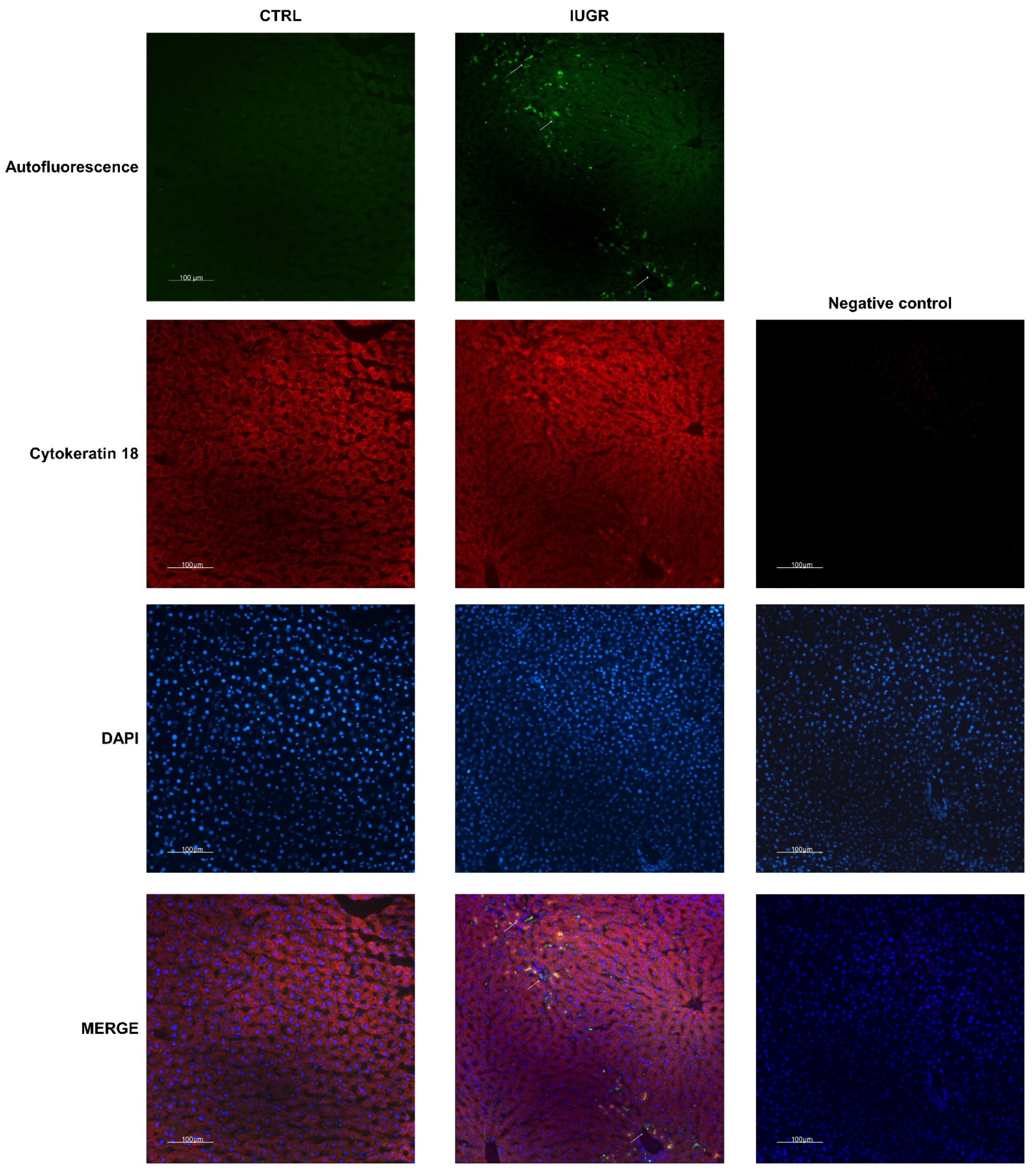
2.10. Histological Detection of Cell Senescence
2.11. PCR-Selected cDNA Subtraction Kit
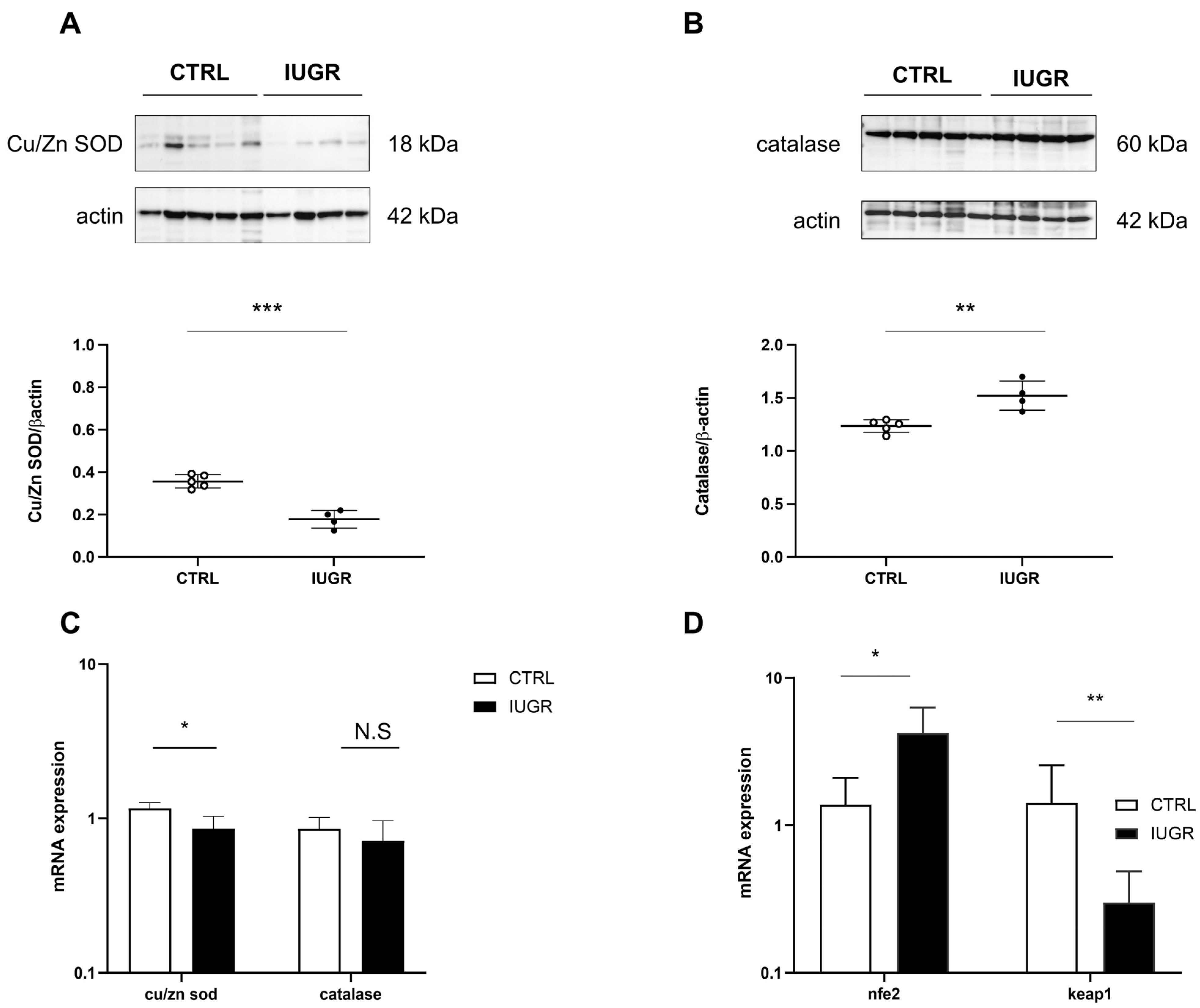
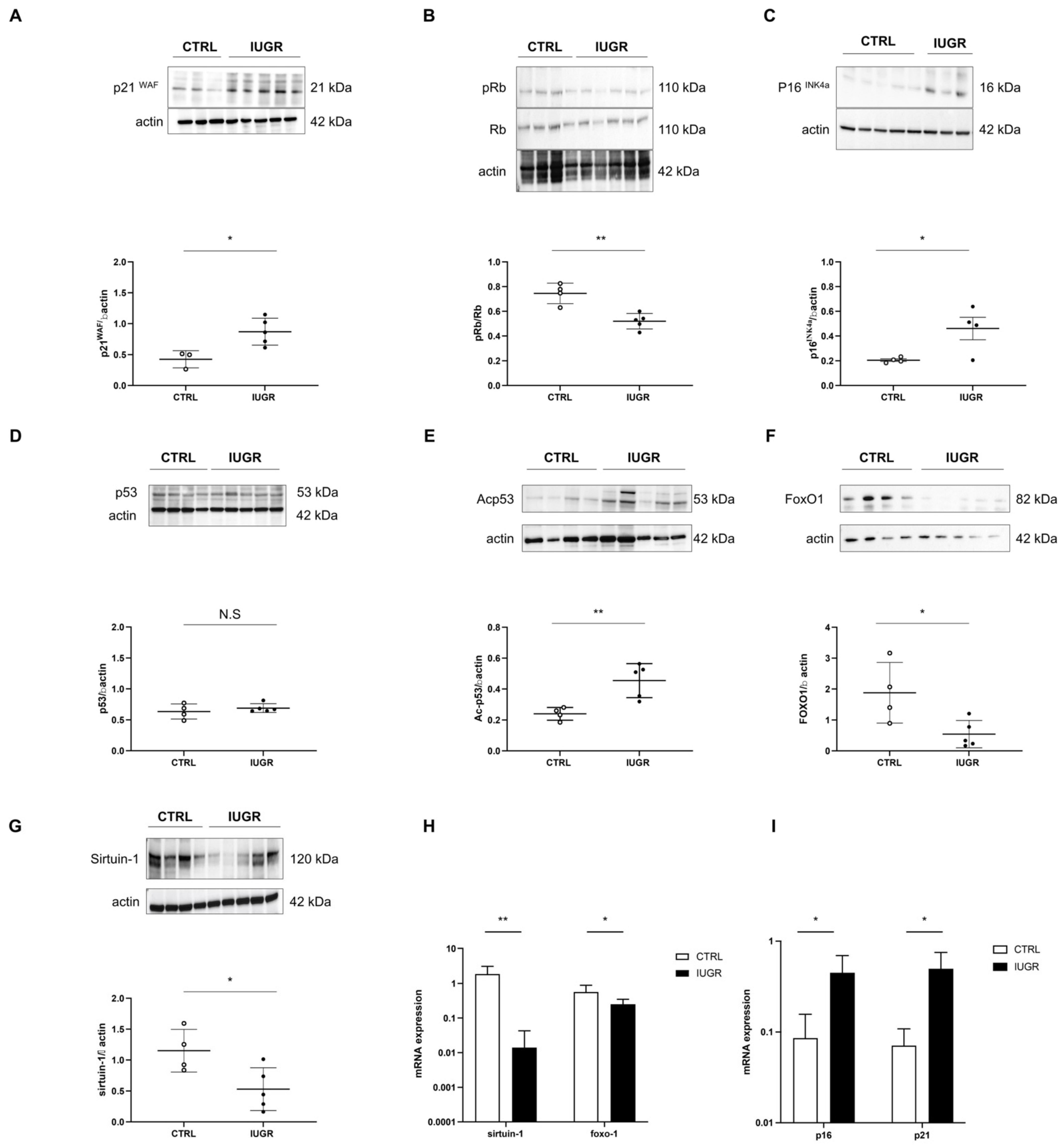
2.12. Protein Expression Measurement Using Western Blotting
2.13. Statistical Analyses
3. Results
3.1. Intrauterine Growth Worsened Glucose Intolerance and Increased Visceral Fat Mass
3.2. Intrauterine Growth Restriction Leads to Liver Function and Structure Alterations
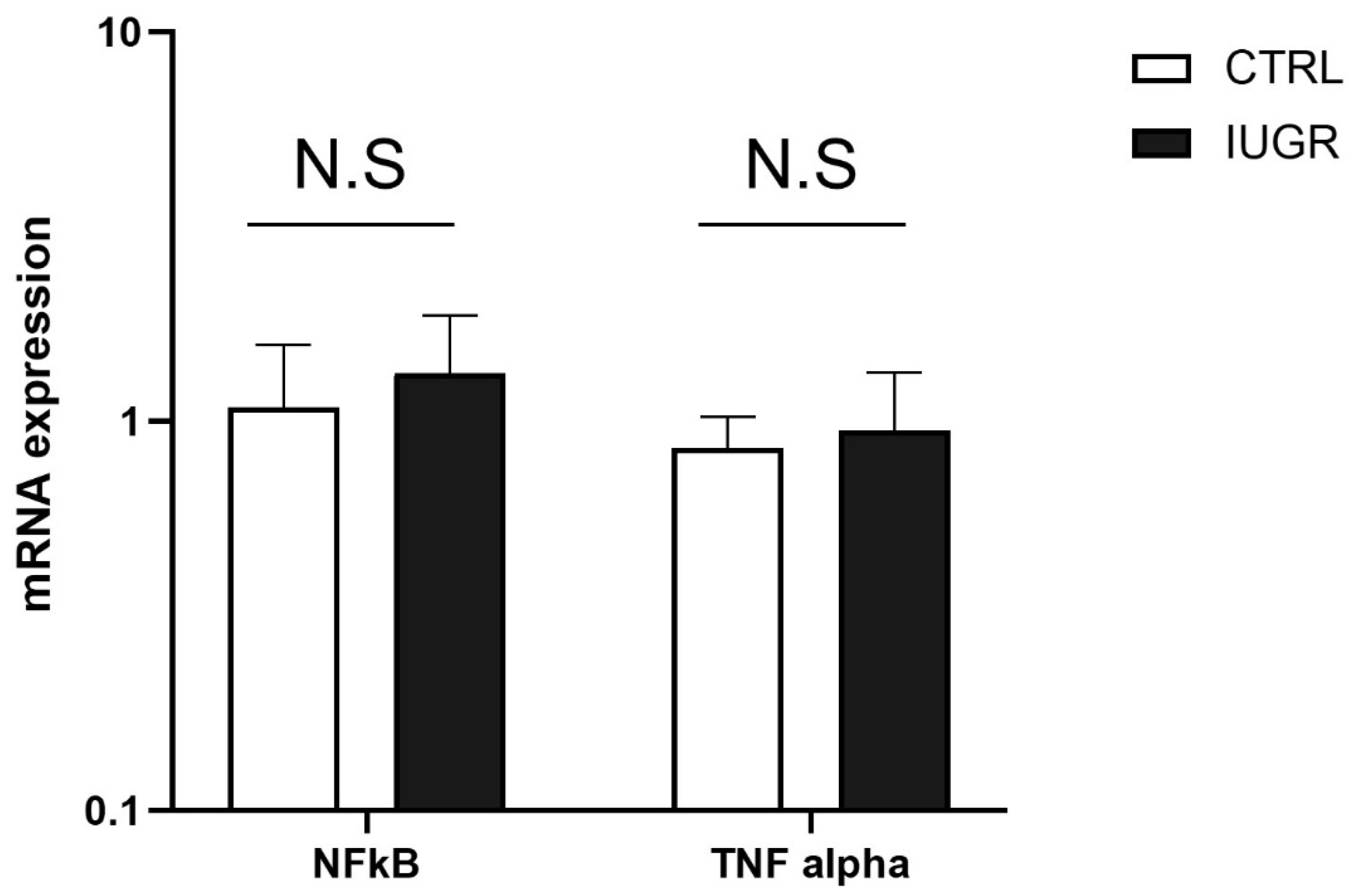
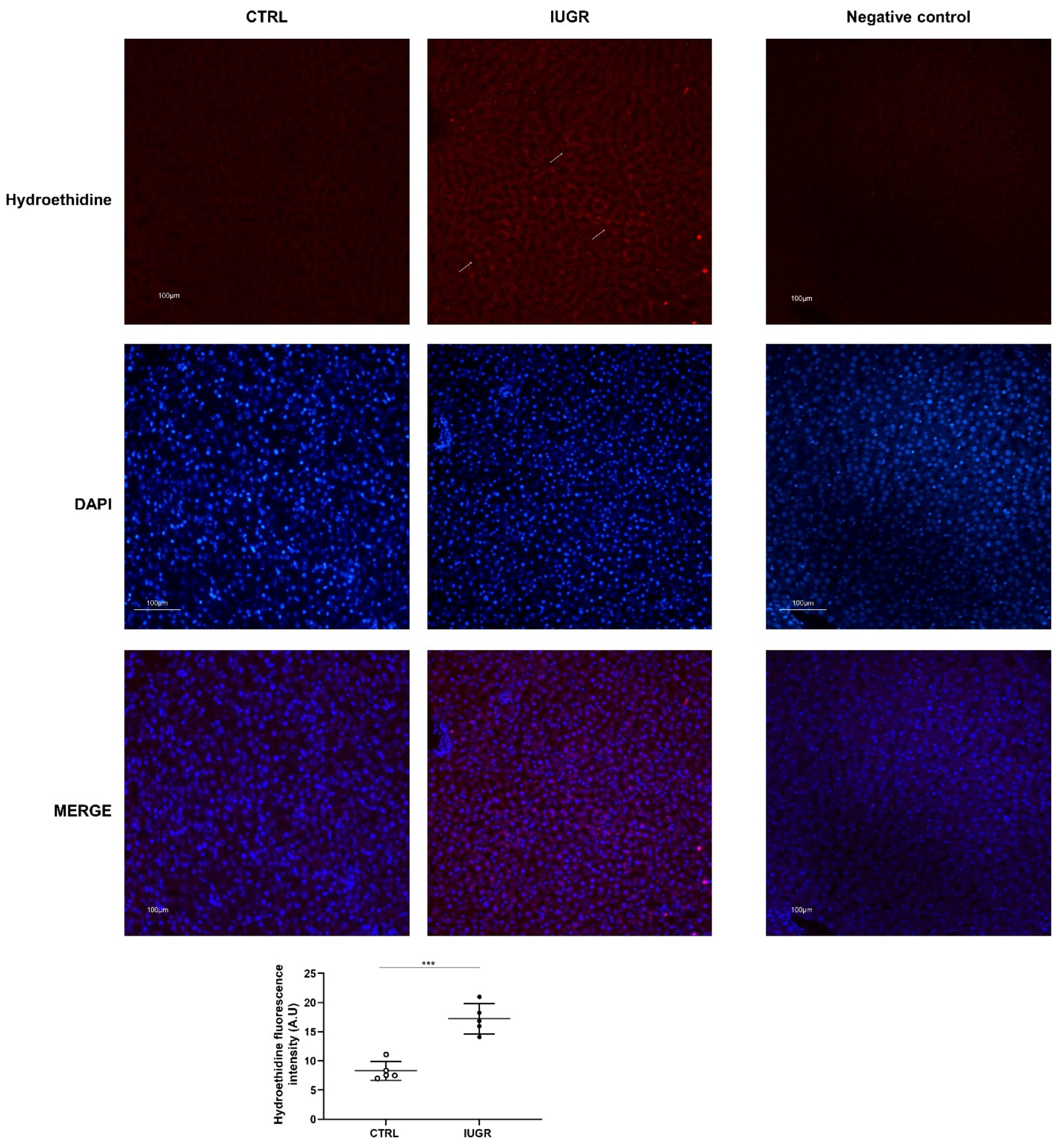
3.3. Oxidative Stress Was Observed in the IUGR Male Livers at Adulthood
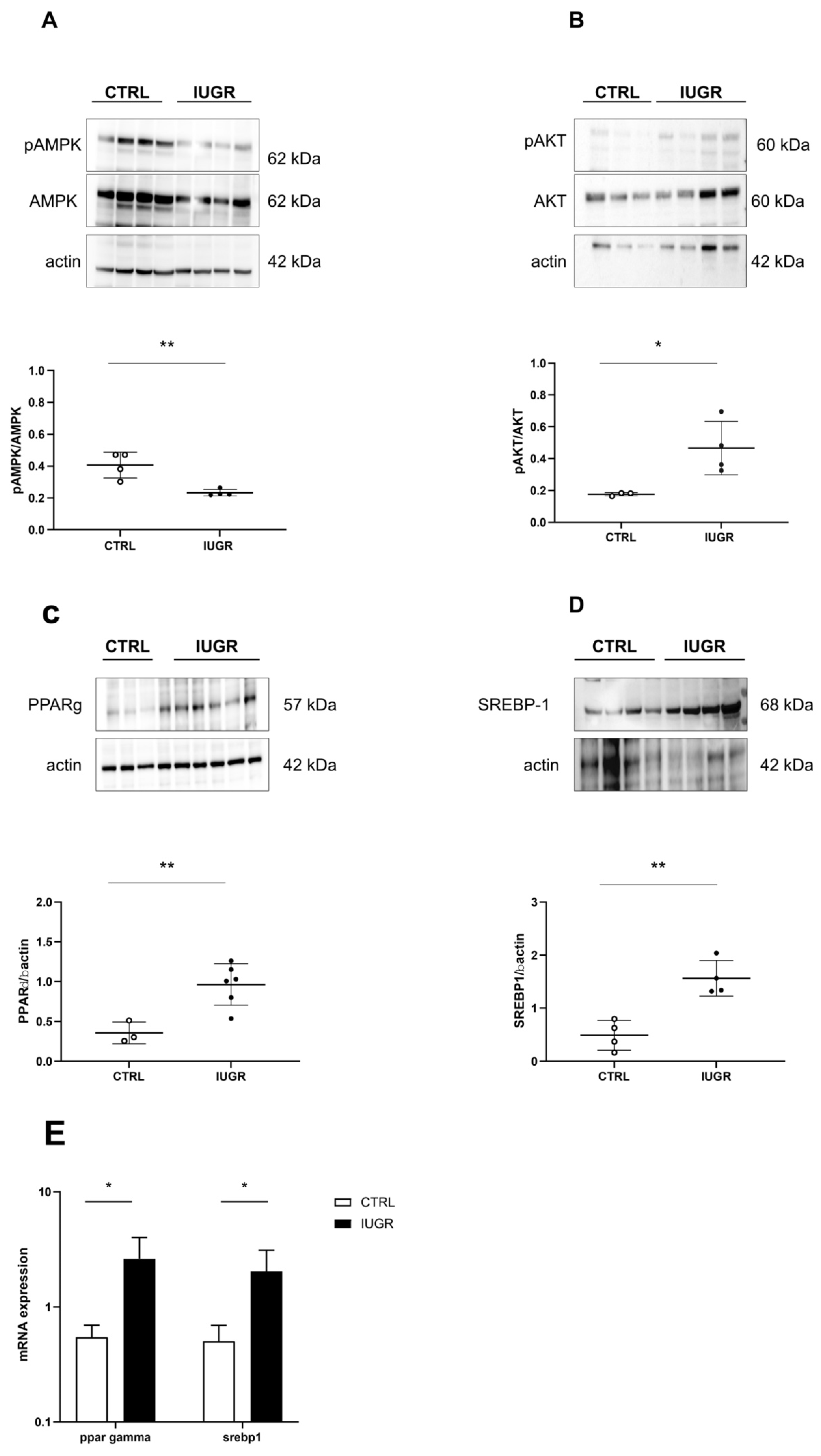
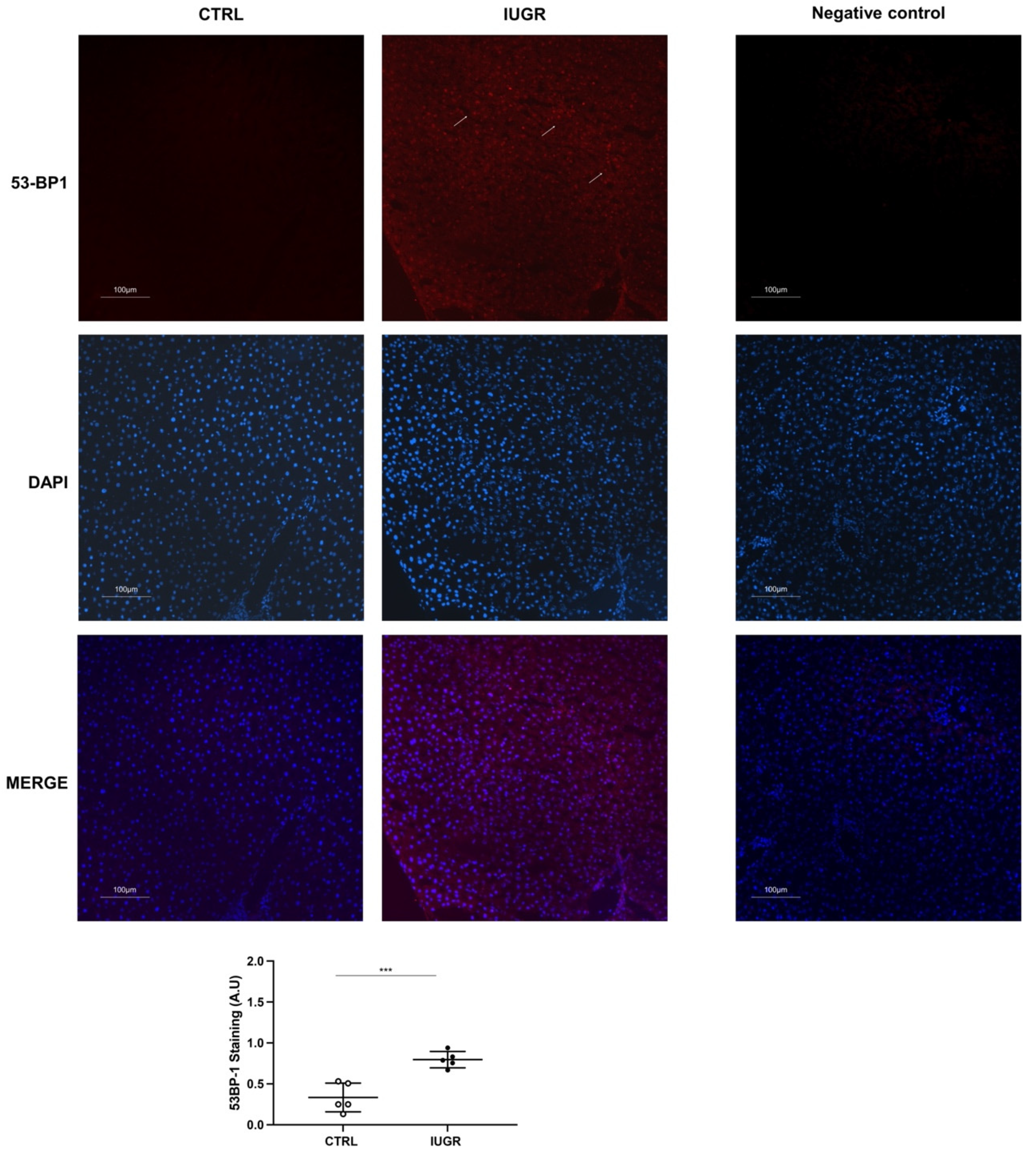
3.4. Stress-Induced Premature Senescence Was Observed in IUGR Males
4. Discussion
5. Conclusions/Perspectives
5.1. Conclusions
5.2. Limitations/Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peyter, A.C.; Armengaud, J.B.; Guillot, E.; Yzydorczyk, C. Endothelial Progenitor Cells Dysfunctions and Cardiometabolic Disorders: From Mechanisms to Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 6667. [Google Scholar] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [PubMed]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic Syndrome, Aging and Involvement of Oxidative Stress. Aging Dis. 2015, 6, 109–120. [Google Scholar]
- Stefan, N.; Haring, H.U.; Cusi, K. Non-Alcoholic Fatty Liver Disease: Causes, Diagnosis, Cardiometabolic Consequences, and Treatment Strategies. Lancet Diabetes Endocrinol 2019, 7, 313–324. [Google Scholar] [PubMed]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar]
- Eckel, R.H.; Alberti, K.G.; Grundy, S.M.; Zimmet, P.Z. The Metabolic Syndrome. Lancet 2010, 375, 181–183. [Google Scholar]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The Metabolic Syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar]
- Samson, S.L.; Garber, A.J. Metabolic Syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar]
- Hales, C.N.; Barker, D.J.; Clark, P.M.; Cox, L.J.; Fall, C.; Osmond, C.; Winter, P.D. Fetal and Infant Growth and Impaired Glucose Tolerance at Age 64. BMJ 1991, 303, 1019–1022. [Google Scholar]
- Zarrati, M.; Shidfar, F.; Razmpoosh, E.; Nezhad, F.N.; Keivani, H.; Hemami, M.R.; Asemi, Z. Does Low Birth Weight Predict Hypertension and Obesity in Schoolchildren? Ann. Nutr. Metab. 2013, 63, 69–76. [Google Scholar]
- Ramadhani, M.K.; Grobbee, D.E.; Bots, M.L.; Cabezas, M.C.; Vos, L.E.; Oren, A.; Uiterwaal, C.S. Lower Birth Weight Predicts Metabolic Syndrome in Young Adults: The Atherosclerosis Risk in Young Adults (Arya)-Study. Atherosclerosis 2006, 184, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Sandboge, S.; Perala, M.M.; Salonen, M.K.; Blomstedt, P.A.; Osmond, C.; Kajantie, E.; Barker, D.J.; Eriksson, J.G. Early Growth and Non-Alcoholic Fatty Liver Disease in Adulthood-the Nafld Liver Fat Score and Equation Applied on the Helsinki Birth Cohort Study. Ann. Med. 2013, 45, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Brunetti, G.; Ventura, A.; D’Aniello, M.; Pepe, T.; Giordano, P.; Monteduro, M.; Cavallo, L. Nonalcoholic Fatty Liver Disease in Prepubertal Children Born Small for Gestational Age: Influence of Rapid Weight Catch-up Growth. Horm. Res. Paediatr. 2013, 79, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.; Ebrahim, S.; Smith, G.D.; Lawlor, D.A. The Associations between Height Components (Leg and Trunk Length) and Adult Levels of Liver Enzymes. J. Epidemiol. Community Health 2008, 62, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Suomela, E.; Oikonen, M.; Pitkanen, N.; Ahola-Olli, A.; Virtanen, J.; Parkkola, R.; Jokinen, E.; Laitinen, T.; Hutri-Kahonen, N.; Kahonen, M.; et al. Childhood Predictors of Adult Fatty Liver. The Cardiovascular Risk in Young Finns Study. J. Hepatol. 2016, 65, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Marcellini, M.; Marchesini, G.; Vanni, E.; Manco, M.; Villani, A.; Bugianesi, E. Intrauterine Growth Retardation, Insulin Resistance, and Nonalcoholic Fatty Liver Disease in Children. Diabetes Care 2007, 30, 2638–2640. [Google Scholar] [CrossRef]
- Hattersley, A.T.; Tooke, J.E. The Fetal Insulin Hypothesis: An Alternative Explanation of the Association of Low Birthweight with Diabetes and Vascular Disease. Lancet 1999, 353, 1789–1792. [Google Scholar] [CrossRef]
- Urakawa, H.; Katsuki, A.; Sumida, Y.; Gabazza, E.C.; Murashima, S.; Morioka, K.; Maruyama, N.; Kitagawa, N.; Tanaka, T.; Hori, Y.; et al. Oxidative Stress Is Associated with Adiposity and Insulin Resistance in Men. J. Clin. Endocrinol. Metab. 2003, 88, 4673–4676. [Google Scholar] [CrossRef]
- Hong, T.; Chen, Y.; Li, X.; Lu, Y. The Role and Mechanism of Oxidative Stress and Nuclear Receptors in the Development of Nafld. Oxid. Med. Cell. Longev. 2021, 2021, 6889533. [Google Scholar] [CrossRef]
- Armutcu, F.; Ataymen, M.; Atmaca, H.; Gurel, A. Oxidative Stress Markers, C-Reactive Protein and Heat Shock Protein 70 Levels in Subjects with Metabolic Syndrome. Clin. Chem. Lab. Med. 2008, 46, 785–790. [Google Scholar] [CrossRef]
- Palmieri, V.O.; Grattagliano, I.; Portincasa, P.; Palasciano, G. Systemic Oxidative Alterations Are Associated with Visceral Adiposity and Liver Steatosis in Patients with Metabolic Syndrome. J. Nutr. 2006, 136, 3022–3026. [Google Scholar] [CrossRef] [PubMed]
- Hansel, B.; Giral, P.; Nobecourt, E.; Chantepie, S.; Bruckert, E.; Chapman, M.J.; Kontush, A. Metabolic Syndrome Is Associated with Elevated Oxidative Stress and Dysfunctional Dense High-Density Lipoprotein Particles Displaying Impaired Antioxidative Activity. J. Clin. Endocrinol. Metab. 2004, 89, 4963–4971. [Google Scholar] [CrossRef] [PubMed]
- Perticone, F.; Ceravolo, R.; Candigliota, M.; Ventura, G.; Iacopino, S.; Sinopoli, F.; Mattioli, P.L. Obesity and Body Fat Distribution Induce Endothelial Dysfunction by Oxidative Stress: Protective Effect of Vitamin C. Diabetes 2001, 50, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Celermajer, D.S.; Sorensen, K.E.; Spiegelhalter, D.J.; Georgakopoulos, D.; Robinson, J.; Deanfield, J.E. Aging Is Associated with Endothelial Dysfunction in Healthy Men Years before the Age-Related Decline in Women. J. Am. Coll. Cardiol. 1994, 24, 471–476. [Google Scholar] [CrossRef]
- Erusalimsky, J.D.; Kurz, D.J. Cellular Senescence in Vivo: Its Relevance in Ageing and Cardiovascular Disease. Exp. Gerontol. 2005, 40, 634–642. [Google Scholar] [CrossRef]
- Weinsaft, J.W.; Edelberg, J.M. Aging-Associated Changes in Vascular Activity: A Potential Link to Geriatric Cardiovascular Disease. Am. J. Geriatr. Cardiol. 2001, 10, 348–354. [Google Scholar] [CrossRef]
- Panasiuk, A.; Dzieciol, J.; Panasiuk, B.; Prokopowicz, D. Expression of P53, Bax and Bcl-2 Proteins in Hepatocytes in Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2006, 12, 6198–6202. [Google Scholar] [CrossRef]
- Ping, F.; Li, Z.Y.; Lv, K.; Zhou, M.C.; Dong, Y.X.; Sun, Q.; Li, Y.X. Deoxyribonucleic Acid Telomere Length Shortening Can Predict the Incidence of Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus. J. Diabetes Investig. 2017, 8, 174–180. [Google Scholar] [CrossRef]
- Laish, I.; Mannasse-Green, B.; Hadary, R.; Biron-Shental, T.; Konikoff, F.M.; Amiel, A.; Kitay-Cohen, Y. Telomere Dysfunction in Nonalcoholic Fatty Liver Disease and Cryptogenic Cirrhosis. Cytogenet. Genome Res. 2016, 150, 93–99. [Google Scholar] [CrossRef]
- Yzydorczyk, C.; Li, N.; Chehade, H.; Mosig, D.; Bidho, M.; Keshavjee, B.; Armengaud, J.B.; Nardou, K.; Siddeek, B.; Benahmed, M.; et al. Transient Postnatal Overfeeding Causes Liver Stress-Induced Premature Senescence in Adult Mice. Sci. Rep. 2017, 7, 12911. [Google Scholar] [CrossRef]
- Guvendag Guven, E.S.; Karcaaltincaba, D.; Kandemir, O.; Kiykac, S.; Mentese, A. Cord Blood Oxidative Stress Markers Correlate with Umbilical Artery Pulsatility in Fetal Growth Restriction. J. Matern. Fetal. Neonatal. Med. 2013, 26, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.M.; Cook, L.G.; Danchuk, S.; Puschett, J.B. Uncoupled Endothelial Nitric Oxide Synthase and Oxidative Stress in a Rat Model of Pregnancy-Induced Hypertension. Am. J. Hypertens. 2007, 20, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Oke, S.L.; Hardy, D.B. The Role of Cellular Stress in Intrauterine Growth Restriction and Postnatal Dysmetabolism. Int. J. Mol. Sci. 2021, 22, 6986. [Google Scholar] [CrossRef]
- Karowicz-Bilinska, A.; Suzin, J.; Sieroszewski, P. Evaluation of Oxidative Stress Indices During Treatment in Pregnant Women with Intrauterine Growth Retardation. Med. Sci. Monit. 2002, 8, CR211-6. [Google Scholar] [PubMed]
- Saker, M.; Mokhtari, N.S.; Merzouk, S.A.; Merzouk, H.; Belarbi, B.; Narce, M. Oxidant and Antioxidant Status in Mothers and Their Newborns According to Birthweight. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 141, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, O.; Durak, I. Role of Oxidative Stress in Intrauterine Growth Restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Narang, M.; Banerjee, B.D.; Basu, S. Oxidative Stress in Term Small for Gestational Age Neonates Born to Undernourished Mothers: A Case Control Study. BMC Pediatr. 2004, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biron-Shental, T.; Sukenik-Halevy, R.; Sharon, Y.; Goldberg-Bittman, L.; Kidron, D.; Fejgin, M.D.; Amiel, A. Short Telomeres May Play a Role in Placental Dysfunction in Preeclampsia and Intrauterine Growth Restriction. Am. J. Obstet. Gynecol. 2010, 202, 381.e1–381.e7. [Google Scholar] [CrossRef] [PubMed]
- Biron-Shental, T.; Kidron, D.; Sukenik-Halevy, R.; Goldberg-Bittman, L.; Sharony, R.; Fejgin, M.D.; Amiel, A. Terc Telomerase Subunit Gene Copy Number in Placentas from Pregnancies Complicated with Intrauterine Growth Restriction. Early Hum. Dev. 2011, 87, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, P.F.; Simoncini, S.; Ligi, I.; Chateau, A.L.; Bachelier, R.; Robert, S.; Morere, J.; Fernandez, S.; Guillet, B.; Marcelli, M.; et al. Accelerated Senescence of Cord Blood Endothelial Progenitor Cells in Premature Neonates Is Driven by Sirt1 Decreased Expression. Blood 2014, 123, 2116–2126. [Google Scholar] [CrossRef]
- Simoncini, S.; Coppola, H.; Rocca, A.; Bachmann, I.; Guillot, E.; Zippo, L.; Dignat-George, F.; Sabatier, F.; Bedel, R.; Wilson, A.; et al. Endothelial Colony-Forming Cells Dysfunctions Are Associated with Arterial Hypertension in a Rat Model of Intrauterine Growth Restriction. Int. J. Mol. Sci. 2021, 22, 10159. [Google Scholar] [CrossRef] [PubMed]
- Yzydorczyk, C.; Li, N.; Rigal, E.; Chehade, H.; Mosig, D.; Armengaud, J.B.; Rolle, T.; Krishnasamy, A.; Orozco, E.; Siddeek, B.; et al. Calorie Restriction in Adulthood Reduces Hepatic Disorders Induced by Transient Postnatal Overfeeding in Mice. Nutrients 2019, 11, 2796. [Google Scholar] [CrossRef] [PubMed]
- Gianfranceschi, G.; Caragnano, A.; Piazza, S.; Manini, I.; Ciani, Y.; Verardo, R.; Toffoletto, B.; Finato, N.; Livi, U.; Beltrami, C.A.; et al. Critical Role of Lysosomes in the Dysfunction of Human Cardiac Stem Cells Obtained from Failing Hearts. Int. J. Cardiol. 2016, 216, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, Y.; Okada, C.; Kawabe, N.; Sasaki, A.; Tsukamoto, H.; Nagao, R.; Osawa, M. Myocardial Lipofuscin Accumulation in Ageing and Sudden Cardiac Death. Sci. Rep. 2019, 9, 3304. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.A.; Templeton, L.J.; Gertz, S.J. Intrauterine Growth Retardation Leads to the Development of Type 2 Diabetes in the Rat. Diabetes 2001, 50, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal Visceral and Subcutaneous Adipose Tissue Compartments: Association with Metabolic Risk Factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef]
- Armani, A.; Berry, A.; Cirulli, F.; Caprio, M. Molecular Mechanisms Underlying Metabolic Syndrome: The Expanding Role of the Adipocyte. FASEB J. 2017, 31, 4240–4255. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.; Guo, Z.; Johnson, C.M.; Hensrud, D.D.; Jensen, M.D. Splanchnic Lipolysis in Human Obesity. J. Clin. Investig. 2004, 113, 1582–1588. [Google Scholar] [CrossRef]
- Desai, M.; Hales, C.N. Role of Fetal and Infant Growth in Programming Metabolism in Later Life. Biol. Rev. Camb. Philos. Soc. 1997, 72, 329–348. [Google Scholar] [CrossRef]
- McMillen, I.C.; Robinson, J.S. Developmental Origins of the Metabolic Syndrome: Prediction, Plasticity, and Programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef]
- Hales, C.N.; Desai, M.; Ozanne, S.E. The Thrifty Phenotype Hypothesis: How Does It Look after 5 Years? Diabet. Med. 1997, 14, 189–195. [Google Scholar] [CrossRef]
- Seckl, J.R.; Meaney, M.J. Glucocorticoid Programming. Ann. N. Y. Acad. Sci. 2004, 1032, 63–84. [Google Scholar] [CrossRef] [PubMed]
- Sasser, T.A.; Chapman, S.E.; Li, S.; Hudson, C.; Orton, S.P.; Diener, J.M.; Gammon, S.T.; Correcher, C.; Leevy, W.M. Segmentation and Measurement of Fat Volumes in Murine Obesity Models Using X-ray Computed Tomography. J. Vis. Exp. 2012, 62, e3680. [Google Scholar] [CrossRef]
- Desai, M.; Gayle, D.; Babu, J.; Ross, M.G. Programmed Obesity in Intrauterine Growth-Restricted Newborns: Modulation by Newborn Nutrition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R91–R96. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Gayle, D.; Babu, J.; Ross, M.G. Permanent Reduction in Heart and Kidney Organ Growth in Offspring of Undernourished Rat Dams. Am. J. Obstet. Gynecol. 2005, 193 Pt 2, 1224–1232. [Google Scholar] [CrossRef]
- Coupe, B.; Amarger, V.; Grit, I.; Benani, A.; Parnet, P. Nutritional Programming Affects Hypothalamic Organization and Early Response to Leptin. Endocrinology 2010, 151, 702–713. [Google Scholar] [CrossRef]
- Jia, Y.; Nguyen, T.; Desai, M.; Ross, M.G. Programmed Alterations in Hypothalamic Neuronal Orexigenic Responses to Ghrelin following Gestational Nutrient Restriction. Reprod. Sci. 2008, 15, 702–709. [Google Scholar] [CrossRef]
- Khandelwal, P.; Jain, V.; Gupta, A.K.; Kalaivani, M.; Paul, V.K. Association of Early Postnatal Growth Trajectory with Body Composition in Term Low Birth Weight Infants. J. Dev. Orig. Health Dis. 2014, 5, 189–196. [Google Scholar] [CrossRef]
- Carolan-Olah, M.; Duarte-Gardea, M.; Lechuga, J. A Critical Review: Early Life Nutrition and Prenatal Programming for Adult Disease. J. Clin. Nurs. 2015, 24, 3716–3729. [Google Scholar] [CrossRef]
- Intapad, S.; Tull, F.L.; Brown, A.D.; Dasinger, J.H.; Ojeda, N.B.; Fahling, J.M.; Alexander, B.T. Renal Denervation Abolishes the Age-Dependent Increase in Blood Pressure in Female Intrauterine Growth-Restricted Rats at 12 Months of Age. Hypertension 2013, 61, 828–834. [Google Scholar] [CrossRef]
- Saget, S.; Cong, R.; Decourtye, L.; Endale, M.L.; Martinerie, L.; Girardet, C.; Perret, C.; Clemessy, M.; Leneuve, P.; Dinard, L.; et al. Changes in Circulating Mirna19a-3p Precede Insulin Resistance Programmed by Intra-Uterine Growth Retardation in Mice. Mol. Metab. 2020, 42, 101083. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C.E.; Ozanne, S.E. Sex Differences in Developmental Programming Models. Reproduction 2013, 145, R1–R13. [Google Scholar] [CrossRef] [PubMed]
- Tarrade, A.; Panchenko, P.; Junien, C.; Gabory, A. Placental Contribution to Nutritional Programming of Health and Diseases: Epigenetics and Sexual Dimorphism. J. Exp. Biol. 2015, 218 Pt 1, 50–58. [Google Scholar] [CrossRef]
- Lefebvre, P.; Staels, B. Hepatic Sexual Dimorphism—Implications for Non-Alcoholic Fatty Liver Disease. Nat. Rev. Endocrinol. 2021, 17, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic Review: The Epidemiology and Natural History of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis in Adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.S.; Hvid, H.; Damgaard, J.; Nygaard, H.; Ingvorsen, C.; Wulff, E.M.; Lykkesfeldt, J.; Fledelius, C. Dietary Fat Stimulates Development of Nafld More Potently Than Dietary Fructose in Sprague-Dawley Rats. Diabetol. Metab. Syndr. 2018, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Deng, X.; Liu, Y.; Tan, Q.; Huang, G.; Che, Q.; Guo, J.; Su, Z. Kupffer Cells in Non-Alcoholic Fatty Liver Disease: Friend or Foe? Int. J. Biol. Sci. 2020, 16, 2367–2378. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Schwabe, R.F. Nf-Kappab in the Liver–Linking Injury, Fibrosis and Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef]
- Ferreira-Gonzalez, S.; Rodrigo-Torres, D.; Gadd, V.L.; Forbes, S.J. Cellular Senescence in Liver Disease and Regeneration. Semin. Liver Dis. 2021, 41, 50–66. [Google Scholar] [CrossRef]
- Martin-Ruiz, C.; Saretzki, G.; Petrie, J.; Ladhoff, J.; Jeyapalan, J.; Wei, W.; Sedivy, J.; von Zglinicki, T. Stochastic Variation in Telomere Shortening Rate Causes Heterogeneity of Human Fibroblast Replicative Life Span. J. Biol. Chem. 2004, 279, 17826–17833. [Google Scholar] [CrossRef]
- Swanlund, J.M.; Kregel, K.C.; Oberley, T.D. Autophagy following Heat Stress: The Role of Aging and Protein Nitration. Autophagy 2008, 4, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Cogger, V.C.; Svistounov, D.; Warren, A.; Zykova, S.; Melvin, R.G.; Solon-Biet, S.M.; O’Reilly, J.N.; McMahon, A.C.; Ballard, J.W.; de Cabo, R.; et al. Liver Aging and Pseudocapillarization in a Werner Syndrome Mouse Model. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Cogger, V.C.; Markus, A.M.; Harvey, P.J.; Yin, Z.L.; Ansselin, A.D.; McLean, A.J. Pseudocapillarization and Associated Energy Limitation in the Aged Rat Liver. Hepatology 2001, 33, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulou, E.A.; Tsimaratou, K.; Evangelou, K.; Marcos, P.J.F.; Zoumpourlis, V.; Trougakos, I.P.; Kletsas, D.; Bartek, J.; Serrano, M.; Gorgoulis, V.G. Specific Lipofuscin Staining as a Novel Biomarker to Detect Replicative and Stress-Induced Senescence. A Method Applicable in Cryo-Preserved and Archival Tissues. Aging 2013, 5, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Schmucker, D.L.; Sachs, H. Quantifying Dense Bodies and Lipofuscin during Aging: A Morphologist’s Perspective. Arch. Gerontol. Geriatr. 2002, 34, 249–261. [Google Scholar] [CrossRef]
- Jung, T.; Bader, N.; Grune, T. Lipofuscin: Formation, Distribution, and Metabolic Consequences. Ann. N. Y. Acad. Sci. 2007, 1119, 97–111. [Google Scholar] [CrossRef]
- Braidy, N.; Guillemin, G.J.; Mansour, H.; Chan-Ling, T.; Poljak, A.; Grant, R. Age Related Changes in Nad+ Metabolism Oxidative Stress and Sirt1 Activity in Wistar Rats. PLoS ONE 2011, 6, e19194. [Google Scholar] [CrossRef]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a Promising Target in Slowing Down the Ageing Process. Biogerontology 2017, 18, 447–476. [Google Scholar] [CrossRef]
- Langley, E.; Pearson, M.; Faretta, M.; Bauer, U.M.; Frye, R.A.; Minucci, S.; Pelicci, P.G.; Kouzarides, T. Human Sir2 Deacetylates P53 and Antagonizes Pml/P53-Induced Cellular Senescence. EMBO J. 2002, 21, 2383–2396. [Google Scholar] [CrossRef]
- Papatheodoridi, A.M.; Chrysavgis, L.; Koutsilieris, M.; Chatzigeorgiou, A. The Role of Senescence in the Development of Nonalcoholic Fatty Liver Disease and Progression to Nonalcoholic Steatohepatitis. Hepatology 2020, 71, 363–374. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Leclerc, J.; Hebrard, S.; Lantier, L.; Mounier, R.; Andreelli, F.; Foretz, M. Amp-Activated Protein Kinase in the Regulation of Hepatic Energy Metabolism: From Physiology to Therapeutic Perspectives. Acta Physiol. 2009, 196, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.; Prentki, M. Amp Kinase and Malonyl-Coa: Targets for Therapy of the Metabolic Syndrome. Nat. Rev. Drug Discov. 2004, 3, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tai, H.; Wang, X.; Wang, Z.; Zhou, J.; Wei, X.; Ding, Y.; Gong, H.; Mo, C.; Zhang, J.; et al. Ampk Activation Protects Cells from Oxidative Stress-Induced Senescence via Autophagic Flux Restoration and Intracellular Nad(+) Elevation. Aging Cell 2016, 15, 416–427. [Google Scholar] [CrossRef]
- Xu, X.J.; Gauthier, M.S.; Hess, D.T.; Apovian, C.M.; Cacicedo, J.M.; Gokce, N.; Farb, M.; Valentine, R.J.; Ruderman, N.B. Insulin Sensitive and Resistant Obesity in Humans: Ampk Activity, Oxidative Stress, and Depot-Specific Changes in Gene Expression in Adipose Tissue. J. Lipid Res. 2012, 53, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Horman, S.; Leclerc, J.; Lantier, L.; Foretz, M.; Billaud, M.; Giri, S.; Andreelli, F. Ampk Inhibition in Health and Disease. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Boudaba, N.; Marion, A.; Huet, C.; Pierre, R.; Viollet, B.; Foretz, M. Ampk Re-Activation Suppresses Hepatic Steatosis but Its Downregulation Does Not Promote Fatty Liver Development. EBioMedicine 2018, 28, 194–209. [Google Scholar] [CrossRef]
- Liang, Z.; Li, T.; Jiang, S.; Xu, J.; Di, W.; Yang, Z.; Hu, W.; Yang, Y. Ampk: A Novel Target for Treating Hepatic Fibrosis. Oncotarget 2017, 8, 62780–62792. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Li, C.F.; Cai, Z.; Zhang, X.; Jin, G.; Zhang, W.N.; Xu, C.; Wang, C.Y.; Morrow, J.; Zhang, S.; et al. The Critical Role of Ampk in Driving Akt Activation under Stress, Tumorigenesis and Drug Resistance. Nat. Commun. 2018, 9, 4728. [Google Scholar] [CrossRef]
- Yamazaki, S.; Satoh, H.; Watanabe, T. Liraglutide Enhances Insulin Sensitivity by Activating Amp-Activated Protein Kinase in Male Wistar Rats. Endocrinology 2014, 155, 3288–3301. [Google Scholar] [CrossRef]
- Li, S.; Ogawa, W.; Emi, A.; Hayashi, K.; Senga, Y.; Nomura, K.; Hara, K.; Yu, D.; Kasuga, M. Role of S6k1 in Regulation of Srebp1c Expression in the Liver. Biochem. Biophys. Res. Commun. 2011, 412, 197–202. [Google Scholar] [CrossRef]
- Kim, Y.M.; Shin, H.T.; Seo, Y.H.; Byun, H.O.; Yoon, S.H.; Lee, I.K.; Hyun, D.H.; Chung, H.Y.; Yoon, G. Sterol Regulatory Element-Binding Protein (Srebp)-1-Mediated Lipogenesis Is Involved in Cell Senescence. J. Biol. Chem. 2010, 285, 29069–29077. [Google Scholar] [CrossRef]
- Shimano, H.; Horton, J.D.; Shimomura, I.; Hammer, R.E.; Brown, M.S.; Goldstein, J.L. Isoform 1c of Sterol Regulatory Element Binding Protein Is Less Active Than Isoform 1a in Livers of Transgenic Mice and in Cultured Cells. J. Clin. Investig. 1997, 99, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Shimano, H.; Yahagi, N.; Amemiya-Kudo, M.; Hasty, A.H.; Osuga, J.; Tamura, Y.; Shionoiri, F.; Iizuka, Y.; Ohashi, K.; Harada, K.; et al. Sterol Regulatory Element-Binding Protein-1 as a Key Transcription Factor for Nutritional Induction of Lipogenic Enzyme Genes. J. Biol. Chem. 1999, 274, 35832–35839. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.A.; Griffiths, B.; Santos, C.R.; Pende, M.; Schulze, A. Regulation of the Srebp Transcription Factors by Mtorc1. Biochem. Soc. Trans. 2011, 39, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A. The Integral Role of Mtor in Lipid Metabolism. Cell Cycle 2011, 10, 861–862. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.Y.; Kim, D.Y.; Kim, S.J.; Jo, H.K.; Kim, G.W.; Chung, S.H. Betulinic Acid Alleviates Non-Alcoholic Fatty Liver by Inhibiting Srebp1 Activity Via the Ampk-Mtor-Srebp Signaling Pathway. Biochem. Pharmacol. 2013, 85, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.; Guo, H.; Yuan, Z.; Wang, T.; Zhang, L.; Jiang, Z. Emodin Alleviates Hepatic Steatosis by Inhibiting Sterol Regulatory Element Binding Protein 1 Activity by Way of the Calcium/Calmodulin-Dependent Kinase Kinase-Amp-Activated Protein Kinase-Mechanistic Target of Rapamycin-P70 Ribosomal S6 Kinase Signaling Pathway. Hepatol. Res. 2017, 47, 683–701. [Google Scholar]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.L.; Schulze, A. Srebp Activity Is Regulated by Mtorc1 and Contributes to Akt-Dependent Cell Growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Gan, Q.; Huang, J.; Zhou, R.; Niu, J.; Zhu, X.; Wang, J.; Zhang, Z.; Tong, T. Ppar{Gamma} Accelerates Cellular Senescence by Inducing P16ink4{Alpha} Expression in Human Diploid Fibroblasts. J. Cell Sci. 2008, 121 Pt 13, 2235–2245. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Puigserver, P. Fasting-Dependent Glucose and Lipid Metabolic Response through Hepatic Sirtuin 1. Proc. Natl. Acad. Sci. USA 2007, 104, 12861–12866. [Google Scholar] [CrossRef]
- Pfluger, P.T.; Herranz, D.; Velasco-Miguel, S.; Serrano, M.; Tschop, M.H. Sirt1 Protects against High-Fat Diet-Induced Metabolic Damage. Proc. Natl. Acad. Sci. USA 2008, 105, 9793–9798. [Google Scholar] [CrossRef]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-Specific Deletion of Sirt1 Alters Fatty Acid Metabolism and Results in Hepatic Steatosis and Inflammation. Cell Metab. 2009, 9, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Marazita, M.C.; Dugour, A.; Marquioni-Ramella, M.D.; Figueroa, J.M.; Suburo, A.M. Oxidative Stress-Induced Premature Senescence Dysregulates Vegf and Cfh Expression in Retinal Pigment Epithelial Cells: Implications for Age-Related Macular Degeneration. Redox. Biol. 2016, 7, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lee, G.; Heo, S.Y.; Roh, Y.S. Oxidative Stress Is a Key Modulator in the Development of Nonalcoholic Fatty Liver Disease. Antioxidants 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.T.; Fu, S.; Stocker, R.; Davies, M.J. Biochemistry and Pathology of Radical-Mediated Protein Oxidation. Biochem. J. 1997, 324 Pt 1, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C. “Sly as a Foxo”: New Paths with Forkhead Signaling in the Brain. Curr. Neurovasc. Res. 2007, 4, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C. Outfoxoing Disease and Disability: The Therapeutic Potential of Targeting Foxo Proteins. Trends Mol. Med. 2008, 14, 219–227. [Google Scholar] [CrossRef]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-Dependent Regulation of Foxo Transcription Factors by the Sirt1 Deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Hou, S.; Jiang, J.; Sekutowicz, M.; Kelly, J.; Bacskai, B.J. Rapid Cell Death Is Preceded by Amyloid Plaque-Mediated Oxidative Stress. Proc. Natl. Acad. Sci. USA 2013, 110, 7904–7909. [Google Scholar] [CrossRef]
- Xiong, S.; Salazar, G.; Patrushev, N.; Alexander, R.W. Foxo1 Mediates an Autofeedback Loop Regulating Sirt1 Expression. J. Biol. Chem. 2011, 286, 5289–5299. [Google Scholar] [CrossRef]
- Jaiswal, A.K. Nrf2 Signaling in Coordinated Activation of Antioxidant Gene Expression. Free Radic. Biol. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, S.; Long, D.J., 2nd; Jaiswal, A.K. Antioxidant Regulation of Genes Encoding Enzymes That Detoxify Xenobiotics and Carcinogens. Curr. Top. Cell. Regul. 2000, 36, 201–216. [Google Scholar] [PubMed]
- Zhang, D.D. Mechanistic Studies of the Nrf2-Keap1 Signaling Pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamamoto, M. Nrf2-Keap1 Regulation of Cellular Defense Mechanisms against Electrophiles and Reactive Oxygen Species. Adv. Enzyme Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef]
- Huang, J.; Tabbi-Anneni, I.; Gunda, V.; Wang, L. Transcription Factor Nrf2 Regulates Shp and Lipogenic Gene Expression in Hepatic Lipid Metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1211–G1221. [Google Scholar] [CrossRef]
- Kim, S.; Sohn, I.; Ahn, J.I.; Lee, K.H.; Lee, Y.S.; Lee, Y.S. Hepatic Gene Expression Profiles in a Long-Term High-Fat Diet-Induced Obesity Mouse Model. Gene 2004, 340, 99–109. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 Represses Nuclear Activation of Antioxidant Responsive Elements by Nrf2 through Binding to the Amino-Terminal Neh2 Domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [Green Version]
| - | CODE | U8959P | U8959P |
|---|---|---|---|
| - | VERSION | 1 | 40 |
| - | FORMULA INGREDIENTS G/KG | 210 Control | Hypoproteic 8% |
| 61250A99 | dextrose | 380 | 380 |
| 63400A99 | casein | 230 | 90 |
| 60366A99 | pregelatinized cornstarch | 200 | 345 |
| 91101A99 | pre-mixture of minerals PM 205B 7% | 70 | 70 |
| 64254A99 | crude cellulose | 60 | 60 |
| 61964A99 | lard | 30 | 30 |
| 61910A99 | colza oil | 10 | 10 |
| 61930A99 | corn oil | 10 | 10 |
| 90513A99 | pre-mixture of vitamins PV 200 1% (casein) | 10 | 5 |
| 61254A99 | sucrose | 0 | 0 |
| 65884A99 | choline | 0 | 0 |
| 65815A99 | vitamin e | 0 | 0 |
| 65300A99 | calcium carbonate | 0 | 0 |
| 65805A99 | inositol | 0 | 0 |
| 65841A99 | nicotinic acid | 0 | 0 |
| 65806A99 | para-amino-acid ben | 0 | 0 |
| 65876A99 | vitamin b12 | 0 | 0 |
| 65828A99 | vitamin k3 mnb | 0 | 0 |
| 65801A99 | vitamin a | 0 | 0 |
| 65831A99 | vitamin b1 thiamin | 0 | 0 |
| 65836A99 | riboflavin | 0 | 0 |
| 65856A99 | vitamin b6 pyridoxine | 0 | 0 |
| 65851A99 | vitamin b5 | 0 | 0 |
| 65811A99 | vitamin d3 | 0 | 0 |
| 65861A99 | folic acid | 0 | 0 |
| 65880A99 | vitamin c | 0 | 0 |
| 65873A99 | biotin | 0 | 0 |
| Primers | Forward Primer | Reverse Primer |
|---|---|---|
| Actin NM031144.3 | AACACCCCAGCCATGTACG | GCATGAGGGAGCGCGTAAC |
| Foxo1 NM001191846.3 | CGTCCTCGAACCAGCTCAA | TTGGCGGTGCAAATGAATAG |
| nfe2 NM001012224.1 | CTAGTTCGGGACATCCGTCG | TCTCGCTCCAACTGCACAAT |
| keap1 NM057152.2 | CTTCGGGGAGGAGGAGTTCT | ATTTGACCCAGTCGATGCAC |
| sirtuin-1 NM001372090.1 | TGTGCAGTGGAAGGAAAGCA | CTGCAACCTGCTCCAAGGTA |
| Tnf-alpha NM012675.3 | CATCCGTTCTCTACCCAGCC | GGGCTCTGAGGAGTAGACGA |
| Nfκb NM001276711.1 | ACCACTGTCAACAGATGGCCC | TTCGCACACGTAGCGGAATC |
| Srebf1 NM001276707.1 | CGCTCTTGACCGACATCGAA | GAGGCCACAGTGCTCATTCT |
| ppar gamma NM013124.3 | TCGCTGATGCACTGCCTATG | TGATTCCGAAGTTGGTGGGC |
| p 16 NM031550.2 | AACACTTTCGGTCGTACCCC | CCCAGCGGAGGAGAGTAGAT |
| p 21 NM080782.4 | ATGTCCGACCTGTTCCACAC | ATCGGCGCTTGGAGTGATAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keshavjee, B.; Lambelet, V.; Coppola, H.; Viertl, D.; Prior, J.O.; Kappeler, L.; Armengaud, J.-B.; Chouraqui, J.-P.; Chehade, H.; Vanderriele, P.-E.; et al. Stress-Induced Premature Senescence Related to Oxidative Stress in the Developmental Programming of Nonalcoholic Fatty Liver Disease in a Rat Model of Intrauterine Growth Restriction. Antioxidants 2022, 11, 1695. https://doi.org/10.3390/antiox11091695
Keshavjee B, Lambelet V, Coppola H, Viertl D, Prior JO, Kappeler L, Armengaud J-B, Chouraqui J-P, Chehade H, Vanderriele P-E, et al. Stress-Induced Premature Senescence Related to Oxidative Stress in the Developmental Programming of Nonalcoholic Fatty Liver Disease in a Rat Model of Intrauterine Growth Restriction. Antioxidants. 2022; 11(9):1695. https://doi.org/10.3390/antiox11091695
Chicago/Turabian StyleKeshavjee, Basile, Valentine Lambelet, Hanna Coppola, David Viertl, John O. Prior, Laurent Kappeler, Jean-Baptiste Armengaud, Jean-Pierre Chouraqui, Hassib Chehade, Paul-Emmanuel Vanderriele, and et al. 2022. "Stress-Induced Premature Senescence Related to Oxidative Stress in the Developmental Programming of Nonalcoholic Fatty Liver Disease in a Rat Model of Intrauterine Growth Restriction" Antioxidants 11, no. 9: 1695. https://doi.org/10.3390/antiox11091695
APA StyleKeshavjee, B., Lambelet, V., Coppola, H., Viertl, D., Prior, J. O., Kappeler, L., Armengaud, J.-B., Chouraqui, J.-P., Chehade, H., Vanderriele, P.-E., Allouche, M., Balsiger, A., Sarre, A., Peyter, A.-C., Simeoni, U., & Yzydorczyk, C. (2022). Stress-Induced Premature Senescence Related to Oxidative Stress in the Developmental Programming of Nonalcoholic Fatty Liver Disease in a Rat Model of Intrauterine Growth Restriction. Antioxidants, 11(9), 1695. https://doi.org/10.3390/antiox11091695








