Abstract
Background/Objectives: Duchenne muscular dystrophy (DMD) is often discussed in the literature with regard to physical impairments. This narrative review aims to show that living with DMD involves psychological, psychosocial, and cognitive aspects in addition to the well-known physical complications. Methods: Firstly, this review examines the main cognitive functions affecting subjects with DMD and the possible role of dystrophin gene mutations on the central nervous system. Secondly, it analyzes the comorbidity between DMD, neurodevelopmental disorders (autism spectrum disorders, attention-deficit/hyperactivity disorder, obsessive–compulsive disorder) and psychopathological traits (anxiety and/or depressive symptoms). Finally, the review addresses the relatively sparse literature investigating the positive aspects associated with the experience of DMD, like psychosocial resources, resilience, subjective well-being, positive individual and social functioning, and social support. Results: DMD has a significant impact on cognitive areas, probably due to dystrophin deficiency in the brain. The prevalence of neurodevelopmental comorbidities and psychopathological symptoms is also higher in people with DMD than in the general population. Despite these challenges, emerging studies highlight the role of psychosocial and environmental resources, including resilience and supportive social relations, in promoting a good quality of life and successful adaptation to disease progression. Conclusions: Early recognition of the above difficulties and strengths could ensure better care and promote an overall better quality of life for people with DMD and their families, physically, psychologically, and socially. Preclinical and clinical research is moving in the direction of finding new therapies, treatments, and psychosocial interventions to pursue these goals.
1. Introduction
Duchenne muscular dystrophy (DMD) is a progressive, X-linked inherited disease caused by mutations in the dystrophin gene. These mutations lead to the absence or deficiency of the dystrophin protein, which is responsible for maintaining the integrity of muscle cells, resulting in the continuous degeneration of muscle fibers. It is characterized by progressive muscular weakness, cardiomyopathy, and respiratory failure. DMD has an estimated worldwide incidence of 1/3300 births, affecting males and, in rare cases, females, who usually exhibit a milder phenotype. The earliest symptoms, presenting at around 2–3 years of age, are usually difficulties with maintaining balance, climbing stairs, a waddling gait, and frequent falls. Most people with DMD become wheelchair-dependent at around 10–12 years of age and need assisted ventilation at around 20 years of age []. Due to advances in medical care, including improved respiratory and cardiac management and steroid therapy, survival rates have increased, and many individuals with DMD now live into their 30s or beyond [,,,]. These improvements in care present new demands. On the one hand, there is the management of typical adulthood issues, such as independence, leaving the family of origin and the management of new health problems (physical and psychological); on the other hand, there is also the necessary acceptance of the fact that, despite the progress in therapeutic research, at the moment, the majority of elderly individuals with DMD (or simply those confined to wheelchairs) do not have access or have very few possibilities of accessing experimental therapies or innovative treatments. As previously defined, DMD is caused by mutations in the DMD gene, a huge gene containing 79 exons and 8 promoters that is expressed in skeletal, cardiac, smooth muscle tissues and also in the human central nervous system. In the central nervous system, the full-length dystrophin isoform is expressed (Dp427). The dystrophin gene has at least four internal promoters, giving rise to shorter dystrophin products (Dp260, Dp140, Dp116, and Dp71) [,]. DMD gene mutations result in a loss of the full-length dystrophin protein (Dp427). Dystrophin Dp427 plays a critical role in maintaining the structure and stability of muscle and nerve cells, and it is altered in all individuals with DMD. There are three major isoforms of Dp427: Dp427-M (muscle), expressed mainly in skeletal and cardiac muscle; Dp427-B (brain), expressed in various areas of the central nervous system; Dp427-P (Purkinje), expressed mainly in Purkinje cells of the cerebellum. The Dp427B isoform and two short brain isoforms expressed by internal promoters, Dp140 and Dp71, are thought to be expressed throughout the cerebral cortex with the highest expression in the temporal and frontal cortex, the amygdala, and the hippocampus [,]. These regions are important in central nervous system development, neuroplasticity, and synaptogenesis, and them alterations can lead to an increased risk of developing cognitive, neuropsychological and/or neuropsychiatric disorders [,]. The hippocampus and Purkinje cell structures are part of a network that connects with the frontal lobe, which plays an important role in human memory and is responsible for executive functions, such as decision-making skills, judgment, attention span, and inhibition []. An important role is played by the position of the gene’s mutation: mutations near the beginning of the gene will affect only the longest isoforms, which are expressed mainly in skeletal and cardiac muscle; mutations progressively further along the gene (downstream of exon 44 and onwards) affect other isoforms that are expressed particularly in the brain, such as Dp140 and Dp71 []. As observed in several studies, mutations in the dystrophin gene, particularly in the distal portion, are often associated with specific cognitive deficits or neurodevelopmental disorders [,]. Altered synaptic function [], alterations in the blood–brain barrier [], neuroinflammation [], altered calcium homeostasis and, more generally, altered ionic homeostasis and ion channel function [,] may all contribute to the neurological manifestation of DMD, but further researches are needed. While the neuropsychological and psychological aspects of individuals with DMD may have negative consequences for them and their families, resilience and well-being may play a crucial role in helping them cope with daily difficulties. However, there is limited evidence on the positive factors contributing to mental health, quality of life, and effective adaptation strategies among those living with DMD. For this reason, this narrative review aims to highlight the cognitive, psychological, and psychosocial resources present in individuals with DMD, which are not always considered.
To achieve our aim, we searched PubMed from 2000 to 2025 to analyze the most recent literature corresponding with our focus. We excluded papers that were not written in English, that dealt with other diagnoses, or that were off-topic.
2. Cognitive Abilities
In 1861, describing the first clinical case of a boy with muscular dystrophy, Duchenne used the definition of “caractere obtus”, effectively suggesting a cognitive impairment.
Throughout history, the main focus in DMD has been on muscle impairment, neglecting the cognitive manifestations of the disease [].
Only in the last 20 years has it been possible to recognize DMD as a condition with significant neurological and cognitive implications. This has led to more attention being paid to the cognitive profile of these individuals, suggesting that cognitive impairment is non-progressive and not related to the severity of the muscle disease [].
Dystrophin isoforms expressed in the central nervous system, particularly Dp140 and Dp71, play a relevant role in the brain and in the development of cognitive abilities in individuals with DMD []. The Dp140, encoded by an internal promoter of the DMD gene located in intron 44, is expressed primarily in the central nervous system, particularly during fetal brain development. Its amount decreases in the adult brain.
Individuals with mutations downstream of exon 44 are more likely to experience problems with fine motor and visual perceptual skills, speech, and probably some locomotor aspects. This suggests a role for brain dystrophin isoforms in the development of coordination and motor dysfunction []. Research conducted by Pane and colleagues found that younger subjects, assessed with the Griffiths scale, had a lower developmental quotient (DQ) and had difficulties in hearing, speech and in performance tasks that older individuals with similar gene mutations were able to perform. This suggests that these difficulties were not mutation-dependent, but rather age-dependent, suggesting a delay in developmental milestones rather than general disability and a longer latency in acquiring those specific skills []. Several authors showed a close association between the increased risk of cognitive impairment and deletions in the distal portion of the DMD gene (ranging from exon 45 to exon 60), specifically those involving the Dp140 isoform [,]. In particular, children with DMD who do not express Dp140 tend to have a lower intelligence quotient (IQ) and greater cognitive difficulties than those who retain this isoform []. Some authors reported that individuals lacking the Dp140 isoform show deficits in verbal working memory and processing speed [,]. The biochemical and tissue characteristics of Dp140 suggest that this isoform plays an important function in normal cognitive development, highlighting a potential association between the preservation of these regulatory sequences and the maintenance of normal cognitive function in people with DMD []. Dp71 is the shortest isoform and is encoded by a promoter far downstream, near the 3′ end of the DMD gene (starting at exon 63). It is implicated in multiple cellular processes, and it gradually increases from the embryonic stage to the adult stage. Several studies describe mutations in these isoforms as a factor contributing to the severity of cognitive impairment [,]. The absence or reduction of these isoforms has been linked to a distinct cognitive profile observed in individuals with DMD.
In individuals with mutations affecting all distal isoforms, there is a strong association with severe learning disability and an IQ that is two standard deviations (SD) lower than normal; in individuals with mutations affecting only those isoforms upstream of exon 30, a minimal frequency of intellectual impairment is observed [].
A recent meta-analysis has investigated the cognitive profile of individuals with DMD, highlighting that the mean full-scale IQ is approximately one SD below the average norm []. In another review, intellectual disability in individuals with DMD was observed in 22% of the patients, with a lower prevalence in patients with the exclusive involvement of Dp427 and higher rates in those with additional Dp140 or Dp71 involvement []. In line with this result, D’Angelo and colleagues showed that children with DMD have an average IQ that is about one SD lower than the population average. They also found a discrepancy between the verbal intelligence quotient (VIQ) and the performance intelligence quotient (PIQ), showing that VIQ is more affected than PIQ []. The above discrepancy is well established and broadly acknowledged in the current literature [].
These difficulties in verbal competence seem to be associated with specific neuropsychological deficits; children with DMD may have difficulties in the maintenance and manipulation of verbal information, immediate verbal memory, verbal working memory, verbal comprehension, vocabulary, and verbal learning and encoding.
A study conducted by Lo Russo and colleagues showed that verbal deficits tend to be more evident in receptive speech than in expressive language []. Poor verbal skills seem to play a role in learning abilities. These children may have problems with processing large amounts of information; for example, when following a lesson in the classroom. As a result, they would benefit from breaking information and tasks into concise and simple steps []. Furthermore, Lo Russo’s study showed the presence of reading difficulties in boys with DMD, essentially involving speed parameters []. The type of language spoken by the individuals plays an important role: in orthographically transparent languages, such as Italian, the letter-to-sound conversion is relatively intact but, for example, in French, individuals have greater reading disabilities because of their opaque language.
It has been observed in the literature that reading disabilities and reduced arithmetic skills are prevalent in individuals with DMD, particularly in those with mutations affecting both Dp140 and Dp71 [].
Despite normal IQ scores in some individuals, deficits in working memory and executive functions, including inhibition, switching, problem-solving, and planning, are commonly reported [,].
The main cognitive domains that could be affected in individuals with DMD are illustrated in Figure 1.
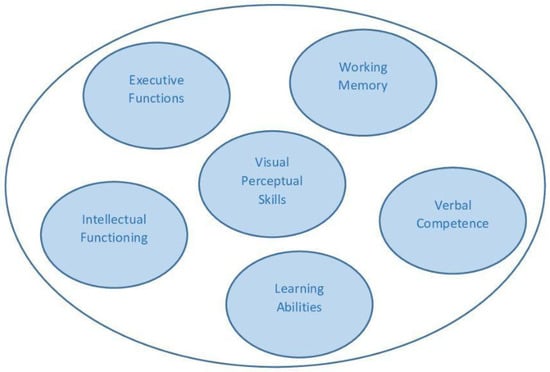
Figure 1.
Main cognitive abilities affected in DMD.
The disruption of the dystrophin gene has an impact on brain functions, but the specific relationship between the loss of dystrophin isoforms in the brain and mental functioning has not been clarified yet.
Significant neurobiological advances in understanding the role of dystrophins in the brain have largely stemmed from studies using dystrophic mouse models []. The mdx mouse, which lacks the Dp427 isoform, demonstrates specific deficits in learning and memory, as well as marked abnormalities in emotional and social behaviors. One of the most notable features of this model is an increased sensitivity to stress, often expressed as exaggerated unconditioned fear responses to mild stimuli. This phenotype has been associated with amygdala dysfunction and reduced GABAergic inhibition []. Such heightened stress reactivity is consistently observed across various dystrophic animal models and mirrors the increased emotional vulnerability frequently reported in people with DMD [,,]. However, while some of the behavioral traits observed in mice are clearly recognizable in affected individuals, the expression of these traits in humans is more heterogeneous, with considerable variability in their severity and prevalence [].
Brain morphology and metabolic studies have not reveal structural abnormalities but they show specific alterations in people with DMD compared with healthy controls, such as total brain volume and cerebral gray-matter atrophy. Furthermore, reduced gray-matter volume and cerebral blood flow were observed in children lacking both Dp427 and Dp140 [,].
Abnormal dendrite development, myelin damage, or altered structural/functional connectivity are assumed to play a role in altered brain development in subjects with DMD []. Most studies on brain alterations in DMD focus on the correlation between brain abnormalities and the clinical phenotype and compare subgroups of patients based on mutations.
Doorenweerd and colleagues showed through pseudo-continuous arterial spin-labeling MRI that there was lower cerebral blood flow (CBF) in DMD subjects than in control subjects; moreover, DMD individuals lacking Dp140 had a lower CBF than the ones with preserved Dp140 [].
Recent studies using diffusion tensor imaging (DTI) have revealed elevated diffusivities in the prefrontal cortex of individuals with DMD. This finding is accompanied by increased taurine levels in the same cerebral area and a decrease in fractional anisotropy in the hippocampus, along with metabolic changes. These changes suggest potential impairments in axonal and myelin integrity, as well as reduced neuronal density in the white matter [,,].
Most previous studies focused on the brain of DMD patients in comparison with healthy controls and did not account for possible comorbidities such as cognitive impairment and/or neurodevelopmental disorders.
Given the complex comorbidities in DMD, at our institute, Peruzzo et al. recently used a multimodal, multivariate approach to analyze DMD-related effects in the central nervous system (CNS) to identify a pattern of brain structural alterations uniquely associated with the disease. A group of people with DMD were compared with a control group that included not only healthy people but also participants diagnosed with cognitive impairment and autism spectrum disorder (ASD) without DMD gene alterations. Through this method of analysis, we were able to show that white-matter alterations, particularly of long fiber bundles, were selectively observed in individuals with DMD, suggesting the presence of a specific dystrophin-related pattern [].
Recent therapeutic advances have made it clear how important early diagnosis and evaluations are. A detailed assessment as early as possible would identify the child’s difficulties and suggest the most appropriate intervention.
Given the non-progressive nature of cognitive deficits in DMD, early recognition and intervention are critical for optimizing developmental outcomes and improving the quality of life of affected individuals and their families.
Pharmacological and Gene Therapy
In recent years, remarkable progress has been made in the therapeutic landscape for DMD, offering new strategies for disease modification and symptom control. Gene therapy is considered one of the most transformative of these developments. Delandistrogene moxeparvovec is the most widely adeno-associated-virus-delivered micro-dystrophin gene therapy. Despite its promise, recent reports of treatment-associated acute liver failure and fatalities have raised safety concerns, emphasizing the importance of strict patient monitoring and risk mitigation strategies [].
Equally important are exon-skipping therapies with antisense oligonucleotides that restore a partially functioning dystrophin by bypassing specific mutations [].
Despite their potential for the treatment of DMD, the effects of gene therapies and exon-skipping approaches on cognitive and neuropsychological functions are not yet fully understood.
Recently, interventional studies in mdx mouse models, such as antisense oligonucleotide therapy, have shown restoration of dystrophin in the mouse brain and partial improvement of cognitive impairment [].
Pharmacological treatments aimed at modulating inflammation and fibrosis are also central to current care [] and may play a role in cognitive function [,,].
Lopez et al. reported that increasing endogenous nitric oxide levels restored calcium homeostasis, decreased reactive oxygen species production, and improved cognitive function in mdx mice [].
These preliminary results seem to be very promising and open up a new era of intervention in DMD individuals that addresses not only muscle function but also “brain” function. However, we still need to be very cautious.
3. Psychopathological Aspects
As life expectancy has increased, more attention has been paid to the health-related quality of life of people with DMD, their psychological and psychosocial care, and their transition to adulthood. As a result, the goal of care has evolved from simply prolonging life to improving the quality of life, particularly in terms of mental health and psychosocial functioning [,].
Research showed that children and adults with DMD have a higher risk of developing symptoms of neurodevelopmental disorders and psychopathological traits than the general population. The most frequent ones are autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), obsessive–compulsive disorder (OCD), and emotional and behavioral disorders, including depression, aggression, and anxiety [,,].
Several studies have investigated the frequency of neurodevelopmental disorders and psychopathological traits, particularly internalizing traits, in individuals with DMD reporting similar prevalence rates. The variability in the rates probably reflects the different methods used, such as the age of the sample; for example, when the study takes into account the entire age range of DMD symptomatology, the measure of the frequency of these traits might be more accurate because the onset of symptoms can be spread over a large time span [,].
The prevalence of these disorders and traits in individuals with DMD appears to be higher than the incidence rate in the general population. Autism spectrum disorder (ASD) occurs in 3–20% of cases, in contrast to the general pediatric population, which is about 0.6% [,,,,,].
Attention-deficit hyperactivity disorder (ADHD) is one of the most observed psychiatric comorbidities among children with DMD, with 11–32% of observed cases, showing a higher rate than in the general population, which is about 6–7% [,].
The frequency of ADHD in DMD may vary due to several aspects, including the method used for screening or the different manifestations of symptomatology. For example, children with DMD may not show the same kind of symptoms of hyperactivity and agitation. It is important to note that in children with DMD, these behaviors may be less noticeable due to their motor problems, muscle weakness, and physical limitations, and therefore, measures of physical movement may not be positively evaluated. It is equally important to consider that some of the cognitive patterns observed in DMD, such as short-term memory and attention problems, or behavioral problems resulting from the side effects of steroid therapy may lead to a misdiagnosis of ADHD in the child [,].
Evidence shows that the incidence of obsessive–compulsive disorder (OCD) in individuals with DMD is around 5–11%, with higher prevalence than in the general population, which is about 2–3%. Boys with DMD frequently have a recognizable obsessive compulsive phenotype with associated anxiety [,].
Finally, features consistent with anxiety have been found in 24–29% of individuals with DMD, a higher rate compared with the 10–30% estimated in the general pediatric population. Depressive symptoms are evident in 17–27% [,,,,,]. Young children may develop emotional disorders that manifest as oppositional behaviors or anger outbursts rather than the classic clinical presentation of depression or anxiety. In contrast, regarding externalizing aspects, there are no clear and uniform data from the literature.
Most studies on the psychopathology of individuals with DMD rely on clinical assessments or parent-reported questionnaires. This may result in a difference in the assessment of symptomatology from what is actually perceived by the children themselves. These discrepancies may be explained by differences between the home environment, in which the parent sees their child, and the actual experience of the child when they are alone; differences in the attribution of their behavior—for example, due to temperament or derived from an emotionally charged situation; and by the person’s psychopathology. It is noteworthy that the self-perception of many children and adolescents with DMD is more positive than what their caregivers describe [,].
Regarding the risk of developing comorbidities with neurodevelopmental disorders and behavioral and emotional difficulties, the literature suggests a possible correlation with genetic mutations, indicating a role for Dp427, Dp140, and Dp71 [,,]. It appears that individuals with mutations that affect multiple brain isoforms or with complete and shorter brain isoforms have more severe cognitive and behavioral problems [].
Individuals with mutations downstream of exon 30 appear to have a higher risk of developing these comorbidities than those whose mutation affects only the longer isoform of Dp427 [,]. It is important to underline that the isoforms associated with a higher risk of developing comorbidities with neurodevelopmental disorders are the same ones associated with a higher risk of cognitive impairment; that is, Dp71 and Dp140.
Therefore, the increased risk of ADHD, ASD, anxiety, and OCD in boys with DMD may be related to the lack of dystrophin expression in the brain or to the effect of dystrophin on other central nervous system proteins []. In contrast to neurodevelopmental features, behavioral and emotional features such as obsessive/compulsive features, anxiety, and depressed mood appear to be well distributed among the genotype subgroups. The relatively uniform distribution of these symptoms suggests that the pathogenic mechanisms underlying emotional and behavioral symptoms may differ from those responsible for neurodevelopmental symptoms. Indeed, it is conceivable that mutations that alter the expression of longer dystrophin isoforms—including those located far upstream, near the 5′ end of the dystrophin gene—contribute to the development of these symptoms [].
Instead, studies investigating the association between ASD and genetics have provided mixed results. Some of these studies have demonstrated possible alterations in a specific region of the dystrophin gene, located near but not within it; for example, mutations that interrupt the Dp140 isoform of dystrophin. This would explain the coexistence of double diagnoses in the same patient [,,]. If this transcript is found in the muscle, it gives rise to DMD, whereas if it is found in the central nervous system, it gives rise to neuronal dysfunction, with the possible consequence of ASD [].
In contrast, the meta-analysis conducted by Pascual-Morena’s research group suggests that the Dp71 isoform modulates the risk of developmental disorders in DMD. Although the meta-analysis did not show a statistically significant association for ASD, a clear trend toward a deleterious association in DMD was observed when Dp71 was involved. From their work, it appears that Dp140 has no influence on ASD []. Although the association between dystrophin isoforms and ASD remains unclear, these studies demonstrate a potential genetic link between DMD and ASD.
On the other hand, evidence of the association between ADHD and possible genetic mutations seems to be more consistent. Research suggests the possible involvement of Dp140 and Dp71 isoforms, although the study by Pascual-Morena et al. seems to show a less significant association with Dp140 [,].
Studies have shown that ADHD is more frequently associated with mutations affecting the expression of Dp140 (mutations in exons 45–55) and mutations affecting all short isoforms of dystrophin, including Dp71 (mutations in exons 62 and 63). Therefore, a higher frequency is observed in individuals with mutations affecting the expression of dystrophin in the brain [,,]. However, other genetic and/or environmental modifiers may play a role in determining the development, phenotype severity, and phenotypic spectrum of the neurocognitive profile in ADHD. Sometimes patients with identical mutations do not exhibit the same cognitive or behavioral phenotype [,,]. Regarding emotional-related disorders, the research does not suggest an association between the isoforms involved and the prevalence risk of emotional and behavioral dysregulation, depression, anxiety disorders, and OCD []. It cannot be ruled out that mood disorders could also develop as a result of becoming aware of the disease, and living with the disease itself could also be a risk factor for the onset of internalizing traits.
However, the association between neuropsychiatric disorders and the number of dystrophin isoforms involved, summarized in Table 1, is not distinctly clear. Therefore, further research is needed.

Table 1.
Overview of psychopathological features, neurodevelopmental disorders, and genetics.
Given the high incidence of psychiatric or neurodevelopmental comorbidities, it is important for people with DMD to be evaluated by a mental health professional so that necessary and more appropriate treatments can be identified. Mild symptoms may benefit from psychosocial and/or psychotherapeutic interventions. If the severity of symptoms is greater and, consequently, this type of intervention is not sufficient and effective, psychopharmacological treatment may be necessary []. However, research studies have highlighted a significant gap in the knowledge of psychopharmacological treatments for mental disorders in people with neuromuscular diseases. Therefore, the overall low level of evidence from published data does not allow for the formulation of recommendations that clinicians can rely upon [].
Steroid therapy is the gold-standard therapy for stabilizing muscle strength, extending walking and standing abilities, and supporting respiratory function in people with DMD [,]; nevertheless, boys taking steroids show more externalizing behavioral problems, i.e., aggressive behaviors, than boys not taking steroids [,]. The impact of corticosteroids on behavior remains controversial; long-term exposure may be associated with initial mood swings, as well as memory and attention difficulties, which may stabilize over time [,]. Effects of medications usually prescribed by first-line providers to manage acute and chronic medical conditions may worsen psychiatric symptoms in DMD individuals [,].
The use of psychotropic drugs for psychiatric manifestations in people with neuromuscular diseases may be hindered by multiple factors, including the ability to monitor their efficacy and safety and the risk exposure to multisystem complications, such as weight gain or the occurrence of heart disease. The latter may represent a contraindication to the administration of antidepressant, atypical antipsychotic, or CNS stimulant psychotropic drugs [,].
Despite the possible presence of psychiatric and neurodevelopmental comorbidities, people with DMD and their families may use specific coping strategies that help them deal with illness and stressful situations and, thus, make their emotional and behavioral difficulties milder [].
Evidence suggests that most children with DMD cope relatively well with the disorder; in particular, overall adjustment improves as they get older [,,]. The term “psychosocial adjustment” is used in reference to emotional, behavioral, and social functioning; it is a central aspect of quality of life and provides a good estimate of how an individual copes with DMD [].
However, relationships with peers become more problematic with advancing age, as declining physical functioning and health may result in reduced access to social and recreational opportunities. In fact, children with DMD exhibit unexpectedly high levels of social and communication difficulties, regardless of a diagnosis of ASD. Other difficulties are observed in children with ADHD comorbidity, who report significantly worse psychosocial adjustment than children with DMD without an ADHD diagnosis. Behaviors commonly associated with ADHD, including poor emotional regulation, low frustration tolerance, aggression, oppositional/argumentative behavior, and mental rigidity/inflexibility, can be significantly problematic and stressful for families with DMD [,].
Symptoms of anxiety and depression affect quality of life and are often associated with poor adjustment and coping skills []. Personal resources of people with DMD and their family members may moderate the relationship between illness and psychological adjustment. Caregivers adopting maladaptive coping strategies, such as avoidance, report higher levels of stress, distress, frequent feelings of guilt, low self-esteem, sadness, and depression related to their care recipient’s condition. In contrast, caregivers showing high self-esteem, a sense of support, and a positive perception of their own experience tend to have better coping skills, accepting reality rather than denying it, and positively reinterpreting the experience. This could lead to better long-term emotional regulation, with a positive influence on the care recipient’s emotional well-being and stress levels. Therefore, caregivers’ use of avoidant coping strategies is associated with an increased risk of emotional/behavioral problems in individuals with DMD [].
5. Conclusions
This narrative review highlights how DMD is a multifaceted condition that includes progressive muscle degeneration, which is now well documented, and cognitive, psychopathological, and psychosocial aspects. These latter aspects are less known but equally crucial, as they have a profound impact on the quality of life of people with DMD and their families.
Genetic alterations responsible for the loss of specific dystrophin isoforms play a key role in the development of cognitive and neuropsychological deficits, such as IQ, working memory, language, executive functions, and academic skills. In contrast, the link between specific genetic mutations and psychopathological aspects is only partially understood. On the one hand, the presence of neurodevelopmental disorders seems to be more related to the lack of specific dystrophin isoforms; on the other hand, emotional and behavioral symptoms may be the result of a more multifactorial interaction between biological, psychological, coping strategies and environmental factors.
This dual mechanism of genetic susceptibility and environmental influences emphasizes the need for comprehensive and multidisciplinary care strategies.
Recently available treatments for DMD include pharmacological and genetic therapies that aim to modify dystrophin levels in the central nervous system. To date, the impact that these therapies could have on the cognitive abilities and behavior of these individuals is still not fully known.
As research develops new insights into the effects of gene therapies, it is important to consider several aspects when planning targeted educational and therapeutic interventions. These include deficits in verbal processing and memory, as they hinder academic performance and adaptive behavior, and cognitive impairment that does not progress over time, suggesting a developmental origin, as this may impact daily functioning.
At the same time, we emphasize the importance of psychological and social resources in promoting well-being despite the challenges of DMD. Resilience and psychological flexibility together with social support from family, friends, and community were found to be key protective factors for maintaining a satisfactory quality of life. It is of particular significance that, in many cases, the quality of life reported by people with DMD is higher than that reported by their caregivers.
This discrepancy underscores the need to listen to and to incorporate the perspective of those affected into care planning by recognizing their capacity for positive adaptation and their internal resources. An approach focused only on pathological aspects would not adequately capture the complexity of the experience lived by people with DMD. For this reason, it is important to develop interventions that also take into account positive dimensions such as psychological well-being, social relationships, and adaptability. A holistic approach that takes into account both the challenges and strengths of patients and their families as well as neuropsychological, psychopathological, and psychosocial aspects, together with early diagnosis, allows for the development of more targeted and personalized interventions. This allows for an increase in the quality of life and general well-being of patients and their families and opens up new perspectives for research and clinical practice.
Author Contributions
Contributed to the conception of the work, critically reviewed the first draft, and approved the final version of the manuscript: M.G.D., A.D.F., and M.N. Wrote the manuscript: F.T., G.C., and D.D.L. Read and agreed to the published version of the manuscript: F.T., G.C., A.D.F., D.D.L., M.L.L., M.N., and M.G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health (grant number: Ricerca Corrente 2024-2026) and by Telethon-UILDM (starting grant: GUP 24011).
Acknowledgments
The Department of Pathophysiology and Transplantation, University of Milan, is funded by the Italian Ministry of Education and Research (MUR): Dipartimenti di Eccellenza Program 2023 to 2027. We thank all patients and families who contributed to all the cited studies. We thank our Scientific Director (Bassi Maria Teresa) for support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| DMD | Duchenne Muscular Dystrophy |
| IQ | Intelligence Quotient |
| SD | Standard Deviation |
| VIQ | Verbal Intelligence Quotient |
| PIQ | Performance Intelligence Quotient |
| CBF | Cerebral Blood Flow |
| DTI | Diffusion Tensor Imaging |
| CNS | Central Nervous System |
| ASD | Autism Spectrum Disorder |
| ADHD | Attention-Deficit/Hyperactivity Disorder |
| OCD | Obsessive–Compulsive Disorder |
| WHO | World Health Organization |
| QoL | Quality of Life |
| HRQoL | Health-Related Quality of Life |
| CBT | Cognitive–Behavioral Therapy |
| ACT | Acceptance and Commitment Therapy |
References
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- LoMauro, A.; Gandossini, S.; Russo, A.; Velardo, D.; Comi, G.P.; Turconi, A.C.; Bresolin, N.; Aliverti, A.; D’Angelo, M.G. A Multidisciplinary Evaluation of Patients with DMD in An Italian Tertiary Care Center. J. Neuromuscul. Dis. 2021, 8, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Guglieri, M.; Bushby, K.; McDermott, M.P.; Hart, K.A.; Tawil, R.; Martens, W.B.; Herr, B.E.; McColl, E.; Speed, C.; Wilkinson, J.; et al. Effect of Different Corticosteroid Dosing Regimens on Clinical Outcomes in Boys with Duchenne Muscular Dystrophy: A Randomized Clinical Trial. JAMA 2022, 327, 1456–1468. [Google Scholar] [CrossRef] [PubMed]
- Bello, L.; Gordish-Dressman, H.; Morgenroth, L.P.; Henricson, E.K.; Duong, T.; Hoffman, E.P.; Cnaan, A.; McDonald, C.M. Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study. Neurology 2015, 85, 1048–1055. [Google Scholar] [CrossRef]
- Banihani, R.; Smile, S.; Yoon, G.; Dupuis, A.; Mosleh, M.; Snider, A.; McAdam, L. Cognitive and Neurobehavioral Profile in Boys with Duchenne Muscular Dystrophy. J. Child. Neurol. 2015, 30, 1472–1482. [Google Scholar] [CrossRef]
- Ervasti, J.M. Dystrophin, its interactions with other proteins, and implications for muscular dystrophy. Biochim. Biophys. Acta 2007, 1772, 108–117. [Google Scholar] [CrossRef]
- Hellebrekers, D.M.J.; van Abeelen, S.A.M.; Catsman, C.E.; van Kuijk, S.M.J.; Laridon, A.M.; Klinkenberg, S.; Hendriksen, J.G.M.; Vles, J.S.H. Cognitive and behavioral functioning in two neurogenetic disorders; how different are these aspects in Duchenne muscular dystrophy and Neurofibromatosis type 1? PLoS ONE 2022, 17, e0275803. [Google Scholar] [CrossRef]
- Doorenweerd, N. Combining genetics, neuropsychology and neuroimaging to improve understanding of brain involvement in Duchenne muscular dystrophy—A narrative review. Neuromuscul. Disord. 2020, 30, 437–442. [Google Scholar] [CrossRef]
- Pascual-Morena, C.; Cavero-Redondo, I.; Sequí-Domínguez, I.; Rodríguez-Gutiérrez, E.; Visier-Alfonso, M.E.; Martínez-Vizcaíno, V. Intelligence quotient-genotype association in dystrophinopathies: A systematic review and meta-analysis. Neuropathol. Appl. Neurobiol. 2023, 49, e12914. [Google Scholar] [CrossRef]
- Rae, M.G.; O’Malley, D. Cognitive dysfunction in Duchenne muscular dystrophy: A possible role for neuromodulatory immune molecules. J. Neurophysiol. 2016, 116, 1304–1315. [Google Scholar] [CrossRef]
- Ricotti, V.; Roberts, R.G.; Muntoni, F. Dystrophin and the brain. Dev. Med. Child. Neurol. 2011, 53, 12. [Google Scholar] [CrossRef] [PubMed]
- Pane, M.; Lombardo, M.E.; Alfieri, P.; D’Amico, A.; Bianco, F.; Vasco, G.; Piccini, G.; Mallardi, M.; Romeo, D.M.; Ricotti, V.; et al. Attention deficit hyperactivity disorder and cognitive function in Duchenne muscular dystrophy: Phenotype-genotype correlation. J. Pediatr. 2012, 161, 705–709.e1. [Google Scholar] [CrossRef] [PubMed]
- Ricotti, V.; Mandy, W.P.; Scoto, M.; Pane, M.; Deconinck, N.; Messina, S.; Mercuri, E.; Skuse, D.H.; Muntoni, F. Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev. Med. Child. Neurol. 2016, 58, 77–84. [Google Scholar] [CrossRef]
- De Stefano, M.E.; Ferretti, V.; Mozzetta, C. Synaptic alterations as a neurodevelopmental trait of Duchenne muscular dystrophy. Neurobiol. Dis. 2022, 168, 105718. [Google Scholar] [CrossRef]
- Belmaati Cherkaoui, M.; Vacca, O.; Izabelle, C.; Boulay, A.C.; Boulogne, C.; Gillet, C.; Barnier, J.V.; Rendon, A.; Cohen-Salmon, M.; Vaillend, C. Dp71 contribution to the molecular scaffold anchoring aquaporine-4 channels in brain macroglial cells. Glia 2021, 69, 954–970. [Google Scholar] [CrossRef]
- Comim, C.M.; Ventura, L.; Freiberger, V.; Dias, P.; Bragagnolo, D.; Dutra, M.L.; Amaral, R.A.; Camargo-Fagundes, A.L.S.; Reis, P.A.; Castro-Faria-Neto, H.C.; et al. Neurocognitive Impairment in mdx Mice. Mol. Neurobiol. 2019, 56, 7608–7616. [Google Scholar] [CrossRef]
- Lopez, J.R.; Kolster, J.; Uryash, A.; Estève, E.; Altamirano, F.; Adams, J.A. Dysregulation of Intracellular Ca2+ in Dystrophic Cortical and Hippocampal Neurons. Mol. Neurobiol. 2018, 55, 603–618. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Belosludtsev, K.N. Ion Channels of the Sarcolemma and Intracellular Organelles in Duchenne Muscular Dystrophy: A Role in the Dysregulation of Ion Homeostasis and a Possible Target for Therapy. Int. J. Mol. Sci. 2023, 24, 2229. [Google Scholar] [CrossRef]
- Nardes, F.; Araújo, A.P.; Ribeiro, M.G. Mental retardation in Duchenne muscular dystrophy. J. Pediatr. (Rio J.) 2012, 88, 6–16. [Google Scholar] [CrossRef]
- Cotton, S.M.; Voudouris, N.J.; Greenwood, K.M. Association between intellectual functioning and age in children and young adults with Duchenne muscular dystrophy: Further results from a meta-analysis. Dev. Med. Child. Neurol. 2005, 47, 257–265. [Google Scholar] [CrossRef]
- Taylor, P.J.; Betts, G.A.; Maroulis, S.; Gilissen, C.; Pedersen, R.L.; Mowat, D.R.; Johnston, H.M.; Buckley, M.F. Dystrophin gene mutation location and the risk of cognitive impairment in Duchenne muscular dystrophy. PLoS ONE 2010, 5, e8803. [Google Scholar] [CrossRef] [PubMed]
- Pane, M.; Scalise, R.; Berardinelli, A.; D’Angelo, G.; Ricotti, V.; Alfieri, P.; Moroni, I.; Hartley, L.; Pera, M.C.; Baranello, G.; et al. Early neurodevelopmental assessment in Duchenne muscular dystrophy. Neuromuscul. Disord. 2013, 23, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Felisari, G.; Martinelli Boneschi, F.; Bardoni, A.; Sironi, M.; Comi, G.P.; Robotti, M.; Turconi, A.C.; Lai, M.; Corrao, G.; Bresolin, N. Loss of Dp140 dystrophin isoform and intellectual impairment in Duchenne dystrophy. Neurology 2000, 55, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Bardoni, A.; Felisari, G.; Sironi, M.; Comi, G.; Lai, M.; Robotti, M.; Bresolin, N. Loss of Dp140 regulatory sequences is associated with cognitive impairment in dystrophinopathies. Neuromuscul. Disord. 2000, 10, 194–199. [Google Scholar] [CrossRef]
- Chamova, T.; Guergueltcheva, V.; Raycheva, M.; Todorov, T.; Genova, J.; Bichev, S.; Bojinova, V.; Mitev, V.; Tournev, I.; Todorova, A. Association between loss of dp140 and cognitive impairment in duchenne and becker dystrophies. Balkan J. Med. Genet. 2013, 16, 21–30. [Google Scholar] [CrossRef]
- Hellebrekers, D.M.J.; Doorenweerd, N.; Sweere, D.J.J.; van Kuijk, S.M.J.; Aartsma-Rus, A.M.; Klinkenberg, S.; Vles, J.S.H.; Hendriksen, J.G.M. Longitudinal follow-up of verbal span and processing speed in Duchenne muscular dystrophy. Eur. J. Paediatr. Neurol. 2020, 25, 120–126. [Google Scholar] [CrossRef]
- Tyagi, R.; Arvind, H.; Goyal, M.; Anand, A.; Mohanty, M. Working Memory Alterations Plays an Essential Role in Developing Global Neuropsychological Impairment in Duchenne Muscular Dystrophy. Front. Psychol. 2021, 11, 613242. [Google Scholar] [CrossRef]
- Wingeier, K.; Giger, E.; Strozzi, S.; Kreis, R.; Joncourt, F.; Conrad, B.; Gallati, S.; Steinlin, M. Neuropsychological impairments and the impact of dystrophin mutations on general cognitive functioning of patients with Duchenne muscular dystrophy. J. Clin. Neurosci. 2011, 18, 90–95. [Google Scholar] [CrossRef]
- Daoud, F.; Angeard, N.; Demerre, B.; Martie, I.; Benyaou, R.; Leturcq, F.; Cossée, M.; Deburgrave, N.; Saillour, Y.; Tuffery, S.; et al. Analysis of Dp71 contribution in the severity of mental retardation through comparison of Duchenne and Becker patients differing by mutation consequences on Dp71 expression. Hum. Mol. Genet. 2009, 18, 3779–3794. [Google Scholar] [CrossRef]
- Weerkamp, P.M.M.; Mol, E.M.; Sweere, D.J.J.; Schrans, D.G.M.; Vermeulen, R.J.; Klinkenberg, S.; Hurks, P.P.M.; Hendriksen, J.G.M. Wechsler Scale Intelligence Testing in Males with Dystrophinopathies: A Review and Meta-Analysis. Brain Sci. 2022, 12, 1544. [Google Scholar] [CrossRef]
- D’Angelo, M.G.; Lorusso, M.L.; Civati, F.; Comi, G.P.; Magri, F.; Del Bo, R.; Guglieri, M.; Molteni, M.; Turconi, A.C.; Bresolin, N. Neurocognitive profiles in Duchenne muscular dystrophy and gene mutation site. Pediatr. Neurol. 2011, 45, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, M.L.; Civati, F.; Molteni, M.; Turconi, A.C.; Bresolin, N.; D’Angelo, M.G. Specific profiles of neurocognitive and reading functions in a sample of 42 Italian boys with Duchenne Muscular Dystrophy. Child. Neuropsychol. 2013, 19, 350–369. [Google Scholar] [CrossRef] [PubMed]
- Perumal, A.R.; Rajeswaran, J.; Nalini, A. Neuropsychological profile of duchenne muscular dystrophy. Appl. Neuropsychol. Child. 2015, 4, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Fee, R.J.; Montes, J.; Stewart, J.L.; Hinton, V.J. Executive Skills and Academic Achievement in the Dystrophinopathies. J. Int. Neuropsychol. Soc. 2018, 24, 928–938. [Google Scholar] [CrossRef]
- Battini, R.; Lenzi, S.; Lucibello, S.; Chieffo, D.; Moriconi, F.; Cristofani, P.; Bulgheroni, S.; Cumbo, F.; Pane, M.; Baranello, G.; et al. Longitudinal data of neuropsychological profile in a cohort of Duchenne muscular dystrophy boys without cognitive impairment. Neuromuscul. Disord. 2021, 31, 319–327. [Google Scholar] [CrossRef]
- Vaillend, C.; Aoki, Y.; Mercuri, E.; Hendriksen, J.; Tetorou, K.; Goyenvalle, A.; Muntoni, F. Duchenne muscular dystrophy: Recent insights in brain related comorbidities. Nat. Commun. 2025, 16, 1298. [Google Scholar] [CrossRef]
- Vaillend, C.; Chaussenot, R. Relationships linking emotional, motor, cognitive and GABAergic dysfunctions in dystrophin-deficient mdx mice. Hum. Mol. Genet. 2017, 26, 1041–1055. [Google Scholar] [CrossRef]
- Darmahkasih, A.J.; Rybalsky, I.; Tian, C.; Shellenbarger, K.C.; Horn, P.S.; Lambert, J.T.; Wong, B.L. Neurodevelopmental, behavioral, and emotional symptoms common in Duchenne muscular dystrophy. Muscle Nerve 2020, 61, 466–474. [Google Scholar] [CrossRef]
- Maresh, K.; Papageorgiou, A.; Ridout, D.; Harrison, N.A.; Mandy, W.; Skuse, D.; Muntoni, F. Startle responses in Duchenne muscular dystrophy: A novel biomarker of brain dystrophin deficiency. Brain 2023, 146, 252–265. [Google Scholar] [CrossRef]
- Caudal, D.; François, V.; Lafoux, A.; Ledevin, M.; Anegon, I.; Le Guiner, C.; Larcher, T.; Huchet, C. Characterization of brain dystrophins absence and impact in dystrophin-deficient Dmdmdx rat model. PLoS ONE 2020, 15, e0230083. [Google Scholar] [CrossRef]
- Doorenweerd, N.; Straathof, C.S.; Dumas, E.M.; Spitali, P.; Ginjaar, I.B.; Wokke, B.H.; Schrans, D.G.; van den Bergen, J.C.; van Zwet, E.W.; Webb, A.; et al. Reduced cerebral gray matter and altered white matter in boys with Duchenne muscular dystrophy. Ann. Neurol. 2014, 76, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Xu, H.; Zhou, H.; Guo, Y.; Roberts, N.; Li, N.; Hu, X.; Chen, X.; Xu, K.; Lan, Y.; et al. Connectomic disturbances in Duchenne muscular dystrophy with mild cognitive impairment. Cereb. Cortex 2023, 33, 6785–6791. [Google Scholar] [CrossRef] [PubMed]
- Doorenweerd, N.; Dumas, E.M.; Ghariq, E.; Schmid, S.; Straathof, C.S.; Roest, A.A.; Wokke, B.H.; van Zwet, E.W.; Webb, A.G.; Hendriksen, J.G.; et al. Decreased cerebral perfusion in Duchenne muscular dystrophy patients. Neuromuscul. Disord. 2017, 27, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.; Pons, R.; Nikas, I.; Papadopoulos, G.; Verganelakis, D.A.; Kolovou, G.; Chrousos, G.P. Brain and heart magnetic resonance imaging/spectroscopy in duchenne muscular dystrophy. Eur. J. Clin. Investig. 2017, 47, 12842. [Google Scholar] [CrossRef]
- Preethish-Kumar, V.; Shah, A.; Kumar, M.; Ingalhalikar, M.; Polavarapu, K.; Afsar, M.; Rajeswaran, J.; Vengalil, S.; Nashi, S.; Thomas, P.T.; et al. In Vivo Evaluation of White Matter Abnormalities in Children with Duchenne Muscular Dystrophy Using DTI. AJNR Am. J. Neuroradiol. 2020, 41, 1271–1278. [Google Scholar] [CrossRef]
- Fu, Y.; Dong, Y.; Zhang, C.; Sun, Y.; Zhang, S.; Mu, X.; Wang, H.; Xu, W.; Wu, S. Diffusion tensor imaging study in Duchenne muscular dystrophy. Ann. Transl. Med. 2016, 4, 109. [Google Scholar] [CrossRef]
- Peruzzo, D.; Ciceri, T.; Mascheretti, S.; Lampis, V.; Arrigoni, F.; Agarwal, N.; Giubergia, A.; Villa, F.M.; Crippa, A.; Nobile, M.; et al. Brain Alteration Patterns in Children with Duchenne Muscular Dystrophy: A Machine Learning Approach to Magnetic Resonance Imaging. J. Neuromuscul. Dis. 2024, 1–13, preprint. [Google Scholar] [CrossRef]
- Mendell, J.R.; Sahenk, Z.; Lehman, K.J.; Lowes, L.P.; Reash, N.F.; Iammarino, M.A.; Alfano, L.N.; Lewis, S.; Church, K.; Shell, R.; et al. Long-term safety and functional outcomes of delandistrogene moxeparvovec gene therapy in patients with Duchenne muscular dystrophy: A phase 1/2a nonrandomized trial. Muscle Nerve 2024, 69, 93–98. [Google Scholar] [CrossRef]
- Takeda, S.; Clemens, P.R.; Hoffman, E.P. Exon-Skipping in Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2021, 8, S343–S358. [Google Scholar] [CrossRef]
- Zarrouki, F.; Relizani, K.; Bizot, F.; Tensorer, T.; Garcia, L.; Vaillend, C.; Goyenvalle, A. Partial Restoration of Brain Dystrophin and Behavioral Deficits by Exon Skipping in the Muscular Dystrophy X-Linked (mdx) Mouse. Ann. Neurol. 2022, 92, 213–229. [Google Scholar] [CrossRef]
- Mercuri, E.; Vilchez, J.J.; Boespflug-Tanguy, O.; Zaidman, C.M.; Mah, J.K.; Goemans, N.; Müller-Felber, W.; Niks, E.H.; Schara-Schmidt, U.; Bertini, E.; et al. EPIDYS Study Group. Safety and efficacy of givinostat in boys with Duchenne muscular dystrophy (EPIDYS): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2024, 23, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.J.; Khom, S.; Bajo, M.; Vlkolinsky, R.; Polis, I.; Cates-Gatto, C.; Roberto, M.; Gruol, D.L. Increased IL-6 expression in astrocytes is associated with emotionality, alterations in central amygdala GABAergic transmission, and excitability during alcohol withdrawal. Brain Behav. Immun. 2019, 82, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Tuon, L.; Comim, C.M.; Fraga, D.B.; Scaini, G.; Rezin, G.T.; Baptista, B.R.; Streck, E.L.; Vainzof, M.; Quevedo, J. Mitochondrial respiratory chain and creatine kinase activities in mdx mouse brain. Muscle Nerve 2010, 41, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.R.; Uryash, A.; Kolster, J.; Estève, E.; Zhang, R.; Adams, J.A. Enhancing Endogenous Nitric Oxide by Whole Body Periodic Acceleration Elicits Neuroprotective Effects in Dystrophic Neurons. Mol. Neurobiol. 2018, 55, 8680–8694. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Colvin, M.K.; Cripe, L.; Herron, A.R.; Kennedy, A.; Kinnett, K.; et al. DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 3: Primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol. 2018, 17, 445–455. [Google Scholar] [CrossRef]
- Brusa, C.; Gadaleta, G.; D’Alessandro, R.; Urbano, G.; Vacchetti, M.; Davico, C.; Vitiello, B.; Ricci, F.S.; Mongini, T.E. Psychopharmacological Treatments for Mental Disorders in Patients with Neuromuscular Diseases: A Scoping Review. Brain Sci. 2022, 12, 176. [Google Scholar] [CrossRef]
- Fujino, H.; Saito, T.; Matsumura, T.; Shibata, S.; Iwata, Y.; Fujimura, H.; Imura, O. Autism spectrum disorders are prevalent among patients with dystrophinopathies. Neurol. Sci. 2018, 39, 1279–1282. [Google Scholar] [CrossRef]
- Gosar, D.; Košmrlj, L.; Musek, P.L.; Meško, T.; Stropnik, S.; Krkoč, V.; Golli, T.; Butenko, T.; Loboda, T.; Osredkar, D. Adaptive skills and mental health in children and adolescents with neuromuscular diseases. Eur. J. Paediatr. Neurol. 2021, 30, 134–143. [Google Scholar] [CrossRef]
- Colombo, P.; Nobile, M.; Tesei, A.; Civati, F.; Gandossini, S.; Mani, E.; Molteni, M.; Bresolin, N.; D’Angelo, G. Assessing mental health in boys with Duchenne muscular dystrophy: Emotional, behavioural and neurodevelopmental profile in an Italian clinical sample. Eur. J. Paediatr. Neurol. 2017, 21, 639–647. [Google Scholar] [CrossRef]
- Lee, A.J.; Buckingham, E.T.; Kauer, A.J.; Mathews, K.D. Descriptive Phenotype of Obsessive Compulsive Symptoms in Males with Duchenne Muscular Dystrophy. J. Child. Neurol. 2018, 33, 572–579. [Google Scholar] [CrossRef]
- Pascual-Morena, C.; Cavero-Redondo, I.; Martínez-Vizcaíno, V.; Sequí-Domínguez, I.; Fernández-Bravo-Rodrigo, J.; Jiménez-López, E. Dystrophin Genotype and Risk of Neuropsychiatric Disorders in Dystrophinopathies: A Systematic Review and Meta-Analysis. J. Neuromuscul. Dis. 2023, 10, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Kuban, K.C.; Allred, E.; Shapiro, F.; Darras, B.T. Association of Duchenne muscular dystrophy with autism spectrum disorder. J. Child. Neurol. 2005, 20, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Takeshita, E.; Komaki, H.; Nishino, I.; Sasaki, M. Determining neurodevelopmental manifestations in Duchenne muscular dystrophy using a battery of brief tests. J. Neurol. Sci. 2022, 440, 120340. [Google Scholar] [CrossRef] [PubMed]
- Poysky, J. Behavior patterns in Duchenne muscular dystrophy: Report on the Parent Project Muscular Dystrophy behavior workshop 8–9 of December 2006, Philadelphia, USA. Neuromuscul. Disord. 2007, 17, 986–994. [Google Scholar] [CrossRef]
- Tesei, A.; Nobile, M.; Colombo, P.; Civati, F.; Gandossini, S.; Mani, E.; Molteni, M.; Bresolin, N.; D’Angelo, G. Mental health and coping strategies in families of children and young adults with muscular dystrophies. J. Neurol. 2020, 267, 2054–2069. [Google Scholar] [CrossRef]
- Pascual-Morena, C.; Cavero-Redondo, I.; Reina-Gutiérrez, S.; Saz-Lara, A.; López-Gil, J.F.; Martínez-Vizcaíno, V. Prevalence of Neuropsychiatric Disorders in Duchenne and Becker Muscular Dystrophies: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2022, 103, 2444–2453. [Google Scholar] [CrossRef]
- Negri, L.; Buzzi, A.; Aru, A.B.; Cannavò, A.; Castegnaro, C.; Fasulo, M.R.; Lassandro, G.; Rocino, A.; Santoro, C.; Sottilotta, G.; et al. Perceived well-being and mental health in haemophilia. Psychol. Health Med. 2020, 25, 1062–1072. [Google Scholar] [CrossRef]
- Diener, E.; Pressman, S.D.; Hunter, J.; Delgadillo-Chase, D. If, Why, and When Subjective Well-Being Influences Health, and Future Needed Research. Appl. Psychol. Health Well Being 2017, 9, 133–167. [Google Scholar] [CrossRef]
- Hsieh, C.M. Importance is Not Unimportant: The Role of Importance Weighting in QOL Measures. Soc. Indic. Res. 2011, 109, 267–278. [Google Scholar] [CrossRef]
- Diener, E.; Chan, M.Y. Happy People Live Longer: Subjective Well-Being Contributes to Health and Longevity. Appl. Psychol. Health Well Being 2011, 3, 1–43. [Google Scholar] [CrossRef]
- Pakenham, K.I.; Cox, S. The dimensional structure of benefit finding in multiple sclerosis and relations with positive and negative adjustment: A longitudinal study. Psychol. Health 2009, 24, 373–393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delle Fave, A. Meaning in Life: Structure, Sources, and Relations with Mental and Physical Health. Acta Philos. 2020, 29, 19–32. [Google Scholar] [CrossRef]
- Wong, P.T.; Bowers, V. Mature Happiness and Global Wellbeing in Difficult Times. In Scientific Concepts Behind Happiness, Kindness, and Empathy in Contemporary Society; Nava, R.S., Ed.; IGI Global: Hershey, PA, USA, 2019; pp. 112–134. [Google Scholar] [CrossRef]
- Ryff, C.D. Psychological well-being revisited: Advances in the science and practice of eudaimonia. Psychother. Psychosom. 2014, 83, 10–28. [Google Scholar] [CrossRef]
- Bassi, M.; Falautano, M.; Cilia, S.; Goretti, B.; Grobberio, M.; Pattini, M.; Pietrolongo, E.; Viterbo, R.G.; Amato, M.P.; Benin, M.; et al. The coexistence of well- and ill-being in persons with multiple sclerosis, their caregivers and health professionals. J. Neurol. Sci. 2014, 337, 67–73. [Google Scholar] [CrossRef]
- Cavazza, M.; Kodra, Y.; Armeni, P.; De Santis, M.; López-Bastida, J.; Linertová, R.; Oliva-Moreno, J.; Serrano-Aguilar, P.; Posada-de-la-Paz, M.; Taruscio, D.; et al. Social/economic costs and health-related quality of life in patients with Duchenne muscular dystrophy in Europe. Eur. J. Health Econ. 2016, 17, 19–29. [Google Scholar] [CrossRef]
- Huang, M.; Chen, T.; Wang, Y.; Zhou, C.; Cao, J.; Lu, X.; Zeng, H. Chronic pain, psychological distress, and quality of life in males with Duchenne muscular dystrophy. Dev. Med. Child. Neurol. 2023, 65, 640–654. [Google Scholar] [CrossRef]
- Gocheva, V.; Schmidt, S.; Orsini, A.L.; Hafner, P.; Schaedelin, S.; Rueedi, N.; Weber, P.; Fischer, D. Association Between Health-Related Quality of Life and Motor Function in Ambulant and Nonambulant Duchenne Muscular Dystrophy Patients. J. Child. Neurol. 2019, 34, 873–885. [Google Scholar] [CrossRef]
- Sanzo’, S.; Tizzoni, F.; Previtali, S.C.; Berardinelli, A.; Nobile, M.; Molteni, M.; Manzoni, M.; Tarabelloni, A.; Russo, A.; Delle Fave, A.; et al. Psychosocial resources and psychopathology among persons with neuromuscular disorders during the COVID-19 pandemic. BMC Psychol. 2024, 12, 243. [Google Scholar] [CrossRef]
- Bendixen, R.M.; Senesac, C.; Lott, D.J.; Vandenborne, K. Participation and quality of life in children with Duchenne muscular dystrophy using the International Classification of Functioning, Disability, and Health. Health Qual. Life Outcomes 2012, 10, 43. [Google Scholar] [CrossRef]
- McDonald, C.M.; McDonald, D.A.; Bagley, A.; Sienko Thomas, S.; Buckon, C.E.; Henricson, E.; Nicorici, A.; Sussman, M.D. Relationship between clinical outcome measures and parent proxy reports of health-related quality of life in ambulatory children with Duchenne muscular dystrophy. J. Child. Neurol. 2010, 25, 1130–1144. [Google Scholar] [CrossRef]
- Baiardini, I.; Minetti, C.; Bonifacino, S.; Porcu, A.; Klersy, C.; Petralia, P.; Balestracci, S.; Tarchino, F.; Parodi, S.; Canonica, G.W.; et al. Quality of life in Duchenne muscular dystrophy: The subjective impact on children and parents. J. Child. Neurol. 2011, 26, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Lue, Y.J.; Chen, S.S.; Lu, Y.M. Quality of life of patients with Duchenne muscular dystrophy: From adolescence to young men. Disabil. Rehabil. 2017, 39, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Fee, R.J.; Hinton, V.J. Resilience in children diagnosed with a chronic neuromuscular disorder. J. Dev. Behav. Pediatr. 2011, 32, 644–650. [Google Scholar] [CrossRef]
- Houwen-van Opstal, S.L.; Jansen, M.; van Alfen, N.; De Groot, I.J. Health-related quality of life and its relation to disease severity in boys with Duchenne muscular dystrophy: Satisfied boys, worrying parents--a case-control study. J. Child. Neurol. 2014, 29, 1486–1495. [Google Scholar] [CrossRef]
- Pangalila, R.F.; Van Den Bos, G.A.; Bartels, B.; Bergen, M.P.; Kampelmacher, M.J.; Stam, H.J.; Roebroeck, M.E. Quality of life of adult men with Duchenne muscular dystrophy in the Netherlands: Implications for care. J. Rehabil. Med. 2015, 47, 161–166. [Google Scholar] [CrossRef]
- Landfeldt, E.; Lindgren, P.; Bell, C.F.; Guglieri, M.; Straub, V.; Lochmüller, H.; Bushby, K. Health-related quality of life in patients with Duchenne muscular dystrophy: A multinational, cross-sectional study. Dev. Med. Child. Neurol. 2016, 58, 508–515. [Google Scholar] [CrossRef]
- Jacques, M.F.; Stockley, R.C.; Onambele-Pearson, G.L.; Reeves, N.D.; Stebbings, G.K.; Dawson, E.A.; Groves, L.; Morse, C.I. Quality of life in adults with muscular dystrophy. Health Qual. Life Outcomes 2019, 17, 121. [Google Scholar] [CrossRef]
- Jesus, A.; Bennett, C.; Masterson, C.; Brenner, L.; Scharf, R. Self- and Caregiver-Reported Participation, Quality of Life, and Related Mood and Behavior Challenges in People Living with Dystrophinopathies. Pediatr. Neurol. 2024, 151, 37–44. [Google Scholar] [CrossRef]
- Messina, S.; Vita, G.L.; Sframeli, M.; Mondello, S.; Mazzone, E.; D’Amico, A.; Berardinelli, A.; La Rosa, M.; Bruno, C.; Distefano, M.G.; et al. Health-related quality of life and functional changes in DMD: A 12-month longitudinal cohort study. Neuromuscul. Disord. 2016, 26, 189–196. [Google Scholar] [CrossRef]
- Simon, V.A.; Resende, M.B.; Simon, M.A.; Zanoteli, E.; Reed, U.C. Duchenne muscular dystrophy: Quality of life among 95 patients evaluated using the Life Satisfaction Index for Adolescents. Arq. Neuropsiquiatr. 2011, 69, 19–22. [Google Scholar] [CrossRef]
- Otto, C.; Steffensen, B.F.; Højberg, A.L.; Barkmann, C.; Rahbek, J.; Ravens-Sieberer, U.; Mahoney, A.; Vry, J.; Gramsch, K.; Thompson, R.; et al. Predictors of Health-Related Quality of Life in boys with Duchenne muscular dystrophy from six European countries. J. Neurol. 2017, 264, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Bray, P.; Bundy, A.C.; Ryan, M.M.; North, K.N.; Everett, A. Health-related quality of life in boys with Duchenne muscular dystrophy: Agreement between parents and their sons. J. Child. Neurol. 2010, 25, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Kohler, M.; Clarenbach, C.F.; Böni, L.; Brack, T.; Russi, E.W.; Bloch, K.E. Quality of life, physical disability, and respiratory impairment in Duchenne muscular dystrophy. Am. J. Respir. Crit. Care Med. 2005, 172, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Speechley, K.N.; Zou, G.; Campbell, C. The relationship between quality of life and health-related quality of life in young males with Duchenne muscular dystrophy. Dev. Med. Child. Neurol. 2017, 59, 1152–1157. [Google Scholar] [CrossRef]
- Liang, R.; Chan, S.H.S.; Ho, F.K.W.; Tang, O.C.; Cherk, S.W.W.; Ip, P.; Lau, E.Y.Y. Health-related quality of life in Chinese boys with Duchenne muscular dystrophy and their families. J. Child. Health Care 2019, 23, 495–506. [Google Scholar] [CrossRef]
- Zamani, G.; Heidari, M.; Azizi Malamiri, R.; Ashrafi, M.R.; Mohammadi, M.; Shervin Badv, R.; Hosseini, S.A.; Salehi, S.; Shahrokhi, A.; Qorbani, M.; et al. The quality of life in boys with Duchenne muscular dystrophy. Neuromuscul. Disord. 2016, 26, 423–427. [Google Scholar] [CrossRef]
- Graham, C.D.; Rose, M.R. What explains high life satisfaction in men living with Duchenne muscular dystrophy? A preliminary study to inform psychological intervention. Muscle Nerve 2017, 56, 163–166. [Google Scholar] [CrossRef]
- Elsenbruch, S.; Schmid, J.; Lutz, S.; Geers, B.; Schara, U. Self-reported quality of life and depressive symptoms in children, adolescents, and adults with Duchenne muscular dystrophy: A cross-sectional survey study. Neuropediatrics 2013, 44, 257–264. [Google Scholar] [CrossRef]
- Walklet, E.; Muse, K.; Meyrick, J.; Moss, T. Do Psychosocial Interventions Improve Quality of Life and Wellbeing in Adults with Neuromuscular Disorders? A Systematic Review and Narrative Synthesis. J. Neuromuscul. Dis. 2016, 3, 347–362. [Google Scholar] [CrossRef]
- Bozdoğan, Y.; Ergun, E. Exploring the experiences of parents of children with Duchenne muscular dystrophy in Turkey: A descriptive phenomenological study. J. Pediatr. Nurs. 2025, 82, 65–74. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).