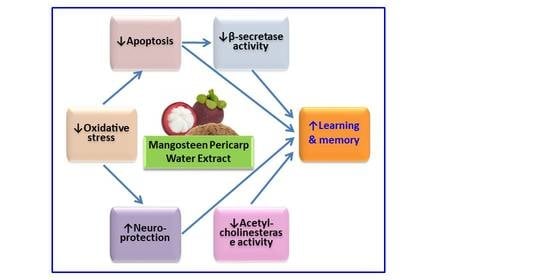
Memory-Enhancing Effects of Mangosteen Pericarp Water Extract through Antioxidative Neuroprotection and Anti-Apoptotic Action
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation and Analysis of MPW
2.4. Primary Cultures of Rat Cerebrocortical Cells
2.5. Treatment of Cells with Various Types of Insults and Assessment of Cell Viability
2.6. Measurement of Intracellular ROS Levels
2.7. TUNEL Assay
2.8. Western Blotting
2.9. Assessment of the LPO Levels in Rat Brain Homogenates
2.10. DPPH Radical Scavenging Activity Assay
2.11. In Vitro Assessment of β-Secretase Activity
2.12. In Vitro Assessment of Acetylcholinesterase (AChE) Activity
2.13. The Morris Water Maze (MWM) Test
2.14. Statistical Analysis
3. Results
3.1. Preparation and Analysis of MPW
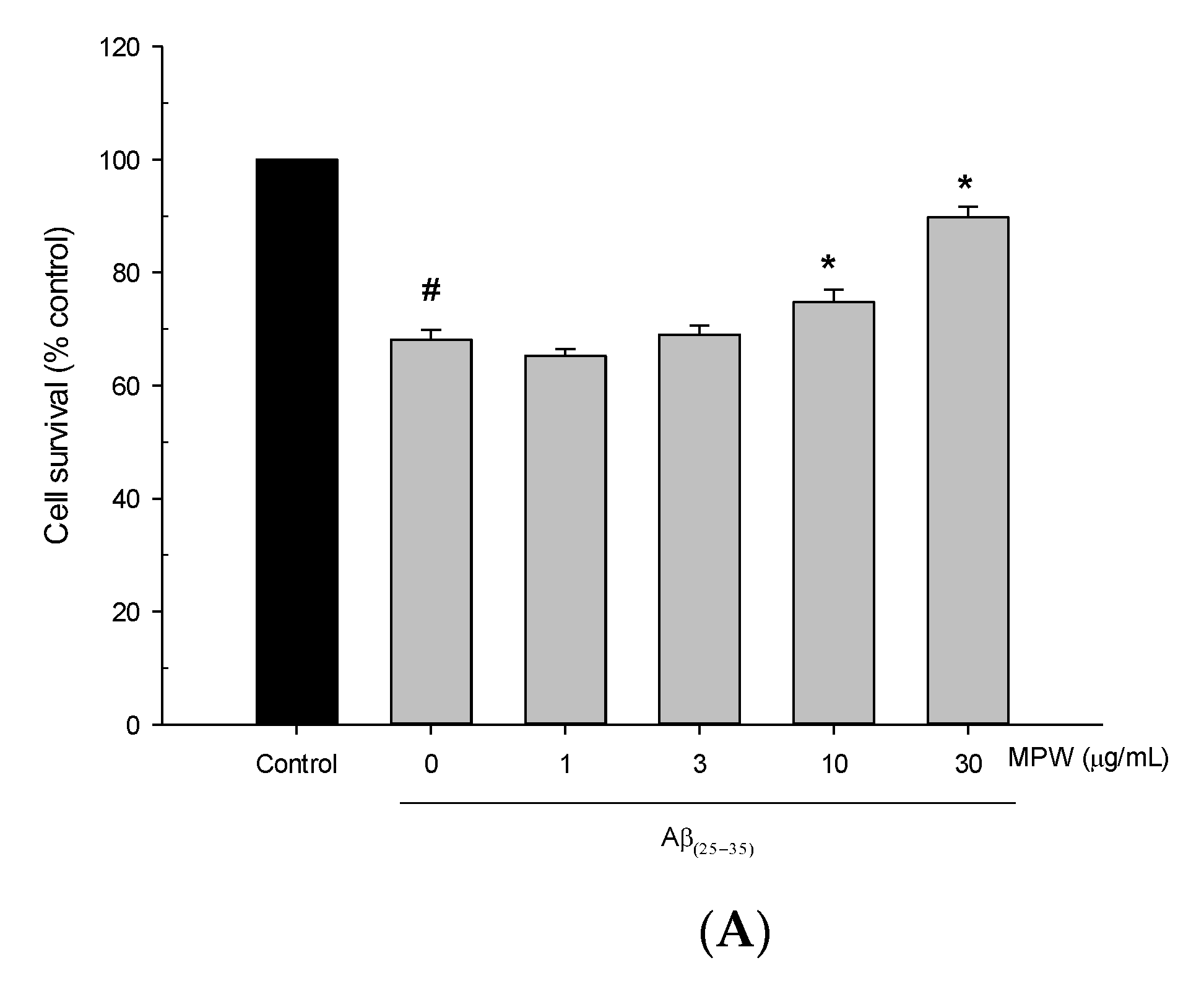
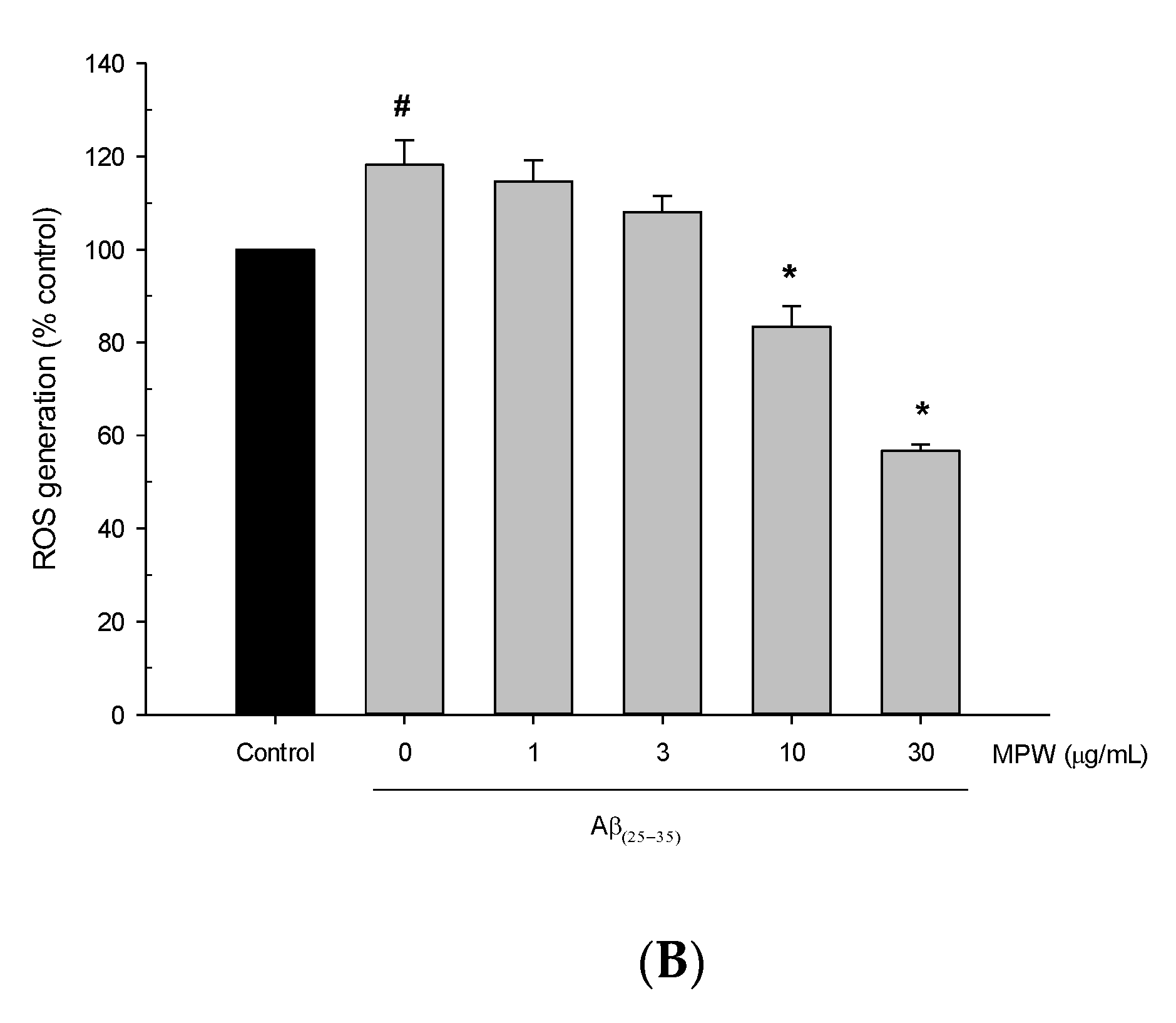
3.2. Effects of MPW on Aβ(25–35)-Induced Neurotoxicity and ROS Generation in Primary Rat Cortical Cells
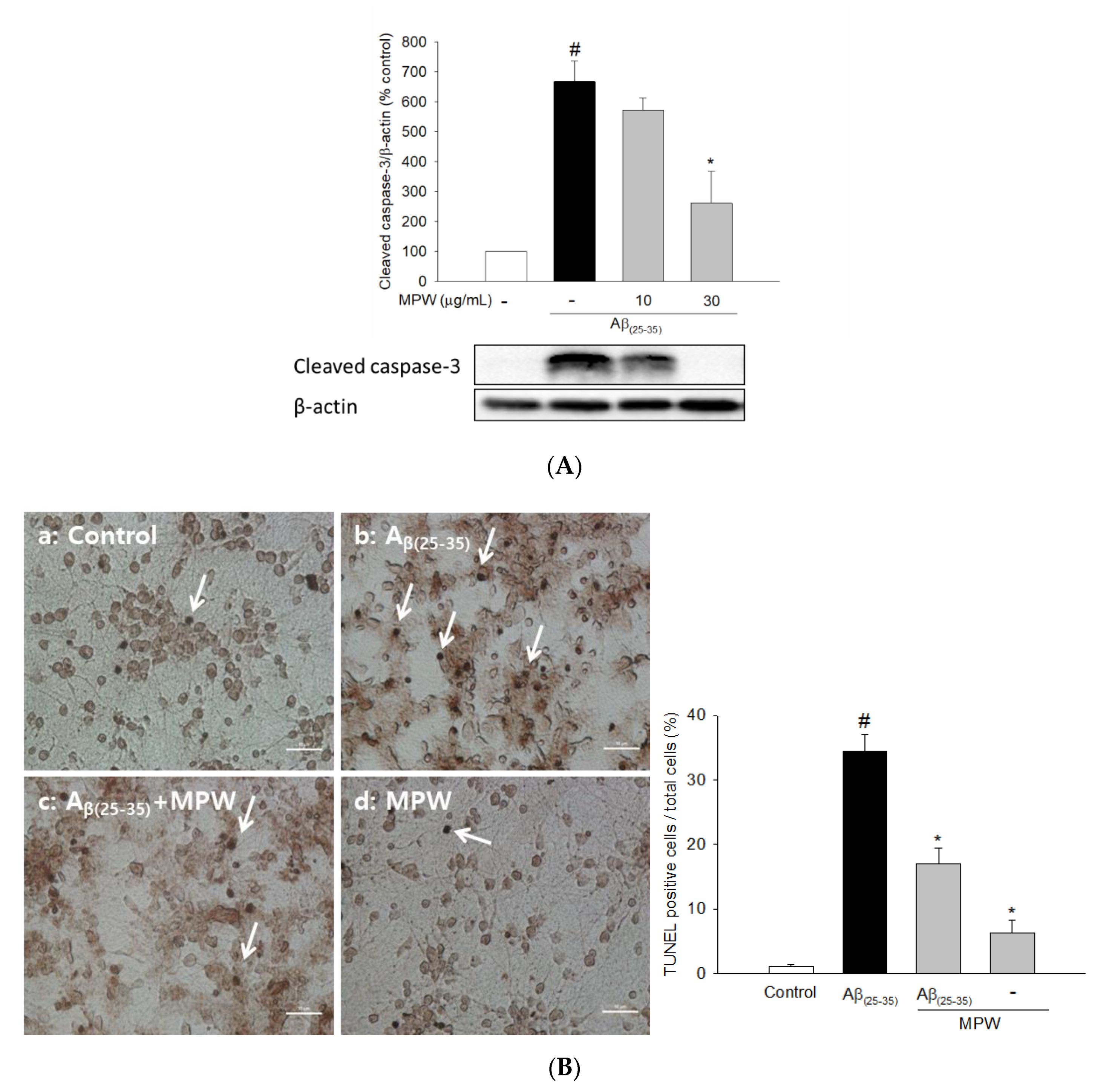
3.3. Effects of MPW on Aβ(25–35)-Induced Caspase 3 Activation and DNA Fragmentation in Primary Rat Cortical Cells
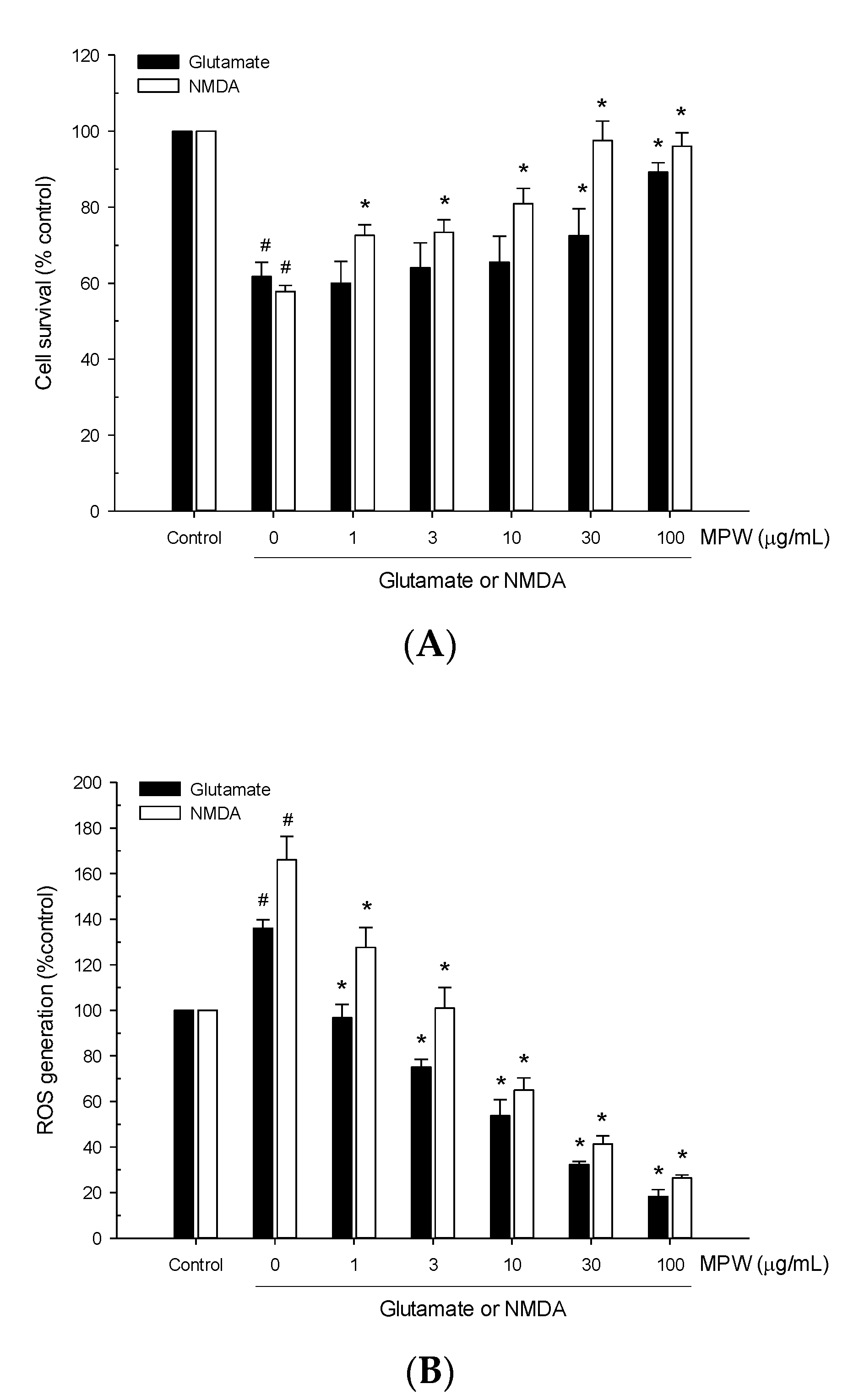
3.4. Effects of MPW on Glutamate- or NMDA-Induced Neurotoxicity and ROS Generation in Primary Rat Cortical Cells
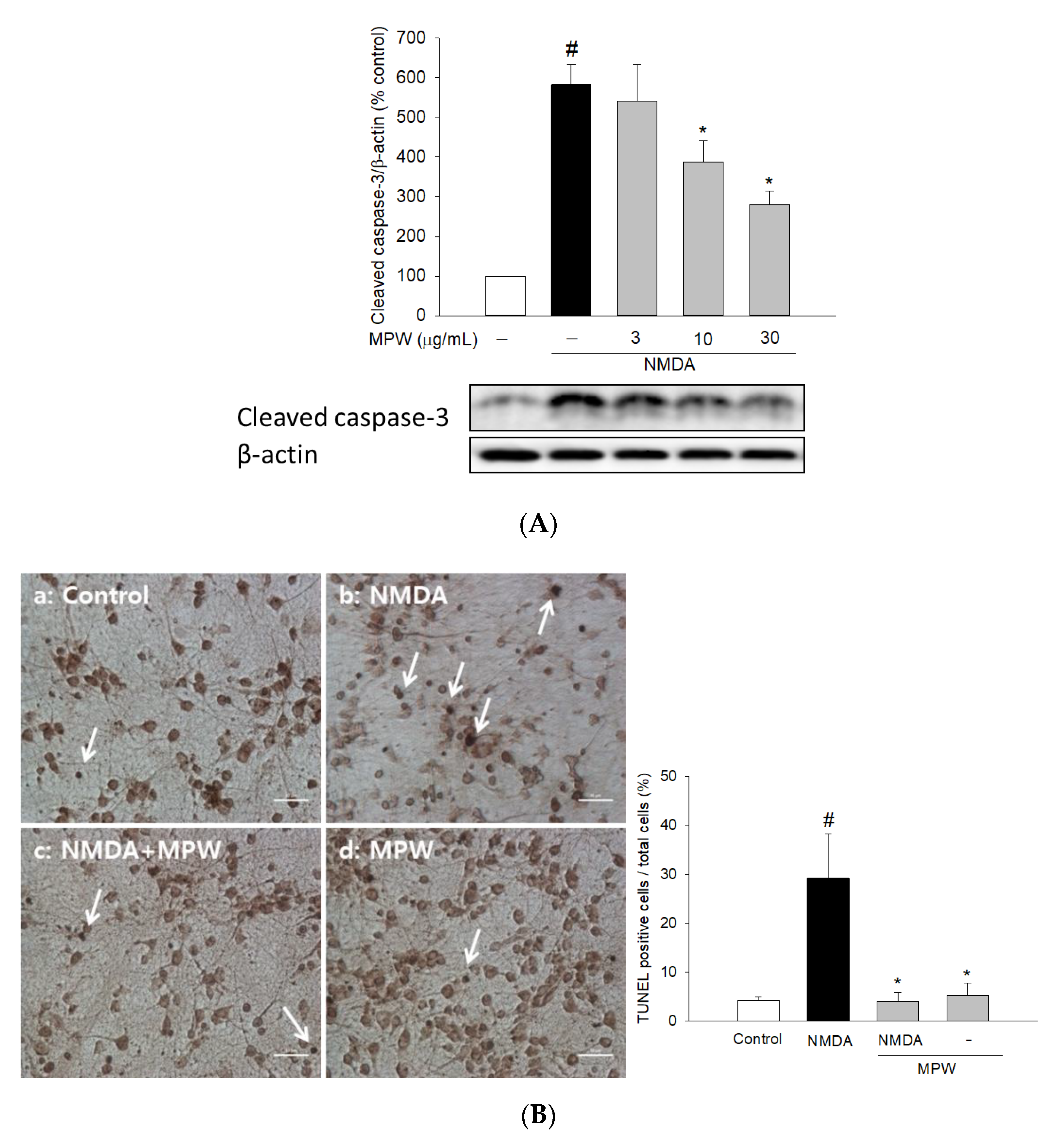
3.5. Effects of MPW on NMDA-Induced Caspase 3 Activation and DNA Fragmentation in Primary Rat Cortical Cells
3.6. Effects of MPW on LPO and DPPH Radical Formation
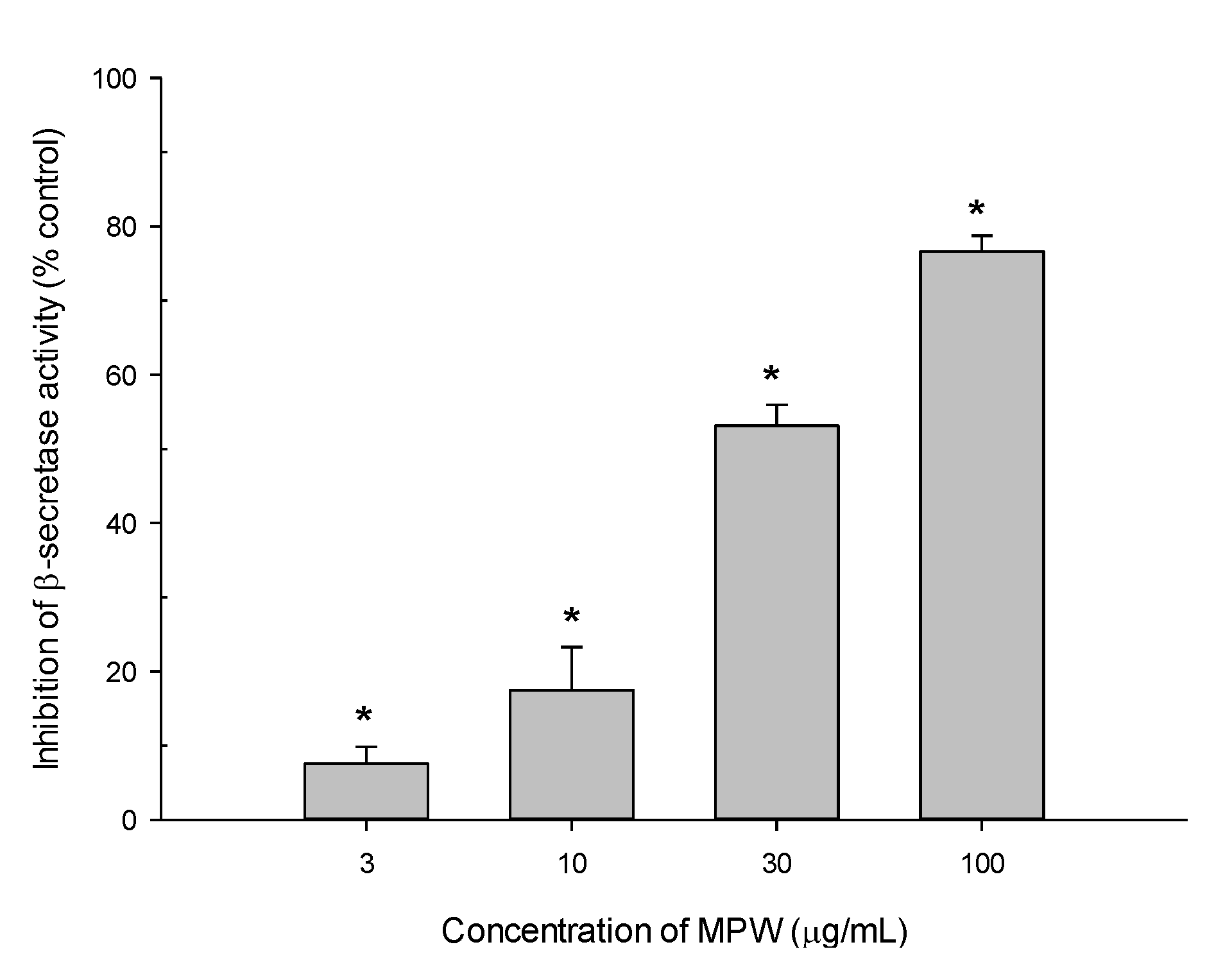
3.7. Effect of MPW on β-Secretase Activity
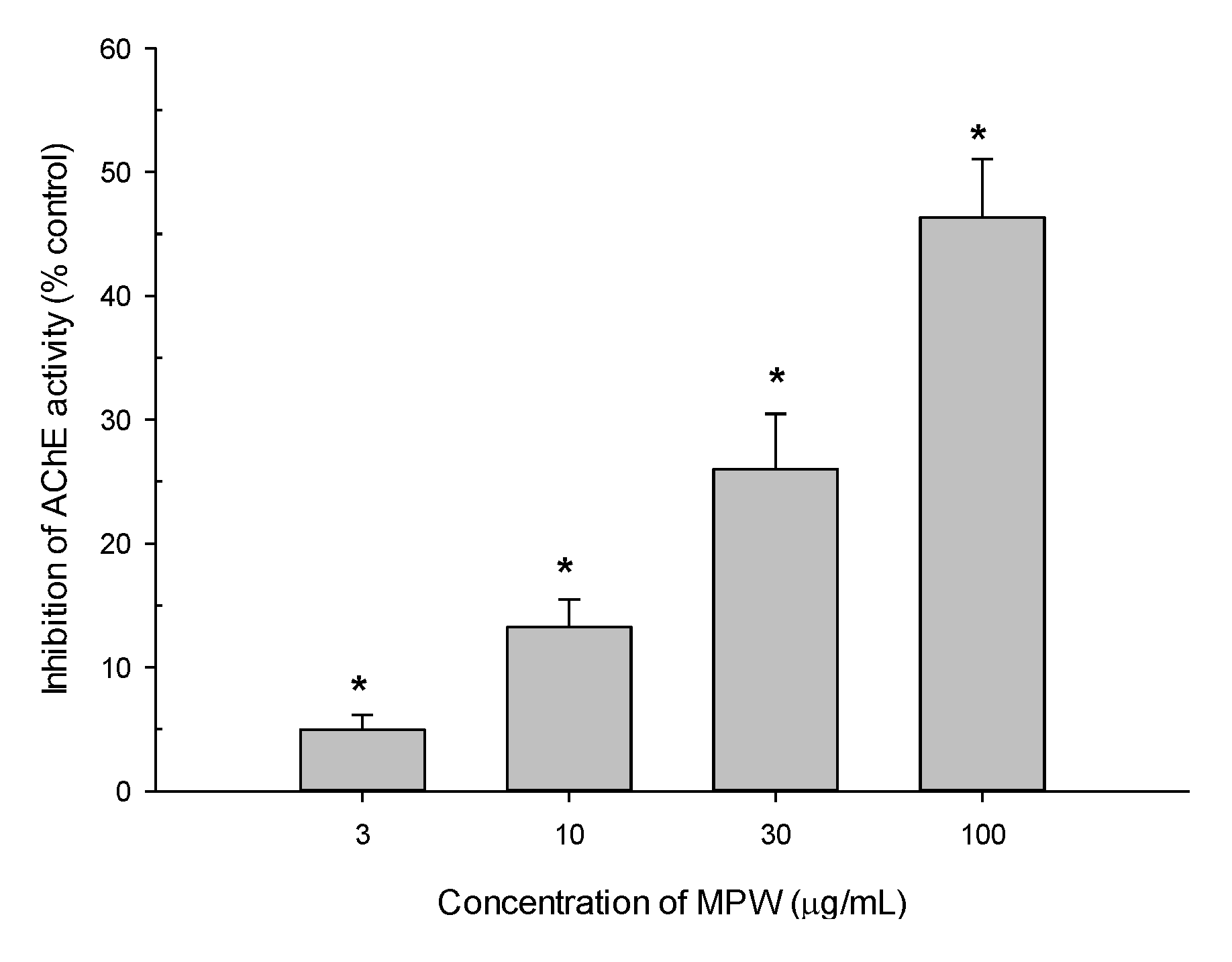
3.8. Effect of MPW on AChE Activity
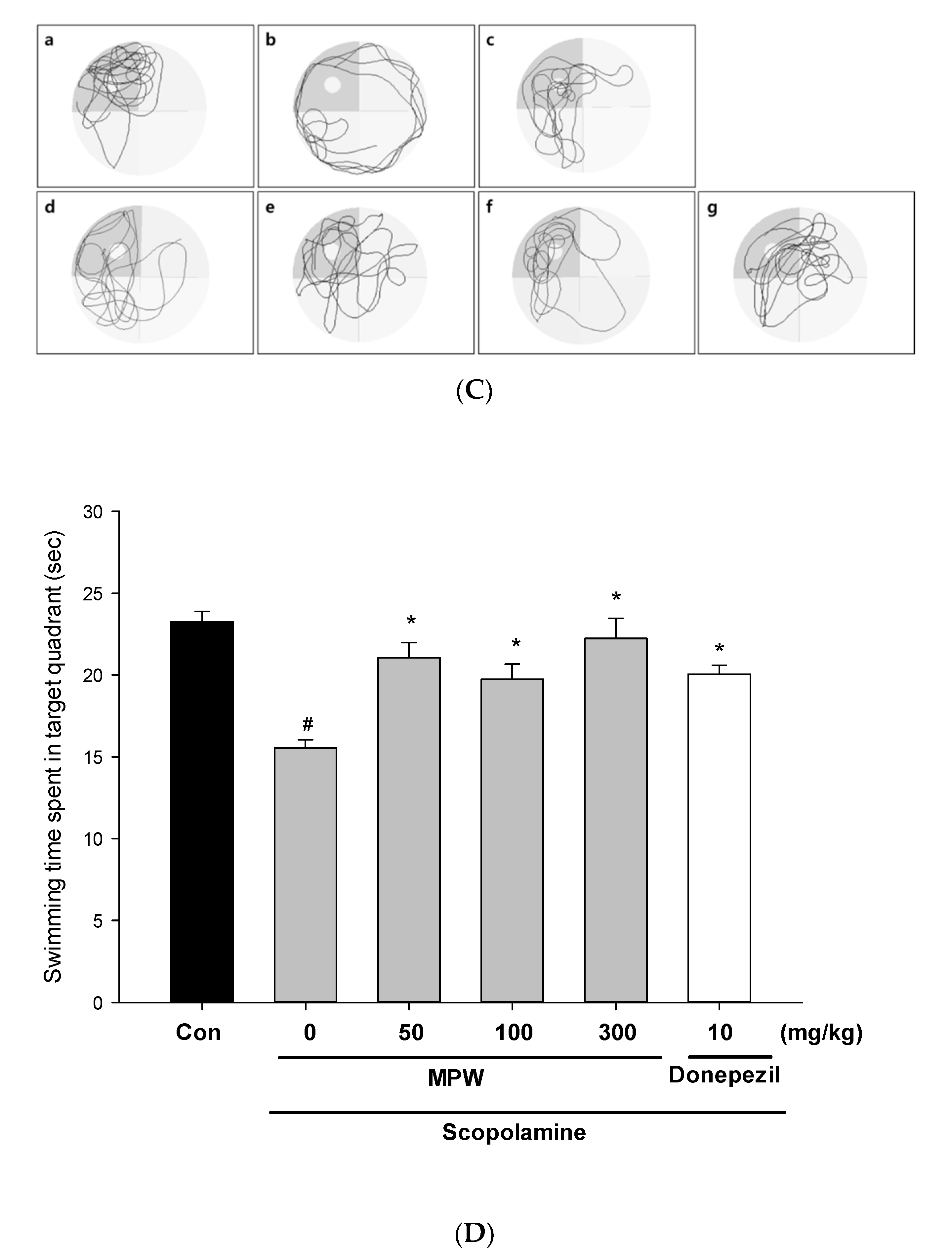
3.9. Effect of MPW on Scopolamine-Induced Spatial Learning and Memory Impairment in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vassar, R.; Kandalepas, P.C. The β-secretase enzyme BACE1 as a therapeutic target for Alzheimer’s disease. Alzheimers Res. Ther. 2011, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Findeis, M.A. The role of amyloid beta peptide 42 in Alzheimer’s disease. Pharmacol. Ther. 2007, 116, 266–286. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef]
- Panza, F.; Seripa, D.; Solfrizzi, V.; Imbimbo, B.P.; Lozupone, M.; Leo, A.; Sardone, R.; Gagliardi, G.; Lofano, L.; Creanza, B.C.; et al. Emerging drugs to reduce abnormal β-amyloid protein in Alzheimer’s disease patients. Expert Opin. Emerg. Drugs 2016, 21, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Butterfield, D.A.; Hall, N.; Cole, P.; Subramaniam, R.; Mark, R.; Mattson, M.P.; Markesbery, W.R.; Harris, M.E.; Aksenov, M. Reactive oxygen species as causal agents in the neurotoxicity of the Alzheimer’s disease-associated amyloid beta peptide. Ann. N. Y. Acad. Sci. 1996, 786, 120–134. [Google Scholar] [CrossRef]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Pike, C.J.; Ramezan-Arab, N.; Cotman, C.W. Beta-amyloid neurotoxicity in vitro: Evidence of oxidative stress but not protection by antioxidants. J. Neurochem. 1997, 69, 1601–1611. [Google Scholar] [CrossRef]
- Persson, T.; Popescu, B.O.; Cedazo-Minguez, A. Oxidative stress in Alzheimer’s disease: Why did antioxidant therapy fail? Oxid. Med. Cell. Longev. 2014, 2014, 427318. [Google Scholar] [CrossRef]
- Doble, A. The role of excitotoxicity in neurodegenerative disease: Implications for therapy. Pharmacol. Ther. 1999, 81, 163–221. [Google Scholar] [CrossRef]
- McEntee, W.J.; Crook, T.H. Glutamate: Its role in learning, memory, and the aging brain. Psychopharmacology 1993, 111, 391–401. [Google Scholar] [CrossRef]
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Al-Abdulla, N.A.; Brambrink, A.M.; Kirsch, J.R.; Sieber, F.E.; Portera-Cailliau, C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res. Bull. 1998, 46, 281–309. [Google Scholar] [CrossRef]
- Dong, X.X.; Wang, Y.; Qin, Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A. The molecular basis of memantine action in Alzheimer’s disease and other neurologic disorders: Low-affinity, uncompetitive antagonism. Curr. Alzheimer Res. 2005, 2, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ovalle-Magallanes, B.; Eugenio-Pérez, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017, 109, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Ashton, M.M.; Dean, O.M.; Walker, A.J.; Bortolasci, C.C.; Ng, C.H.; Hopwood, M.; Harvey, B.H.; Möller, M.; McGrath, J.J.; Marx, W.; et al. The Therapeutic Potential of Mangosteen Pericarp as an Adjunctive Therapy for Bipolar Disorder and Schizophrenia. Front. Psychiatry 2019, 10, 115. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Siriwatanametanon, N.; Heinrich, M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother. Res. 2009, 23, 1047–1065. [Google Scholar] [CrossRef]
- Chin, Y.-W.; Kinghorn, A.D. Structural Characterization, Biological Effects, and Synthetic Studies on Xanthones from Mangosteen (Garcinia mangostana), a Popular Botanical Dietary Supplement. Mini Rev. Org. Chem. 2008, 5, 355–364. [Google Scholar] [CrossRef]
- Gutierrez-Orozco, F.; Failla, M.L. Biological activities and bioavailability of mangosteen xanthones: A critical review of the current evidence. Nutrients 2013, 5, 3163–3183. [Google Scholar] [CrossRef]
- Pedraza-Chaverrí, J.; Reyes-Fermín, L.M.; Nolasco-Amaya, E.G.; Orozco-Ibarra, M.; Medina-Campos, O.N.; González-Cuahutencos, O.; Rivero-Cruz, I.; Mata, R. ROS scavenging capacity and neuroprotective effect of alpha-mangostin against 3-nitropropionic acid in cerebellar granule neurons. Exp. Toxicol. Pathol. 2009, 61, 491–501. [Google Scholar] [CrossRef]
- Moongkarndi, P.; Kosem, N.; Kaslungka, S.; Luanratana, O.; Pongpan, N.; Neungton, N. Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J. Ethnopharmacol. 2004, 90, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Al-Abd, A.M.; El-Halawany, A.M.; Abdallah, H.M.; Ibrahim, S.R.M. New xanthones and cytotoxic constituents from Garcinia mangostana fruit hulls against human hepatocellular, breast, and colorectal cancer cell lines. J. Ethnopharmacol. 2017, 198, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Weecharangsan, W.; Opanasopit, P.; Sukma, M.; Ngawhirunpat, T.; Sotanaphun, U.; Siripong, P. Antioxidative and neuroprotective activities of extracts from the fruit hull of mangosteen (Garcinia mangostana Linn.). Med. Princ. Pract. 2006, 15, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, S.; Oh, Y.; Kim, Y.-M.; Chin, Y.-W.; Cho, J. Inhibition of Oxidative Neurotoxicity and Scopolamine-Induced Memory Impairment by γ-Mangostin: In Vitro and In Vivo Evidence. Oxid. Med. Cell. Longev. 2019, 2019, 3640753. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Kong, J.Y.; Jeong, D.Y.; Lee, K.D.; Lee, D.U.; Kang, B.S. NMDA recepter-mediated neuroprotection by essential oils from the rhizomes of Acorus gramineus. Life Sci. 2001, 68, 1567–1573. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Cho, J. Korean red ginseng extract exhibits neuroprotective effects through inhibition of apoptotic cell death. Biol. Pharm. Bull. 2014, 37, 938–946. [Google Scholar] [CrossRef]
- Cho, J.; Joo, N.E.; Kong, J.Y.; Jeong, D.Y.; Lee, K.D.; Kang, B.S. Inhibition of excitotoxic neuronal death by methanol extract of Acori graminei rhizoma in cultured rat cortical neurons. J. Ethnopharmacol. 2000, 73, 31–37. [Google Scholar] [CrossRef]
- Cho, J.; Kim, H.M.; Ryu, J.-H.; Jeong, Y.S.; Lee, Y.S.; Jin, C. Neuroprotective and antioxidant effects of the ethyl acetate fraction prepared from Tussilago farfara L. Biol. Pharm. Bull. 2005, 28, 455–460. [Google Scholar] [CrossRef]
- Liu, X.; Xu, K.; Yan, M.; Wang, Y.; Zheng, X. Protective effects of galantamine against Abeta-induced PC12 cell apoptosis by preventing mitochondrial dysfunction and endoplasmic reticulum stress. Neurochem. Int. 2010, 57, 588–599. [Google Scholar] [CrossRef]
- Kim, S.; Chin, Y.W.; Cho, J. Protection of Cultured Cortical Neurons by Luteolin against Oxidative Damage through Inhibition of Apoptosis and Induction of Heme Oxygenase-1. Biol. Pharm. Bull. 2017, 40, 256–265. [Google Scholar] [CrossRef]
- Cho, J.; Gruol, D.L. The chemokine CCL2 activates p38 mitogen-activated protein kinase pathway in cultured rat hippocampal cells. J. Neuroimmunol. 2008, 199, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Lee, H.-K. Wogonin inhibits excitotoxic and oxidative neuronal damage in primary cultured rat cortical cells. Eur. J. Pharmacol. 2004, 485, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeon, S.J.; Son, K.H.; Jung, J.W.; Lee, S.; Yoon, B.H.; Lee, J.-J.; Cho, Y.-W.; Cheong, J.H.; Ko, K.H.; et al. The ameliorating effect of oroxylin A on scopolamine-induced memory impairment in mice. Neurobiol. Learn. Mem. 2007, 87, 536–546. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Do, H.T.T.; Cho, J. Mangosteen Pericarp and Its Bioactive Xanthones: Potential Therapeutic Value in Alzheimer’s Disease, Parkinson’s Disease, and Depression with Pharmacokinetic and Safety Profiles. Int. J. Mol. Sci. 2020, 21, 6211. [Google Scholar] [CrossRef]
- Hardy, J. The amyloid hypothesis for Alzheimer’s disease: A critical reappraisal. J. Neurochem. 2009, 110, 1129–1134. [Google Scholar] [CrossRef]
- Kaminsky, Y.G.; Marlatt, M.W.; Smith, M.A.; Kosenko, E.A. Subcellular and metabolic examination of amyloid-beta peptides in Alzheimer disease pathogenesis: Evidence for Aβ(25–35). Exp. Neurol. 2010, 221, 26–37. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Howson, P.A.; Xia, Z.; Zhou, S.; Wu, E.; Xia, Z.; Hu, Y. Smilagenin attenuates beta amyloid (25–35)-induced degeneration of neuronal cells via stimulating the gene expression of brain-derived neurotrophic factor. Neuroscience 2012, 210, 275–285. [Google Scholar] [CrossRef]
- Aliaga, E.; Silhol, M.; Bonneau, N.; Maurice, T.; Arancibia, S.; Tapia-Arancibia, L. Dual response of BDNF to sublethal concentrations of beta-amyloid peptides in cultured cortical neurons. Neurobiol. Dis. 2010, 37, 208–217. [Google Scholar] [CrossRef]
- Hughes, E.; Burke, R.M.; Doig, A.J. Inhibition of toxicity in the beta-amyloid peptide fragment β-(25–35) using N-methylated derivatives: A general strategy to prevent amyloid formation. J. Biol. Chem. 2000, 275, 25109–25115. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014, 42 (Suppl. 3) (Suppl. 3), S125–S152. [Google Scholar] [CrossRef]
- Behl, C.; Moosmann, B. Oxidative nerve cell death in Alzheimer’s disease and stroke: Antioxidants as neuroprotective compounds. Biol. Chem. 2002, 383, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Fatokun, A.A.; Stone, T.W.; Smith, R.A. Oxidative stress in neurodegeneration and available means of protection. Front. Biosci. 2008, 13, 3288–3311. [Google Scholar] [CrossRef]
- Doble, A. Excitatory amino acid receptors and neurodegeneration. Therapie 1995, 50, 319–337. [Google Scholar]
- Lipton, S.A. Paradigm shift in neuroprotection by NMDA receptor blockade: Memantine and beyond. Nat. Rev. Drug Discov. 2006, 5, 160–170. [Google Scholar] [CrossRef]
- De Felice, F.G.; Velasco, P.T.; Lambert, M.P.; Viola, K.; Fernandez, S.J.; Ferreira, S.T.; Klein, W.L. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J. Biol. Chem. 2007, 282, 11590–11601. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Alvarez, X.A.; Cacabelos, R.; Quack, G. Neuroprotection by memantine against neurodegeneration induced by β-amyloid(1-40). Brain Res. 2002, 958, 210–221. [Google Scholar] [CrossRef]
- Mattson, M.P.; Cheng, B.; Davis, D.; Bryant, K.; Lieberburg, I.; Rydel, R.E. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 1992, 12, 376–389. [Google Scholar] [CrossRef]
- Schubert, D.; Piasecki, D. Oxidative glutamate toxicity can be a component of the excitotoxicity cascade. J. Neurosci. 2001, 21, 7455–7462. [Google Scholar] [CrossRef] [PubMed]
- Moongkarndi, P.; Srisawat, C.; Saetun, P.; Jantaravinid, J.; Peerapittayamongkol, C.; Soi-ampornkul, R.; Junnu, S.; Sinchaikul, S.; Chen, S.T.; Charoensilp, P.; et al. Protective effect of mangosteen extract against beta-amyloid-induced cytotoxicity, oxidative stress and altered proteome in SK-N-SH cells. J. Proteom. Res. 2010, 9, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.N.; Li, Q.; Jing, M.H.; Alba, E.; Yang, X.H.; Sabaté, R.; Han, Y.F.; Pi, R.B.; Lan, W.J.; Yang, X.B.; et al. Natural Xanthones from Garcinia mangostana with Multifunctional Activities for the Therapy of Alzheimer’s Disease. Neurochem. Res. 2016, 41, 1806–1817. [Google Scholar] [CrossRef]
- Chin, Y.W.; Jung, H.A.; Chai, H.; Keller, W.J.; Kinghorn, A.D. Xanthones with quinone reductase-inducing activity from the fruits of Garcinia mangostana (Mangosteen). Phytochemistry 2008, 69, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Vassar, R. Targeting the β secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014, 13, 319–329. [Google Scholar] [CrossRef]
- Zhao, L.X.; Wang, Y.; Liu, T.; Wang, Y.X.; Chen, H.Z.; Xu, J.R.; Qiu, Y. α-Mangostin decreases β-amyloid peptides production via modulation of amyloidogenic pathway. CNS Neurosci. Ther. 2017, 23, 526–534. [Google Scholar] [CrossRef]
- Giacobini, E. Cholinergic function and Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2003, 18, S1–S5. [Google Scholar] [CrossRef]
- Lovestone, S.; Howard, R. Alzheimer’s disease: A treatment in sight? J. Neurol. Neurosurg. Psychiatry 1995, 59, 566–567. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Sattayasai, J.; Chaonapan, P.; Arkaravichie, T.; Soi-Ampornkul, R.; Junnu, S.; Charoensilp, P.; Samer, J.; Jantaravinid, J.; Masaratana, P.; Suktitipat, B.; et al. Protective effects of mangosteen extract on H2O2-induced cytotoxicity in SK-N-SH cells and scopolamine-induced memory impairment in mice. PLoS ONE 2013, 8, e85053. [Google Scholar] [CrossRef]
- Bakshi, V.; Avinash, P.; Reddy, A.; Begum, N. Neuroprotective Effect of Garcinia Mangostana on Streptozotocin Induced Sporadic Type Alzheimer’s Disease in Mice. Int. J. Appl. Pharm. Sci. Res. 2016, 1, 8–15. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Choi, S.B.; Tan, S.C.; Wahab, H.A.; Chan, K.L.; Murugaiyah, V. Prenylated xanthones from mangosteen as promising cholinesterase inhibitors and their molecular docking studies. Phytomed. Int. J. phytother. Phytopharmacol. 2014, 21, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Schmeller, T.; Sporer, F.; Sauerwein, M.; Wink, M. Binding of tropane alkaloids to nicotinic and muscarinic acetylcholine receptors. Pharmazie 1995, 50, 493–495. [Google Scholar] [PubMed]
- Giridharan, V.V.; Thandavarayan, R.A.; Sato, S.; Ko, K.M.; Konishi, T. Prevention of scopolamine-induced memory deficits by schisandrin B, an antioxidant lignan from Schisandra chinensis in mice. Free Radic. Res. 2011, 45, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Pachauri, S.D.; Tota, S.; Khandelwal, K.; Verma, P.R.; Nath, C.; Hanif, K.; Shukla, R.; Saxena, J.K.; Dwivedi, A.K. Protective effect of fruits of Morinda citrifolia L. on scopolamine induced memory impairment in mice: A behavioral, biochemical and cerebral blood flow study. J. Ethnopharmacol. 2012, 139, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Budzynska, B.; Boguszewska-Czubara, A.; Kruk-Slomka, M.; Skalicka-Wozniak, K.; Michalak, A.; Musik, I.; Biala, G. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology (Berl) 2015, 232, 931–942. [Google Scholar] [CrossRef]
- Flood, J.F.; Cherkin, A. Scopolamine effects on memory retention in mice: A model of dementia? Behav. Neural Biol. 1986, 45, 169–184. [Google Scholar] [CrossRef]
- Huang, H.J.; Chen, W.L.; Hsieh, R.H.; Hsieh-Li, H.M. Multifunctional Effects of Mangosteen Pericarp on Cognition in C57BL/6J and Triple Transgenic Alzheimer’s Mice. Evid. Based Complement. Altern. Med. 2014, 2014, 813672. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, Y.; Do, H.T.T.; Kim, S.; Kim, Y.-M.; Chin, Y.-W.; Cho, J. Memory-Enhancing Effects of Mangosteen Pericarp Water Extract through Antioxidative Neuroprotection and Anti-Apoptotic Action. Antioxidants 2021, 10, 34. https://doi.org/10.3390/antiox10010034
Oh Y, Do HTT, Kim S, Kim Y-M, Chin Y-W, Cho J. Memory-Enhancing Effects of Mangosteen Pericarp Water Extract through Antioxidative Neuroprotection and Anti-Apoptotic Action. Antioxidants. 2021; 10(1):34. https://doi.org/10.3390/antiox10010034
Chicago/Turabian StyleOh, Yeonsoo, Ha Thi Thu Do, Sunyoung Kim, Young-Mi Kim, Young-Won Chin, and Jungsook Cho. 2021. "Memory-Enhancing Effects of Mangosteen Pericarp Water Extract through Antioxidative Neuroprotection and Anti-Apoptotic Action" Antioxidants 10, no. 1: 34. https://doi.org/10.3390/antiox10010034
APA StyleOh, Y., Do, H. T. T., Kim, S., Kim, Y.-M., Chin, Y.-W., & Cho, J. (2021). Memory-Enhancing Effects of Mangosteen Pericarp Water Extract through Antioxidative Neuroprotection and Anti-Apoptotic Action. Antioxidants, 10(1), 34. https://doi.org/10.3390/antiox10010034