The Role and Mechanisms of Selenium Supplementation on Fatty Liver-Associated Disorder
Abstract
:1. Introduction
2. Regulatory Mechanisms of Selenium
2.1. The Effect of Selenium on Insulin Resistance
2.2. The Regulation of Selenoproteins as Endogenous Antioxidant Defense
2.3. The Modulation of Selenium on Inflammation
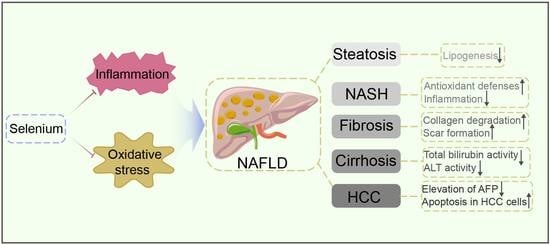
3. The Role of Selenium on the Progression of NAFLD
3.1. Selenium and Lipid Metabolism in the Liver
3.2. Selenium and Inflammatory Response in Liver
3.3. Selenium and Hepatic Fibrosis Formation
3.4. Selenium and Liver Cirrhosis Development
3.5. Selenium and Hepatocellular Carcinoma
4. Discussion and Conclusions
5. Challenges and Outlooks
Author Contributions
Funding
Conflicts of Interest
References
- Wang, N.; Tan, H.Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxid. Med. Cell. Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef] [PubMed]
- Pinsent, J. The need for selenite and molybdate in the formation of formic dehydrogenase by members of the Coli-aerogenes group of bacteria. Biochem. J. 1954, 57, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, C.; Perkins, A.V. Selenoproteins in the Human Placenta: How Essential Is Selenium to a Healthy Start to Life? Nutrients 2022, 14, 628. [Google Scholar] [CrossRef] [PubMed]
- Nosewicz, J.; Spaccarelli, N.; Roberts, K.M.; Hart, P.A.; Kaffenberger, J.A.; Trinidad, J.C.; Kaffenberger, B.H. The epidemiology, impact, and diagnosis of micronutrient nutritional dermatoses part 1: Zinc, selenium, copper, vitamin A., and vitamin C. J. Am. Acad. Dermatol. 2022, 86, 267–278. [Google Scholar] [CrossRef]
- Tóth, R.J.; Csapó, J. The role of selenium in nutrition—A review. Acta Univ. Sapientiae Aliment. 2018, 11, 128–144. [Google Scholar] [CrossRef] [Green Version]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Qataya, P.O.; Elsayed, N.M.; Elguindy, N.M.; Ahmed Hafiz, M.; Samy, W.M. Selenium: A sole treatment for erosive oral lichen planus (Randomized controlled clinical trial). Oral Dis. 2020, 26, 789–804. [Google Scholar] [CrossRef]
- Dennert, G.; Horneber, M. Selenium for alleviating the side effects of chemotherapy, radiotherapy and surgery in cancer patients. Cochrane Database Syst Rev. 2006, CD005037. [Google Scholar] [CrossRef]
- Liu, T.; Zeng, L.; Jiang, W.; Fu, Y.; Zheng, W.; Chen, T. Rational design of cancer-targeted selenium nanoparticles to antagonize multidrug resistance in cancer cells. Nanomedicine 2015, 11, 947–958. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [CrossRef] [Green Version]
- Burk, R.F.; Norsworthy, B.K.; Hill, K.E.; Motley, A.K.; Byrne, D.W. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol. Biomark. Prev. 2006, 15, 804–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuoka, H.C.; Chalasani, N. Nonalcoholic fatty liver disease: An emerging threat to obese and diabetic individuals. Ann. N. Y. Acad. Sci. 2013, 1281, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.; Choi, K.C.; Wong, G.L.; Zhang, Y.; Chan, H.L.; Luk, A.O.; Shu, S.S.; Chan, A.W.; Yeung, M.W.; Chan, J.C.; et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: A prospective cohort study. Gut 2016, 65, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, J.; Yang, X.; Wang, K.; Sun, K.; Yang, Z.; Zhang, L.; Yang, L.; Gu, C.; Huang, X.; et al. Dysregulated m6A Modification Promotes Lipogenesis and Development of Non-alcoholic Fatty Liver Disease and Hepatocellular Carcinoma. Mol. Ther. 2022. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Candia, R.; Zapata, R.; Munoz, C.; Arancibia, J.P.; Poniachik, J.; Soza, A.; Fuster, F.; Brahm, J.; Sanhueza, E.; et al. Management of nonalcoholic fatty liver disease: An evidence-based clinical practice review. World J. Gastroenterol. 2014, 20, 12182–12201. [Google Scholar] [CrossRef] [PubMed]
- Thuluvath, P.J.; Triger, D.R. Selenium in chronic liver disease. J. Hepatol. 1992, 14, 176–182. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Arner, P. Insulin resistance in type 2 diabetes: Role of fatty acids. Diabetes Metab. Res. Rev. 2002, 18 (Suppl. S2), S5–S9. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K.; Pettiti, M.; Hardies, J.; Miyazaki, Y.; Berria, R.; Buzzigoli, E.; Sironi, A.M.; Cersosimo, E.; Ferrannini, E.; et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007, 133, 496–506. [Google Scholar] [CrossRef]
- Feng, W.; Cui, X.; Liu, B.; Liu, C.; Xiao, Y.; Lu, W.; Guo, H.; He, M.; Zhang, X.; Yuan, J.; et al. Association of urinary metal profiles with altered glucose levels and diabetes risk: A population-based study in China. PLoS ONE 2015, 10, e0123742. [Google Scholar] [CrossRef] [PubMed]
- Barakat, G.M.; Moustafa, M.E.; Bikhazi, A.B. Effects of selenium and exendin-4 on glucagon-like peptide-1 receptor, IRS-1, and Raf-1 in the liver of diabetic rats. Biochem. Genet. 2012, 50, 922–935. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, D.; Vahl, T.; Prigeon, R. Effects of glucagon-like peptide 1 on the hepatic glucose metabolism. Horm. Metab. Res. 2004, 36, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Jun, H.S. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism 2014, 63, 9–19. [Google Scholar] [CrossRef]
- Barakat, G.; Moustafa, M.E.; Khalifeh, I.; Hodroj, M.H.; Bikhazi, A.; Rizk, S. Effects of exendin-4 and selenium on the expression of GLP-1R, IRS-1, and preproinsulin in the pancreas of diabetic rats. J. Physiol. Biochem. 2016, 73, 387–394. [Google Scholar] [CrossRef]
- Iizuka, Y.; Ueda, Y.; Yagi, Y.; Sakurai, E. Significant improvement of insulin resistance of GK rats by treatment with sodium selenate. Biol. Trace Elem. Res. 2010, 138, 265–271. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung. Cell Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [Green Version]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigo, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [Green Version]
- Brenneisen, P.; Steinbrenner, H.; Sies, H. Selenium, oxidative stress, and health aspects. Mol. Aspects Med. 2005, 26, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, G.F.; Gross, E.G.; et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996, 276, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.G.; Cheng, W.H.; McClung, J.P. Metabolic regulation and function of glutathione peroxidase-1. Annu. Rev. Nutr. 2007, 27, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef]
- Lillig, C.H.; Holmgren, A. Thioredoxin and related molecules—From biology to health and disease. Antioxid. Redox Signal. 2007, 9, 25–47. [Google Scholar] [CrossRef]
- Burke-Gaffney, A.; Callister, M.E.; Nakamura, H. Thioredoxin: Friend or foe in human disease? Trends Pharmacol. Sci. 2005, 26, 398–404. [Google Scholar] [CrossRef]
- Vunta, H.; Belda, B.J.; Arner, R.J.; Channa Reddy, C.; Vanden Heuvel, J.P.; Sandeep Prabhu, K. Selenium attenuates pro-inflammatory gene expression in macrophages. Mol. Nutr. Food Res. 2008, 52, 1316–1323. [Google Scholar] [CrossRef]
- Vunta, H.; Davis, F.; Palempalli, U.D.; Bhat, D.; Arner, R.J.; Thompson, J.T.; Peterson, D.G.; Reddy, C.C.; Prabhu, K.S. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages. J. Biol. Chem. 2007, 282, 17964–17973. [Google Scholar] [CrossRef] [Green Version]
- Kretz-Remy, C.; Arrigo, A.P. Selenium: A key element that controls NF-kappa B activation and I kappa B alpha half life. Biofactors 2001, 14, 117–125. [Google Scholar] [CrossRef]
- Yu, S.S.; Du, J.L. Selenoprotein S: A therapeutic target for diabetes and macroangiopathy? Cardiovasc. Diabetol. 2017, 16, 101. [Google Scholar] [CrossRef] [Green Version]
- Benedict, M.; Zhang, X. Non-alcoholic fatty liver disease: An expanded review. World J. Hepatol. 2017, 9, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, B.; Schulz, S.; Hiller, F.; Hesse, D.; Schumacher, F.; Kleuser, B.; Geisel, J.; Obeid, R.; Grune, T.; Kipp, A.P. Selenium increases hepatic DNA methylation and modulates one-carbon metabolism in the liver of mice. J. Nutr. Biochem. 2017, 48, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, C.; Cuzzocrea, S. The role of endogenous and exogenous ligands for the peroxisome proliferator-activated receptor alpha (PPAR-alpha) in the regulation of inflammation in macrophages. Shock 2009, 32, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Karamali, M.; Dastyar, F.; Badakhsh, M.H.; Aghadavood, E.; Amirani, E.; Asemi, Z. The Effects of Selenium Supplementation on Gene Expression Related to Insulin and Lipid Metabolism, and Pregnancy Outcomes in Patients with Gestational Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2020, 195, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.S.; Bollineni, J.S. Selenium-deficient diet induces renal oxidative stress and injury via TGF-beta1 in normal and diabetic rats. Kidney Int. 2001, 59, 1342–1353. [Google Scholar] [CrossRef] [Green Version]
- Sakiyama, H.; Fujiwara, N.; Yoneoka, Y.; Yoshihara, D.; Eguchi, H.; Suzuki, K. Cu, Zn-SOD deficiency induces the accumulation of hepatic collagen. Free Radic. Res. 2016, 50, 666–677. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, Z.Y.; Zhou, P.H.; Zhou, X.L.; Zhang, D.L.; He, N.; Zhang, J.; Liu, Y.H.; Gu, Q. Effects of oral selenium and magnesium co-supplementation on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in rats fed a high-fat diet. Lipids Health Dis. 2018, 17, 165. [Google Scholar] [CrossRef] [Green Version]
- Shidfar, F.; Faghihi, A.; Amiri, H.L.; Mousavi, S.N. Regression of Nonalcoholic Fatty Liver Disease with Zinc and Selenium Co-supplementation after Disease Progression in Rats. Iran. J. Med. Sci. 2018, 43, 26–31. [Google Scholar]
- Del Campo, J.A.; Gallego, P.; Grande, L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J. Hepatol. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Tang, C.; Li, S.; Zhang, K.; Li, J.; Han, Y.; Zhan, T.; Zhao, Q.; Guo, X.; Zhang, J. Selenium deficiency-induced redox imbalance leads to metabolic reprogramming and inflammation in the liver. Redox Biol. 2020, 36, 101519. [Google Scholar] [CrossRef]
- Al-Dossari, M.H.; Fadda, L.M.; Attia, H.A.; Hasan, I.H.; Mahmoud, A.M. Curcumin and Selenium Prevent Lipopolysaccharide/Diclofenac-Induced Liver Injury by Suppressing Inflammation and Oxidative Stress. Biol. Trace Elem. Res. 2020, 196, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.C.; Chu, J.H.; Chen, X.W.; Li, L.X.; Fan, R.F. Selenium alleviates mercury chloride-induced liver injury by regulating mitochondrial dynamics to inhibit the crosstalk between energy metabolism disorder and NF-kappaB/NLRP3 inflammasome-mediated inflammation. Ecotoxicol. Environ. Saf. 2021, 228, 113018. [Google Scholar] [CrossRef] [PubMed]
- Reja, M.; Makar, M.; Visaria, A.; Marino, D.; Rustgi, V. Increased serum selenium levels are associated with reduced risk of advanced liver fibrosis and all-cause mortality in NAFLD patients: National Health and Nutrition Examination Survey (NHANES) III. Ann. Hepatol. 2020, 19, 635–640. [Google Scholar] [CrossRef] [PubMed]
- George, J. Determination of selenium during pathogenesis of hepatic fibrosis employing hydride generation and inductively coupled plasma mass spectrometry. Biol. Chem. 2018, 399, 499–509. [Google Scholar] [CrossRef]
- Ding, M.; Potter, J.J.; Liu, X.; Torbenson, M.S.; Mezey, E. Selenium supplementation decreases hepatic fibrosis in mice after chronic carbon tetrachloride administration. Biol. Trace Elem. Res. 2010, 133, 83–97. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xu, J.Y.; Wei, Y.; Gao, S.L.; Wang, L.; Zheng, J.Y.; Gu, M.; Qin, L.Q. Protective Effect of Selenium-Enriched Green Tea on Carbon Tetrachloride-Induced Liver Fibrosis. Biol. Trace Elem. Res. 2021, 200, 2233–2238. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Ye, G.; Khan, A.; Liu, J.; Gan, F.; Zhang, X.; Kumbhar, S.; Huang, K. Protective effects of Selenium-enriched probiotics on carbon tetrachloride-induced liver fibrosis in rats. J. Agric. Food Chem. 2015, 63, 242–249. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Hesketh, J.; Huang, D.; Gan, F.; Hao, S.; Tang, S.; Guo, Y.; Huang, K. Protective effects of selenium-glutathione-enriched probiotics on CCl4-induced liver fibrosis. J. Nutr. Biochem. 2018, 58, 138–149. [Google Scholar] [CrossRef]
- Shen, X.H.; Cheng, W.F.; Li, X.H.; Sun, J.Q.; Li, F.; Ma, L.; Xie, L.M. Effects of dietary supplementation with vitamin E and selenium on rat hepatic stellate cell apoptosis. World J. Gastroenterol. 2005, 11, 4957–4961. [Google Scholar] [CrossRef]
- Nangliya, V.; Sharma, A.; Yadav, D.; Sunder, S.; Nijhawan, S.; Mishra, S. Study of trace elements in liver cirrhosis patients and their role in prognosis of disease. Biol. Trace Elem. Res. 2015, 165, 35–40. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Minuk, G.Y. Effects of hepatic stimulator substance, herbal medicine, selenium/vitamin E, and ciprofloxacin on cirrhosis in the rat. Gastroenterology 1996, 110, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.N.; Mahboob, T. Role of selenium in protection of liver cirrhosis. Pak. J. Pharm. Sci. 2013, 26, 1097–1102. [Google Scholar] [PubMed]
- Zhang, J.; Wang, H.; Yu, H. Thioacetamide-induced cirrhosis in selenium-adequate mice displays rapid and persistent abnormity of hepatic selenoenzymes which are mute to selenium supplementation. Toxicol. Appl. Pharmacol. 2007, 224, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Gong, Y.; Dong, F.; Geng, Y.; Zhuang, H.; Ma, Z.; Zhou, Z.; Huang, B.; Sun, Z.; Hou, B. Selenium concentration, dietary intake and risk of hepatocellular carcinoma—A systematic review with meta-analysis. Nutr. Hosp. 2019, 36, 1430–1437. [Google Scholar] [CrossRef]
- Zhang, Z.; Bi, M.; Liu, Q.; Yang, J.; Xu, S. Meta-analysis of the correlation between selenium and incidence of hepatocellular carcinoma. Oncotarget 2016, 7, 77110–77116. [Google Scholar] [CrossRef]
- Rohr-Udilova, N.; Sieghart, W.; Eferl, R.; Stoiber, D.; Bjorkhem-Bergman, L.; Eriksson, L.C.; Stolze, K.; Hayden, H.; Keppler, B.; Sagmeister, S.; et al. Antagonistic effects of selenium and lipid peroxides on growth control in early hepatocellular carcinoma. Hepatology 2012, 55, 1112–1121. [Google Scholar] [CrossRef]
- Yang, T.; Huo, J.; Xu, R.; Su, Q.; Tang, W.; Zhang, D.; Zhu, M.; Zhan, Y.; Dai, B.; Zhang, Y. Selenium sulfide disrupts the PLAGL2/C-MET/STAT3-induced resistance against mitochondrial apoptosis in hepatocellular carcinoma. Clin. Transl. Med. 2021, 11, e536. [Google Scholar] [CrossRef]
- Elkhamesy, A.; Refaat, M.; Gouida, M.S.O.; Alrdahe, S.S.; Youssef, M.M. Diminished CCl4-induced hepatocellular carcinoma, oxidative stress, and apoptosis by co-administration of curcumin or selenium in mice. J. Food Biochem. 2021, e13845. [Google Scholar] [CrossRef]
- Luo, M.; Huang, S.; Zhang, J.; Zhang, L.; Mehmood, K.; Jiang, J.; Zhang, N.; Zhou, D. Effect of selenium nanoparticles against abnormal fatty acid metabolism induced by hexavalent chromium in chicken’s liver. Environ. Sci. Pollut. Res. Int. 2019, 26, 21828–21834. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Zavos, C. Nonalcoholic fatty liver disease: The pathogenetic roles of insulin resistance and adipocytokines. Curr. Mol. Med. 2009, 9, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Reimers, E.; Monedero-Prieto, M.J.; Gonzalez-Perez, J.M.; Duran-Castellon, M.C.; Galindo-Martin, L.; Abreu-Gonzalez, P.; Sanchez-Perez, M.J.; Santolaria-Fernandez, F. Relative and combined effects of selenium, protein deficiency and ethanol on hepatocyte ballooning and liver steatosis. Biol. Trace Elem. Res. 2013, 154, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Adipokines in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1062–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Liang, H.; Yi, J.; Tan, W.; He, S.; Wang, S.; Li, F.; Wu, X.; Ma, J.; Shi, X.; et al. Long-Term Selenium-Deficient Diet Induces Liver Damage by Altering Hepatocyte Ultrastructure and MMP1/3 and TIMP1/3 Expression in Growing Rats. Biol. Trace Elem. Res. 2017, 175, 396–404. [Google Scholar] [CrossRef]
- Wasser, S.; Lim, G.Y.; Ong, C.N.; Tan, C.E. Anti-oxidant ebselen causes the resolution of experimentally induced hepatic fibrosis in rats. J. Gastroenterol. Hepatol. 2001, 16, 1244–1253. [Google Scholar] [CrossRef]
- Jacobs, E.T.; Lance, P.; Mandarino, L.J.; Ellis, N.A.; Chow, H.S.; Foote, J.; Martinez, J.A.; Hsu, C.P.; Batai, K.; Saboda, K.; et al. Selenium supplementation and insulin resistance in a randomized, clinical trial. BMJ Open Diabetes Res. Care 2019, 7, e000613. [Google Scholar] [CrossRef]
- Kim, I.W.; Bae, S.M.; Kim, Y.W.; Liu, H.B.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; Chaturvedi, P.K.; Battogtokh, G.; Ahn, W.S. Serum selenium levels in Korean hepatoma patients. Biol. Trace Elem. Res. 2012, 148, 25–31. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, C.; Liu, G.; Niu, Y.; Zhang, W.; Lu, S.; Li, X.; Zhang, H.; Ning, G.; Fan, J.; et al. Plasma selenium levels and nonalcoholic fatty liver disease in Chinese adults: A cross-sectional analysis. Sci. Rep. 2016, 6, 37288. [Google Scholar] [CrossRef]
- Hladun, K.R.; Smith, B.H.; Mustard, J.A.; Morton, R.R.; Trumble, J.T. Selenium toxicity to honey bee (Apis mellifera L.) pollinators: Effects on behaviors and survival. PLoS ONE 2012, 7, e34137. [Google Scholar] [CrossRef]
- Franz, E.D.; Wiramanaden, C.I.; Janz, D.M.; Pickering, I.J.; Liber, K. Selenium bioaccumulation and speciation in Chironomus dilutus exposed to water-borne selenate, selenite, or seleno-DL-methionine. Environ. Toxicol. Chem. 2011, 30, 2292–2299. [Google Scholar] [CrossRef]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- See, K.A.; Lavercombe, P.S.; Dillon, J.; Ginsberg, R. Accidental death from acute selenium poisoning. Med. J. Aust. 2006, 185, 388–389. [Google Scholar] [CrossRef] [PubMed]

| Component | Animal | Model | Route of Administration | Main Outcome | Ref |
|---|---|---|---|---|---|
| Sodium selenite | Rats | High-fat diet induced hyperlipidemic | Oral administration | Attenuated liver steatosis | [47] |
| Sodium selenite | Mice | Carbon tetrachloride (CCl4) induced hepatic fibrosis | Intraperitoneal injection | Decreased hepatic fibrosis after CCl4 | [55] |
| Selenium-enriched green tea | Mice | Carbon tetrachloride (CCl4) induced hepatic fibrosis | Oral administration | Improved liver fibrosis | [56] |
| Selenium-glutathione-enriched probiotics | Rats | Carbon tetrachloride (CCl4) induced hepatic fibrosis | Oral administration | Attenuated liver fibrosis | [58] |
| Selenium and vitamin E | Rats | Carbon tetrachloride (CCl4) induced hepatic fibrosis | Oral administration | Decreased the degree of hepatic fibrosis and promote the recovery process | [59] |
| Selenium and vitamin E | Rats | Carbon tetrachloride (CCl4)/ethanol-induced cirrhosis | Oral administration | Decreased the amount of hepatic fibrosis | [61] |
| Sodium selenite | Rats | Thioacetamide induced cirrhosis | Intraperitoneal injection | Attenuated liver cirrhosis. | [62] |
| Sodium selenite | Mice | Thioacetamide induced cirrhosis | Oral administration Intraperitoneal injection | Could not restore hepatic glutathione peroxidase | [63] |
| Sodium selenite | Rats | Diethylnitrosamin (DEN) induced hepatocellular carcinoma | Oral administration | Accelerated the growth of hepatocellular carcinoma | [67] |
| Selenium | Mice | Carbon tetrachloride (CCl4) induced hepatocellular carcinoma | Oral administration | Protected against liver damage | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Lu, Y.; Wang, N.; Feng, Y. The Role and Mechanisms of Selenium Supplementation on Fatty Liver-Associated Disorder. Antioxidants 2022, 11, 922. https://doi.org/10.3390/antiox11050922
Xu L, Lu Y, Wang N, Feng Y. The Role and Mechanisms of Selenium Supplementation on Fatty Liver-Associated Disorder. Antioxidants. 2022; 11(5):922. https://doi.org/10.3390/antiox11050922
Chicago/Turabian StyleXu, Lin, Yuanjun Lu, Ning Wang, and Yibin Feng. 2022. "The Role and Mechanisms of Selenium Supplementation on Fatty Liver-Associated Disorder" Antioxidants 11, no. 5: 922. https://doi.org/10.3390/antiox11050922
APA StyleXu, L., Lu, Y., Wang, N., & Feng, Y. (2022). The Role and Mechanisms of Selenium Supplementation on Fatty Liver-Associated Disorder. Antioxidants, 11(5), 922. https://doi.org/10.3390/antiox11050922