ER-Mitochondria Calcium Flux by β-Sitosterol Promotes Cell Death in Ovarian Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Incubation
2.3. Western Blot
2.4. Annexin V and PI Staining
2.5. JC-1 Staining
2.6. Measurement of ROS Production
2.7. Cytosolic Ca2+ Level Analysis
2.8. Mitochondrial Ca2+ Level Analysis
2.9. 3D Cell Culture Assay
2.10. Proliferation Assay
2.11. Migration Assay
2.12. Statistical Analysis
3. Results
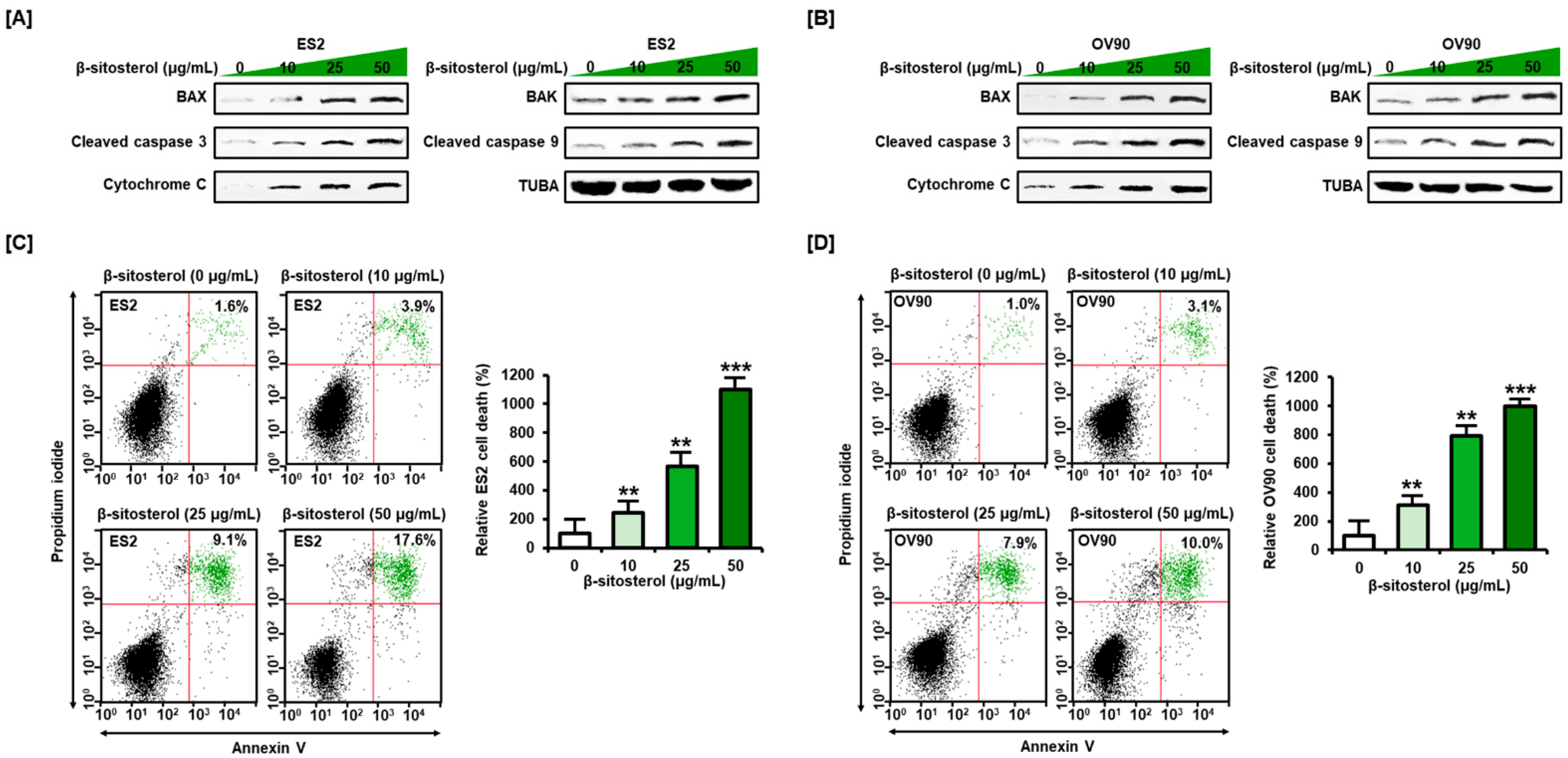
3.1. Activation of Cell Death Signals and Cell Apoptosis by β-Sitosterol in Human Ovarian Cancer Cells
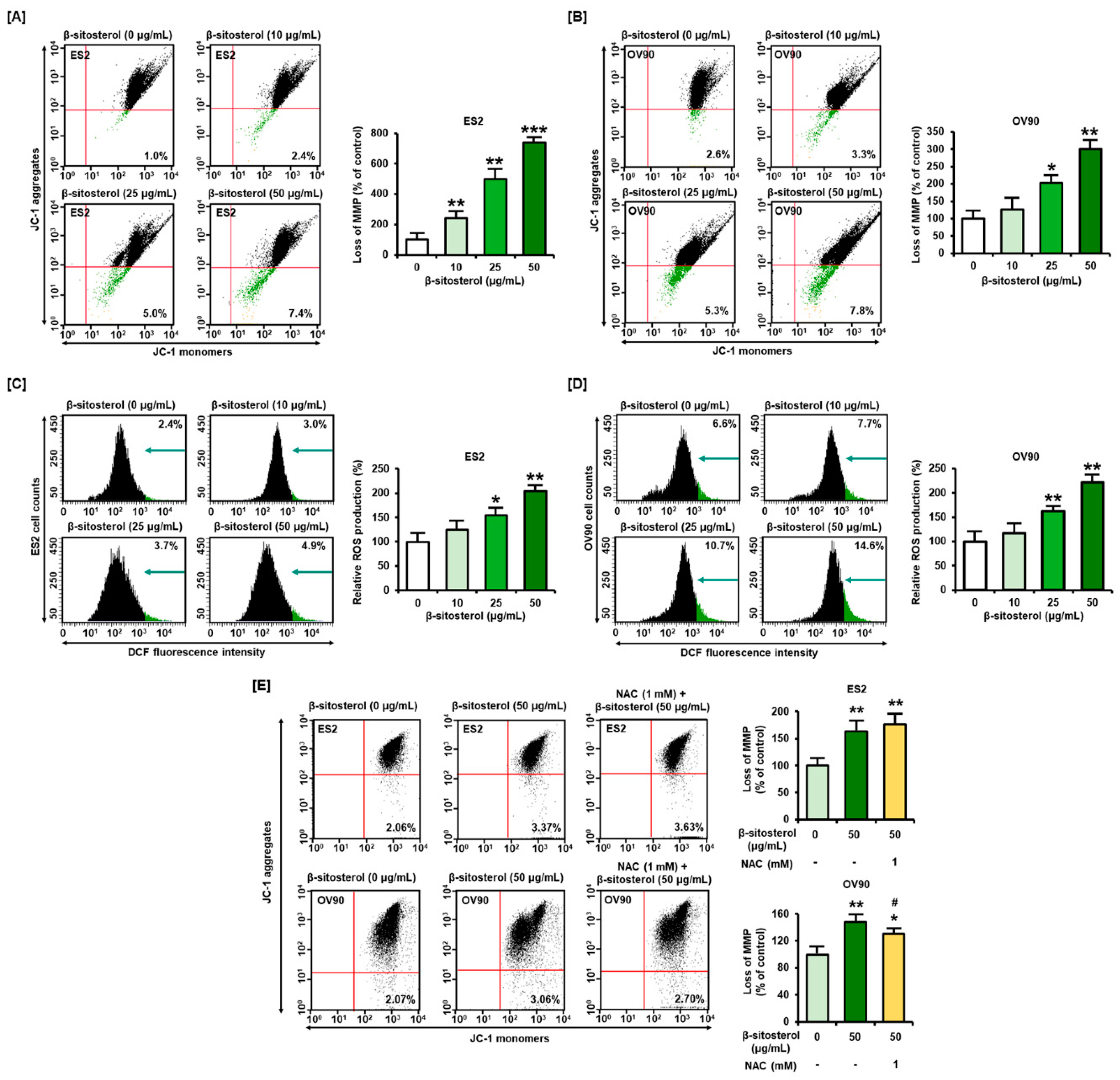
3.2. Changes in Mitochondrial Membrane Potential (MMP) and ROS Levels Caused by β-Sitosterol in Ovarian Cancer Cells
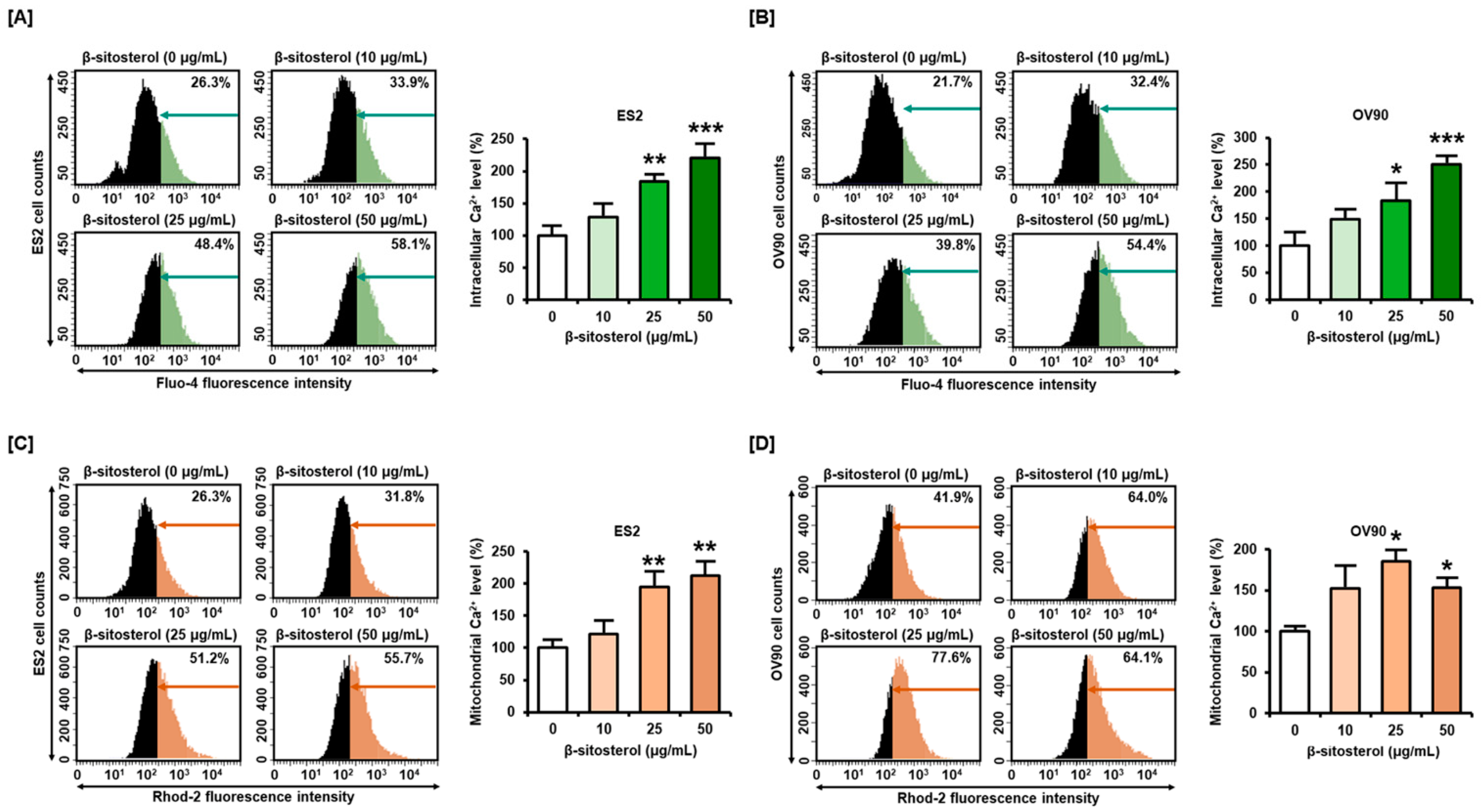
3.3. Excessive Overload of Cytosolic and Mitochondrial Calcium Levels in β-Sitosterol-Treated Ovarian Cancer Cells
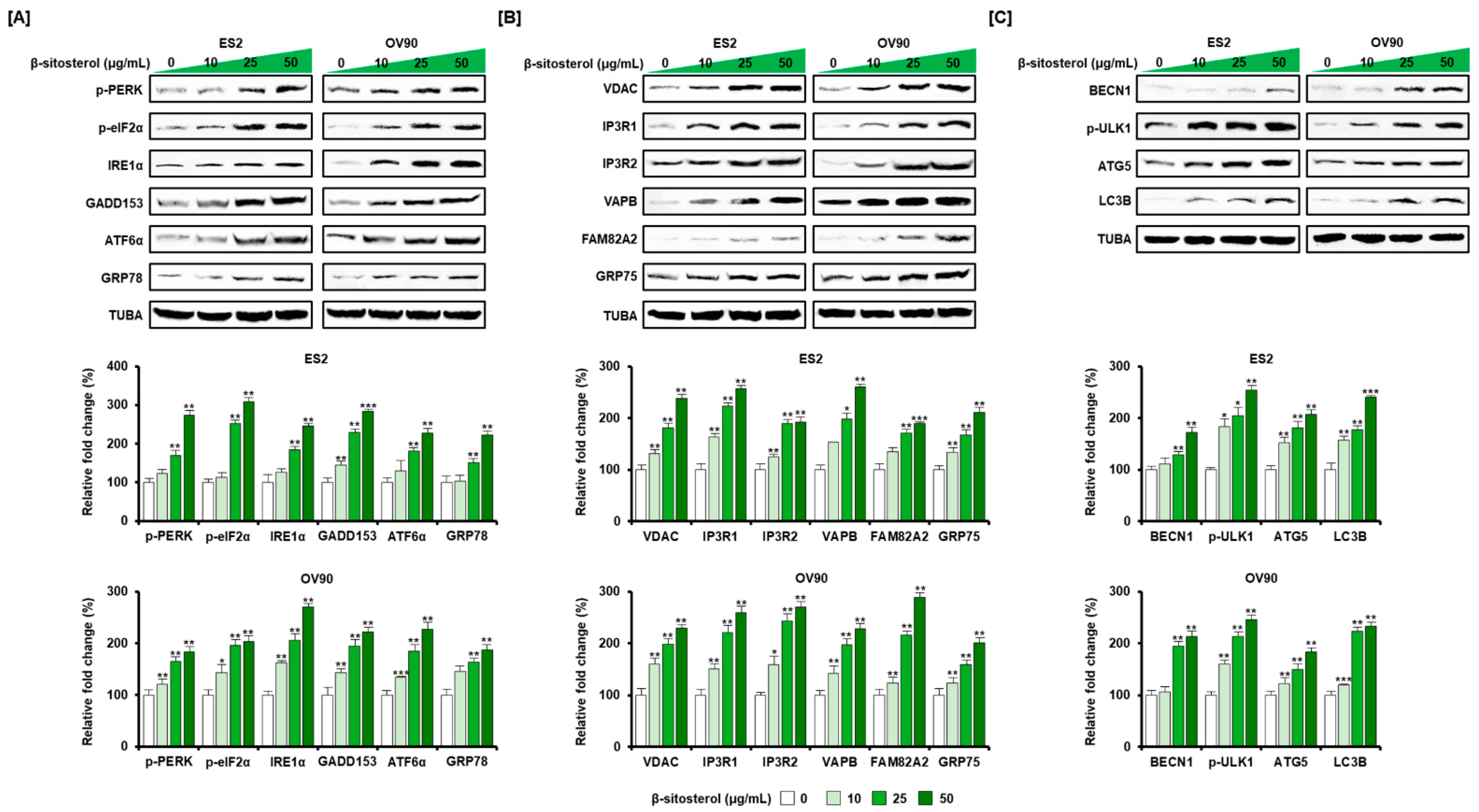
3.4. Regulation of ER Stress and of the ER-Mitochondrial Axis by β-Sitosterol in the Two Cell Lines
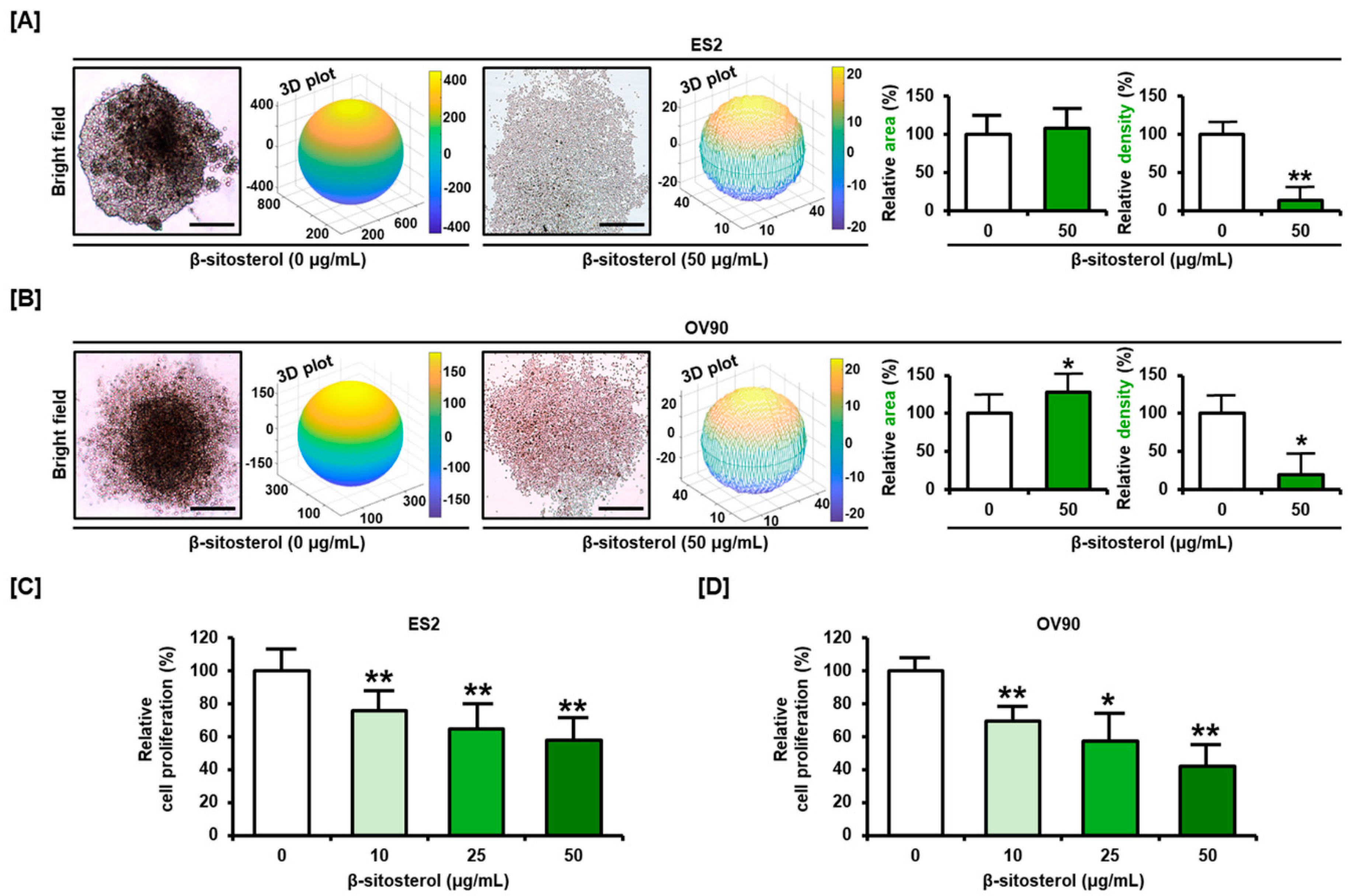
3.5. Restriction of Cell Growth and Cell Cycle by β-Sitosterol in Ovarian Cancer Cells
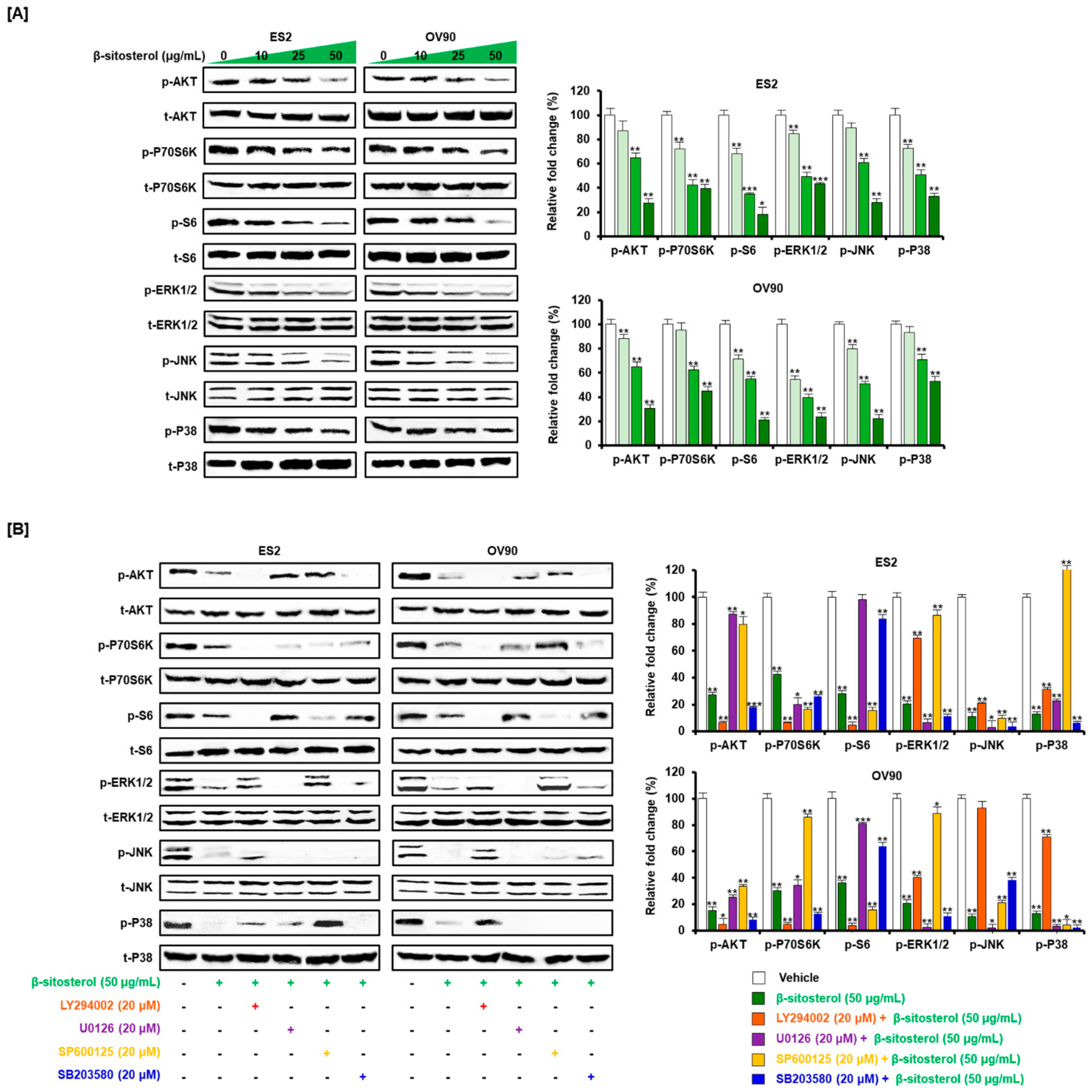
3.6. Inactivated Intracellular Signals Caused by β-Sitosterol in Ovarian Cancer Cells
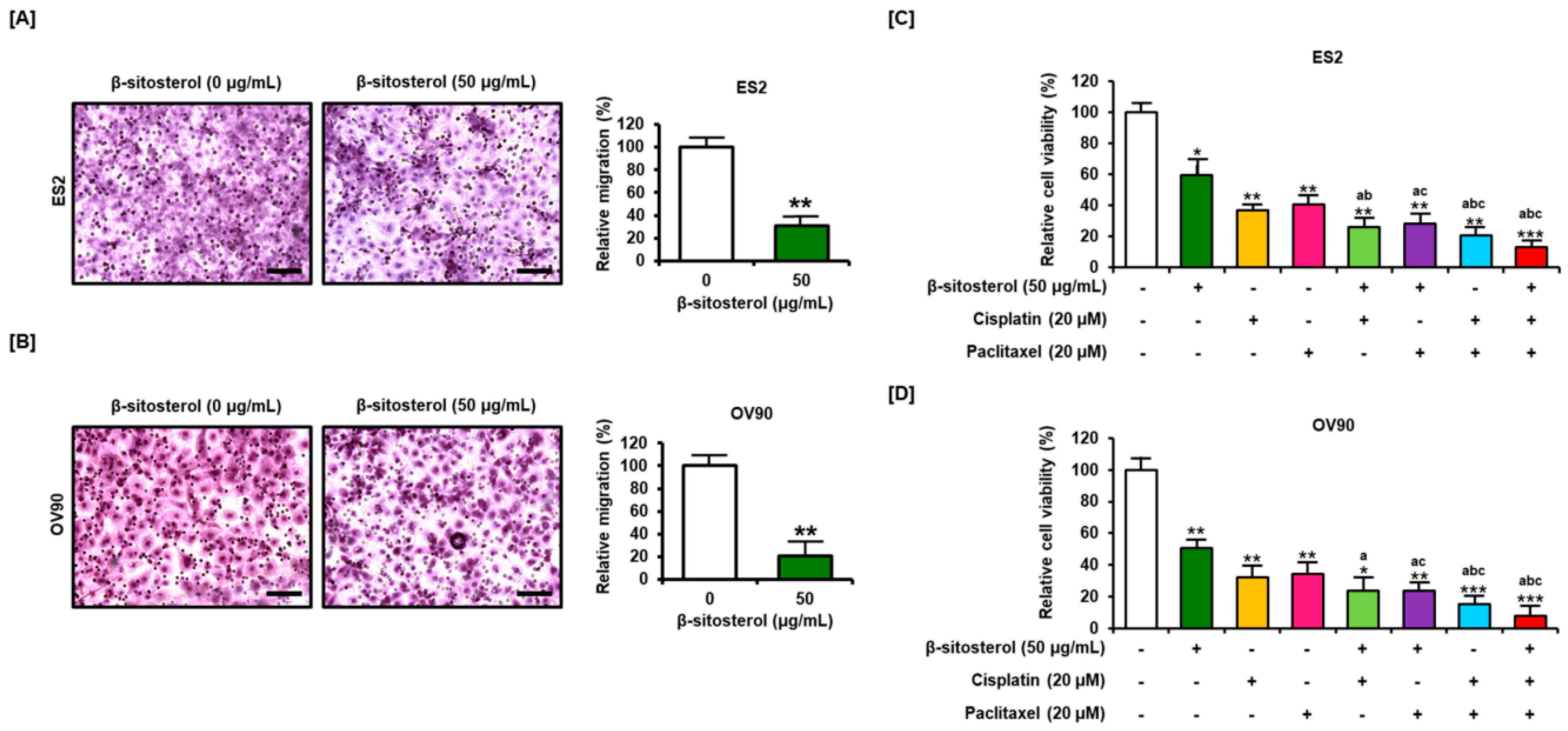
3.7. Suppression of Cell Migration and Enhancement of the Anti-Proliferation Effect of Existing Conventional Chemotherapeutic Agents by β-Sitosterol
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebell, M.H.; Culp, M.B.; Radke, T. A systematic review of symptoms for the diagnosis of ovarian cancer. Am. J. Prev. Med. 2016, 50, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Iida, Y.; Okamoto, A.; Hollis, R.L.; Gourley, C.; Herrington, C.S. Clear cell carcinoma of the ovary: A clinical and molecular perspective. Int. J. Gynecol. Cancer 2020, 31, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liu, Y.; Wang, X.; Guan, L.; Chen, W.; Jiang, H.; Lu, Y. Clear cell carcinoma of the ovary. Medicine 2018, 97, e10881. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, D.L.; Kabarowski, K.A.; Porubsky, V.; Kreeger, P.K. High-grade serous ovarian cancer cell lines exhibit heterogeneous responses to growth factor stimulation. Cancer Cell Int. 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Ling, W.; Jones, P. Dietary phytosterols: A review of metabolism, benefits and side effects. Life Sci. 1995, 57, 195–206. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lim, H.-J.; Kim, M.-S.; Lee, M.S. Dietary supplements for benign prostatic hyperplasia: An overview of systematic reviews. Maturitas 2012, 73, 180–185. [Google Scholar] [CrossRef]
- Wilt, T.J.; Ishani, A.; MacDonald, R.; Stark, G.; Mulrow, C.D.; Lau, J. Beta-sitosterols for benign prostatic hyperplasia. Cochrane Database Syst. Rev. 1999, 2011, CD001043. [Google Scholar] [CrossRef]
- Patel, M.D.; Thompson, P.D. Phytosterols and vascular disease. Atherosclerosis 2006, 186, 12–19. [Google Scholar] [CrossRef]
- Moon, D.-O.; Lee, K.-J.; Choi, Y.H.; Kim, G.-Y. β-Sitosterol-induced-apoptosis is mediated by the activation of ERK and the downregulation of Akt in MCA-102 murine fibrosarcoma cells. Int. Immunopharmacol. 2007, 7, 1044–1053. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kong, K.R.; Kim, Y.-A.; Jung, K.-O.; Kil, J.-H.; Rhee, S.-H.; Park, K.-Y. Induction of Bax and activation of caspases during beta-sitosterol-mediated apoptosis in human colon cancer cells. Int. J. Oncol. 2003, 23, 1657–1662. [Google Scholar]
- Awad, A.B.; Williams, H.; Fink, C.S. Phytosterols reduce in vitro metastatic ability of MDA-MB-231 human breast cancer cells. Nutr. Cancer 2001, 40, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Roy, R.; Fink, C. β-sitosterol, a plant sterol, induces apoptosis and activates key caspases in MDA-MB-231 human breast cancer cells. Oncol. Rep. 2003, 10, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.B.; Burr, A.T.; Fink, C.S. Effect of resveratrol and β-sitosterol in combination on reactive oxygen species and prostaglandin release by PC-3 cells. Prostaglandins Leukot. Essent. Fat. Acids 2005, 72, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.B.; Barta, S.L.; Fink, C.S.; Bradford, P.G. β-Sitosterol enhances tamoxifen effectiveness on breast cancer cells by affecting ceramide metabolism. Mol. Nutr. Food Res. 2008, 52, 419–426. [Google Scholar] [CrossRef] [PubMed]
- A Woyengo, T.; Ramprasath, V.R.; Jones, P.J.H. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009, 63, 813–820. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. In The Protein Protocols Handbook; Springer: Cham, Switzerland; Humana Press: Totowa, NJ, USA, 1994; Volume 32, pp. 9–16. [Google Scholar] [CrossRef]
- Bae, H.; Park, S.; Yang, C.; Song, G.; Lim, W. Disruption of endoplasmic reticulum and ROS production in human ovarian cancer by campesterol. Antioxidants 2021, 10, 379. [Google Scholar] [CrossRef]
- Reers, M.; Smiley, S.T.; Mottola-Hartshorn, C.; Chen, A.; Lin, M.; Chen, L.B. [29] Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995, 260, 406–417. [Google Scholar] [CrossRef]
- Kim, H.; Xue, X. Detection of total reactive oxygen species in adherent cells by 2′,7′-Dichlorodihydrofluorescein diacetate staining. J. Vis. Exp. 2020, e60682. [Google Scholar] [CrossRef]
- Tymianski, M.; Bernstein, G.; Abdel-Hamid, K.; Sattler, R.; Velumian, A.; Carlen, P.; Razavi, H.M.; Jones, O. A novel use for a carbodiimide compound for the fixation of fluorescent and non-fluorescent calcium indicators in situ following physiological experiments. Cell Calcium 1997, 21, 175–183. [Google Scholar] [CrossRef]
- Jean-Quartier, C.; Bondarenko, O.; Alam, M.R.; Trenker, M.; Waldeck-Weiermair, M.; Malli, R.; Graier, W.F. Studying mitochondrial Ca2+ uptake—A revisit. Mol. Cell. Endocrinol. 2012, 353, 114–127. [Google Scholar] [CrossRef]
- Paredes, R.M.; Etzler, J.C.; Watts, L.T.; Zheng, W.; Lechleiter, J.D. Chemical calcium indicators. Methods 2008, 46, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Casanova, J.C.; Uribe, V.; Badia-Careaga, C.; Giovinazzo, G.; Torres, M.; Sanz-Ezquerro, J.J. Apical ectodermal ridge morphogenesis in limb development is controlled by Arid3b-mediated regulation of cell movements. Development 2011, 138, 1195–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, R.-D. Anticancer compounds and sphingolipid metabolism in the colon. Vivo 2005, 19, 293–300. [Google Scholar]
- Awad, A.B.; Fink, C.S.; Williams, H.; Kim, U. In vitro and in vivo (SCID mice) effects of phytosterols on the growth and dissemination of human prostate cancer PC-3 cells. Eur. J. Cancer Prev. 2001, 10, 507–513. [Google Scholar] [CrossRef]
- Vundru, S.S.; Kale, R.K.; Singh, R.P. β-sitosterol induces G1 arrest and causes depolarization of mitochondrial membrane potential in breast carcinoma MDA-MB-231 cells. BMC Complement. Altern. Med. 2013, 13, 280. [Google Scholar] [CrossRef] [Green Version]
- Perry, A.; Jenkins, R.B.; O’Fallon, J.R.; Schaefer, P.L.; Kimmel, D.W.; Mahoney, M.R.; Scheithauer, B.W.; Smith, S.M.; Hill, E.M.; Sebo, T.J.; et al. Clinicopathologic study of 85 similarly treated patients with anaplastic astrocytic tumors. Cancer 1999, 86, 672–683. [Google Scholar] [CrossRef]
- Ziliotto, L.; Barbisan, L.F.; Rodrigues, M.A.M. Lack of chemoprevention of dietary Agaricus blazei against rat colonic aberrant crypt foci. Hum. Exp. Toxicol. 2008, 27, 505–511. [Google Scholar] [CrossRef]
- Awad, A.; Chinnam, M.; Fink, C.; Bradford, P. β-Sitosterol activates Fas signaling in human breast cancer cells. Phytomedicine 2007, 14, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Bin Sayeed, M.S.; Ameen, S.S. Beta-sitosterol: A promising but orphan nutraceutical to fight against cancer. Nutr. Cancer 2015, 67, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azimi, I.; Roberts-Thomson, S.; Monteith, G. Calcium influx pathways in breast cancer: Opportunities for pharmacological intervention. Br. J. Pharmacol. 2014, 171, 945–960. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-G.; Pathan, N.; Ethell, I.M.; Krajewski, S.; Yamaguchi, Y.; Shibasaki, F.; McKeon, F.; Bobo, T.; Franke, T.F.; Reed, J.C. Ca2+-Induced apoptosis through calcineurin dephosphorylation of BAD. Science 1999, 284, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)—Bioenerg. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef]
- Larman, M.; Lucy, J. Meeting report. Mol. Membr. Biol. 1999, 16, 313–316. [Google Scholar] [CrossRef]
- Limonta, P.; Moretti, R.M.; Marzagalli, M.; Fontana, F.; Raimondi, M.; Marelli, M.M. Role of endoplasmic reticulum stress in the anticancer activity of natural compounds. Int. J. Mol. Sci. 2019, 20, 961. [Google Scholar] [CrossRef] [Green Version]
- Doghman, M.; Lalli, E. ER-mitochondria interactions: Both strength and weakness within cancer cells. Biochim. Biophys. Acta (BBA)—Bioenerg. 2019, 1866, 650–662. [Google Scholar] [CrossRef]
- Gomez-Suaga, P.; Paillusson, S.; Stoica, R.; Noble, W.; Hanger, D.; Miller, C.C. The ER-mitochondria tethering complex VAPB-PTPIP51 regulates autophagy. Curr. Biol. 2017, 27, 371–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazaz, I.O.; Demir, S.; Yulug, E.; Colak, F.; Bodur, A.; Yaman, S.O.; Karaguzel, E.; Mentese, A. N-acetylcysteine protects testicular tissue against ischemia/reperfusion injury via inhibiting endoplasmic reticulum stress and apoptosis. J. Pediatr. Urol. 2019, 15, 253.e1–253.e8. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Trejo, O.E.; Reyes-Fermín, L.M.; Briones-Herrera, A.; Tapia, E.; León-Contreras, J.C.; Hernández-Pando, R.; Sanchez-Lozada, L.-G.; Pedraza-Chaverri, J. Protective effects of N-acetyl-cysteine in mitochondria bioenergetics, oxidative stress, dynamics and S-glutathionylation alterations in acute kidney damage induced by folic acid. Free. Radic. Biol. Med. 2018, 130, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Osaki, M.; Oshimura, M.; Ito, H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis 2004, 9, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.G.; Ali, R.A.; El-Monem, D.D.A.; El-Magd, M.A. Graviola leaves extract enhances the anticancer effect of cisplatin on various cancer cell lines. Mol. Cell. Toxicol. 2020, 16, 1–15. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Bae, H.; Yang, C.; Park, S.; Youn, B.-S.; Kim, H.-S.; Song, G.; Lim, W. Eupatilin promotes cell death by calcium influx through ER-mitochondria axis with SERPINB11 inhibition in epithelial ovarian cancer. Cancers 2020, 12, 1459. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A comprehensive review on MAPK: A promising therapeutic target in cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, H.; Park, S.; Ham, J.; Song, J.; Hong, T.; Choi, J.-H.; Song, G.; Lim, W. ER-Mitochondria Calcium Flux by β-Sitosterol Promotes Cell Death in Ovarian Cancer. Antioxidants 2021, 10, 1583. https://doi.org/10.3390/antiox10101583
Bae H, Park S, Ham J, Song J, Hong T, Choi J-H, Song G, Lim W. ER-Mitochondria Calcium Flux by β-Sitosterol Promotes Cell Death in Ovarian Cancer. Antioxidants. 2021; 10(10):1583. https://doi.org/10.3390/antiox10101583
Chicago/Turabian StyleBae, Hyocheol, Sunwoo Park, Jiyeon Ham, Jisoo Song, Taeyeon Hong, Jin-Hee Choi, Gwonhwa Song, and Whasun Lim. 2021. "ER-Mitochondria Calcium Flux by β-Sitosterol Promotes Cell Death in Ovarian Cancer" Antioxidants 10, no. 10: 1583. https://doi.org/10.3390/antiox10101583
APA StyleBae, H., Park, S., Ham, J., Song, J., Hong, T., Choi, J.-H., Song, G., & Lim, W. (2021). ER-Mitochondria Calcium Flux by β-Sitosterol Promotes Cell Death in Ovarian Cancer. Antioxidants, 10(10), 1583. https://doi.org/10.3390/antiox10101583






