Investigation of Biochemical Alterations in Ischemic Stroke Using Fourier Transform Infrared Imaging Spectroscopy—A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Block and Tissue Preparation
2.3. Transmission Electron Microscopy
2.4. FTIR Data Acquisition and Pre-Processing
2.5. Statistical Analysis
3. Results
3.1. Transmission Electron Microscope Studies to Define Ultrastructural Changes
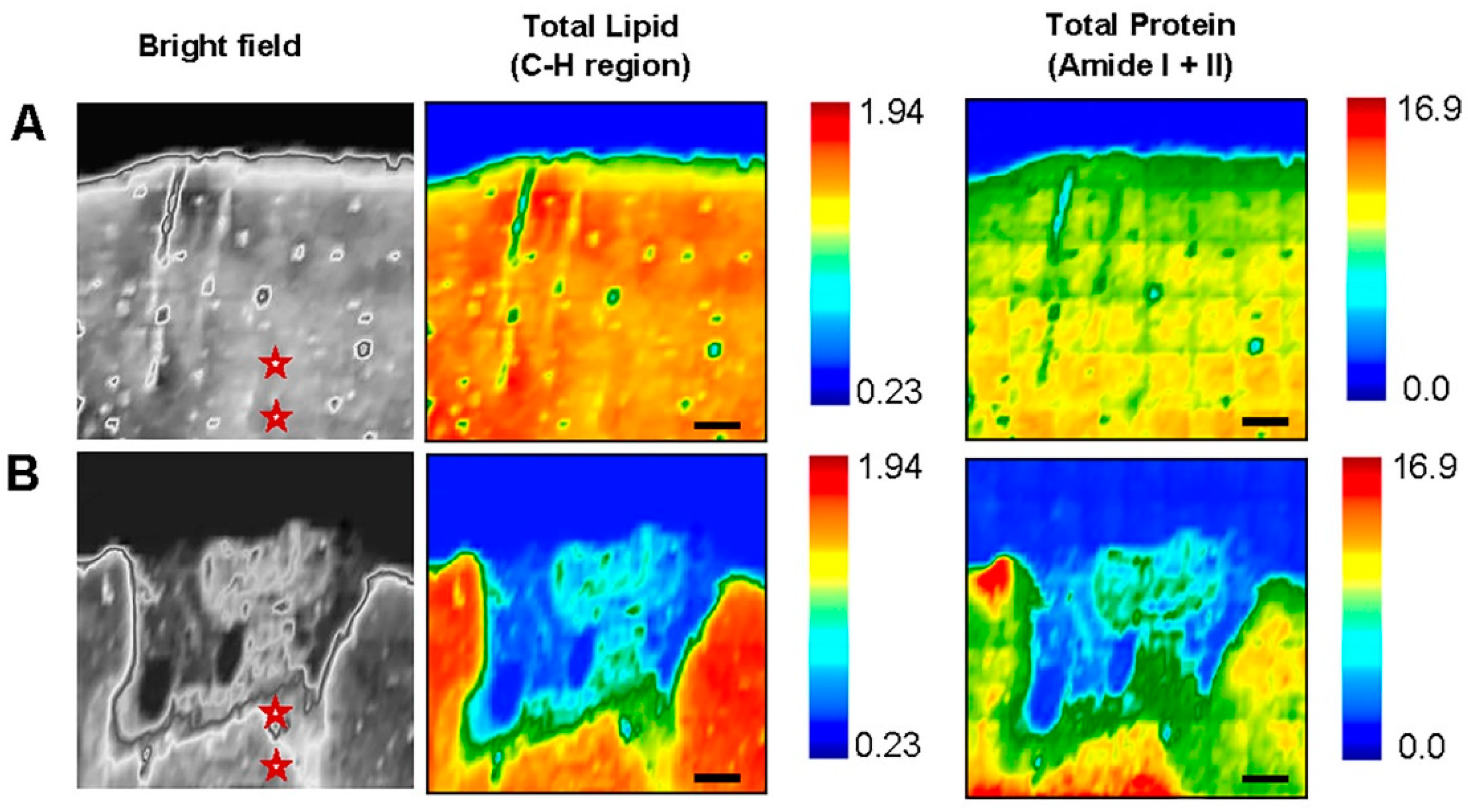
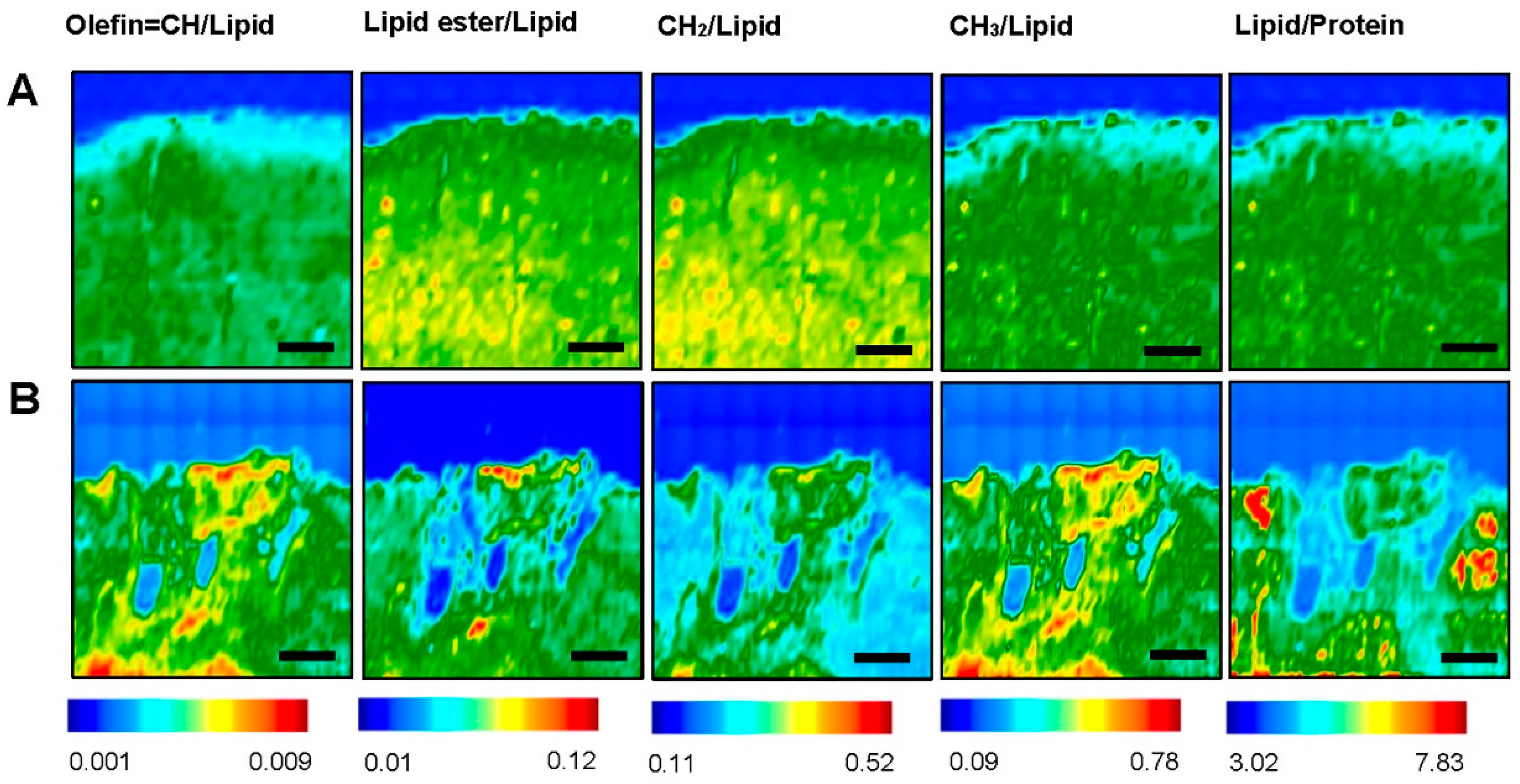
3.2. Fourier Transform Infrared Imaging Spectroscopic Studies to Identify Biochemical and Molecular Changes
3.2.1. Contra-Lesional WM vs Ipsi-Lesional WM
3.2.2. Contra-Lesional GM vs Ipsi-Lesional PIZ
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hossmann, K.-A. Pathophysiology and therapy of experimental stroke. Cell. Mol. Neurobiol. 2006, 26, 1055–1081. [Google Scholar] [CrossRef]
- Sommer, C.J. Ischemic stroke: Experimental models and reality. Acta Neuropathol. 2017, 133, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Mergenthaler, P.; Dirnagl, U.; Meisel, A. Pathophysiology of stroke: Lessons from animal models. Metab. Brain Dis. 2004, 19, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Ali, M.H.M.; Rakib, F.; Abdelalim, E.M.; Limbeck, A.; Mall, R.; Ullah, E.; Mesaeli, N.; McNaughton, D.; Ahmed, T.; Al-Saad, K. Fourier-Transform Infrared Imaging Spectroscopy and Laser Ablation -ICPMS New Vistas for Biochemical Analyses of Ischemic Stroke in Rat Brain. Front. Mol. Neurosci. 2018, 12, 647. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003, 4, 399–414. [Google Scholar] [CrossRef]
- Siesjö, B.K. Acidosis and ischemic brain damage. Neurochem. Pathol. 1988, 9, 31–88. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Thundyil, J.; Tang, S.-C.; Sobey, C.G.; Taylor, S.M.; Arumugam, T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011, 6, 11. [Google Scholar] [CrossRef]
- Pekny, M.; Nilsson, M. Astrocyte activation and reactive gliosis. Glia 2005, 50, 427–434. [Google Scholar] [CrossRef]
- Wan, J.; Ren, H.; Wang, J. Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc. Neurol. 2019, 4, 93–95. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Westlake, K.P.; Nagarajan, S.S. Functional Connectivity in Relation to Motor Performance and Recovery After Stroke. Front. Syst. Neurosci. 2011, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Dijkhuizen, R.M.; van der Marel, K.; Otte, W.M.; Hoff, E.I.; van der Zijden, J.P.; van der Toorn, A.; van meer, M.P.A. Functional MRI and Diffusion Tensor Imaging of Brain Reorganization After Experimental Stroke. Transl. Stroke Res. 2012, 3, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, N.; Cullen, B.M.; King, M.D.; Doran, M.; Williams, S.R.; Gadian, D.G.; Cremer, J.E. T2- and diffusion-weighted magnetic resonance imaging of a focal ischemic lesion in rat brain. Stroke 1992, 23, 576–582. [Google Scholar] [CrossRef]
- Dijkhuizen, R.M.; Singhal, A.B.; Mandeville, J.B.; Wu, O.; Halpern, E.F.; Finklestein, S.P.; Rosen, B.R.; Lo, E.H. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: A functional magnetic resonance imaging study. J. Neurosci. 2003, 23, 510–517. [Google Scholar] [CrossRef]
- Dijkhuizen, R.M.; Beekwilder, J.P.; Van Der Worp, H.B.; Van Der Sprenkel, J.W.B.; Tulleken, K.A.F.; Nicolay, K. Correlation between tissue depolarizations and damage in focal ischemic rat brain. Brain Res. 1999, 840, 194–205. [Google Scholar] [CrossRef]
- Dietrich, W.D.; Ginsberg, M.D.; Busto, R.; Watson, B.D. Photochemically induced cortical infarction in the Rat. 2. Acute and subacute alterations in local glucose utilization. Br. J. Pharmacol. 1986, 6, 195–202. [Google Scholar] [CrossRef]
- Pevsner, P.H.; Eichenbaum, J.W.; Miller, D.C.; Pivawer, G.; Eichenbaum, K.D.; Stern, A.; Zakian, K.L.; Koutcher, J.A. A photothrombotic model of small early ischemic infarcts in the rat brain with histologic and MRI correlation. J. Pharmacol. Toxicol. Methods 2001, 45, 227–233. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Xi, G.; Hua, Y.; Nagaraja, T.N.; Fenstermacher, J.D.; Keep, R.F. Development of a rat model of photothrombotic ischemia and infarction within the caudoputamen. Stroke 2009, 40, 248–253. [Google Scholar] [CrossRef]
- Ali, M.H.M.; Rakib, F.; Al-Saad, K.; Al-Saady, R.; Goormaghtigh, E. An Innovative Platform Merging Elemental Analysis and Ftir Imaging for Breast Tissue Analysis. Sci. Rep. 2019, 9, 9854. [Google Scholar] [CrossRef]
- Levin, I.W.; Bhargava, R. Fourier transform infrared vibrational spectroscopic imaging: Integrating microscopy and molecular recognition. Annu. Rev. Phys. Chem. 2005, 56, 429–474. [Google Scholar] [CrossRef] [PubMed]
- Caine, S.; Hackett, M.J.; Hou, H.; Kumar, S.; Maley, J.; Ivanishvili, Z.; Suen, B.; Szmigielski, A.; Jiang, Z.; Sylvain, N.J.; et al. A novel multi-modal platform to image molecular and elemental alterations in ischemic stroke. Neurobiol. Dis. 2016, 91, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.H.M.; Rakib, F.; Nischwitz, V.; Ullah, E.; Mall, R.; Shraim, A.M.; Ahmad, M.I.; Ghouri, Z.K.; McNaughton, D.; Küppers, S.; et al. Application of FTIR and LA-ICPMS Spectroscopies as a Possible Approach for Biochemical Analyses of Different Rat Brain Regions. Appl. Sci. 2018, 8, 2436. [Google Scholar] [CrossRef]
- Petibois, C.; Wehbe, K.; Belbachir, K.; Noreen, R.; Déléris, G. Current trends in the development of FTIR imaging for the quantitative analysis of biological samples. Acta Phys. Pol. A 2009, 115, 507. [Google Scholar] [CrossRef]
- Miller, L.M.; Bourassa, M.W.; Smith, R.J. FTIR spectroscopic imaging of protein aggregation in living cells. Biochim. et Biophys. Acta Bioenergy 2013, 1828, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.S.; Adamcik, J.; Jeong, J.S.; Lashuel, H.A.; Mezzenga, R.; Dietler, G. Influence of the β-Sheet Content on the Mechanical Properties of Aggregates during Amyloid Fibrillization. Angew. Chem. Int. Ed. 2015, 54, 2462–2466. [Google Scholar] [CrossRef]
- Hackett, M.J.; Aitken, J.B.; El-Assaad, F.; McQuillan, J.A.; Carter, E.A.; Ball, H.J.; Tobin, M.J.; Paterson, D.; De Jonge, M.D.; Siegele, R.; et al. Mechanisms of murine cerebral malaria: Multimodal imaging of altered cerebral metabolism and protein oxidation at hemorrhage sites. Sci. Adv. 2015, 1, e1500911. [Google Scholar] [CrossRef]
- Szczerbowska-Boruchowska, M.; Dumas, P.; Kastyak, M.Z.; Chwiej, J.; Lankosz, M.; Adamek, D.; Krygowska-Wajs, A. Biomolecular investigation of human substantia nigra in Parkinson’s disease by synchrotron radiation Fourier transform infrared microspectroscopy. Arch. Biochem. Biophys. 2007, 459, 241–248. [Google Scholar] [CrossRef]
- Sarroukh, R.; Goormaghtigh, E.; Ruysschaert, J.-M.; Raussens, V. ATR-FTIR: A “rejuvenated” tool to investigate amyloid proteins. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2328–2338. [Google Scholar] [CrossRef]
- Choo, L.-P.; Mansfield, J.R.; Pizzi, N.; Somorjai, R.L.; Jackson, M.; Halliday, W.C.; Mantsch, H.H. Infrared spectra of human central nervous system tissue: Diagnosis of alzheimer’s disease by multivariate analyses. Biospectroscopy 1995, 1, 141–148. [Google Scholar] [CrossRef]
- Maquelin, K.; Kirschner, C.; Choo-Smith, L.-P.; Ngo-Thi, N.A.; Van Vreeswijk, T.; Stämmler, M.; Endtz, H.P.; Bruining, H.A.; Naumann, D.; Puppels, G.J. Prospective study of the performance of vibrational spectroscopies for rapid identification of bacterial and fungal pathogens recovered from blood cultures. J. Clin. Microbiol. 2003, 41, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, S.T. Cellular and molecular mechanisms of neural repair after stroke: Making waves. Ann. Neurol. 2006, 59, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001, 2, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Niwa, S.; Tanaka, Y. Molecular motors in neurons: Transport mechanisms and roles in brain function, development, and disease. Neuron 2010, 68, 610–638. [Google Scholar] [CrossRef]
- Hackett, M.J.; Sylvain, N.J.; Hou, H.; Caine, S.; Alaverdashvili, M.; Pushie, M.J.; Kelly, M.E. Concurrent glycogen and lactate imaging with FTIR spectroscopy to spatially localize metabolic parameters of the glial response following brain ischemia. Anal. Chem. 2016, 88, 10949–10956. [Google Scholar] [CrossRef]
- Gazi, E.; Baker, M.; Dwyer, J.; Lockyer, N.P.; Gardner, P.; Shanks, J.H.; Reeve, R.S.; Hart, C.A.; Clarke, N.W.; Brown, M.D. A Correlation of FTIR Spectra Derived from Prostate Cancer Biopsies with Gleason Grade and Tumour Stage. Eur. Urol. 2006, 50, 750–761. [Google Scholar] [CrossRef]
- Sahu, R.; Mordechai, S. Fourier transform infrared spectroscopy in cancer detection. Futur. Oncol. 2005, 1, 635–647. [Google Scholar] [CrossRef]
- Richter, T.; Steiner, G.; Abu-Id, M.H.; Salzer, R.; Bergmann, R.; Rodig, H.; Johannsen, B. Identification of tumor tissue by FTIR spectroscopy in combination with positron emission tomography. Vib. Spectrosc. 2002, 28, 103–110. [Google Scholar] [CrossRef]
- Uzdensky, A.B. Photothrombotic Stroke as a Model of Ischemic Stroke. Transl. Stroke Res. 2018, 9, 437–451. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Yang, S.; Huang, J.; Xue, X.; Zheng, Y.; Shang, G.; Tao, J.; Chen, L. Electroacupunctre improves motor impairment via inhibition of microglia-mediated neuroinflammation in the sensorimotor cortex after ischemic stroke. Life Sci. 2016, 151, 313–322. [Google Scholar] [CrossRef]
- Sinke, M.R.T.; Otte, W.M.; van Meer, M.P.A.; van der Toorn, A.; Dijkhuizen, R.M. Modified structural network backbone in the contralesional hemisphere chronically after stroke in rat brain. J. Cereb. Blood Flow Metab. 2018, 38, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
- Grefkes, C.; Fink, G.R. Reorganization of cerebral networks after stroke: New insights from neuroimaging with connectivity approaches. Brain 2011, 134, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, G.; Miller, L.M.; Zorlu, F.; Severcan, F. Amifostine, a radioprotectant agent, protects rat brain tissue lipids against ionizing radiation induced damage: An FTIR microspectroscopic imaging study. Arch. Biochem. Biophys. 2012, 520, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Petibois, C.; Desbat, B. Clinical application of FTIR imaging: New reasons for hope. Trends Biotechnol. 2010, 28, 495–500. [Google Scholar] [CrossRef]
- Hackett, M.J.; DeSouza, M.; Caine, S.; Bewer, B.; Nichol, H.; Paterson, P.G.; Colbourne, F. A new method to image heme-Fe, total Fe, and aggregated protein levels after intracerebral hemorrhage. ACS Chem. Neurosci. 2015, 6, 761–770. [Google Scholar] [CrossRef]
- Shirley, R.; Ord, E.N.J.; Work, L.M. Oxidative stress and the use of antioxidants in stroke. Antioxidants 2014, 3, 472–501. [Google Scholar] [CrossRef]
- Cipolla, M.J.; Crete, R.; Vitullo, L.; Rix, R.D. Transcellular transport as a mechanism of blood-brain barrier disruption during stroke. Front. Biosci. 2004, 9, 777–785. [Google Scholar] [CrossRef]
- Solenski, N.J.; Dipierro, C.G.; Trimmer, P.A.; Kwan, A.-L.; Helms, G.A. Ultrastructural changes of neuronal mitochondria after transient and permanent cerebral ischemia. Stroke 2002, 33, 816–824. [Google Scholar] [CrossRef]
- Szalontai, B.; Nishiyama, Y.; Gombos, Z.; Murata, N. Membrane dynamics as seen by Fourier transform infrared spectroscopy in a cyanobacterium, Synechocystis PCC 6803—The effects of lipid unsaturation and the protein-to-lipid ratio. Biochim. Biophys. Acta Biomembr. 2000, 1509, 409–419. [Google Scholar] [CrossRef]
- Toyran, N.; Zorlu, F.; Dönmez, G.; Öǧe, K.; Severcan, F. Chronic hypoperfusion alters the content and structure of proteins and lipids of rat brain homogenates: A Fourier transform infrared spectroscopy study. Eur. Biophys. J. 2004, 33, 549–554. [Google Scholar] [CrossRef]
- Schreibelt, G.; Kooij, G.; Reijerkerk, A.; Van Doorn, R.; Gringhuis, S.I.; Van Der Pol, S.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Piontek, J.; et al. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007, 21, 3666–3676. [Google Scholar] [CrossRef] [PubMed]
- Magura, I.S.; Rozhmanova, O.M. Oxidative stress and neurodegenerative disorders. Biopolym. Cell 1997, 13, 513–515. [Google Scholar] [CrossRef]
- Emerit, J.; Edeas, M.; Bricaire, F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004, 58, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Lamba, O.P.; Borchman, D.; Garner, W.H. Spectral characterization of lipid peroxidation in rabbit lens membranes induced by hydrogen peroxide in the presence of Fe2+ Fe3+ cations: A site-specific catalyzed oxidation. Free Radic. Biol. Med. 1994, 16, 591–601. [Google Scholar] [CrossRef]
- De Zwart, L.L.; Meerman, J.H.N.; Commandeur, J.N.M.; Vermeulen, N.P.E. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic. Biol. Med. 1999, 26, 202–226. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J.F. Altered Lipid Metabolism in Brain Injury and Disorders. In Lipids in Health and Disease; Springer: Dordrecht, The Netherlands, 2008; pp. 241–268. [Google Scholar]
- Romont, C.; Marie, C.; Bralet, J. Increased lipid peroxidation in vulnerable brain regions after transient forebrain ischemia in rats. Stroke 1989, 20, 918–924. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Slater, T.F. Free-radical mechanisms in tissue injury. Biochem. J. 1984, 222, 1–15. [Google Scholar] [CrossRef]
- Riley, P. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Boil. 1994, 65, 27–33. [Google Scholar] [CrossRef]
- Spitz, D.R.; Azzam, E.I.; Li, J.J.; Gius, D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev. 2004, 23, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Sowa, M.G.; Mantsch, H.H. Infrared spectroscopy: A new frontier in medicine. Biophys. Chem. 1997, 68, 109–125. [Google Scholar] [CrossRef]
- Xing, C.; Arai, K.; Lo, E.H.; Hommel, M. Pathophysiologic cascades in ischemic stroke. Int. J. Stroke 2012, 7, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar] [CrossRef]
- Sen, N.; Hara, M.R.; Ahmad, A.S.; Cascio, M.B.; Kamiya, A.; Ehmsen, J.T.; Aggrawal, N.; Hester, L.; Doré, S.; Snyder, S.H.; et al. GOSPEL: A neuroprotective protein that binds to GAPDH upon S-Nitrosylation. Neuron 2009, 63, 709. [Google Scholar] [CrossRef]
- Squitti, R.; Siotto, M.; Assenza, G.; Giannantoni, N.M.; Rongioletti, M.; Zappasodi, F.; Tecchio, F. Prognostic value of serum copper for post-stroke clinical recovery: A pilot study. Front. Neurol. 2018, 9, 333. [Google Scholar] [CrossRef]
- DeGregorio-Rocasolano, N.; Martí-Sistac, O.; Ponce, J.; Castelló-Ruiz, M.; Millán, M.; Guirao, V.; García-Yébenes, I.; Salom, J.B.; Ramos-Cabrer, P.; Alborch, E.; et al. Iron-loaded transferrin (Tf) is detrimental whereas iron-free Tf confers protection against brain ischemia by modifying blood Tf saturation and subsequent neuronal damage. Redox Biol. 2018, 15, 143–158. [Google Scholar] [CrossRef]
- Qin, A.-P.; Zhang, H.-L.; Qin, Z.-H. Mechanisms of lysosomal proteases participating in cerebral ischemia-induced neuronal death. Neurosci. Bull. 2008, 24, 117–123. [Google Scholar] [CrossRef][Green Version]
- Ozek, N.S.; Tuna, S.; Erson-Bensan, A.E.; Severcan, F. Characterization of microRNA-125b expression in MCF7 breast cancer cells by ATR-FTIR spectroscopy. Analyst 2010, 135, 3094–3102. [Google Scholar] [CrossRef]
- Wang, Q.; Kretlow, A.; Beekes, M.; Naumann, D.; Miller, L. In situ characterization of prion protein structure and metal accumulation in scrapie-infected cells by synchrotron infrared and X-ray imaging. Vib. Spectrosc. 2005, 38, 61–69. [Google Scholar] [CrossRef]


| Infrared Band Assignment | Spectral Range (cm−1) | Comments | |
|---|---|---|---|
| Amide I + II | 1700–1500 | Total protein region | |
| Protein components | Amide I | 1700–1600 | Specifically sensitive to protein secondary structure |
| Amide II | 1555–1535 | Mainly ν(C–N) associated with proteins | |
| β-sheet | 1610–1635 | Functional protein, protein secondary structure | |
| α-helix | 1660–1650 | Functional protein, protein secondary structure | |
| Random coil | 1645–1630 | protein secondary structure | |
| C-H region | 3100–2800 | Total lipid region | |
| Lipid component | νsCH2 | 2852–2800 | to measure lipid acyl chain length |
| νasCH2 | 2915–2930 | ||
| νasCH3 | 2950–2960 | to measure concentration of methyl content | |
| ν (C = O) | 1755–1715 | to measure oxidative stress | |
| ν (olefinic = CH) | 3000–3027 | to measure unsaturated lipid |
| ROI | Total Lipid | Total Protein | Lipid/Protein | CH2/Lipid | CH3/Lipid | Olefin/Lipid | Lipid Ester/Lipid | |
|---|---|---|---|---|---|---|---|---|
| Contra-lesional | GM | 0.69 ± 0.022 * | 14.881 ± 0.043 * | 5.887 ± 0.0013 ** | 0.233 ± 0.0001 ** | 0.165 ± 0.0003 ** | 0.0032 ± 0.0003 ** | 0.063 ± 0.0051 ** |
| WM | 1.39 ± 0.072 * | 9.061 ± 0.0014 * | 6.991 ± 0.0013 ** | 0.341 ± 0.0002 ** | 0.281 ± 0.0002 ** | 0.0045 ± 0.0003 ** | 0.086 ± 0.0031 ** | |
| Ipsi-lesional | PIZ | 0.59 ± 0.032 ** | 11.003 ± 0.012 ** | 5.256 ± 0.0033 ** | 0.194 ± 0.0002 ** | 0.394 ± 0.0003 ** | 0.0052 ± 0.0005 ** | 0.057 ± 0.0062 ** |
| WM | 0.93 ± 0.052 ** | 8.995 ± 0.0014 ** | 6.367 ± 0.0013 ** | 0.125 ± 0.0001 ** | 0.399 ± 0.0006 ** | 0.0062 ± 0.0003 ** | 0.067 ± 0.0033 ** |
| Location of Brain | Contra-Lesional GM | Ipsi-Lesional PIZ | |
|---|---|---|---|
| Ratios | |||
| α-helix/Amide I | 51% * | 32% * | |
| β-sheet/Amide I | 37% * | 66% * | |
| Random coil/Amide I | 12% * | 10% * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakib, F.; Ali, C.M.; Yousuf, M.; Afifi, M.; Bhatt, P.R.; Ullah, E.; Al-Saad, K.; Ali, M.H.M. Investigation of Biochemical Alterations in Ischemic Stroke Using Fourier Transform Infrared Imaging Spectroscopy—A Preliminary Study. Brain Sci. 2019, 9, 293. https://doi.org/10.3390/brainsci9110293
Rakib F, Ali CM, Yousuf M, Afifi M, Bhatt PR, Ullah E, Al-Saad K, Ali MHM. Investigation of Biochemical Alterations in Ischemic Stroke Using Fourier Transform Infrared Imaging Spectroscopy—A Preliminary Study. Brain Sciences. 2019; 9(11):293. https://doi.org/10.3390/brainsci9110293
Chicago/Turabian StyleRakib, Fazle, Carmen M. Ali, Mohammed Yousuf, Mohammed Afifi, Pooja R. Bhatt, Ehsan Ullah, Khalid Al-Saad, and Mohamed H. M. Ali. 2019. "Investigation of Biochemical Alterations in Ischemic Stroke Using Fourier Transform Infrared Imaging Spectroscopy—A Preliminary Study" Brain Sciences 9, no. 11: 293. https://doi.org/10.3390/brainsci9110293
APA StyleRakib, F., Ali, C. M., Yousuf, M., Afifi, M., Bhatt, P. R., Ullah, E., Al-Saad, K., & Ali, M. H. M. (2019). Investigation of Biochemical Alterations in Ischemic Stroke Using Fourier Transform Infrared Imaging Spectroscopy—A Preliminary Study. Brain Sciences, 9(11), 293. https://doi.org/10.3390/brainsci9110293






