Abstract
The aim of the study was to evaluate the impact of early pregnancy and exposure to tobacco smoke on antioxidant status and copper, zinc, and cadmium concentrations in the blood of non-smoking and smoking, as well as non-pregnant or pregnant women. The study included 213 women. More specifically, 150 women in first trimester of pregnancy and 63 non-pregnant women. Women were divided into subgroups according to exposure to tobacco smoke. Pregnancy significant influences higher copper and lower zinc concentration in the serum, whereas exposure to tobacco smoke during pregnancy is mainly associated with an elevation in cadmium and zinc concentration. It seems that metallothionein, superoxide dismutase, and glutathione peroxidase are the important antioxidants during early pregnancy, when exposure to tobacco smoke occurs, whereas the pregnancy itself is associated with a higher concentration of metallothionein and activity of catalase. Both pregnancy in the first trimester and exposure to tobacco smoke decrease glutathione concentration. In addition, active and passive maternal smoking have a similarly negative effect on antioxidant status in the first trimester. Early pregnancy as well as exposure to tobacco smoke is associated with significant alteration in antioxidant status and copper, zinc, and cadmium concentration. Due to a small number of smoking subjects (11 cases of non-pregnant, active smokers and 14 pregnant active smokers), the obtained results should be treated as a pilot, and this should be considered for future studies.
1. Introduction
Pregnancy is a physiological state associated with increased metabolism and demand for oxygen, which can cause excessive production of reactivity oxygen species (ROS) and deficiency of antioxidants [1]. Oxidative stress has been associated with many reproductive and pregnancy disorders, including pregnancy loss [2,3]. Between 10 and 12 weeks of gestation, the trophoblast plugs are dislodged from maternal spiral arteries, filling the intervillous space with maternal blood [4]. This is accompanied by a sharp rise in oxygen tension, marking the establishment of full maternal arterial circulation to the placenta associated with an increase in ROS and oxidative stress [5]. Exposure to tobacco smoke during pregnancy intensifies metabolic and biochemical disorders and adaptive responses in both the fetus and the mother [6]. The impact of tobacco smoke on fetal growth may be observed from the beginning of pregnancy [7]. In our earlier study, we revealed that the trophoblast was significantly smallest in smoking pregnant women and its lower volume was correlated with higher cotinine and cadmium (Cd) concentration [7]. In addition, chronic exposure to tobacco smoke during pregnancies complicated by intrauterine growth restriction (IUGR) impaired flows in fetal arteries and veins and increased the risk of hypoxia in decompensation phase [8]. Furthermore, we observed in the group of women with diagnosed oligohydramnios or premature rupture of membranes that exposure to tobacco smoke altered the distribution of Cd, lead (Pb), and zinc (Zn), which probably affected placental tissue and fetal membranes [9].
The smoking pregnant woman exposes her fetus to a variety of harmful chemicals [10] which disturb, inter alia, the homeostasis of trace elements, such as Zn and Cu [11]. Previous studies showed that the optimum concentration of Zn and Cu, as well as the correct value of the Cu/Zn ratio, plays a crucial role in fertility and reproductive outcomes [12]. Physiological and metabolic changes of pregnant women as well as increased demands for the important elements such as Zn and Cu by the developing fetus, decrease their concentrations in maternal blood, and lead to pregnancy complications [13,14]. Zn is an essential trace element required by the human body for a variety of biological functions and is required for reproduction and promotes healthy growth [13]. While Zn deficiency was associated with a lower implantation rate, abnormal ovarian development, ovarian follicular growth, and oocyte maturation [15], too high Cu concentrations are associated with the promotion of oxidative stress [16]. Contemporary medicine indicates that lower maternal plasma Cu concentrations in the first trimester of pregnancy is a better protection against risk for any pregnancy complication when compared with women with high plasma Cu [17,18]. It was also claimed that Cu may be more important for placentation rather than conception [17]. In the first trimester of pregnancy, antioxidants protect trophoblast cells against oxidative stress [16,19]. One of the important non-enzymatic antioxidants is metallothionein (MT), a low molecular weight protein which mainly scavenges O2•− or •OH [20]. MT binds, but also is induced by, both essential (for example Zn and Cu) and toxic metals (for example Cd) [21]. The presence of MT was demonstrated in the human placenta and fetal membranes [22,23,24]. Furthermore, glutathione (GSH) is also one of the first-line non-enzymatic antioxidants which detoxify O2•− or •OH [25]. It was shown that Zn deficiency is accompanied by an increase in oxidants which decreases GSH concentration [20]. Cu and Zn are also required for superoxide dismutase (SOD) activity, a universal antioxidant enzyme, which catalyzes O2•− to hydrogen peroxide (H2O2) and molecular oxygen (O2) [26]. Hydrogen peroxide is still highly toxic, but less reactive than O2•− [27] and is further converted by catalase (CAT) or selenium-dependent glutathione peroxidase (GPx) to water (H2O) [28].
Alterations in nonenzymatic and enzymatic antioxidants during pregnancy was demonstrated in many investigations. Changes in MT concentration could be associates with disorders in homeostasis of Zn and Cu as well as detoxification of Cd both in maternal and umbilical cord blood, which could negatively affect the fetus development [1,29,30]. The drop in SOD activity was found during pregnancy complicated by preeclampsia [31], IUGR (1), in the blood of women with recurrent miscarriage [14] or missed abortion [32]. Moreover, reduced activity of GPx during pregnancy could be associated with hypertension of preeclampsia [33] and spontaneous abortion [34], while decreased activity of CAT was proposed as a promising predictor for preeclampsia [35].
To sum up, our study aimed to assess the effect of pregnancy and exposure to tobacco smoke during the first trimester of pregnancy on the status of non-enzymatic- or enzymatic antioxidants and Zn, Cu, and Cd concentrations in the blood of women. Our investigations used blood samples from non-pregnant women and women in the first trimester of pregnancy, exposed and not exposed to tobacco smoke.
2. Materials and Methods
The study included 150 women in weeks 11 to 14 of pregnancy: 52 of them were exposed to tobacco smoke (38 passive tobacco smokers and 14 active tobacco smokers) and 98 were non-smoking healthy pregnant women without any complications during pregnancy. The main inclusion criterion was singleton pregnancy. Women with any complications during their current or previous pregnancy (for example, diabetes, pregnancy-induced hypertension, preeclampsia, fetal or uterine malformations; pregnancies resulting from infertility treatment, vaginal bleeding) were excluded from the study. Control subjects were 63 young non-pregnant women, not exposed to tobacco smoke (n = 52) or actively smoking (n = 11). Pregnant women were of similar age and had similar BMI values. Women from the control group also were of similar age and had similar BMI values. The characteristics of pregnant women and the control group were summarized in Table 1. Pregnant and non-pregnant women were divided into subgroups according to cotinine (metabolite of nicotine) concentration and information was obtained in a personal interview. Biochemical parameters of both the study group and the control group were assayed in the Department of Biomedical and Environmental Analysis, Wroclaw Medical University. Blood collection, as well as clinical investigations of pregnant women, were performed in the 2nd Department and Clinic of Obstetrics and Gynaecology, Wroclaw Medical University. The study was approved by the Local Bioethics Committee of Wroclaw Medical University (KBN-152/2015). All pregnant women had undergone the prenatal examination in weeks 11–14 according to Fetal Medicine Foundation guidelines and were qualified for prenatal examination due to the mother’s age. The inclusion criteria, detailed characteristics, and conducted procedures including 3D ultrasound examinations and Doppler flow imaging were published earlier [7]. Also, in the control group, women taking any medicines including oral contraception/supplements were excluded from the study.

Table 1.
Characteristics of studied women.
Cotinine concentration was assayed using a commercially available test (ref. No. 40-101-325056; GenWay Biotech Inc, San Diego, CA, USA). Serum Zn and Cu concentrations were determined with the use of flame atomic absorption spectrometry using SOLAAR M6, Thermo Elemental Co. Cd in whole blood was assayed using graphite furnace atomic absorption spectroscopy (GFAAS), with an absorbency measured at λ = 228.8 nm, using Zeeman background correction. Elements were assayed in a certified Atomic Absorption Spectroscopy Laboratory of the Department and Clinic of Internal and Occupational Diseases, Wroclaw Medical University.
The concentration of MT in plasma and erythrocyte lysate was measured by the two-step direct enzyme-linked immunosorbent assay (ELISA) using the method developed in our laboratory [36]. The concentration of GSH in whole blood was determined using a spectrophotometric method based on the reaction of GSH with alloxan and the formation of a complex with an absorbance maximum at 305 nm [37]. Superoxide dismutase activity in plasma was determined with Superoxide Dismutase Assay Kit (ref. No. 706002, Cayman Chemicals, USA). Plasma catalase activity was measured using the Catalase Assay Kit (ref. No. 707002, Cayman Chemicals, Ann Arbor, MI, USA). The assay of GPx activity in plasma was performed with the Glutathione Peroxide Assay Kit (ref. No. 703102, Cayman Chemicals, Ann Arbor, MI, USA).
Statistical analysis was performed using the Statistica software package, version 13.3 (Polish version; StatSoft, Kraków, Poland). Values were shown as mean ± SD and 1st quartile, median, 3rd quartile. The normality of the variables was tested with the use of the Shapiro-Wilk test. The homogeneity of variance was assessed using Levene’s test. The Student’s t-test was used when the normality of distribution and equality of variance were satisfied. The non-parametric Mann–Whitney U test was used when a lack of normal distribution and variance uniformity were revealed. Correlation was checked using the Pearson product-moment correlation coefficient or Spearman’s rank-order correlation coefficient. The value of p lower than 0.05 was considered significant.
3. Results
3.1. The Impact of Early Pregnancy on Metal Concentration and Antioxidant Status
3.1.1. Metal Concentrations
A significantly lower Zn concentration was found in the group of non-smoking pregnant women when compared to the non-smoking control group. Cu concentration, as well as the value of Cu/Zn ratio, in both non-smoking and actively smoking pregnant women was significantly higher than in both groups of non-pregnant women. A higher Cd concentration was observed in the group of non-smoking pregnant women when compared to non-smoking, non-pregnant women. Zn and Cd concentrations were similar in both groups of actively smoking non-pregnant and pregnant women (Table 2 and Table 3).

Table 2.
Values of selected antioxidative parameters, and metals in nonsmokers.

Table 3.
Values of selected antioxidative parameters, and metals in smokers.
3.1.2. Non-Enzymatic Antioxidant Status
In the group of pregnant smoking women, GSH concentration was the lowest and almost 5-fold lower when compared to smoking non-pregnant women. Also, GSH concentration in the group of non-smoking pregnant women was lower when compared to non-smoking non-pregnant women. Plasma MT concentration in both groups of pregnant women was higher than in both groups of non-pregnant women, while MT concentration in erythrocyte lysate was similar in non-smoking pregnant women and non-smoking non-pregnant women (Table 2 and Table 3).
3.1.3. Enzymatic Antioxidant Status
Significantly lower plasma SOD activity was revealed in both groups of pregnant women when compared to non-pregnant women (Table 2 and Table 3). Higher plasma CAT activity and lower GPx activity were found in the group of non-smoking pregnant women when compared to non-smoking non-pregnant women, whereas lower plasma CAT activity was observed in the group of actively smoking pregnant women when compared to actively smoking non-pregnant women (Table 2 and Table 3).
3.2. The Impact of Exposure to Tobacco Smoke during the First Trimester of Pregnancy on Copper, Zinc, and Cadmium Concentration and Blood Antioxidant Status
3.2.1. Metal Concentrations
We revealed a statistically significantly higher serum Zn and whole blood Cd concentrations in the group of pregnant women exposed to tobacco smoke when compared to non-exposed pregnant women. Active exposure to tobacco smoke further increased Zn and Cd concentrations. Cu concentration was similar in all pregnant women. A lower value of Cu/Zn ratio was found in the group of pregnant women exposed to tobacco smoke when compared to non-exposed pregnant women (Table 4 and Table 5).

Table 4.
Values of selected antioxidative parameters, and metals in pregnant women non-exposed or exposed (passive or active) to tobacco smoke.

Table 5.
Values of selected antioxidative parameters, and metals in studied subjects.
3.2.2. Non-Enzymatic Antioxidant Status
Significantly higher plasma and erythrocyte lysate MT concentrations and lower GSH concentrations were found only in the group of pregnant women exposed to tobacco smoke when compared to the non-exposed group. The type of exposure to tobacco smoke did not influence MT and GSH concentrations (Table 4 and Table 5).
3.2.3. Enzymatic Antioxidant Status
In the group of exposed pregnant women, significantly higher plasma SOD and GPx activity, and lower CAT activity was observed when compared to non-exposed pregnant women. The activity of SOD, GPx, and CAT was similar in both groups of passively and actively smoking pregnant women (Table 4 and Table 5).
3.3. Correlation Coefficient
Correlation coefficients in the group of non-smoking, passively, and actively smoking pregnant women are summarized in Table 6.

Table 6.
Significant correlation coefficients in the group of pregnant women.
3.3.1. In Non-Smoking Pregnant Women
Increased lysate MT concentration was associated with higher concentrations of Cu and higher values of the Cu/Zn ratio. GSH concentration was positively correlated with GPx activity as well as negatively with CAT activity.
3.3.2. In Passively Smoking Pregnant Women
A negative correlation between GSH concentration and activity of CAT was observed. In addition, GPx activity was negatively correlated with Cu concentration, whereas higher CAT activity was associated with lower Zn concentration.
3.3.3. In Actively Smoking Pregnant Women
GSH concentration was positively correlated with the activity of SOD or GPx. In addition, increased SOD activity was associated with higher Zn concentration, whereas a lower GSH concentration was associated with higher values of the Cu/Zn ratio.
4. Discussion
Little is known about the impact of pregnancy, especially in the first trimester, and exposure to tobacco smoke on antioxidant status in pregnant women, therefore in our study we assessed the levels of non-enzymatic (MT, GSH) and enzymatic antioxidants (SOD, CAT, GPx) in the blood of non-smoking and smoking women who were not pregnant or in the first trimester of pregnancy. In addition, we evaluated the influence of those factors on Zn, Cu, and Cd concentrations in the blood of the investigated women.
Regardless of exposure to tobacco smoke, higher concentrations of Cu and values of Cu/Zn were found in the serum of non-smoking and smoking pregnant women when compared to non-smoking and smoking non-pregnant women, respectively. Our results clearly show that pregnancy itself is associated with higher Cu concentration, whereas exposure to tobacco smoke, independently of type (passive or active smoking), did not influence Cu concentrations in the pregnant group, which coincides with our earlier study conducted in the group of pregnant women with IUGR [1] and with results obtained by other authors [10]. In addition, similar to our previous study, the average Cu concentration in the serum of pregnant women was higher than the reference range of the used method [1].
Increased Cu concentrations in pregnancy was also demonstrated by other authors [16], which was explained by an increase in its carrier proteins, ceruloplasmin, and elevated levels of maternal estrogens. It was also shown that, in serum of women with normal fetal development, Cu concentration was increased when compared to women with fetal developmental disorders [38]. On the other hand, it was demonstrated, that women at early stage of pregnancy with lower plasma Cu concentrations were better protected against risk for any pregnancy complication when compared with women with high plasma Cu [17], because higher concentration of Cu is associated not only with oxidative stress, but also with inflammation.
It was well documented that higher concentration of Cu could have teratogenic consequences [39] due to excessive cellular oxidative damage [40]. Oxidized reactions are greatly accelerated by the presence of Cu in the Fenton reaction [38]. Copper is one of the most important transition metals involved in the production of •OH [16,38]. In the presence of a higher H2O2 concentration (especially when a lower activity of CAT or GPx occurs), Cu can generate •OH, which is the most harmful free radical in the human body. In addition, a higher concentration of H2O2 can release iron ions from a range of haem proteins and further increase the production of •OH and enhance oxidative stress.
In the case of Zn concentration, we revealed a significant drop in the serum of pregnant women when compared to that of non-pregnant women, but this finding was only observed in the group of non-exposed women. The literature has also shown lower Zn concentrations in the serum of pregnant women when compared to non-pregnant women [14,41]. During pregnancy, there is a drop in circulating Zn and a decrease also occurs as the pregnancy progresses, possibly due to a decrease in Zn binding and increased transfer of Zn from the mother to the fetus [41]. In addition, Zn does not undergo redox changes and as a consequence, it cannot be involved in electron transfer reactions, which is why the cellular toxicity of Zn is lower than that of Cu [42].
When we take into consideration the concentration of Zn only in the group of pregnant women, we found concentration in the group of smoking women to be significantly higher (with the highest value in the group of active smokers) than in the non-smoking women, which presumably could be associated with the presence of Zn in cigarettes [43]. The differences in Zn and Cu concentrations could be also associated with the age of pregnant women. The main features of pregnant women involved in our study were physiological single pregnancy and maternal age around 35 years old. The literature also indicates that aging is associated with decreased Zn status and an increased value of the Cu/Zn ratio [44], which can also explain the lower Zn concentration and higher value of the Cu/Zn ratio in our group of pregnant women.
In our study higher concentration of Cd was associated not only with exposure to tobacco smoke but also with pregnancy. In addition, active exposure to tobacco smoke in the first trimester increased Cd concentration more than passive exposure. Previous studies showed that higher Cd concentration in maternal blood was associated with decreased birth weight [45,46] and this effect was independent of cotinine-defined smoking status [47]. Furthermore, in the group of smokers, higher Zn concentration can protect against Cd toxicity [48] and those metals compete for the same binding targets, and Zn ions can reduce the adverse effect of Cd due to Zn induction of MT synthesis [49]. Increased placenta concentrations of Cd, Zn [23,50], and MT was earlier reported in smoking pregnant women [50]. In addition, MT is involved in the cellular storage and/or delivery of Cu ions to cuproenzymes [51], which potentially explains a positive correlation between the concentration of MT and Cu or the value of the Cu/Zn ratio.
In both groups of non-smoking and smoking pregnant women we found higher plasma MT concentrations, and almost 4-fold lower plasma SOD activity when compared to non-pregnant non-smoking or smoking women, respectively. SOD is another such parameter associated with Zn and Cu homeostasis [52,53,54]. Lower plasma SOD activity could result from the replacement of Cu from the active center of the enzyme. Also, a higher concentration of Cd can decrease SOD activity, which was earlier claimed in an experimental study by other authors [55]. Moreover, SOD scavenges O2•− which is also removed by MT, which could suggest that MT plays a crucial role as an antioxidant during pregnancy. A similar effect of exposure to tobacco smoke on higher MT concentration in the blood of pregnant women with intrauterine growth restriction was observed in our earlier study [1]. In addition, we revealed in a previous study that MT concentration in the first trimester was lower when compared to the third trimester [30]. The kind of exposure to tobacco smoke (passive or active smoking) during pregnancy did not influence MT concentration either in plasma and lysate or plasma SOD activity, whereas exposure to tobacco smoke during pregnancy resulted in a higher MT concentration and SOD activity. We found higher SOD activity in the plasma of pregnant women exposed to tobacco smoke when compared to non-exposed pregnant women, but we did not reveal any significant difference in SOD activity between actively and passively smoking pregnant women. There are fewer data concerning the influence of exposure to tobacco smoke during pregnancy on SOD activity. A previous study has shown a higher SOD activity in the group of smoking pregnant women when compared to non-smoking subjects [56], which was explained by an elevation in Zn concentration, which was also increased in serum of women exposed to tobacco smoke. The same correlation was obtained in the present study, where we found a positive correlation between SOD activity and Zn concentration in the group of actively smoking pregnant women (r = 0.68).
Another important non-enzymatic antioxidant which acts as a major intracellular defense against oxidative stress is GSH [57] which is consumed by this process resulting in lowered intracellular GSH concentration. Also, presumably in our study, higher oxidative stress associated with pregnancy and/or exposure to tobacco smoke significantly decreased the concentration of GSH. Reduced concentration of GSH was also observed by other authors when exposure to tobacco smoke occurs [58] or during pregnancy, which was explained by oxidative stress and increases in plasma volume [59]. Unfortunately, when we evaluated the effect of exposure to tobacco smoke in each trimester of pregnancy in our previous study, we did not reveal any significant differences between non-smoking and smoking pregnant women [30].
It seems that H2O2 scavenging was dependent on exposure to tobacco smoke. After the conversion of O2•− to H2O2, hydrogen peroxide is decomposed to water by either CAT or GPx enzymes [60]. We found higher activity of CAT only in the group of non-smoking pregnant women when compared to non-smoking non-pregnant women, whereas in other cases we observed significantly lower activity of CAT (in the group of actively smoking pregnant women when compared to actively smoking non-pregnant women or non-smoking and smoking pregnant women (passive or active smoking), which suggests that pregnancy itself is associated with an elevation in CAT activity, whereas exposure to tobacco smoke during pregnancy decreases CAT activity. In contrast, in the group with lower CAT activity, higher GPx activity was observed which confirmed that those two enzymatic antioxidants complemented each other. GPx is the most important enzyme to eliminate peroxides in the cells of mammals and needs GSH as a cofactor which is oxidized in the presence of free radicals [61]. This finding was confirmed in our study by a positive correlation between GSH concentration and GPx activity in the group of non-smoking and actively smoking pregnant women. The results of our study suggest that GPx plays a crucial role as a scavenger of H2O2 in the group of pregnant women exposed to tobacco smoke and the results are similar to other investigations [56]. Chełchowska et al. also demonstrated, at the beginning of pregnancy, an elevation in activity of GPx, but only in the smoking group, whereas the activity of CAT was increased in the non-smoking group [56].
On the other hand, higher activity of CAT could be associated with a reduced risk of many diseases during pregnancy, when exposure to tobacco smoke did not occur and that decrease in CAT activity during pregnancy in women exposed to tobacco smoke may be indicative of early-onset preeclampsia [36], which confirmed the results of a meta-analysis conducted by Wei et al. [62]. In addition, lower CAT activity in early pregnancy may be related to preterm birth [63], which could be enhanced by exposure to tobacco smoke [11,64]. Probably, in our study lower CAT activity was compensated by higher activity of GPx which should be sufficient to convert H2O2 to H2O [65], even if a higher concentration of Cu occurs.
It is possible that in the first trimester of pregnancy, the antioxidant system is sufficient to prevent oxidative stress, but many diseases develop in the later stages of pregnancy, but to confirm these findings, further investigation on larger sample size is needed.
Our results suggest that MT, SOD, and GPx are important antioxidants during pregnancy when exposure to tobacco smoke occurs, whereas pregnancy itself is associated with a higher concentration of MT and increased activity of CAT. Both exposure to tobacco smoke and pregnancy decrease the concentration of GSH.
Our study also confirmed that active and passive maternal smoking have a similarly negative effect on antioxidant status in the first trimester.
Some limitations should be considered when interpreting these study results. Due to a small number of smoking subjects (11 cases of non-pregnant, active smokers and 14 pregnant active smokers), the obtained results should be treated as a pilot, and should be considered for future studies.
5. Conclusions
Active and passive maternal smoking has a similarly negative effect on antioxidant status in the first trimester. Early pregnancy itself significantly increased serum Cu concentration, while decreased Zn concentration and activity of SOD. In addition, an elevation in activity of CAT in plasma of pregnant women was found, but when exposure to tobacco smoke did not occur. An inverse effect was observed in case of GPx activity. Both investigated factors, i.e., early pregnancy and exposure to tobacco smoke, were related to reduced concentration of GSH. It seems that plasma MT plays a crucial role as an antioxidant during pregnancy (Figure 1 and Figure 2 summarize the main findings).
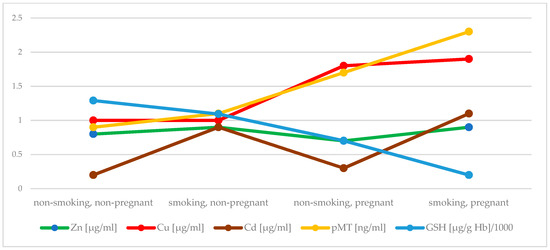
Figure 1.
Comparison of the concentartion of Zn, Cu, Cd, MT in plasma and GSH between groups.
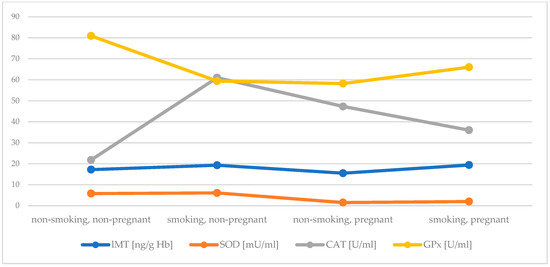
Figure 2.
Comparison of the concentartion of MT in lysate and the activity of SOD, CAT and GPx between groups.
Author Contributions
Conceptualization, A.B. and H.M.; methodology, A.B. and K.K.-P.; software, A.B.; validation, A.B.; formal analysis, A.B. and H.M.; investigation, A.B. and K.K.-P.; resources, H.M. and E.M.-N.; data curation, E.M.-N.; writing—original draft preparation, A.B.; writing—review and editing, A.B., H.M., and E.M.-N.; supervision, H.M. and E.M.-N.; project administration, H.M. and E.M.-N.; funding acquisition, H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the SUB.D170.21.042, Wroclaw Medical University.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Local Bioethics Committee of Wroclaw Medical University (KBN-152/2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bizoń, A.; Milnerowicz-Nabzdyk, E.; Zalewska, M.; Zimmer, M.; Milnerowicz, H. Changes in pro/antioxidant balance in smoking and non-smoking pregnant women with intrauterine growth restriction. Reprod. Toxicol. Elmsford N 2011, 32, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.A.M.; Schetinger, M.R.C.; Leal, D.B.R.; Morsch, V.M.; da Silva, A.S.; Rezer, J.F.P.; de Bairros, A.V.; Jaques, J.A.D.S. Oxidative stress and antioxidant defenses in pregnant women. Redox Rep. 2011, 16, 230–236. [Google Scholar] [CrossRef]
- Duhig, K.; Chappell, L.C.; Shennan, A.H. Oxidative stress in pregnancy and reproduction. Obstet. Med. 2016, 9, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Sundl, M.; Glasner, A.; Huppertz, B.; Moser, G. The trophoblast plug during early pregnancy: A deeper insight. Histochem. Cell Biol. 2016, 146, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Rua, E.d.A.O.; Porto, M.L.; Ramos, J.P.L.; Nogueira, B.V.; Meyrelles, S.S.; Vasquez, E.C.; Pereira, T.C. Effects of tobacco smoking during pregnancy on oxidative stress in the umbilical cord and mononuclear blood cells of neonates. J. Biomed. Sci. 2014, 21, 105. [Google Scholar] [CrossRef]
- Milnerowicz-Nabzdyk, E.; Bizoń, A.; Zimmer, M. How Does Tobacco Smoke Affect Fetal Growth Potential in the First Trimester of Pregnancy as Measured by Volume Parameters of the Fetus, Trophoblast, and Gestational Sac? Reprod. Sci. Thousand Oaks Calif. 2017, 24, 548–559. [Google Scholar] [CrossRef]
- Milnerowicz-Nabzdyk, E.; Bizoń, A. Effect of cigarette smoking on vascular flows in pregnancies complicated by intrauterine growth restriction. Reprod. Toxicol. Elmsford N 2014, 50, 27–35. [Google Scholar] [CrossRef]
- Milnerowicz, H.; Zalewski, J.; Geneja, R.; Milnerowicz-Nabzdyk, E.; Woytoń, J. Levels of Cd, Pb in blood and Zn, Cu, Cd, Pb in amniotic fluid of tobacco smoking women during pregnancy complicated oligohydramnios or premature rupture of membranes. Ginekol. Pol. 2000, 71, 311–316. [Google Scholar]
- Pizent, A.; Lazarus, M.; Kovačić, J.; Lovaković, B.T.; Karačonji, I.B.; Semren, T.Ž.; Sekovanić, A.; Orct, T.; Branović-Čakanić, K.; Brajenović, N.; et al. Cigarette Smoking during Pregnancy: Effects on Antioxidant Enzymes, Metallothionein and Trace Elements in Mother-Newborn Pairs. Biomolecules 2020, 10, 892. [Google Scholar] [CrossRef]
- Milnerowicz, H.; Zalewski, J.; Geneja, R.; Milnerowicz-Nabzdyk, E.; Zasławski, R.; Woytoń, J. Effects of exposure to tobacco smoke in pregnancies complicated by oligohydramnios and premature rupture of the membranes. I. Concentration of Cd and Pb in blood and Zn, Cu, Cd and Pb in amniotic fluid. Int. J. Occup. Med. Environ. Health 2000, 13, 185–193. [Google Scholar]
- Thaker, R.; Oza, H.; Shaikh, I.; Kumar, S. Correlation of Copper and Zinc in Spontaneous Abortion. Int. J. Fertil. Steril. 2019, 13, 97–101. [Google Scholar]
- Ikeh-Tawari, E.P.; Anetor, J.I.; Charles-Davies, M.A. Cadmium level in pregnancy, influence on neonatal birth weight and possible amelioration by some essential trace elements. Toxicol. Int. 2013, 20, 108–112. [Google Scholar] [CrossRef]
- Ghneim, H.K.; Al-Sheikh, Y.A.; Alshebly, M.M.; Aboul-Soud, M.A.M. Superoxide dismutase activity and gene expression levels in Saudi women with recurrent miscarriage. Mol. Med. Rep. 2016, 13, 2606–2612. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Anthony, K.; Neuberger, T.; Diaz, F.J. Preconception zinc deficiency disrupts postimplantation fetal and placental development in mice. Biol. Reprod. 2014, 90, 83. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.; Sajdak, S.; Marciniak, W.; Lubiński, J. First Trimester Serum Copper or Zinc Levels, and Risk of Pregnancy-Induced Hypertension. Nutrients 2019, 11, 2479. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.A.; Grzeskowiak, L.E.; Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Jankovic-Karasoulos, T.; Perkins, A.V.; Norman, R.J.; Dekker, G.A.; Roberts, C.T. Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility. Nutrients 2019, 11, 1609. [Google Scholar] [CrossRef]
- Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Grzeskowiak, L.E.; Dekker, G.A.; Roberts, C.T. Early pregnancy maternal trace mineral status and the association with adverse pregnancy outcome in a cohort of Australian women. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2018, 46, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Tian, F.-J.; Lin, Y. Oxidative Stress in Placenta: Health and Diseases. BioMed Res. Int. 2015, 2015, 293271. [Google Scholar] [CrossRef]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef]
- Lau, J.C.; Joseph, M.G.; Cherian, M.G. Role of placental metallothionein in maternal to fetal transfer of cadmium in genetically altered mice. Toxicology 1998, 127, 167–178. [Google Scholar] [CrossRef]
- Goyer, R.A.; Cherian, M.G. Role of metallothionein in human placenta and rats exposed to cadmium. IARC Sci. Publ. 1992, 239–247. [Google Scholar]
- Milnerowicz, H. Influence of tobacco smoking on metallothionein isoforms contents in human placenta, amniotic fluid and milk. Int. J. Occup. Med. Environ. Health 1997, 10, 395–403. [Google Scholar] [PubMed]
- Milnerowicz, H.; Jacyszyn, K.; Bakońska, E. Isolation of metallothionein from the human placenta. Folia Med. Cracov. 1987, 28, 105–116. [Google Scholar]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Case, A.J. On the Origin of Superoxide Dismutase: An Evolutionary Perspective of Superoxide-Mediated Redox Signaling. Antioxidants 2017, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C.; Raes, M.; Toussaint, O.; Remacle, J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic. Biol. Med. 1994, 17, 235–248. [Google Scholar] [CrossRef]
- Caulfield, L.E.; Donangelo, C.M.; Chen, P.; Junco, J.; Merialdi, M.; Zavaleta, N. Red blood cell metallothionein as an indicator of zinc status during pregnancy. Nutr. Burbank Los Angel. Cty. Calif. 2008, 24, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Milnerowicz, H.; Wrześniak, M.; Królik, M.; Kowalska, K. Influence of tobacco smoke on zinc, cadmium, iron, iron-binding proteins, and low-weight anti-oxidant status in pregnancy. Inhal. Toxicol. 2018, 30, 534–541. [Google Scholar] [CrossRef]
- Bakacak, M.; Kılınç, M.; Serin, S.; Ercan, Ö.; Köstü, B.; Avcı, F.; Kıran, H.; Kıran, G. Changes in Copper, Zinc, and Malondialdehyde Levels and Superoxide Dismutase Activities in Pre-Eclamptic Pregnancies. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 2414–2420. [Google Scholar]
- Zhu, L.-J.; Chen, Y.-P.; Chen, B.-J.; Mei, X.-H. Changes in reactive oxygen species, superoxide dismutase, and hypoxia-inducible factor-1α levels in missed abortion. Int. J. Clin. Exp. Med. 2014, 7, 2179–2184. [Google Scholar] [PubMed]
- Mistry, H.D.; Wilson, V.; Ramsay, M.M.; Symonds, M.E.; Broughton Pipkin, F. Reduced selenium concentrations and glutathione peroxidase activity in preeclamptic pregnancies. Hypertens. Dallas Tex 1979 2008, 52, 881–888. [Google Scholar] [CrossRef]
- Zachara, B.A.; Dobrzyński, W.; Trafikowska, U.; Szymański, W. Blood selenium and glutathione peroxidases in miscarriage. BJOG Int. J. Obstet. Gynaecol. 2001, 108, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.M.; Zimmerman, M.C.; Moore, T.A. Oxidative stress in early pregnancy and the risk of preeclampsia. Pregnancy Hypertens. 2019, 18, 99–102. [Google Scholar] [CrossRef]
- Milnerowicz, H.; Bizoń, A. Determination of metallothionein in biological fluids using enzyme-linked immunoassay with commercial antibody. Acta Biochim. Pol. 2010, 57, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.W.; Lazarow, A. Determination of Glutathione. In Methods of Biochemical Analysis; Glick, D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 259–278. ISBN 978-0-471-30459-3. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Kwiatkowski, S.; Kosik-Bogacka, D. The Role of Fe, Zn, and Cu in Pregnancy. Biomolecules 2020, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Keen, C.L.; Uriu-Hare, J.Y.; Hawk, S.N.; Jankowski, M.A.; Daston, G.P.; Kwik-Uribe, C.L.; Rucker, R.B. Effect of copper deficiency on prenatal development and pregnancy outcome. Am. J. Clin. Nutr. 1998, 67, 1003S–1011S. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Kassu, A.; Yabutani, T.; Mulu, A.; Tessema, B.; Ota, F. Serum zinc, copper, selenium, calcium, and magnesium levels in pregnant and non-pregnant women in Gondar, Northwest Ethiopia. Biol. Trace Elem. Res. 2008, 122, 97–106. [Google Scholar] [CrossRef]
- Espart, A.; Artime, S.; Tort-Nasarre, G.; Yara-Varón, E. Cadmium exposure during pregnancy and lactation: Materno-fetal and newborn repercussions of Cd(ii), and Cd-metallothionein complexes. Met. Integr. Biometal Sci. 2018, 10, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Masironi, R. Toxic and trace elements in tobacco and tobacco smoke. Bull. World Health Organ. 1992, 70, 269–275. [Google Scholar]
- Mravunac, M.; Szymlek-Gay, E.A.; Daly, R.M.; Roberts, B.R.; Formica, M.; Gianoudis, J.; O’Connell, S.L.; Nowson, C.A.; Cardoso, B.R. Greater Circulating Copper Concentrations and Copper/Zinc Ratios are Associated with Lower Psychological Distress, But Not Cognitive Performance, in a Sample of Australian Older Adults. Nutrients 2019, 11, 2503. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fan, F.; Wang, L.; Ye, W.; Zhang, Q.; Xie, S. Maternal Cadmium Levels During Pregnancy and the Relationship with Preeclampsia and Fetal Biometric Parameters. Biol. Trace Elem. Res. 2018, 186, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Kippler, M.; Tofail, F.; Gardner, R.; Rahman, A.; Hamadani, J.D.; Bottai, M.; Vahter, M. Maternal cadmium exposure during pregnancy and size at birth: A prospective cohort study. Environ. Health Perspect. 2012, 120, 284–289. [Google Scholar] [CrossRef]
- Johnston, J.E.; Valentiner, E.; Maxson, P.; Miranda, M.L.; Fry, R.C. Maternal cadmium levels during pregnancy associated with lower birth weight in infants in a North Carolina cohort. PLoS ONE 2014, 9, e109661. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Gao, J.; Shahzad, M.; Han, Z.; Wang, Z.; Li, J.; Sjölinder, H. Zinc supplementation protects against cadmium accumulation and cytotoxicity in Madin-Darby bovine kidney cells. PLoS ONE 2014, 9, e103427. [Google Scholar] [CrossRef]
- Richter, P.; Faroon, O.; Pappas, R.S. Cadmium and Cadmium/Zinc Ratios and Tobacco-Related Morbidities. Int. J. Environ. Res. Public. Health 2017, 14, 1154. [Google Scholar] [CrossRef] [PubMed]
- Ronco, A.M.; Garrido, F.; Llanos, M.N. Smoking specifically induces metallothionein-2 isoform in human placenta at term. Toxicology 2006, 223, 46–53. [Google Scholar] [CrossRef]
- Tapia, L.; González-Agüero, M.; Cisternas, M.F.; Suazo, M.; Cambiazo, V.; Uauy, R.; González, M. Metallothionein is crucial for safe intracellular copper storage and cell survival at normal and supra-physiological exposure levels. Biochem. J. 2004, 378, 617–624. [Google Scholar] [CrossRef]
- Kocyigit, A.; Erel, O.; Gur, S. Effects of tobacco smoking on plasma selenium, zinc, copper and iron concentrations and related antioxidative enzyme activities. Clin. Biochem. 2001, 34, 629–633. [Google Scholar] [CrossRef]
- Kocatürk, P.A.; Kavas, G.O.; Erdeve, O.; Siklar, Z. Superoxide dismutase activity and zinc and copper concentrations in growth retardation. Biol. Trace Elem. Res. 2004, 102, 51–59. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Lovásová, E.; Rácz, O.; Cimboláková, I.; Nováková, J.; Dombrovský, P.; Ništiar, F. Effects of chronic low-dose cadmium exposure on selected biochemical and antioxidant parameters in rats. J. Toxicol. Environ. Health A 2013, 76, 1033–1038. [Google Scholar] [CrossRef]
- Chełchowska, M.; Laskowska-Klita, T.; Niemiec, K.T. Activities of superoxide dismutase, glutathione peroxidase and catalase in erythrocytes of women smoking during pregnancy. Przegl. Lek. 2005, 62, 1039–1042. [Google Scholar]
- Adeoye, O.; Olawumi, J.; Opeyemi, A.; Christiania, O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist. Reprod. 2018, 22, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Mons, U.; Muscat, J.E.; Modesto, J.; Richie, J.P.; Brenner, H. Effect of smoking reduction and cessation on the plasma levels of the oxidative stress biomarker glutathione—Post-hoc analysis of data from a smoking cessation trial. Free Radic. Biol. Med. 2016, 91, 172–177. [Google Scholar] [CrossRef]
- Raijmakers, M.T.M.; Roes, E.M.; Poston, L.; Steegers, E.A.P.; Peters, W.H.M. The transient increase of oxidative stress during normal pregnancy is higher and persists after delivery in women with pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 138, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Day, B.J. Catalase and glutathione peroxidase mimics. Biochem. Pharmacol. 2009, 77, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Gopčević, K.R.; Rovčanin, B.R.; Tatić, S.B.; Krivokapić, Z.V.; Gajić, M.M.; Dragutinović, V.V. Activity of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase in different stages of colorectal carcinoma. Dig. Dis. Sci. 2013, 58, 2646–2652. [Google Scholar] [CrossRef]
- Wei, J.; Liu, C.-X.; Gong, T.-T.; Wu, Q.-J.; Wu, L. Cigarette smoking during pregnancy and preeclampsia risk: A systematic review and meta-analysis of prospective studies. Oncotarget 2015, 6, 43667–43678. [Google Scholar] [CrossRef]
- Moore, T.A.; Samson, K.; Ahmad, I.M.; Case, A.J.; Zimmerman, M.C. Oxidative Stress in Pregnant Women Between 12–20 Weeks Gestation and Preterm Birth. Nurs. Res. 2019, 69, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.C.; Wills, A.K.; Bernal, A.L. Environmental Tobacco Smoke Exposure in Pregnancy is Associated With Earlier Delivery and Reduced Birth Weight. Reprod. Sci. Thousand Oaks Calif. 2015, 22, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R. Superoxide dismutase in redox biology: The roles of superoxide and hydrogen peroxide. Anticancer Agents Med. Chem. 2011, 11, 341–346. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
