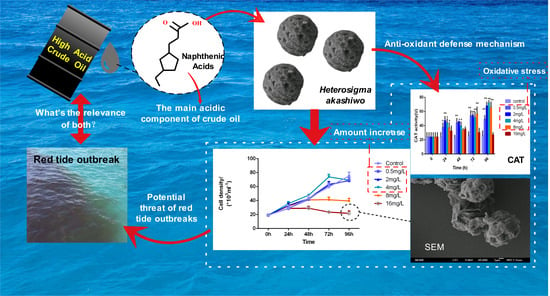
Toxicity of Naphthenic Acids on the Chlorophyll Fluorescence Parameters and Antioxidant Enzyme Activity of Heterosigma akashiwo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Algae and Chemicals
2.2. Culture and Exposure of Microalgae
2.3. Dynamic Monitoring of the Microalgae Population
2.4. Electron Microscopy Analysis
2.5. Determination of the Chlorophyll Fluorescence Parameters
2.6. Determination of the SOD (Superoxide Dismutase) and CAT (Catalase) Activities
2.7. Data Analysis
3. Results
3.1. Effects on the Growth of H. akashiwo
3.2. Effects on Cellular Morphology
3.3. Effects on the ChlF (Chlorophyll Fluorescence) Parameters
3.4. Effects on the SOD and CAT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joye, S.B. Deepwater Horizon, 5 years on. Science 2015, 349, 592–593. [Google Scholar] [CrossRef]
- Zan, S.; Wang, J.; Wang, F.; Han, Y.; Du, M.; Fan, J. Variation and distribution of naphthenic acids in Dalian Bay sediment. Mar. Pollut. Bull. 2019, 140, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Villagomez, J.M.; Peru, K.M.; Edington, C.; Headley, J.V.; Pauli, B.D.; Trudeau, V.L. Naphthenic Acid Mixtures and Acid-Extractable Organics from Oil Sands Process-Affected Water Impair Embryonic Development of Silurana (Xenopus) tropicalis. Environ. Sci. Technol. 2019, 53, 2095–2104. [Google Scholar] [CrossRef]
- Pinzón-Espinosa, A.; Kanda, R. Naphthenic acids are key contributors to toxicity of heavy oil refining effluents. Sci. Total Environ. 2020, 729, 138119. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.C.; MacKinnon, M.D.; Fedorak, P.M. Naphthenic Acids in Athabasca Oil Sands Tailings Waters Are Less Biodegradable than Commercial Naphthenic Acids. Environ. Sci. Technol. 2005, 39, 8388–8394. [Google Scholar] [CrossRef]
- Scott, A.; Whittal, R.; Fedorak, P.M. Coal is a potential source of naphthenic acids in groundwater. Sci. Total Environ. 2009, 407, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.V.; Langford, K.; Petersen, K.; Smith, A.; Tollefsen, K.E. Effect-Directed Identification of Naphthenic Acids as Important in Vitro Xeno-Estrogens and Anti-Androgens in North Sea Offshore Produced Water Discharges. Environ. Sci. Technol. 2009, 43, 8066–8071. [Google Scholar] [CrossRef]
- McKenzie, N.; Yue, S.; Liu, X.; Ramsay, B.A.; Ramsay, J.A. Biodegradation of naphthenic acids in oils sands process waters in an immobilized soil/sediment bioreactor. Chemosphere 2014, 109, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Marentette, J.R.; Frank, R.A.; Bartlett, A.; Gillis, P.L.; Hewitt, L.M.; Peru, K.M.; Headley, J.V.; Brunswick, P.; Shang, D.; Parrott, J.L. Toxicity of naphthenic acid fraction components extracted from fresh and aged oil sands process-affected waters, and commercial naphthenic acid mixtures, to fathead minnow (Pimephales promelas) embryos. Aquat. Toxicol. 2015, 164, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, A.; Scarlett, A.G.; Jha, A.N. Diamondoid naphthenic acids cause in vivo genetic damage in gills and haemocytes of marine mussels. Environ. Sci. Pollut. Res. Int. 2016, 23, 7060–7066. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Cao, X.; Sun, J.; Chai, L.; Huang, Y.; Tang, X. Transcriptional responses of earthworm (Eisenia fetida) exposed to naphthenic acids in soil. Environ. Pollut. 2015, 204, 264–270. [Google Scholar] [CrossRef]
- Marentette, J.R.; Sarty, K.; Cowie, A.M.; Frank, R.A.; Hewitt, L.M.; Parrott, J.L.; Martyniuk, C.J. Molecular responses of Walleye (Sander vitreus) embryos to naphthenic acid fraction components extracted from fresh oil sands process-affected water. Aquat. Toxicol. 2017, 182, 11–19. [Google Scholar] [CrossRef]
- Demeter, M.A.; Lemire, J.; George, I.; Yue, G.; Ceri, H.; Turner, R.J. Harnessing oil sands microbial communities for use in ex situ naphthenic acid bioremediation. Chemosphere 2014, 97, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, J.; Richards, L.C.; Vandergrift, G.W.; Hounjet, L.J.; Stoyanov, S.R.; Gill, C.G.; Krogh, E.T. Direct mass spectrometric analysis of naphthenic acids and polycyclic aromatic hydrocarbons in waters impacted by diluted bitumen and conventional crude oil. Sci. Total Environ. 2021, 765, 144206. [Google Scholar] [CrossRef]
- Headley, J.V.; Du, J.L.; Peru, K.M.; Gurprasad, N.; McMartin, D.W. Evaluation of algal phytodegradation of petroleum naphthenic acids. J. Environ. Sci. Health Part A 2008, 43, 227–232. [Google Scholar] [CrossRef]
- Leung, S.S.; MacKinnon, M.D.; Smith, R.E. The ecological effects of naphthenic acids and salts on phytoplankton from the Athabasca oil sands region. Aquat. Toxicol. 2003, 62, 11–26. [Google Scholar] [CrossRef]
- Woodworth, A.P.; Frank, R.A.; McConkey, B.J.; Müller, K.M. Toxic effects of oil sand naphthenic acids on the biomass accumulation of 21 potential phytoplankton remediation candidates. Ecotoxicol. Environ. Saf. 2012, 86, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Marcellin-Gros, R.; Piganeau, G.; Stien, D. Metabolomic Insights into Marine Phytoplankton Diversity. Mar. Drugs 2020, 18, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olascoaga, M.J. Isolation on the West Florida Shelf with implications for red tides and pollutant dispersal in the Gulf of Mexico. Nonlinear Process Geophys. 2010, 17, 685–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marampouti, C.; Buma, A.G.J.; de Boer, M.K. Mediterranean alien harmful algal blooms: Origins and impacts. Environ. Sci. Pollut. Res. Int. 2021, 28, 3837–3851. [Google Scholar] [CrossRef]
- Almeda, R.; Cosgrove, S.; Buskey, E.J. Oil Spills and Dispersants Can Cause the Initiation of Potentially Harmful Dinoflagellate Blooms (“Red Tides”). Environ. Sci. Technol. 2018, 52, 5718–5724. [Google Scholar] [CrossRef]
- Chen, B.; Dong, D.; Qin, X.; Xu, M.; Chen, X.; Shi, M. Relationship between the Occurrence of Red Tides and the Dynamic Response Mechanisms in the Northern Beibu Gulf. J. Coast. Res. 2019, 93, 185–193. [Google Scholar] [CrossRef]
- Park, B.S.; Erdner, D.L.; Bacosa, H.P.; Liu, Z.; Buskey, E.J. Potential effects of bacterial communities on the formation of blooms of the harmful dinoflagellate Prorocentrum after the 2014 Texas City “Y” oil spill (USA). Harmful Algae 2020, 95, 101802. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Lim, A.S.; Franks, P.J.; Lee, K.H.; Kim, J.H.; Kang, N.S.; Lee, M.J.; Jang, S.H.; Lee, S.Y.; Yoon, E.Y.; et al. A hierarchy of conceptual models of red-tide generation: Nutrition, behavior, and biological interactions. Harmful Algae 2015, 47, 97–115. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tang, X.; Shang, J.; Zhao, X.; Qu, T.; Wang, Y. The effect of naphthenic acids on physiological characteristics of the microalgae Phaeodactylum tricornutum and Platymonas helgolandica var. tsingtaoensis. Environ. Pollut. 2018, 240, 549–556. [Google Scholar] [CrossRef]
- Wang, T.; Ji, Y.; Wang, Y.; Jia, J.; Li, J.; Huang, S.; Han, D.; Hu, Q.; Huang, W.E.; Xu, J. Quantitative dynamics of triacylglycerol accumulation in microalgae populations at single-cell resolution revealed by Raman microspectroscopy. Biotechnol. Biofuels 2014, 7, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodhew, P.J.; Humphreys, J. Electron Microscopy and Analysis; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Park, J.; Dinh, T.B. Contrasting effects of monochromatic LED lighting on growth, pigments and photosynthesis in the commercially important cyanobacterium Arthrospira maxima. Bioresour. Technol. 2019, 291, 121846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, R.; Yu, Z.; Xue, L. Superoxide Dismutase (SOD) and Catalase (CAT) Activity Assay Protocols for Caenorhabditis elegans. Bio-Protocol 2017, 7, e2505. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, H.; Wang, S.; Chen, J.; Xia, Y. The toxicity of ionic liquid 1-decylpyridinium bromide to the algae Scenedesmus obliquus: Growth inhibition, phototoxicity, and oxidative stress. Sci. Total Environ. 2018, 622–623, 1572–1580. [Google Scholar] [CrossRef]
- Cunha, V.; Vogs, C.; Le Bihanic, F.; Dreij, K. Mixture effects of oxygenated PAHs and benzo[a]pyrene on cardiovascular de-velopment and function in zebrafish embryos. Environ. Int. 2020, 143, 105913. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Iavicoli, I.; Calabrese, E.J. The two faces of nanomaterials: A quantification of hormesis in algae and plants. Environ. Int. 2019, 131, 105044. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: A Compelling Platform for Sophisticated Plant Science. Trends Plant Sci. 2019, 24, 318–327. [Google Scholar] [CrossRef]
- Tan, X.; Dai, K.; Parajuli, K.; Hang, X.; Duan, Z.; Hu, Y. Effects of Phenolic Pollution on Interspecific Competition between Microcystis aeruginosa and Chlorella pyrenoidosa and their Photosynthetic Responses. Int. J. Environ. Res. Public Health 2019, 16, 3947. [Google Scholar] [CrossRef] [Green Version]
- Jinliang, P.; Guoan, Y.; Guoxing, S.; Xiaojun, D. Effects of α-naphthol on the gultathione and its reductase in Chlorella. Zhongguo Huanjing Kexue 2001, 21, 140–143. [Google Scholar]
- Huang, R.; Chen, Y.-C. The hormesis effects of low-dose 60Co gamma irradiation on high-temperature tolerance in cultivated Sargassum horneri (Fucales, Phaeophyceae). J. Appl. Phycol. 2018, 30, 3395–3404. [Google Scholar] [CrossRef]
- Fan, H.; Liu, H.; Dong, Y.; Chen, C.; Wang, Z.; Guo, J.; Du, S. Growth inhibition and oxidative stress caused by four ionic liquids in Scenedesmus obliquus: Role of cations and anions. Sci. Total Environ. 2019, 651, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.C.; Bruni, N.; Meineri, G.; Corsi, D.; Calvi, N.; Gastaldi, D.; Dosio, F. Strategies to expand the therapeutic potential of superoxide dismutase by exploiting delivery approaches. Int. J. Biol. Macromol. 2021, 168, 846–865. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, I.A.; Bell, N.B.; Ashmore, M.R.; Cotrozzi, L.; Haiba, N.S.; Basahi, J.M.; Summan, A.; Almeelbi, T.; Ismail, I.M. Radish (Raphanus sativus L.) Cultivar-Specific Response to O3: Patterns of Biochemical and Plant Growth Characteristics. CLEAN–Soil Air Water 2018, 46, 1800124. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Niehus, N.C.; Floeter, C.; Hollert, H.; Witt, G. Miniaturised Marine Algae Test with Polycyclic Aromatic Hydrocarbons—Comparing Equilibrium Passive Dosing and Nominal Spiking. Aquat. Toxicol. 2018, 198, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Delgado, I.C.; Mora-Solarte, D.A.; Velasco-Santamaría, Y.M. Physiological and enzymatic responses of Chlorella vulgaris exposed to produced water and its potential for bioremediation. Environ. Monit. Assess. 2019, 191, 399. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shah, K.; Laforest, S.; Cardinal, E.; Lambert, P.; Hollebone, B.P.; Brown, C.E.; Goldthorp, M.; Waston, K.; Yang, C. Occurrence, characterization, and ecological assessment of petroleum-related hydrocarbons in intertidal marine sediments of Burrard Inlet, Vancouver, British Columbia, Canada. Mar. Pollut. Bull. 2020, 157, 111304. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.M.; El-Shouny, W.A.; Osman, M.E.; El-Gammal, E. Growth and heavy metals removal efficiency of Nostoc muscorum and Anabaena subcylindrica in sewage and industrial wastewater effluents. Environ. Toxicol. Pharmacol. 2005, 19, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Kitao, M. Ethylenediurea Induces Hormesis in Plants. Dose-Response 2018, 16, 1559325818765280. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, M.E.; Castro, P.R.; Azevedo, R.A. Hormesis in plants under Cd exposure: From toxic to beneficial element? J. Hazard. Mater. 2020, 384, 121434. [Google Scholar] [CrossRef]
- Subashchandrabose, S.R.; Venkateswarlu, K.; Venkidusamy, K.; Palanisami, T.; Naidu, R.; Megharaj, M. Bioremediation of soil long-term contaminated with PAHs by algal–bacterial synergy of Chlorella sp. MM3 and Rhodococcus wratislaviensis strain 9 in slurry phase. Sci. Total Environ. 2019, 659, 724–731. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhou, Y.; Kong, Q.; Dong, W.; Lin, Z. Toxicity of Naphthenic Acids on the Chlorophyll Fluorescence Parameters and Antioxidant Enzyme Activity of Heterosigma akashiwo. Antioxidants 2021, 10, 1582. https://doi.org/10.3390/antiox10101582
Zhang H, Zhou Y, Kong Q, Dong W, Lin Z. Toxicity of Naphthenic Acids on the Chlorophyll Fluorescence Parameters and Antioxidant Enzyme Activity of Heterosigma akashiwo. Antioxidants. 2021; 10(10):1582. https://doi.org/10.3390/antiox10101582
Chicago/Turabian StyleZhang, Huanxin, Yumiao Zhou, Qiang Kong, Wenlong Dong, and Zhihao Lin. 2021. "Toxicity of Naphthenic Acids on the Chlorophyll Fluorescence Parameters and Antioxidant Enzyme Activity of Heterosigma akashiwo" Antioxidants 10, no. 10: 1582. https://doi.org/10.3390/antiox10101582
APA StyleZhang, H., Zhou, Y., Kong, Q., Dong, W., & Lin, Z. (2021). Toxicity of Naphthenic Acids on the Chlorophyll Fluorescence Parameters and Antioxidant Enzyme Activity of Heterosigma akashiwo. Antioxidants, 10(10), 1582. https://doi.org/10.3390/antiox10101582