Abstract
Background: The coexistence of extracranial arterial stenoses and intracranial aneurysms presents a unique clinical dilemma. While staged interventions are traditionally preferred to reduce procedural risks, recent advances have enabled single-stage endovascular treatment. This study evaluates the clinical outcomes, procedural strategies, and predictive factors associated with such combined interventions. Methods: This retrospective study included 47 patients treated with single-stage endovascular procedures for concurrent extracranial stenosis and intracranial aneurysm between 2016 and 2024. Clinical, angiographic, and procedural data were collected. Outcomes were assessed using the mmodified Rankin Scale (mRS), and statistical analyses were performed to identify associations between clinical variables and functional outcomes. Results: Of the 47 patients, 85.1% achieved favorable outcomes (mRS 0–2) at ≥6-month follow-up. The most commonly treated arteries were the internal carotid artery (70.2%) and the middle cerebral artery (34%). Stent-assisted coiling or flow diversion was performed in 93.6% of aneurysm cases, while 91.5% underwent carotid or vertebral stenting. Lesion laterality (left-sided aneurysms, p = 0.019) and stenosis length (p = 0.0469) were significantly associated with outcomes. Smoking was linked to multiple stenoses (p = 0.0191). Two patients experienced major complications: one aneurysmal rebleed after stenting, and one intraoperative rupture. Conclusions: Single-stage endovascular treatment for patients with concurrent extracranial stenosis and intracranial aneurysm is technically feasible and clinically effective in selected cases. Lesion configuration, anatomical considerations, and individualized planning are critical in optimizing outcomes.
1. Introduction
The coexistence of extracranial arterial stenosis and intracranial aneurysm presents a complex therapeutic challenge. Historically, these lesions have been addressed separately, typically beginning with carotid endarterectomy or stenting, followed by delayed aneurysm treatment. However, this sequential approach can result in delays, increased procedural burden, and the risk of aneurysm rupture due to hemodynamic changes following revascularization [1,2,3,4,5].
In the era of modern endovascular techniques, single-stage interventions have emerged as a feasible and potentially safer option for selected patients. This strategy not only minimizes anesthesia exposure and hospital stay but also offers a streamlined solution for resource-limited settings, where repeated access procedures are economically burdensome [6,7,8,9].
Despite the increasing application of this approach, there is limited literature addressing patient selection, procedural sequencing, and outcome predictors in such cases. Concerns remain regarding the optimal order of intervention—whether to treat the stenosis first to improve access and reduce ischemic risk, or to secure the aneurysm first to prevent hemorrhagic complications exacerbated by reperfusion [10,11,12,13]. Additionally, few studies systematically evaluate the influence of anatomical configuration (ipsilateral vs. contralateral lesions), lesion length, or hemodynamic factors on functional recovery.
This study aims to assess the safety, feasibility, and clinical outcomes of single-stage endovascular treatment in patients with coexisting extracranial stenosis and intracranial aneurysms. We also propose a treatment algorithm based on real-world decision making and lesion-specific characteristics, contributing practical insights for individualized care planning.
2. Materials and Methods
This retrospective study was conducted at the National Hospital of the Medical Center of the Presidential Affairs Administration of the Republic of Kazakhstan in Almaty, Kazakhstan, and included patients treated between January 2016 and December 2024. Eligible patients had coexisting intracranial aneurysms and extracranial arterial stenoses (internal carotid artery [ICA] or vertebral artery [VA]) and underwent single-stage endovascular treatment.
2.1. Inclusion Criteria Were
- •
- Age ≥ 18 years.
- •
- Confirmed ≥70% extracranial ICA or VA stenosis based on digital subtraction angiography (DSA), CTA, or MRA, evaluated using NASCET criteria.
- •
- At least one saccular or fusiform intracranial aneurysm identified via CTA or DSA.
- •
- Either symptomatic ischemia (e.g., TIA, infarct on DWI) or aneurysm-related symptoms (e.g., headache, SAH).
- •
- Available pre- and post-procedural imaging and clinical records.
Patients with previous intracranial or extracranial interventions, contraindications to dual antiplatelet therapy, or incomplete follow-up were excluded.
2.2. Imaging and Decision Protocol
Pre-treatment imaging included brain MRI with DWI to assess ischemic burden and CTA or DSA to evaluate aneurysm morphology, perfusion patterns, and vessel patency. The decision to treat both lesions in one session was made by a multidisciplinary neurovascular team. In patients with contralateral lesions or ruptured aneurysms, treatment sequencing was individualized. When perfusion through the stenotic segment was severely compromised, revascularization was prioritized. In other cases, aneurysm embolization preceded stenting to minimize rupture risk.
2.3. Procedure
The protocol has remained consistent since 2016; slight updates were implemented in 2020 after the introduction of newer stents. Endovascular procedures were performed under general or local anesthesia using transfemoral access. Aneurysms were treated via coiling, stent-assisted coiling, or flow diversion. Arterial stenoses were treated with balloon angioplasty and/or stenting. In all ICA cases, distal embolic protection devices were employed. Device selection was based on anatomical characteristics and operator preference.
2.4. Periprocedural Management
All patients without subarachnoid hemorrhage (SAH) received dual antiplatelet therapy (aspirin and clopidogrel) at least 5 days before the procedure. In SAH cases, DAPT initiation was delayed or minimized based on hemorrhage severity and urgency of intervention. Intravenous heparin was administered intraoperatively. Postprocedural antiplatelet regimens were adjusted per stent type and patient risk profile.
2.5. Data Collection and Outcomes
Demographic, clinical, radiological, and procedural data were retrospectively collected from electronic health records and imaging archives. Variables included age, sex, comorbidities (hypertension, diabetes, ischemic heart disease, smoking status), aneurysm characteristics (location, size, rupture status), and stenosis features (side, degree, length, symptomatic status).
Procedural details such as stent type, access route, and sequence of interventions were also documented. Modified Rankin Scale (mRS) scores were assessed at baseline and at follow-up by an independent neurologist. The PHASES score was used for aneurysm risk stratification. The primary outcome was a favorable functional outcome at last follow-up, defined as mRS 0–2. Secondary outcomes included periprocedural complications, 30-day mortality, and 30-day re-admission. Complications were categorized as hemorrhagic, ischemic, or technical.
2.6. Statistical Analysis
Descriptive statistics were used for baseline data. Associations between clinical variables and multiple stenoses or aneurysms were assessed using Fisher’s exact test. Logistic regression was employed to evaluate the association between smoking and multiple stenoses. The relationship between anatomical factors (e.g., lesion laterality, stenosis length) and functional outcome (mRS 1–2 vs. mRS 3–6) was also tested. A p-value < 0.05 was considered statistically significant. Analyses were performed using SPSS v26 (IBM Corp., Armonk, NY, USA). Regression analysis was used for exploratory purposes; findings should be interpreted cautiously due to small sample size.
2.7. Ethics Statement
This study was approved by the Institutional Ethics Committee (approval #7, dated 12 December 2024). Patient data were anonymized in compliance with the Declaration of Helsinki.
3. Results
A total of 47 patients (mean age: 67.6 ± 5.9 years; 21 males [44.7%], 26 females [55.3%]) were included. Most patients (95.7%) underwent elective procedures; only two patients (4.3%) presented with ruptured aneurysms requiring urgent intervention. Common presenting symptoms included headache (38.3%), ischemic stroke-related deficits (44.7%), and sensorineural symptoms such as hearing loss (4.3%). One patient presented with Millard–Gubler syndrome.
3.1. Patient Characteristics and Vascular Lesion Profile
In total, 17 patients (36.2%) were active smokers, 11 (23.4%) had diabetes mellitus, and 38 (80.9%) had stage 3 arterial hypertension. Ischemic heart disease was present in 34 patients (72.3%). Aneurysms were most frequently located in the middle cerebral artery (MCA), anterior cerebral artery (ACA), and intracranial ICA segments. The majority of stenoses involved the cervical ICA (57.4%) and vertebral artery (23.4%). Ipsilateral aneurysm–stenosis pairs were observed in 29 patients (61.7%). Patient characteristics can be seen in Table 1.

Table 1.
Patient Characteristics and Treatment Details for Concurrent Intracranial Aneurysms and Arterial Stenoses.
3.2. Risk Factors and Lesion Characteristics
Smoking was significantly associated with the presence of multiple stenoses (75.0% in smokers vs. 25.0% in non-smokers; p = 0.0191, OR = 0.131; 95% CI: 0.023–0.750). Logistic regression confirmed this association, with an odds ratio (OR) of 0.131 (95% CI: 0.023–0.750), indicating that non-smokers were significantly less likely to have multiple stenotic lesions. Other vascular risk factors, including hypertension, diabetes, ischemic heart disease, and obesity, showed no significant association with either multiple stenoses or multiple aneurysms (p > 0.05 for all; Table 2).

Table 2.
Association between clinical factors and occurrence of multiple stenoses and multiple aneurysms.
3.3. Treatment Strategy
The procedural strategy was tailored based on anatomical configuration and clinical presentation. Most cases involved flow diversion or stent-assisted coiling for aneurysm treatment, followed by angioplasty and carotid or vertebral artery stenting. In cases with high-grade ICA stenosis impeding microcatheter navigation, stenting was performed first. In morphologically unstable aneurysms (e.g., wide-neck or high-flow lesions), embolization was prioritized. Dual antiplatelet therapy was used in all elective cases pre- and post-procedurally. No elective patient experienced a deterioration in clinical status postoperatively.
Common devices included Protege (n = 17), ULTIMASTER (n = 8), and CASPER (n = 2). No ischemic periprocedural events were recorded.
3.4. Functional Outcomes and Predictive Factors
At the final follow-up (≥6 months), favorable outcomes (defined as mRS 0–2) were observed in 40 of 47 patients (85.1%). Modified Rankin Scale scores remained stable across the follow-up period for all patients, with no evidence of neurological deterioration. Among the cohort, seven patients (14.9%) had mRS scores of 3–5, indicating moderate to severe disability. No patient experienced mortality or re-admission within 30 days post-procedure.
Notably, left-sided aneurysms were significantly associated with favorable outcomes; all 14 patients with left-sided lesions achieved mRS scores of 1–2 (p = 0.019). However, no significant associations were found between outcome and aneurysm-bearing artery (p = 0.0761), side of extracranial stenosis (p = 0.7017), type of stenotic artery (p = 0.9497), or ipsilateral versus contralateral lesion configuration (p = 0.3108) (Table 3).

Table 3.
Relationship between aneurysm/stenosis localization and mRS outcome.
Among the anatomical predictors, stenosis length showed a statistically significant association with functional outcome (p = 0.0469), with longer stenotic segments correlating with worse mRS scores. In contrast, neither aneurysm size (p = 0.4291) nor stenosis severity as measured by percent narrowing (p = 0.2588) demonstrated predictive value for clinical outcome (Table 4).

Table 4.
Impact of aneurysm size, stenosis length, and degree on mRS outcome.
These findings underscore the importance of lesion morphology—particularly stenosis length—over traditional size-based metrics in predicting recovery. The observed left-side laterality advantage warrants further investigation, potentially reflecting procedural or anatomical factors that influence outcome.
3.5. Complications
Two notable complications were recorded. One patient with a ruptured MCA aneurysm and an ipsilateral critical ICA stenosis developed a subarachnoid hemorrhage after stenting, requiring external ventricular drainage and a decompressive craniectomy; the mRS at 6 months was 5. Another patient experienced an intraoperative aneurysm rupture during coiling (non-ipsilateral), which was managed with intra-arterial nimodipine due to catheter-induced vasospasm; the patient had an mRS of 4 at 4 months.
3.6. Case Examples
Case 1: Simultaneous Treatment of Left MCA Aneurysm and Vertebral Artery Stenosis.
Patient #13 was a 38-year-old male presenting with recurrent occipital headaches, tinnitus, visual disturbances, and transient ischemic attacks. Imaging revealed a saccular aneurysm at the left middle cerebral artery (MCA) bifurcation and an 80% stenosis of the V1 segment of the right vertebral artery. Comorbidities included grade 3 arterial hypertension, impaired glucose tolerance, and grade 1 obesity. A single-stage endovascular procedure was performed. The aneurysm was occluded using balloon-assisted coiling with a LEO stent (2.5 × 18 mm) via the C-stenting technique. In the same session, vertebral artery stenosis was treated with an Ultimaster stent (3.5 × 12 mm). The postoperative course was uneventful, and the patient achieved an mRS of 1 at six months (Figure 1).
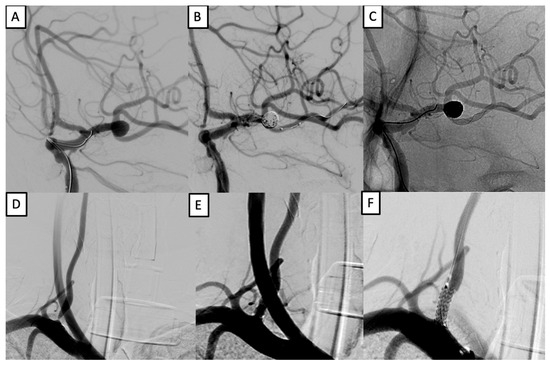
Figure 1.
Simultaneous treatment of left MCA aneurysm and right vertebral artery stenosis. (A–C): balloon-assisted coiling with LEO stent (2.5 × 18 mm) of a left MCA bifurcation aneurysm. (D–F): stenting of right vertebral artery V1 segment using an Ultimaster 3.5 × 12 mm stent.
Case 2: Multivessel Stenosis and Dual Aneurysm Treatment Following SAH.
Patient #12 was a 57-year-old male with a history of smoking and hypertension who initially presented with a subarachnoid hemorrhage (SAH) and a ruptured left ICA aneurysm, treated with coiling. Follow-up imaging showed incomplete occlusion, and additional saccular aneurysms at the left MCA bifurcation and cervical ICA subocclusion were identified.
In a single-stage intervention, balloon angioplasty and stenting of the left cervical ICA were performed using a CASPER 9 × 20 × 143 mm stent, preceded by embolic protection and balloon pre-dilation. Subsequently, the MCA and ICA aneurysms were treated with stent-assisted coiling using a LEO stent (2.5 × 18 mm) and the half-T technique. The final angiography showed successful aneurysm exclusion and vessel patency. At the six-month follow-up, the patient had an mRS of 2 (Figure 2).
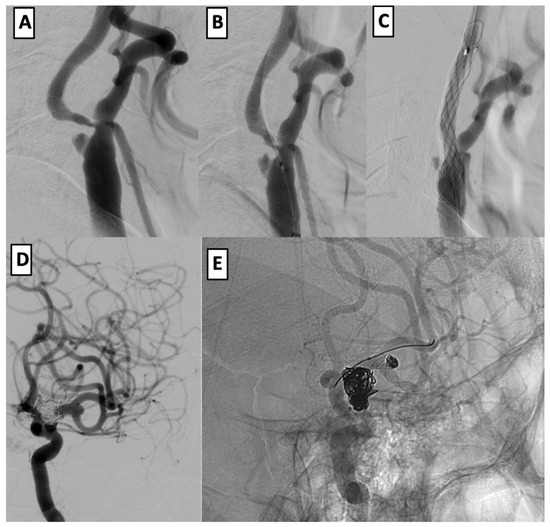
Figure 2.
Endovascular management of dual intracranial aneurysms with cervical ICA stenosis. (A–C): angioplasty and CASPER stenting of the left cervical ICA after subocclusion and embolic protection. (D,E): coil embolization of left MCA and ICA aneurysms using half-T stenting technique with LEO stent (2.5 × 18 mm).
4. Discussion
This study adds to the growing body of the literature supporting the feasibility and safety of single-stage endovascular treatment for patients with coexisting intracranial aneurysms and extracranial arterial stenoses [2,4,7]. In the era of precision medicine, such combined interventions align with the shift toward individualized care that balances procedural efficiency with patient-specific anatomical and hemodynamic considerations [5,6].
All patients were followed for a uniform period of 6 months, capturing intermediate-term recovery and early complications such as in-stent thrombosis or aneurysm recurrence. While longer-term surveillance is needed to evaluate durability, this timeframe offers a reliable snapshot of initial outcomes.
In our cohort, smoking was the only factor significantly associated with the presence of multiple stenoses (p = 0.0191), reinforcing its role as a systemic atherosclerotic risk factor [8]. This finding aligns with the established literature on smoking’s adverse effects on endothelial integrity and vascular remodeling [9]. Other risk factors such as hypertension or ischemic heart disease were not independently predictive, likely reflecting sample size limitations.
Based on our findings and the prior literature, we propose a decision-making algorithm for single-stage intervention, which prioritizes ipsilaterality, symptomatology, lesion size, and anatomical feasibility (Figure 3).
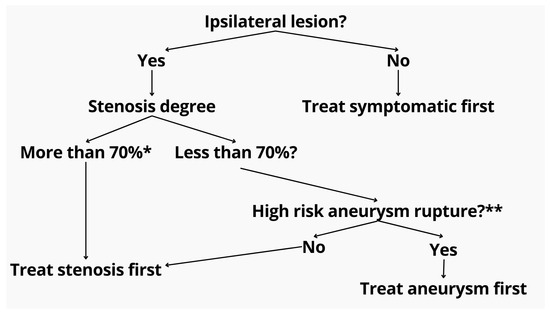
Figure 3.
Proposed treatment algorithm for patients with coexisting extracranial stenosis and intracranial aneurysm. The initial step evaluates lesion ipsilaterality. If non-ipsilateral, the symptomatic pathology is prioritized. For ipsilateral lesions, the degree of stenosis is assessed. Stenoses exceeding 70% (as measured by NASCET criteria *) are treated first to ensure safe access and perfusion. If the stenosis is <70%, aneurysm rupture risk is evaluated using the PHASES score **. High-risk aneurysms are prioritized for embolization, while low-risk cases proceed with stenosis treatment. * North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria. ** Population, hypertension, age, size of aneurysm, earlier SAH, site of aneurysm (PHASES) score.
Our proposed algorithm (Figure 3) also incorporates real-world clinical reasoning suggested by experienced practitioners. Specifically, for symptomatic or >70% asymptomatic stenosis with an unruptured aneurysm—regardless of laterality—ICA stenting is prioritized before aneurysm embolization. The rationale includes the following: (1) reducing hypoperfusion risk during aneurysm navigation, (2) enabling stable catheter positioning distal to the stenosis, and (3) minimizing financial burden in low-resource settings by consolidating procedures and reducing repeated device use. Moreover, treating the aneurysm after stenting may mitigate the risk of pressure-related rupture, also known as normal perfusion breakthrough syndrome. In contrast, in cases of ruptured aneurysm with coexisting symptomatic or severe asymptomatic stenosis, the approach shifts toward initial angioplasty of the ICA to facilitate safe access, followed by aneurysm coiling. If a stent is required for the aneurysm, the ICA stenosis can be addressed concurrently. This sequence minimizes procedural delay in hemorrhagic cases while still preserving cerebral perfusion and catheter stability.
All patients were followed for a uniform period of six months, capturing intermediate-term recovery and procedural complications such as in-stent thrombosis or aneurysm recurrence. Although no patients in our cohort experienced these complications, we have contextualized this in the discussion by referencing the most commonly reported complications—groin hematoma, in-stent thrombosis, and distal embolization—and their incidence rates in the general population. The absence of these events in our group highlights the potential safety of the single-stage strategy, though the relatively small sample limits generalizability.
Balancing ischemic and hemorrhagic risk in these patients remains challenging. Historically, staged approaches like carotid endarterectomy followed by delayed aneurysm embolization were preferred [4,5,7], but they entail logistical and procedural drawbacks. Recent reports suggest that carefully selected patients may benefit from simultaneous interventions [2,8,9].
Case reports highlight the danger of treating carotid stenosis before securing aneurysms. Hartmann et al. [1] described a fatal post-stenting hemorrhage, while Pappada et al. [2] and Adams [5] emphasized the complexity of sequential decisions. Our study supports that, with structured planning, concurrent treatment is not only possible but safe.
Badruddin et al. [8] and Gallego Leon et al. [9] reported strong outcomes in similar cohorts. Our complication rate was low, and factors such as lesion laterality and stenosis length—rather than aneurysm size—were more closely associated with outcome.
We encountered two major complications: aneurysmal rebleeding post-stenting requiring decompressive craniectomy, and intraoperative rupture treated with nimodipine. These underscore the importance of risk stratification, especially in ruptured cases. Hartmann et al. [1] reinforced the theoretical risk of rebleed post-revascularization, validating strategies like coiling first or strict hemodynamic control.
The observed correlation between left-sided aneurysms and favorable outcome was unexpected. Given the complexity of left carotid access and symptomatic burden of left hemispheric strokes [10], this may reflect anatomical variance in our cohort.
These findings suggest that individualized anatomy-driven strategies—potentially aided by AI-based imaging—can enhance procedural outcomes. The lack of correlation between mRS and aneurysm size or stenosis degree suggests that vascular morphology may carry more prognostic value than previously assumed.
Procedurally, our series included patients with ipsilateral, contralateral, symptomatic, and ruptured lesions—all treated in one session. This broad applicability supports single-stage endovascular intervention as a viable alternative to staged approaches in experienced centers [2,4,7,11].
In most cases, CAS was performed first to secure proximal access and reduce embolic load during aneurysm navigation [2,5,12]. When feasible, aneurysm coiling preceded stenting in morphologically unstable lesions. Such flexibility, guided by real-time angiography and preoperative imaging, reflects evolving neurointerventional strategy [6,13].
One high-risk case involved an SAH patient with critical ICA stenosis and impaired perfusion, treated emergently with stenting before embolization. Despite concerns over dual antiplatelet therapy (DAPT) in hemorrhagic states, this decision prioritized cerebral perfusion. Emerging data support cautious DAPT use in such settings [14,15], though we remain conservative and defer stenting when possible in acute SAH.
Limitations
This study has several limitations that must be acknowledged. First, the retrospective and single-center design introduces inherent risks of selection bias and limits the generalizability of the findings. Although our patient population reflects real-world clinical complexity, the absence of randomization restricts the ability to draw causal inferences regarding the superiority of single-stage intervention over staged or conservative approaches.
Second, the relatively small sample size (n = 47) constrains the statistical power, particularly in subgroup analyses such as aneurysm location or lesion-specific characteristics. Larger multicenter cohorts would provide more robust evidence to validate the identified associations—such as the impact of stenosis length and aneurysm laterality on functional outcome.
Third, the follow-up duration, with a minimum of three months, may not be sufficient to capture late complications such as in-stent restenosis, delayed aneurysm recurrence, or long-term neurocognitive deficits. A longer follow-up period is essential for assessing the durability and neurological sequelae of the treatment.
Fourth, while the modified Rankin Scale (mRS) is a widely accepted outcome measure, it may not adequately reflect subtle cognitive or functional impairments, particularly in patients with anterior circulation lesions. Future research should incorporate more detailed neuropsychological and quality-of-life assessments.
Finally, the treatment of multiple aneurysms in a single session alongside extracranial stenosis represents a complex and underexplored subgroup. We plan to collect more cases and conduct focused analysis to better understand outcomes in this patient population.
Despite these limitations, our study offers valuable insights into the evolving paradigm of precision-guided single-session endovascular therapy in complex cerebrovascular disease.
5. Conclusions
This study supports the feasibility and safety of single-stage endovascular treatment in patients with concurrent intracranial aneurysms and extracranial arterial stenoses. Our findings suggest that lesion-specific factors—particularly aneurysm laterality and stenosis length—may influence functional outcomes more significantly than aneurysm size or stenosis degree. Smoking emerged as a strong independent predictor of multiple stenoses, underscoring the importance of modifiable risk factor management in cerebrovascular patients.
Future prospective multicenter studies with longer follow-up and expanded functional outcome measures are warranted to refine treatment algorithms and establish evidence-based protocols for this complex patient population.
Author Contributions
Conceptualization, M.S. and M.B.; methodology, D.D.; software, S.M.; validation, R.B.P., A.Z., and M.B.; formal analysis, D.D.; investigation, A.M.; resources, M.B.; data curation, S.M.; writing—original draft preparation, A.M.; writing—review and editing, R.B.P.; visualization, A.Z.; supervision, M.B.; project administration, M.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of National Hospital of the Medical Center of the Presidential Affairs Administration of the Republic of Kazakhstan (approval #7, dated 12 December 2024).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request. Due to patient privacy regulations and institutional policy, individual-level data cannot be publicly shared but may be provided in anonymized form for academic and research purposes.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACA | Anterior Cerebral Artery |
| Acom | Anterior Communicating Artery |
| BA | Basilar Artery |
| CAS | Carotid Artery Stenting |
| CTA | Computed Tomography Angiography |
| DAPT | Dual Antiplatelet Therapy |
| DSA | Digital Subtraction Angiography |
| DWI | Diffusion-Weighted Imaging |
| ICA | Internal Carotid Artery |
| IDH | Isocitrate Dehydrogenase |
| IHD | Ischemic Heart Disease |
| MCA | Middle Cerebral Artery |
| mRS | Modified Rankin Scale |
| MRI | Magnetic Resonance Imaging |
| SAH | Subarachnoid Hemorrhage |
| TIA | Transient Ischemic Attack |
| VA | Vertebral Artery |
References
- Hartmann, M.; Weber, R.; Zoubaa, S.; Schranz, C.; Knauth, M. Fatal subarachnoid hemorrhage after carotid stenting. J Neuroradiol. 2004, 31, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Pappada, G.; Fiori, L.; Marina, R.; Vaiani, S.; Gaini, S.M. Management of symptomatic carotid stenoses with coincidental intracranial aneurysms. Acta Neurochir. 1996, 138, 1386–1390. [Google Scholar] [CrossRef] [PubMed]
- Kappelle, L.; Eliasziw, M.; Fox, A.J.; Barnett, H.J. Small, unruptured intracranial aneurysms and management of symptomatic carotid artery stenosis: North American Symptomatic Carotid Endarterectomy Trial Group. Neurology 2000, 55, 307–309. [Google Scholar] [PubMed]
- Ladowski, J.S.; Webster, M.W.; Yonas, H.O.; Steed, D.L. Carotid endarterectomy in patients with asymptomatic intracranial aneurysm. Ann. Surg. 1984, 200, 70–73. [Google Scholar] [PubMed]
- Adams, H.P., Jr. Carotid stenosis and coexisting ipsilateral intracranial aneurysm: A problem in management. Arch. Neurol. 1977, 34, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Kaçar, E.; Nas, Ö.F.; Erdoğan, C.; Hakyemez, B. Single-stage endovascular treatment in patients with severe extracranial large vessel stenosis and concomitant ipsilateral unruptured intracranial aneurysm. Diagn. Interv. Radiol. 2015, 21, 476–482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iwata, T.; Mori, T.; Tajiri, H. Successful staged endovascular treatment of a symptomatic cervical bifurcation stenosis coupled with a coincidental unruptured cerebral aneurysm in the carotid distal segment. AJNR Am. J. Neuroradiol. 2008, 29, 1948–1950. [Google Scholar] [CrossRef] [PubMed]
- Badruddin, A.; Teleb, M.S.; Abraham, M.G.; Taqi, M.A.; Zaidat, O.O. Safety and feasibility of simultaneous ipsilateral proximal carotid artery stenting and cerebral aneurysm coiling. Front. Neurol. 2010, 1, 120. [Google Scholar] [CrossRef] [PubMed]
- Gallego León, J.I.; Concepción Aramendía, L.; Ballenilla Marco, F.; Vázquez Suárez, J.C. Concomitant endovascular treatment of concomitant extracranial carotid stenosis and intracranial aneurysm. Our experience. Interv. Neuroradiol. 2009, 15, 53–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ACAS Trial Investigators. Endarterectomy for asymptomatic carotid artery stenosis: Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995, 273, 1421–1428. [Google Scholar] [CrossRef]
- Campos, J.K.; Lin, L.M.; Beaty, N.B.; Bender, M.T.; Jiang, B.; Zarrin, D.A.; Coon, A.L. Tandem cervical carotid stenting for stenosis with flow diversion embolisation for the treatment of intracranial aneurysms. Stroke Vasc. Neurol. 2019, 4, e000187. [Google Scholar] [CrossRef]
- Park, J.C.; Kwon, B.J.; Kang, H.S.; Kim, J.E.; Kim, K.M.; Cho, Y.D.; Han, M.H. Single-stage extracranial carotid artery stenting and intracranial aneurysm coiling: Technical feasibility and clinical outcome. Interv. Neuroradiol. 2013, 19, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Laurent, D.; Lucke-Wold, B.; Leary, O.; Randall, M.H.; Porche, K.; Koch, M.; Chalouhi, N.; Polifka, A.; Hoh, B.L. The evolution of endovascular therapy for intracranial aneurysms: Historical perspective and next frontiers. Neurosci. Insights 2022, 17, 26331055221117560. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lee, C.; Dhillon, P.S.; Kirollos, R.; Nga, V.D.W.; Yeo, T.T.; Henkes, H.; Arthur, A.S.; Yeo, L.L.L.; Bhogal, P. Antiplatelet therapy in aneurysmal subarachnoid hemorrhage: An updated meta-analysis. Neurosurg. Rev. 2023, 46, 221. [Google Scholar] [CrossRef] [PubMed]
- Qoorchi Moheb Seraj, F.; Mirbolouk, M.H.; Vaezi, M. Safety of dual antiplatelet therapy in the acute phase of aneurysmal subarachnoid. Focus 2023, 55, E10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).