Acetone as Indicator of Lipid Oxidation in Stored Margarine
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Margarine Samples and Study Design
2.3. GC/FID Analysis of the Fatty Acid Composition
2.4. Peroxide Value
2.5. Conjugated Dienes
2.6. Analysis of Oxidized Triacylglycerols by Targeted LC-MS/MS
2.7. Oxidative Stability Determined by Rancimat Method
2.8. Headspace SPME-GC-MS Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition of Margarines
3.2. Oxidative Stability of Margarines during Storage
3.2.1. Peroxide Value
3.2.2. Conjugated Dienes
3.2.3. Oxidative Stability Measured by Rancimat Method
3.2.4. Determination of oxTAGs by LC-MS
3.3. Screening of Volatile Products in Stored Margarine
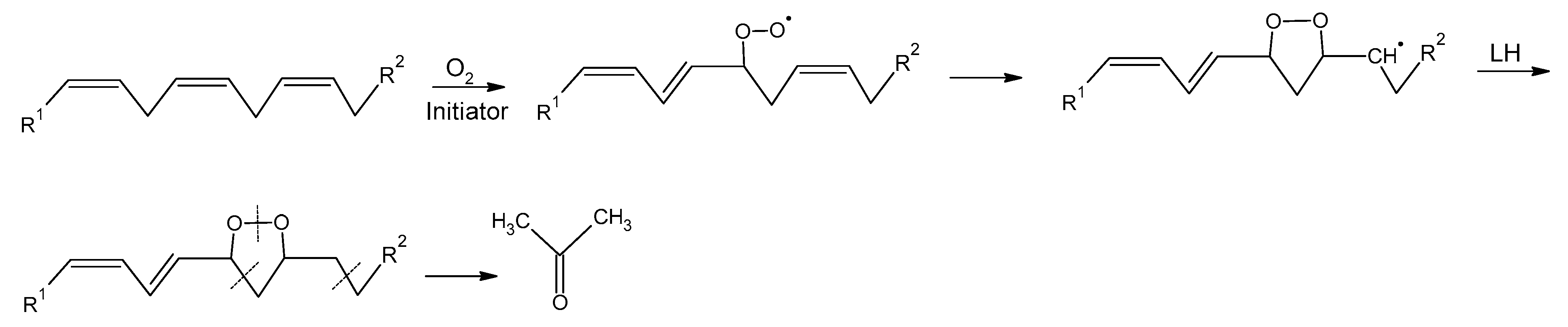
3.4. Acetone Increased during Lipid Oxidation in Margarine
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, D.H.; Vaisey-Genser, M. Role of Margarine in the diet. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Trugo, L., Finglas, P.M., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 3719–3725. [Google Scholar]
- Young, N.; Wassell, P. Margarines and Spreads. In Food Emulsifiers and Their Applications; Hasenhuettl, G.L., Hartel, R.W., Eds.; Springer: New York, NY, USA, 2008. [Google Scholar]
- McClements, D.J.; Decker, E.A. Lipid Oxidation in Oil-in-Water Emulsions: Impact of Molecular Environment on Chemical Reactions in Heterogeneous Food Systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Pokorná, I.; Filip, V.; Šmidrkal, J. Lipid Oxidation in Margarine Emulsions. Czech J. Food Sci. 2004, 22, 140–143. [Google Scholar] [CrossRef]
- Chougui, N.; Djerroud, N.; Naraoui, F.; Hadjal, S.; Aliane, K.; Zeroual, B.; Larbat, R. Physicochemical properties and storage stability of margarine containing Opuntia ficus-indica peel extract as antioxidant. Food Chem. 2015, 173, 382–390. [Google Scholar] [CrossRef]
- Maskan, M.; Öner, M.D.; Kaya, A. Storage stability and accelerated shelf-life testing of margarine samples. J. Food Qual. 1993, 16, 175–186. [Google Scholar] [CrossRef]
- Zaeroomali, M.; Maghsoudlou, Y.; Aryaey, P. The changes of table margarine characterization during storage time. Eur. J. Exp. Biol. 2014, 4, 185–187. [Google Scholar]
- Nogala-Kalucka, M.; Gogolewski, M. Alteration of fatty acid composition, tocopherol content and peroxide value in margarine during storage at various temperatures. Nahrung 2000, 44, 431–433. [Google Scholar] [CrossRef]
- Xia, W.; Budge, S.M. Techniques for the Analysis of Minor Lipid Oxidation Products Derived from Triacylglycerols: Epoxides, Alcohols, and Ketones. Compr. Rev. Food Sci. Food Saf. 2017, 16, 735–758. [Google Scholar] [CrossRef] [PubMed]
- Amlendu, P.; Ashley, Q.; Di, W.; Haojiong, Z.; Mirna, T.; David, J.; Xiaojun, X.; Francis, T.; Nongjian, T.; Erica S, F. Breath Acetone as Biomarker for Lipid Oxidation and Early Ketone Detection. Glob. J. Obes. Diabetes Metab. Syndr. 2014, 1, 12–19. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Fettwissenschaft. Deutsche Einheitsmethoden zur Untersuchung von Fetten, Fettprodukten, Tensiden und Verwandten Stoffen; Wiss. Verlag-Ges.: Berlin, Germay, 2014. [Google Scholar]
- Wheeler, D.H. Peroxide formation as a measure of autoxidative deterioriation. Oil Soap 1932, 9, 89–97. [Google Scholar] [CrossRef]
- Pegg, R.B. Measurement of primary lipid oxidation products. In Handbook of Food Analytical Chemistry: Water, Proteins, Enzymes, Lipids and Carbohydrates; John Wiley & Sons, Inc.: New York, NY, USA, 2005; pp. 515–529. [Google Scholar]
- Grüneis, V.; Fruehwirth, S.; Zehl, M.; Ortner, J.; Schamann, A.; König, J.; Pignitter, M. Simultaneous Analysis of Epoxidized and Hydroperoxidized Triacylglycerols in Canola Oil and Margarine by LC-MS. J. Agric. Food Chem. 2019, 67, 10174–10184. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Ruiz, G.; Jorge, N.; Martín-Polvillo, M.; Dobarganes, M.C. Rapid, quantitative determination of polar compounds in fats and oils by solid-phase extraction and size-exclusion chromatography using monostearin as internal standard. J. Chromatogr. A 1996, 749, 55–60. [Google Scholar] [CrossRef]
- Fruehwirth, S.; Zehentner, S.; Salim, M.; Sterneder, S.; Tiroch, J.; Lieder, B.; Zehl, M.; Somoza, V.; Pignitter, M. In Vitro Digestion of Grape Seed Oil Inhibits Phospholipid-Regulating Effects of Oxidized Lipids. Biomolecules 2020, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2. [Google Scholar] [CrossRef]
- AOCS. Official Methods of Analysis of AOCS International; AOCS: Urbana, IL, USA, 1995. [Google Scholar]
- Aktar, T.; Adal, E. Determining the Arrhenius Kinetics of Avocado Oil: Oxidative Stability under Rancimat Test Conditions. Foods 2019, 8, 236. [Google Scholar] [CrossRef]
- Dadalı, C.; Elmacı, Y. Characterization of Volatile Release and Sensory Properties of Model Margarines by Changing Fat and Emulsifier Content. Eur. J. Lipid Sci. Technol. 2019, 121. [Google Scholar] [CrossRef]
- Shiota, M.; Isogai, T.; Iwasawa, A.; Kotera, M. Model studies on volatile release from different semisolid fat blends correlated with changes in sensory perception. J. Agric. Food Chem. 2011, 59, 4904–4912. [Google Scholar] [CrossRef]
- Amri, I.N. The lauric oil (coconut and palmkernel) oils. In Vegetable Oils in Food Technology: Composition, Properties and Uses, 2nd ed.; Gunstone, F.D., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2011; pp. 169–197. [Google Scholar]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Manzo, N.; Santini, A.; Pizzolongo, F.; Aiello, A.; Romano, R. Effects of α-tocopherol and oleic acid content in sunflower oil subjected to discontinuous and prolonged frying process. Progr. Nutr. 2019, 21, 686–692. [Google Scholar] [CrossRef]
- Giuffre, A.M.; Capocasale, M.; Zappia, C.; Poiana, M. Influence of High Temperature and Duration of Heating on the Sunflower Seed Oil Properties for Food Use and Bio-diesel Production. J. Oleo Sci. 2017, 66, 1193–1205. [Google Scholar] [CrossRef]
- Tan, B.K.; Oh, F.C.H. Oleins and Stearins from Malaysian Palm Oil Chemical and Physical Characteristics; Palm Oil Research Institute of Malaysia: Bandar Baru Bangi, Malaysia, 1981.
- Parsons, S.; Raikova, S.; Chuck, C.J. The viability and desirability of replacing palm oil. Nat. Sustain. 2020, 3, 412–418. [Google Scholar] [CrossRef]
- Mba, O.I.; Dumont, M.-J.; Ngadi, M. Palm oil: Processing, characterisation and utilisation in the food industry—A review. Food Biosci. 2015, 10, 26–41. [Google Scholar] [CrossRef]
- Mansoon, H.L. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef] [PubMed]
- The-Codex-Alimentarius. In Proceedings of the Joint FAO/WHO Food Standard Programme Codex Alimentarius Commission Twenty-Fourth Session, Geneva, Switzerland, 2–7 July 2001; Volum 8.
- Pignitter, M.; Somoza, V. Critical evaluation of methods for the measurement of oxidative rancidity in vegetable oils. J. Food Drug Anal. 2012, 20, 772–777. [Google Scholar] [CrossRef]
- Stangelo, A.J.; Ory, R.L.; Brown, L.E. Comparison of methods for determining peroxidation in processed whole peanut products. J. Am. Oil Chem. Soc. 1975, 52, 34–35. [Google Scholar] [CrossRef]
- Schaich, K.M.; Xie, J.; Bogusz, B.A. Thinking outside the classical chain reaction box of lipid oxidation: Evidence for alternate pathways and the importance of epoxides. Lipid Technol. 2017, 29, 91–96. [Google Scholar] [CrossRef]
- Ibargoitia, M.L.; Sopelana, P.; Guillén, M.D. 1H Nuclear Magnetic Resonance monitoring of the degradation of margarines of varied compositions when heated to high temperature. Food Chem. 2014, 165, 119–128. [Google Scholar] [CrossRef]
- Schaich, K.M. Thinking outside the classical chain reaction box of lipid oxidation. Lipid Technol. 2012, 24, 55–58. [Google Scholar] [CrossRef]
- Khor, Y.P.; Hew, K.S.; Abas, F.; Lai, O.M.; Cheong, L.Z.; Nehdi, I.A.; Sbihi, H.M.; Gewik, M.M.; Tan, C.P. Oxidation and Polymerization of Triacylglycerols: In-Depth Investigations towards the Impact of Heating Profiles. Foods 2019, 8, 475. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Lipids. In Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2009; Volume 4, pp. 158–247. [Google Scholar]
- Mallia, S.; Escher, F.; Schlichtherle-Cerny, H. Aroma-active compounds of butter: A review. Eur. Food Res. Technol. 2007, 226, 315–325. [Google Scholar] [CrossRef]
- Chen, H.; Cui, H.; Zhang, M.; Hayat, K.; Yu, J.; Xia, S.; Zhai, Y.; Zhang, X. Improving the Flavor and Oxidation Resistance of Processed Sunflower Seeds with Maillard Peptides. Food Bioprocess. Technol. 2019, 12, 809–819. [Google Scholar] [CrossRef]
- Schaich, K.M. Chapter 17—Rethinking lipid oxidation. In Food Lipids-Chemistry, Nutrition, and Biotechnology; Akoh, C.C., Ed.; Taylor & Francis Group: London, UK, 2017; Volume 4, pp. 479–498. [Google Scholar]
- Pignitter, M.; Stolze, K.; Gartner, S.; Dumhart, B.; Stoll, C.; Steiger, G.; Kraemer, K.; Somoza, V. Cold fluorescent light as major inducer of lipid oxidation in soybean oil stored at household conditions for eight weeks. J. Agric. Food Chem. 2014, 62, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Pignitter, M.; Dumhart, B.; Gartner, S.; Jirsa, F.; Steiger, G.; Kraemer, K.; Somoza, V. Vitamin A is rapidly degraded in retinyl palmitate-fortified soybean oil stored under household conditions. J. Agric. Food Chem. 2014, 62, 7559–7566. [Google Scholar] [CrossRef] [PubMed]
- Oakley, L.H.; Casadio, F.; Shull, K.R.; Broadbelt, L.J. Examination of Mechanisms for Formation of Volatile Aldehydes from Oxidation of Oil-Based Systems. Ind. Eng. Chem. Res. 2017, 57, 139–149. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Kaneez, F.; Masood, N.; Luqman, S. Quenching of singlet oxygen by natural and synthetic antioxidants and assessment of electronic UV/Visible absorption spectra for alleviating or enhancing the efficacy of photodynamic therapy. Biomed. Res. Ther. 2016, 3, 514–527. [Google Scholar]
- Fakourelis, N.; Lee, E.C.; Min, D.B. Effects of Chlorophyll and p-Carotene on the Oxidation Stability of Olive Oil. J. Food Sci. 1987, 52, 234–235. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.W.; Kanner, J.; German, J.B. Interfacial Phenomena in the evaluation of antioxidants: Bulk oils vs emulsions. J. Agric. Food Chem. 1994, 42, 1054–1059. [Google Scholar] [CrossRef]
- Porter, W.L.; Black, E.D.; Drolet, A.M. Use of Polyamide Oxidative Fluorescence Test on Lipid Emulsions: Contrast in Relative Effectiveness of Antioxidants in Bulk Versus Dispersed Systems. J. Agric. Food Chem. 1989, 37, 615–624. [Google Scholar] [CrossRef]
- Laguerre, M.; Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.C.; Barea, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Chain length affects antioxidant properties of chlorogenate esters in emulsion: The cutoff theory behind the polar paradox. J. Agric. Food Chem. 2009, 57, 11335–11342. [Google Scholar] [CrossRef]
- Balgavý, P.; Devínsky, F. Cut-off effects in biological activities of surfactants. Adv. Colloid Interface Sci. 1996, 66, 23–63. [Google Scholar] [CrossRef]
- Timmins, G.S.; dos Santos, R.E.; Whitwood, A.C.; Catalani, L.H.; Di Mascio, P.; Gilbert, B.C.; Bechara, E.J.H. Lipid Peroxidation-Dependent Chemiluminescence from the Cyclization of Alkylperoxyl Radicals to Dioxetane Radical Intermediates. Chem. Res. Toxicol. 1997, 10, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.; Khorobrykh, S.; Matsui, K.; Iijima, Y.; Sakurai, N.; Suzuki, H.; Shibata, D. Acrolein is formed from trienoic fatty acids in chloroplast: A targeted metabolomics approach. Plant Biotechnol. 2014, 31, 535–543. [Google Scholar] [CrossRef]
- De Dios Alche, J. A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol. 2019, 23. [Google Scholar] [CrossRef]
- Fall, R. Abundant oxygenates in the atmosphere: A biochemic perspective. Chem. Rev. 2003, 103, 4941–4951. [Google Scholar] [CrossRef] [PubMed]

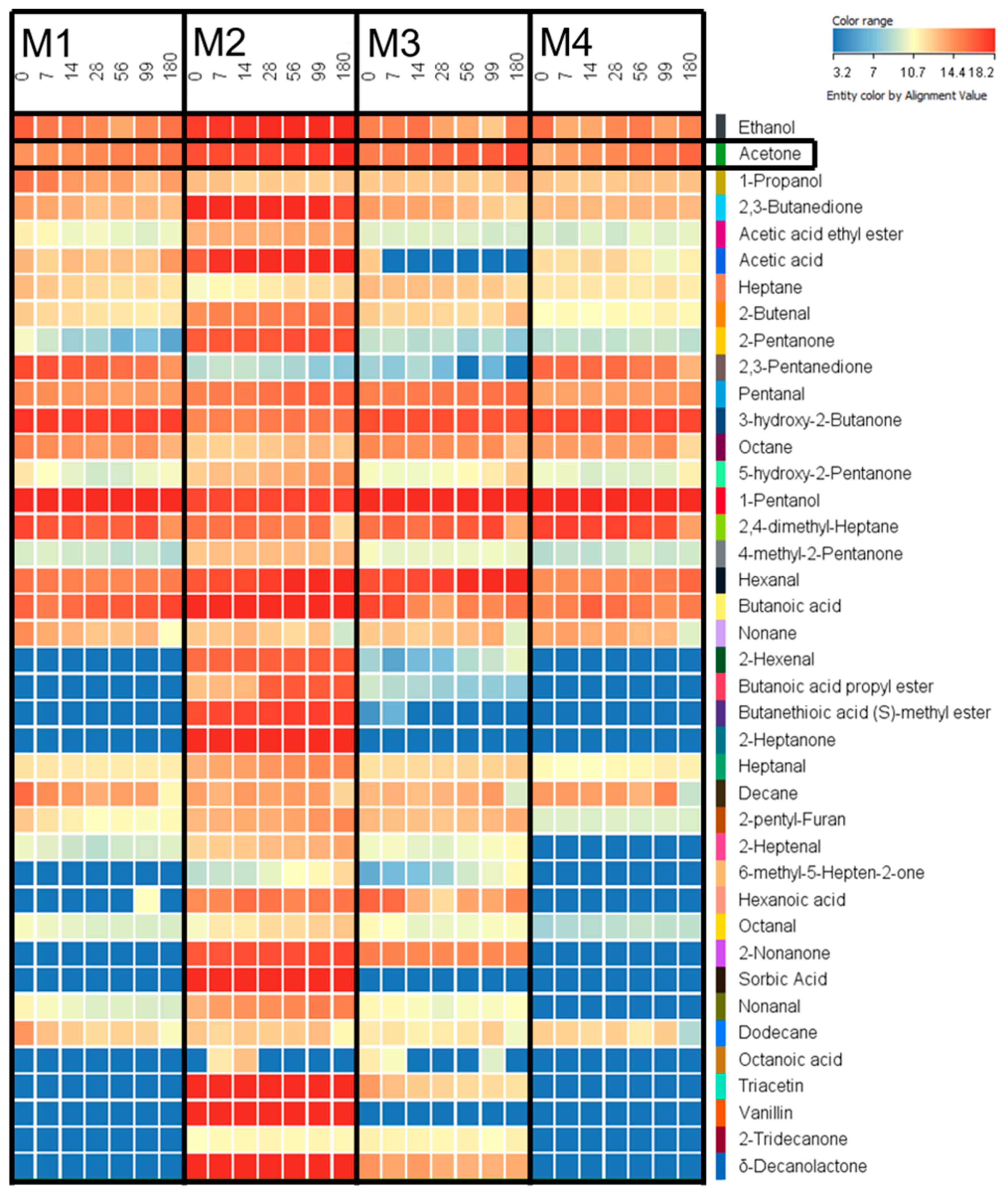

| M1 | M2 | M3 | M4 | |
|---|---|---|---|---|
| Oils | 32% hydrogenated coconut oil | 51% palm oil | 57% palm oil | 32% hydrogenated coconut oil |
| 22% coconut oil | 10% palm oil—free of trans fatty acids, non-hydrogenated | 12% palm stearin | 24% coconut oil | |
| 13% sunflower oil | 8% anhydrous milk fat | 11% rapeseed oil | 14% sunflower oil | |
| 10% palm oil or transesterified palm kernel oil | 7% rapeseed oil | 6% interesterified blend of coconut oil and fully hydrogenated rapeseed oil | ||
| 3% rapeseed oil | 4% coconut oil | 3% rapeseed oil | ||
| Water | 20% | 19% | 20% | 20% |
| Minor ingredients | 0.30% Palsgaar P1388 (mono- and diglycerides of edible fatty acids and polyglycerol esters) | 1% yogurt culture from skimmed milk | 0.18% Dimodan HP (distilled monoglyceride) | 0.30% Palsgaar P1388 (mono- and diglycerides of edible fatty acids and polyglycerol esters) |
| 0.10% salt non-iodized | 0.35% Dimodan HP (distilled monoglyceride) | 0.10% salt non-iodized | 0.10% salt non-iodized | |
| 0.01% butter aroma | 0.11% butter aroma | 0.08% lecithin | 0.01% butter aroma | |
| 0.01% citric acid | 0.10% salt non-iodized | 0.04% butter aroma | 0.01% citric acid | |
| 0.001% β-carotene | 0.08% lecithin | 0.01% citric acid | 0.001% β-carotene | |
| 0.06% citric acid | 0.001% β-carotene | |||
| 0.04% potassium sorbate | ||||
| 0.001% β-carotene |
| − | Day | M1 (meq O2/kg Oil) | Change (%) | M2 (meq O2/kg Oil) | Change (%) | M3 (meq O2/kg Oil) | Change (%) | M4 (meq O2/kg Oil) | Change (%) |
| peroxide value | 0 | 0.33 ± 0.02 | 1.10 ± 0.10 | 0.86 ± 0.06 | 0.40 ± 0.03 | ||||
| 1 | 0.36 ± 0.05 | 9.09 | 1.23 ± 0.10 | 11.8 | 0.89 ± 0.09 | 3.49 | 0.40 ± 0.04 | 0.00 | |
| 7 | 0.41 ± 0.06 | 24.2 | 1.31 ± 0.14 | 19.1 | 0.94 ± 0.10 | 9.30 | 0.46 ± 0.0.5 | 15.0 | |
| 14 | 0.43 ± 0.01 | 30.3 | 1.36 ± 0.23 | 23.6 | 0.97 ± 0.23 | 12.8 | 0.44 ± 0.03 | 10.0 | |
| 28 | 0.56 ± 0.08 | 69.7 | 2.37 ± 0.22 | 115 | 1.24 ± 0.05 | 44.2 | 0.60 ± 0.05 | 50.0 | |
| 56 | 0.82 ± 0.07 | 148 | 3.42 ± 0.28 * | 211 | 1.93 ± 0.38 | 124 | 0.99 ± 0.06 | 148 | |
| 99 | 1.31 ± 0.21 * | 297 | 4.08 ± 0.26 * | 271 | 3.06 ± 0.34 * | 256 | 1.64 ± 0.28 * | 310 | |
| 180 | 2.20 ± 0.67 * | 567 | 4.76 ± 0.92 * | 333 | 3.97 ± 0.51 * | 362 | 2.73 ± 0.27 * | 583 | |
| Day | M1 (µmol/g) | Change (%) | M2 (µmol/g) | Change (%) | M3 (µmol/g) | Change (%) | M4 (µmol/g) | Change (%) | |
| conjugated dienes | 0 | 4.45 ± 0.21 | 11.2 ± 0.68 | 7.75 ± 0.42 | 5.42 ± 0.14 | ||||
| 1 | 4.66 ± 0.15 | 4.72 | 11.3 ± 0.25 | 0.89 | 7.69 ± 0.36 | −0.77 | 3.75 ± 0.61 * | −30.8 | |
| 7 | 4.98 ± 0.40 | 11.9 | 11.3 ± 0.08 | 0.89 | 7.59 ± 0.19 | −2.06 | 5.47 ± 0.27 | 0.92 | |
| 14 | 5.70 ± 0.25 * | 28.1 | 10.7 ± 0.44 | −4.46 | 8.14 ± 0.56 | 5.03 | 5.33 ± 0.62 | −1.66 | |
| 28 | 5.23 ± 0.71 | 17.5 | 12.2 ± 0.20 | 8.93 | 7.81 ± 0.48 | 0.77 | 5.29 ± 0.43 | −2.40 | |
| 56 | 5.80 ± 0.60 * | 30.3 | 12.6 ± 0.23 | 12.5 | 8.50 ± 0.47 | 9.68 | 6.31 ± 0.12 | 16.4 | |
| 99 | 6.12 ± 0.90 * | 37.5 | 12.3 ± 0.42 | 9.82 | 9.96 ± 0.20 * | 28.5 | 5.60 ± 0.59 | 3.32 | |
| 180 | 8.35 ± 0.59 * | 87.6 | 14.7 ± 0.49 * | 31.3 | 9.98 ± 0.73 * | 28.8 | 7.89 ± 0.71 * | 45.6 | |
| Day | M1 (h) | Change (%) | M2 (h) | Change (%) | M3 (h) | Change (%) | M4 (h) | Change (%) | |
| oxidation induction time | 0 | 10.3 ± 0.22 | 13.0 ± 0.23 | 11.7 ± 0.37 | 10.7 ± 0.09 | ||||
| 1 | 11.0 ± 0.88 | 6.80 | 13.2 ± 0.07 | 1.54 | 11.5 ± 0.11 | −1.71 | 10.2 ± 0.07 | −4.67 | |
| 7 | 10.1 ± 0.23 | −1.94 | 12.6 ± 0.15 | −3.08 | 11.4 ± 0.15 | −2.56 | 9.61 ± 0.09 | −10.2 | |
| 14 | 9.39 ± 0.02 | −8.83 | 12.2 ± 0.39 | −6.15 | 10.9 ± 0.20 * | −6.84 | 8.99 ± 0.02 | −16.0 | |
| 28 | 9.64 ± 1.09 | −6.41 | 11.1 ± 0.16 | −14.6 | 10.5 ± 0.11 * | −10.3 | 8.23 ± 0.37 | −23.1 | |
| 56 | 8.02 ± 0.02 | −22.1 | 9.98 ± 0.19 | −23.2 | 9.87 ± 0.10 * | −15.6 | 7.44 ± 0.04 | −30.5 | |
| 99 | 6.83 ± 0.11 | −33.7 | 9.79 ± 0.44 * | −24.7 | 8.93 ± 0.17 * | −23.7 | 6.50 ± 0.22 * | −39.3 | |
| 180 | 5.55 ± 0.01 * | −46.1 | 7.96 ± 0.15 * | −38.8 | 7.71 ± 0.20 * | −34.1 | 5.25 ± 0.20 * | −50.9 |
| M2 | Day 0 (mg Epoxide/kg Margarine) | Day 180 (mg Epoxide/kg Margarine) |
| 54:1 [O] | 0.58 ± 0.03 | 0.24 ± 0.06 |
| 54:2 [O] | 0.19 ± 0.01 | 0.08 ± 0.06 |
| M3 | Day 0 (mg Epoxide/kg Margarine) | Day 180 (mg Epoxide/kg Margarine) |
| 54:1 [O] | 1.06 ± 0.10 | 1.59 ± 0.33 |
| 54:2 [O] | 0.77 ± 0.11 | 1.32 ± 0.33 |
| 54:3 [O] | 0.07 ± 0.04 | 0.27 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fruehwirth, S.; Egger, S.; Flecker, T.; Ressler, M.; Firat, N.; Pignitter, M. Acetone as Indicator of Lipid Oxidation in Stored Margarine. Antioxidants 2021, 10, 59. https://doi.org/10.3390/antiox10010059
Fruehwirth S, Egger S, Flecker T, Ressler M, Firat N, Pignitter M. Acetone as Indicator of Lipid Oxidation in Stored Margarine. Antioxidants. 2021; 10(1):59. https://doi.org/10.3390/antiox10010059
Chicago/Turabian StyleFruehwirth, Sarah, Sandra Egger, Thomas Flecker, Miriam Ressler, Nesrin Firat, and Marc Pignitter. 2021. "Acetone as Indicator of Lipid Oxidation in Stored Margarine" Antioxidants 10, no. 1: 59. https://doi.org/10.3390/antiox10010059
APA StyleFruehwirth, S., Egger, S., Flecker, T., Ressler, M., Firat, N., & Pignitter, M. (2021). Acetone as Indicator of Lipid Oxidation in Stored Margarine. Antioxidants, 10(1), 59. https://doi.org/10.3390/antiox10010059







