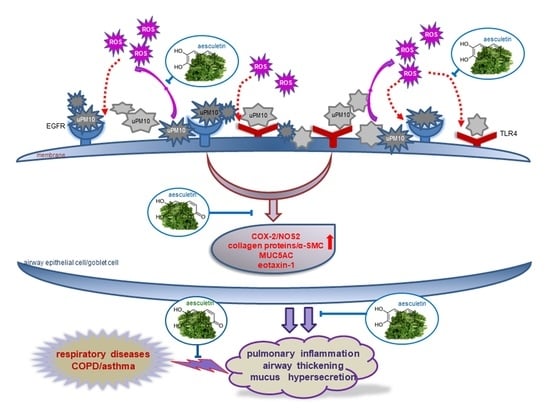
Aesculetin Inhibits Airway Thickening and Mucus Overproduction Induced by Urban Particulate Matter through Blocking Inflammation and Oxidative Stress Involving TLR4 and EGFR
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animal Experiments
2.3. Western Blot Analysis
2.4. Hematoxylin and Eosin (H&E) Staining
2.5. Masson Trichrome Staining
2.6. Alcian Blue-Periodic Acid-Schiff (PAS) Staining
2.7. Dihydroethidium (DHE) Staining for ROS Production
2.8. BEAS-2B Cell Culture
2.9. Statistical Analysis
3. Results
3.1. Inhibitory Effects of Aesculetin on uPM10-Induced Lung Inflammation
3.2. Suppressive Effects of Aesculetin on Thickening of uPM10-Inhaled Airways
3.3. Blockade of uPM10-Induced Airway Mucus Hypersecretion by Aesculetin
3.4. Inhibition of uPM10 Induction of Epithelial Mucin and Collagen by Aesculetin
3.5. Suppression of uPM10-Induced Inflammatory Epithelial Receptors by Aesculetin
3.6. Involvement of Oxidative Stress in Epithelial Receptor Activation by uPM10
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, composition, and lung diseases. Front. Immunol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Ali, M.U.; Liu, G.; Yousaf, B.; Ullah, H.; Abbas, Q.; Munir, M.A.M. A systematic review on global pollution status of particulate matter-associated potential toxic elements and health perspectives in urban environment. Environ. Geochem. Health 2019, 41, 1131–1162. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Yadav, A.; Tsai, C.J.; Kumar, P. A review on recent progress in observations, sources, classification and regulations of PM 2.5 in Asian environments. Environ. Sci. Pollut. Res. Int. 2016, 23, 21165–21175. [Google Scholar] [CrossRef]
- Cooper, D.M.; Loxham, M. Particulate matter and the airway epithelium: The special case of the underground? Eur. Respir. Rev. 2019, 28, 190066. [Google Scholar] [CrossRef]
- Cassee, F.R.; Héroux, M.E.; Gerlofs-Nijland, M.E.; Kelly, F.J. Particulate matter beyond mass: Recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal. Toxicol. 2013, 25, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Fiordelisi, A.; Piscitelli, P.; Trimarco, B.; Coscioni, E.; Iaccarino, G.; Sorriento, D. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail. Rev. 2017, 22, 337–347. [Google Scholar] [CrossRef]
- Losacco, C.; Perillo, A. Particulate matter air pollution and respiratory impact on humans and animals. Environ. Sci. Pollut. Res. Int. 2018, 25, 33901–33910. [Google Scholar] [CrossRef]
- Peters, A.; Veronesi, B.; Calderón-Garcidueñas, L.; Gehr, P.; Chen, L.C.; Geiser, M.; Reed, W.; Rothen-Rutishauser, B.; Schürch, S.; Schulz, H. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part. Fibre Toxicol. 2006, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Suhaimi, N.F.; Jalaludin, J. Biomarker as a research tool in linking exposure to air particles and respiratory health. BioMed Res. Int. 2015, 2015, 962853. [Google Scholar] [CrossRef]
- Korhonen, A.; Lehtomäki, H.; Rumrich, I.; Karvosenoja, N.; Paunu, V.V.; Kupiainen, K.; Sofiev, M.; Palamarchuk, Y.; Kukkonen, J.; Kangas, L.; et al. Influence of spatial resolution on population PM2.5 exposure and health impacts. Air Qual. Atmos. Health 2019, 12, 705–718. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, M.G.; Park, M.K.; Seo, Y.R. Predictive and prognostic biomarkers of respiratory diseases due to particulate matter exposure. J. Cancer Prev. 2017, 22, 6–15. [Google Scholar] [CrossRef]
- Yang, I.A.; Fong, K.M.; Zimmerman, P.V.; Holgate, S.T.; Holloway, J.W. Genetic susceptibility to the respiratory effects of air pollution. Thorax 2008, 63, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Kecorius, S.; Madueño, L.; Löndahl, J.; Vallar, E.; Galvez, M.C.; Idolor, L.F.; Gonzaga-Cayetano, M.; Müller, T.; Birmili, W.; Wiedensohler, A. Respiratory tract deposition of inhaled roadside ultrafine refractory particles in a polluted megacity of South-East Asia. Sci. Total Environ. 2019, 663, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Buonfiglio, L.G.V.; Comellas, A.P. Mechanism of ambient particulate matter and respiratory infections. J. Thorac. Dis. 2020, 12, 134–136. [Google Scholar] [CrossRef]
- Leikauf, G.D.; Kim, S.H.; Jang, A.S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp. Mol. Med. 2020, 52, 329–337. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W.; Donaldson, K. Mechanism of lung injury caused by PM10 and ultrafine particles with special reference to COPD. Eur. Respir. J. 2003, 21, 47s–51s. [Google Scholar] [CrossRef]
- Lodovici, M.; Bigagli, E. Oxidative stress and air pollution exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef]
- Jin, S.P.; Li, Z.; Choi, E.K.; Lee, S.; Kim, Y.K.; Seo, E.Y.; Chung, J.H.; Cho, S. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J. Dermatol. Sci. 2018, 91, 175–183. [Google Scholar] [CrossRef]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of particulate matter air pollution on cardiovascular oxidative stress pathways. Antioxid. Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef]
- Pardo, M.; Qiu, X.; Zimmermann, R.; Rudich, Y. Particulate matter toxicity is NRF2 and mitochondria dependent: The roles of metals and polycyclic aromatic hydrocarbons. Chem. Res. Toxicol. 2020, 33, 1110–1120. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Zaidi, S.R.; Sammani, S.; Siegler, J.; Moreno-Vinasco, L.; Mathew, B.; Natarajan, V.; Garcia, J.G.N. Hydrogen sulfide attenuates particulate matter-induced human lung endothelial barrier disruption via combined reactive oxygen species scavenging and Akt activation. Am. J. Respir. Cell. Mol. Biol. 2012, 47, 491–496. [Google Scholar] [CrossRef]
- Whyand, T.; Hurst, J.R.; Beckles, M.; Caplin, M.E. Pollution and respiratory disease: Can diet or supplements help? A review. Respir. Res. 2018, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Castro-Giner, F.; Kunzli, N.; Sunyer, J. Air pollution, oxidative stress and dietary supplementation: A review. Eur. Respir. J. 2008, 31, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Sun, L.; Chen, Y.; Zhang, L.; Zhang, Z. Therapeutic effects of stemonine on particulate matter 2.5-induced chronic obstructive pulmonary disease in mice. Exp. Ther. Med. 2017, 14, 4453–4459. [Google Scholar] [CrossRef]
- Boo, Y.C. Can plant phenolic compounds protect the skin from airborne particulate matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef]
- Kang, K.S.; Lee, W.; Jung, Y.; Lee, J.H.; Lee, S.; Eom, D.W.; Jeon, Y.; Yoo, H.H.; Jin, M.J.; Song, K.I.; et al. Protective effect of esculin on streptozotocin-induced diabetic renal damage in mice. J. Agric. Food Chem. 2014, 62, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Witaicenis, A.; Seito, L.N.; Di Stasi, L.C. Intestinal anti-inflammatory activity of esculetin and 4-methylesculetin in the trinitrobenzenesulphonic acid model of rat colitis. Chem. Biol. Interact. 2010, 186, 211–218. [Google Scholar] [CrossRef]
- Lee, H.C.; Liu, F.C.; Tsai, C.N.; Chou, A.H.; Liao, C.C.; Yu, H.P. Esculetin ameliorates lipopolysaccharide-induced acute lung injury in mice via modulation of the AKT/ERK/NF-κB and RORγt/IL-17 pathways. Inflammation 2020, 43, 962–974. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, Y.H.; Kang, M.K.; Lee, E.J.; Kim, D.Y.; Oh, H.; Kim, S.I.; Na, W.; Kang, Y.H. Aesculetin attenuates alveolar injury and fibrosis induced by close contact of alveolar epithelial cells with blood-derived macrophages via il-8 signaling. Int. J. Mol. Sci. 2020, 21, 5518. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kang, M.K.; Lee, E.J.; Kim, D.Y.; Oh, H.; Kim, S.I.; Oh, S.Y.; Kim, K.H.; Park, S.J.; Choi, Y.J.; et al. Dried yeast extracts curtails pulmonary oxidative stress, inflammation and tissue destruction in a model of experimental emphysema. Antioxidants 2019, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, Y.J.; Lee, E.J.; Kang, M.K.; Park, S.H.; Kim, D.Y.; Oh, H.; Park, S.J.; Kang, Y.H. Novel glutathione-containing dry-yeast extracts inhibit eosinophilia and mucus overproduction in a murine model of asthma. Nutr. Res. Pract. 2017, 11, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., 3rd; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef]
- Brunekreef, B.; Forsberg, B. Epidemiological evidence of effects of coarse airborne particles on health. Eur. Respir. J. 2005, 26, 309–318. [Google Scholar] [CrossRef]
- Ling, S.H.; van Eeden, S.F. Particulate matter air pollution exposure: Role in the development and exacerbation of chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2009, 4, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.L.; Wang, B.; Chen, H.; Ho, K.F.; Cao, J.; Hai, G.; Jalaludin, B.; Herbert, C.; Thomas, P.S.; Saad, S.; et al. Pulmonary inflammation induced by low-dose particulate matter exposure in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L424–L430. [Google Scholar] [CrossRef]
- Qing, H.; Wang, X.; Zhang, N.; Zheng, K.; Du, K.; Zheng, M.; Li, Y.; Chang, Y.; Zhang, L.; Bachert, C. The effect of fine particulate matter on the inflammatory responses in human upper airway mucosa. Am. J. Respir. Crit. Care Med. 2019, 200, 1315–1318. [Google Scholar] [CrossRef] [PubMed]
- Traboulsi, H.; Guerrina, N.; Iu, M.; Maysinger, D.; Ariya, P.; Baglole, C.J. Inhaled pollutants: The molecular scene behind respiratory and systemic diseases associated with ultrafine particulate matter. Int. J. Mol. Sci. 2017, 18, 243. [Google Scholar] [CrossRef]
- Shi, R.; Su, W.W.; Zhu, Z.T.; Guan, M.Y.; Cheng, K.L.; Fan, W.Y.; Wei, G.Y.; Li, P.B.; Yang, Z.Y.; Yao, H.L. Regulation effects of naringin on diesel particulate matter-induced abnormal airway surface liquid secretion. Phytomedicine 2019, 63, 153004. [Google Scholar] [CrossRef]
- Shoenfelt, J.; Mitkus, R.J.; Zeisler, R.; Spatz, R.O.; Powell, J.; Fenton, M.J.; Squibb, K.A.; Medvedev, A.E. Involvement of TLR2 and TLR4 in inflammatory immune responses induced by fine and coarse ambient air particulate matter. J. Leukoc. Biol. 2009, 86, 303–312. [Google Scholar] [CrossRef]
- He, M.; Ichinose, T.; Yoshida, Y.; Arashidani, K.; Yoshida, S.; Takano, H.; Sun, G.; Shibamoto, T. Urban PM2.5 exacerbates allergic inflammation in the murine lung via a TLR2/TLR4/MyD88-signaling pathway. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Bergin, D.A.; Greene, C.M.; Sterchi, E.E.; Kenna, C.; Geraghty, P.; Belaaouaj, A.; Taggart, C.C.; O’Neill, S.J.; McElvaney, N.G. Activation of the epidermal growth factor receptor (EGFR) by a novel metalloprotease pathway. J. Biol. Chem. 2008, 283, 31736–31744. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Q.; Zhou, X.D.; Kolosov, V.P.; Perelman, J.M. Naringenin attenuates mucous hypersecretion by modulating reactive oxygen species production and inhibiting NF-κB activity via EGFR-PI3K-Akt/ERK MAPKinase signaling in human airway epithelial cells. Mol. Cell. Biochem. 2011, 351, 29–40. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.-Y.; Kim, Y.-H.; Kang, M.-K.; Lee, E.-J.; Kim, D.-Y.; Oh, H.; Kim, S.-I.; Na, W.; Kang, I.-J.; Kang, Y.-H. Aesculetin Inhibits Airway Thickening and Mucus Overproduction Induced by Urban Particulate Matter through Blocking Inflammation and Oxidative Stress Involving TLR4 and EGFR. Antioxidants 2021, 10, 494. https://doi.org/10.3390/antiox10030494
Oh S-Y, Kim Y-H, Kang M-K, Lee E-J, Kim D-Y, Oh H, Kim S-I, Na W, Kang I-J, Kang Y-H. Aesculetin Inhibits Airway Thickening and Mucus Overproduction Induced by Urban Particulate Matter through Blocking Inflammation and Oxidative Stress Involving TLR4 and EGFR. Antioxidants. 2021; 10(3):494. https://doi.org/10.3390/antiox10030494
Chicago/Turabian StyleOh, Su-Yeon, Yun-Ho Kim, Min-Kyung Kang, Eun-Jung Lee, Dong-Yeon Kim, Hyeongjoo Oh, Soo-Il Kim, Woojin Na, Il-Jun Kang, and Young-Hee Kang. 2021. "Aesculetin Inhibits Airway Thickening and Mucus Overproduction Induced by Urban Particulate Matter through Blocking Inflammation and Oxidative Stress Involving TLR4 and EGFR" Antioxidants 10, no. 3: 494. https://doi.org/10.3390/antiox10030494
APA StyleOh, S.-Y., Kim, Y.-H., Kang, M.-K., Lee, E.-J., Kim, D.-Y., Oh, H., Kim, S.-I., Na, W., Kang, I.-J., & Kang, Y.-H. (2021). Aesculetin Inhibits Airway Thickening and Mucus Overproduction Induced by Urban Particulate Matter through Blocking Inflammation and Oxidative Stress Involving TLR4 and EGFR. Antioxidants, 10(3), 494. https://doi.org/10.3390/antiox10030494