1. Introduction
Unruptured intracranial aneurysms (UIAs) are increasingly detected due to the widespread use and improved resolution of non-invasive neuroimaging techniques over the last few years, with a reported prevalence of approximately 3% [
1]. Aneurysms of the anterior communicating artery (AComA) represent 25–30% of all intracranial aneurysms [
2,
3,
4,
5].
Several studies have shown that the natural history of AComA aneurysms is characterized by a higher risk of rupture compared to other aneurysms [
6,
7,
8]. The presence of risk factors further increases the rupture risk [
9,
10,
11]. Rupture of an AComA aneurysm typically results in subarachnoid hemorrhage (SAH), associated with high morbidity and mortality [
12].
The decision regarding treatment of an unruptured AComA aneurysm continues to be challenging and should be individualized according to patient characteristics and aneurysm morphology [
13]. A comprehensive evaluation is required to determine whether an aneurysm can be considered stable or is at risk of rupture. The likelihood of rupture is multifactorial and depends not only on clinical parameters [
14,
15] but also on lifestyle-related risk factors [
16,
17]. In addition, several studies have shown that the morphological characteristics of aneurysms [
18,
19] and hemodynamic parameters [
20] serve as important indicators of rupture risk and should be taken into account [
21].
Balancing the risk of rupture against potential treatment complications is central to decision-making for AComA aneurysms. This risk-benefit assessment directly influences the choice between available treatment strategies, which include microsurgical clipping and various endovascular approaches [
22]. Until the 1990s, microsurgical clipping was the only available treatment modality for AComA aneurysms [
23]. With the introduction of Guglielmi detachable coils (GDCs), endovascular techniques have gained increasing attention and have also become an essential part of aneurysm management [
24]. Since then, various endovascular treatment strategies have emerged, including coiling with balloon or stent assistance, as well as the implantation of Flow Diverters or intrasaccular devices [
25,
26,
27].
Nevertheless, microsurgical clipping remains the treatment modality of choice for many AComA aneurysms, particularly in cases involving complex vascular anatomy, wide necks, or unfavorable dome-to-neck ratios [
28]. The AComA region is anatomically complex, characterized by the presence of highly eloquent perforating arteries, especially the recurrent artery of Heubner [
29]. Frequent anatomical variants such as hypoplastic A1 segments pose significant challenges for both endovascular and microsurgical approaches [
30].
Studies have shown that the projection of the aneurysm dome should influence the choice of treatment modality [
31,
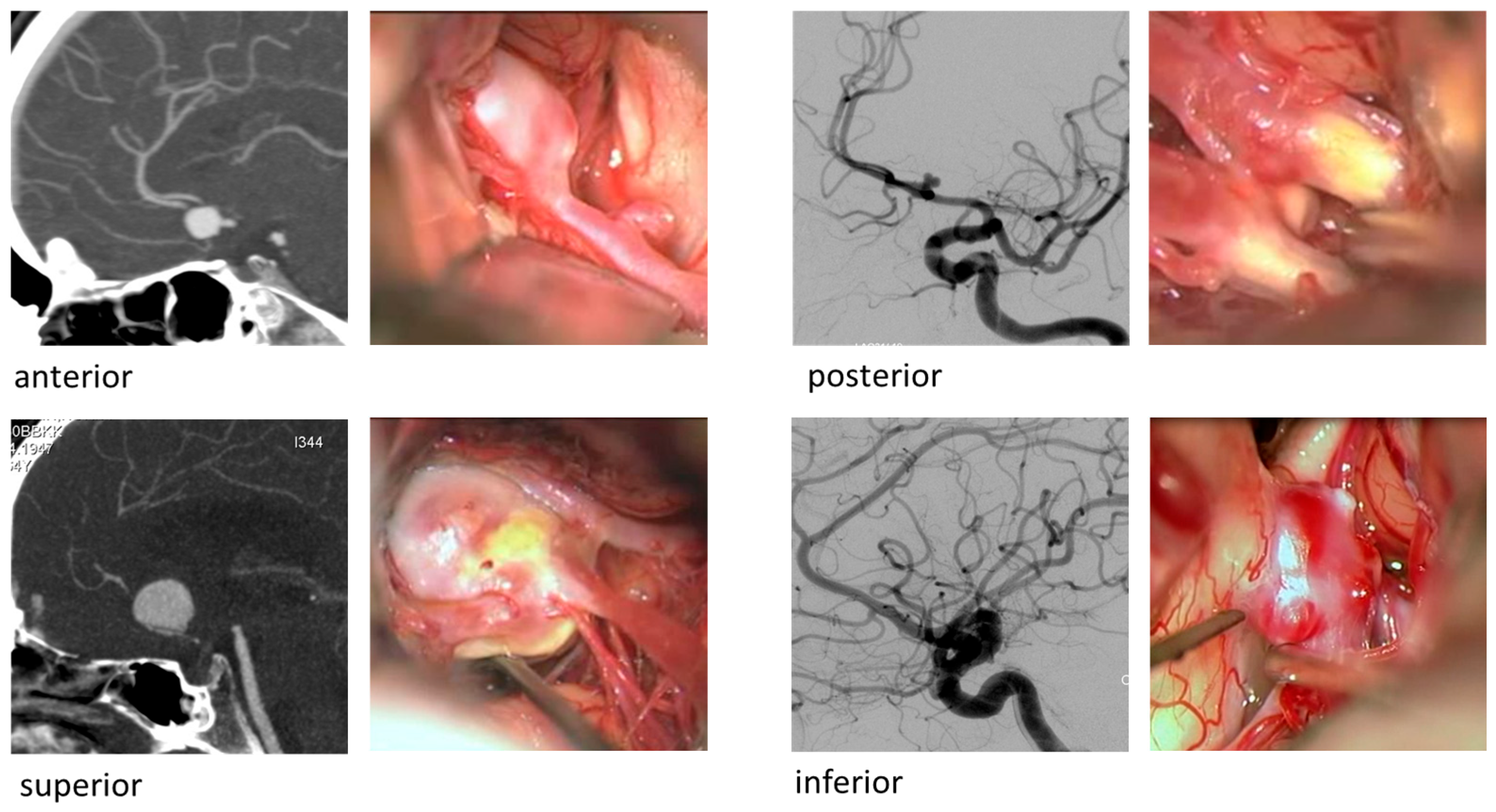
32]. AComA aneurysms may project in various directions—anteriorly, superiorly, inferiorly, or posteriorly (
Figure 1)—resulting in different risk profiles depending on their proximity to perforating arteries and the hypothalamic region [
33].
Despite the growing body of literature on microsurgical and endovascular treatment of AComA aneurysms, robust long-term outcome data, particularly for microsurgical clipping in unruptured cases, remain scarce. This evidence gap complicates treatment decisions, especially in anatomically complex cases where microsurgery may still be preferred. To address this gap and to provide comprehensive data to the ongoing discussion regarding the optimal management of AComA aneurysms and the continued role of microsurgical clipping in selected cases, we present an 18-year single-center analysis of clinical and radiological outcomes following microsurgical clipping of unruptured AComA aneurysms at a high-volume neurosurgical department.
2. Materials and Methods
The retrospective single-center study was conducted at the Department of Neurosurgery, Kepler University Hospital, Linz, Austria, and ethical approval was obtained from the local Ethics Committee of the Federal State of Upper Austria (EK-No.: 1318/2020).
2.1. Patient Selection
All Patients treated for an unruptured AComA aneurysm with microsurgical clipping between January 2002 and December 2020 at the neurosurgical department of the Kepler University Hospital in Linz, Austria were retrospectively identified and systematically entered into a database for subsequent analysis.
Inclusion required the confirmation of an AComA aneurysm by preoperative neuroradiological imaging, such as digital subtraction angiography (DSA) or computed tomography angiography (CTA). Microsurgical clipping had to be recommended as the primary treatment modality by an interdisciplinary cerebrovascular board comprising both endovascular and microsurgical specialists.
Patients were excluded if the aneurysm was previously ruptured or had prior surgical intervention on the same AComA aneurysm. The presence of a coincidental additional aneurysm was not considered an exclusion criterion.
A board-certified neuroradiologist retrospectively reviewed radiological imaging data. In cases of incomplete initial reports, missing findings were subsequently supplemented by the same neuroradiologist.
2.2. Preoperative Parameter
Preoperative data were categorized into patient-specific and aneurysm-specific parameters. Patient-specific variables included sex, age (in years), American Society of Anesthesiologists (ASA) score (ranging from 1 to 5, as a standardized measure of preoperative physical status and comorbidity burden), and relevant comorbidities such as autosomal dominant polycystic kidney disease (ADPKD), history of subarachnoid hemorrhage (SAH), arterial hypertension, diabetes mellitus, smoking status, and alcohol abuse—parameters that impact rupture risk assessment and may affect aneurysm morphology and treatment complexity.
Aneurysm-specific parameters included the projection of the aneurysm fundus, which was assessed using DSA or CTA. Fundus projection was categorized as anterior, superior, inferior, or posterior based on the projection axis. In addition, aneurysm-specific variables comprised the maximum diameter (in millimeters), prior endovascular coiling, presence of intraluminal thrombosis, aneurysm morphology, calcifications, and the number of coincidental aneurysms.
2.3. Surgical Technique and Intraoperative Monitoring
Microsurgical clipping was performed via a frontotemporal/pterional approach in all cases. Surgical planning included assessment of the dominant inflow vessel to maintain physiological flow patterns, guided by preoperative angiography and intraoperative ICG angiography. All procedures were carried out by board-certified neurosurgeons, specialized in cerebrovascular microneurosurgery.
The following intraoperative parameters were systematically documented: side of surgical approach, number of clips applied, necessity for clip repositioning, and occurrence of intraoperative aneurysm rupture. Temporary clipping of the A1 segment of the anterior cerebral artery was employed when required to achieve proximal vascular control.
Additional monitoring parameters were also recorded, including the use of intraoperative neuromonitoring with somatosensory evoked potentials (SSEP) and motor evoked potentials (MEP) to assess neurological integrity, as well as the application of indocyanine green (ICG) videoangiography to verify clip positioning and vessel patency.
Both the subjective intraoperative aneurysm occlusion rate and the radiologically verified occlusion rate were recorded, based on either intraoperative DSA or immediate postoperative DSA.
2.4. Outcome Parameters
Postoperative aneurysm occlusion was evaluated using DSA, either intraoperatively or within the first 24 h postoperatively. The degree of occlusion was classified according to the Raymond–Roy Occlusion Classification (RROC), distinguishing between complete occlusion (Class I), residual neck (Class II), and residual aneurysm (Class III).
In addition, a comprehensive set of postoperative clinical and radiological parameters was assessed: occurrence of postoperative intracerebral hemorrhage (ICH), remote cerebellar hemorrhage (RCH), chronic subdural hematoma (cSDH), and the need for revision surgery. Routine postoperative CT imaging was performed to exclude hematoma or significant brain edema. Ischemic complications such as cerebral infarction were documented, as well as new neurological deficits, which were further differentiated into transient and permanent deficits (defined as persisting beyond four weeks postoperatively). Other recorded complications comprised postoperative seizures, surgical site infections (defined as any wound infection requiring treatment, either antibiotic therapy or surgical intervention), and perioperative mortality.
Furthermore, long-term outcome parameters included the rate of aneurysm recurrence and the necessity for retreatment.
2.5. Statistical Analysis
The statistical analysis was conducted at the Center for Clinical Studies (CCS), Johannes Kepler University in Linz. Statistical analysis included descriptive statistics for all valid observations. For nominal variables, absolute and relative frequencies, and for metric variables, mean and standard deviation (SD), as well as median and range, were computed. Logistic regression models were applied to evaluate the association of preoperative and intraoperative parameters with postoperative outcomes. Variable selection was performed by stepwise forward and backward procedures. In case of quasi-complete separation, penalized likelihood estimation was applied to obtain stable regression estimates.
All statistical analyses were performed using the statistical software R Version 4.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
In this retrospective monocentric study, 106 patients undergoing surgical treatment for AComA aneurysms were included. The mean age was 52.9 years, and 11.3% had a documented history of SAH. Perioperative risk was assessed using the American Society of Anesthesiologists (ASA) physical status at admission in 80 patients, with the majority of patients (n = 47, 58.8%) classified as ASA 2 and only one patient presenting with an ASA score above 3. Baseline patient characteristics are summarized in
Table 1.
Given the relevance of aneurysm projection to surgical approach and outcome, the distribution of projections was examined in detail. The most frequent projection was anterior (n = 50, 47.2%), followed by superior (n = 32, 30.2%), inferior (n = 14, 13.2%), and posterior projection (n = 10, 9.4%). The mean maximal diameter was 5.9 mm (range, 1–19 mm). Seven aneurysms (6.6%) had been previously treated with coiling but required surgical clipping due to recurrence.
Preoperative imaging was performed predominantly via DSA in 91 (85.9%), CTA in 13 (12.3%) and MRA in 2 (1.9%) patients. Bleb formation, defined as focal outpouching of the aneurysm wall suggestive of localized wall weakness, was detected in preoperative imaging in 60 cases (56.6%). Detailed aneurysm-specific parameters are summarized in
Table 1.
3.1. Intraoperative Parameters
The average number of clips applied per aneurysm was 1.3 (range 0–3). Clip repositioning was required in 21 procedures (19.8%), whereas intraoperative rupture occurred in 3 cases (2.8%). Temporary clipping of the parent artery was performed in 12 cases (11.3%). Intraoperative neuromonitoring, including MEP and SSEP, was utilized in 31 interventions (29.3%), and intraoperative ICG angiography was conducted in 41 procedures (38.7%). The surgical approach was predominantly from the right side (65.1%), followed by the left (34.0%), with one midline approach (0.9%). Wrapping of the aneurysm was required in 3 cases (2.8%). Detailed information on the intraoperative characteristics is summarized in
Table 2.
3.2. Main Target Criteria
Complete aneurysm occlusion was confirmed by the surgeon intraoperatively in 101 patients (97.1%). Angiographic control demonstrated complete occlusion in 94 patients (92.2%), with no residual necks or aneurysmal remnants (see
Table 2).
Revision surgery was required in 4 patients (3.8%) and postoperative bleeding occurred in 6 patients (5.7%). Cerebral infarction occurred in 9 (8.5%), new neurological deficits (NND) developed in 18 patients (17.0%), of which 12 were transient (11.3%) and 6 permanent (5.7%). Postoperative epileptic seizures occurred in 7 patients (6.60%). Three patients died during the postoperative course, resulting in an overall mortality of 2.8%. One death was directly related to surgery (fulminant postoperative hemorrhage), while the other two resulted from medical complications unrelated to surgery (heart failure and pulmonary embolism). Notably, univariate analysis, posterior fundus projection showed a higher rate of permanent new neurological deficits (pNND) compared with non-posterior aneurysm locations (20% vs. 4%; OR 5.8, 95% CI 0.9–36.3; Fisher’s exact
p = 0.09). Although this difference did not reach statistical significance, the magnitude of the effect is notable. Detailed information on the postoperative outcome is summarized in
Table 3.
3.3. Logistic Regression Models
Logistic regression analyses were performed for the defined outcome variables (postoperative tNND, pNND, infarction, and postoperative hemorrhage). Results are presented in
Table 4,
Table 5,
Table 6 and
Table 7 for both the full multivariable models and the reduced models obtained through AIC-based stepwise selection. The principal associations identified in the final models are summarized below.
The results have identified a posterior fundus projection as an independent risk factor for postoperative infarction (p = 0.028), whereas the use of intraoperative ICG angiography exerted a protective effect (p = 0.034). The surgical side of approach (p = 0.037) was also protective, while posterior fundus projection (p = 0.030) remained an adverse predictor for the development of a pNND.
For tNND, intraoperative ICG angiography (p = 0.044) and the right-sided approach (p = 0.008) were associated with reduced risk. Postoperative hemorrhage showed significant associations with preoperative ASA (p = 0.016), alcohol consumption (p = 0.036), and diabetes mellitus (p = 0.026).
4. Discussion
This retrospective single-center study analyzed 106 patients who underwent microsurgical treatment for AComA aneurysms, placing it among the larger dedicated series focused exclusively on microsurgical clipping of AComA aneurysms. To date, only Nussbaum et al. [
34] have reported a substantially larger cohort (n = 300), while other notable registries such as those by Kim et al. [
35] (n = 113) and Lai et al. [
36] (n = 115) are of comparable size.
The present findings demonstrate a high rate of complete aneurysm occlusion, with intraoperative confirmation by the operating surgeon in 97.1% of cases and angiographically verified complete exclusion in 92.2%. These results reflect the well-established efficacy of microsurgical clipping in achieving durable aneurysm occlusion and are consistent with occlusion rates reported in the existing literature (92.5–98.4%) [
34,
35,
36,
37]. Smaller studies (n < 40), including those by Nanda et al. [
38] and Moon et al. [
39], have reported lower occlusion rates of 81.3% and 90%, respectively. These results underscore the complete occlusion rate achieved in the current cohort, and suggest a potential relationship between caseload and outcome.
Recently published data on endovascular treatment of AComA aneurysms reveals a wide range of occlusion and retreatment rates depending on the device and technique used. Coiling, including stent- and balloon-assisted variants, remains the most frequently employed modality, yet is associated with moderate rates of incomplete occlusion and recurrence. In the meta-analysis by Sattari et al., complete occlusion was achieved in just 77.3% of cases, with retreatment required in 4.2% overall and 7.5% of patients with unruptured aneurysms [
23]. These findings are consistent with the results reported by Catapano et al., who observed recurrence and retreatment rates of 13.4% and 9.7%, respectively [
40]. The WEB device has emerged as a promising alternative for wide-neck aneurysms, with Adeeb et al. reporting adequate occlusion in 80.6% of treated AComA aneurysms and no major complications [
41]. FDs have demonstrated long-term efficacy in selected anterior circulation aneurysms, with Pagiola et al. reporting complete occlusion rates of up to 86.9% in unruptured ACA aneurysms [
42]. However, FD use in the AComA region remains controversial due to the risk of perforator infarction and delayed rupture. Li et al. emphasized that aneurysms smaller than 4 mm carry a fivefold increased risk of intraoperative rupture during coiling, and highlighted the importance of careful device selection in this anatomically sensitive region [
25]. Our findings demonstrate a strong durability signal for microsurgical clipping, reflected by a retreatment rate of only 0.9%, which aligns with prior microsurgical series and contrasts with higher retreatment rates reported for endovascular techniques.
Postoperative complications were infrequent. Postoperative new neurological deficits were observed in 18 patients, with only six cases (5.7%) resulting in permanent impairment. Logistic regression analysis demonstrated a significant association between posterior aneurysm projection and both infarction and pNND. These findings highlight the critical role of aneurysm projection in surgical planning and risk stratification. Posteriorly projecting aneurysms, due to their anatomical proximity to perforating arteries and the hypothalamic region, pose a higher risk for ischemic complications—likely attributable to limited intraoperative visualisation and increased manipulation of adjacent perforators. Consequently, meticulous dissection and precise clip placement are essential [
33]. Moreover, aneurysm projection should be considered in the decision-making process when choosing between endovascular and microsurgical treatment approaches, reinforcing the need for individualized therapeutic strategies based on anatomical configuration, as previously described in several series [
32,
43,
44]. Unlike endovascular procedures, microsurgical clipping in our cohort was performed without perioperative antiplatelet therapy, eliminating medication-related hemorrhagic risks. In contrast, the mandatory dual antiplatelet regimen for several endovascular techniques introduces additional thromboembolic and bleeding complications.
Intraoperative ICG angiography was employed in 41 of the 106 procedures (38.7%) and intraoperative neuromonitoring in 31 procedures (29.3%). Intraoperative ICG angiography was first introduced into our surgical routine in 2011 with occasional use, and from 2016 onward, both intraoperative ICG angiography and intraoperative Neuromonitoring were implemented as standard modalities in every case. The data suggest a remarkable protective effect of intraoperative ICG angiography, particularly with regard to reducing postoperative infarctions and thereby lowering the risk of tNND. These findings highlight the role of intraoperative ICG angiography as an important adjunct in microsurgical aneurysm treatment, improving both surgical safety and patient outcomes, and are consistent with prior reports demonstrating reduced vascular injury–related morbidity with intraoperative ICG angiography [
45,
46]. In contrast, neuromonitoring with SSEPs and MEPs did not show a significant association with outcome parameters in bivariate or regression analyses. This may reflect limitations in sensitivity, as previously reported in the literature, where the detection of ischemic events by neuromonitoring has been shown to vary considerably depending on monitoring protocols and interpretation thresholds [
47]. Notably, in this subgroup, only one case of pNND due to infarction was observed in 41 aneurysm clippings, highlighting the synergistic potential of integrating both modalities. This stepwise integration likely contributed to the continuous improvement of outcomes over time. In addition, advances in surgical skill, refinements in microsurgical technique, preoperative imaging, and the integration of hybrid operating theatres with high-resolution intraoperative angiography further strengthened perioperative safety.
Taken together, our results corroborate recent reports demonstrating that the combined use of intraoperative ICG angiography and intraoperative neuromonitoring enhances procedural safety in aneurysm surgery, while also reflecting the high procedural safety and favorable outcomes documented in contemporary microsurgical benchmark series [
48].
5. Limitations
This study has several limitations that must be acknowledged. The retrospective design inherently introduces potential biases, including incomplete data capture, as noted in the table descriptions, and variability in documentation quality. Although experienced cerebrovascular neurosurgeons performed all surgical procedures and the indication for clipping was set in an interdisciplinary cerebrovascular board, the single-center nature of the study limits generalizability and may reflect institutional preferences in treatment selection and intraoperative technique.
Furthermore, the cohort likely represents a selected subset of AComA aneurysms—those deemed suitable for microsurgical clipping by an interdisciplinary cerebrovascular board. As such, aneurysms with favorable anatomy for endovascular treatment may have been excluded, potentially skewing the dataset toward more complex cases.
The long observation period spanning nearly two decades introduces heterogeneity in surgical technique, imaging modalities, and perioperative management. While this reflects real-world clinical evolution, it may confound outcome comparisons across time, as the period also encompasses significant advances in both microsurgical and endovascular techniques. No subgroup analysis by time period was performed, which may limit interpretation of temporal trends.
Frequent comparisons with endovascular results from the literature may involve non-contemporaneous comparison bias, which should be considered when interpreting our findings.
Neurocognitive outcomes and quality-of-life measures were not systematically assessed, although these parameters are increasingly recognized as essential endpoints in aneurysm treatment.
6. Conclusions
Our study demonstrates that microsurgical clipping of unruptured AComA aneurysms is a highly effective treatment modality in selected cases, achieving high rates of complete angiographic occlusion and low rates of permanent neurological deficits. The data suggest that aneurysm projection significantly influences postoperative outcomes, with posteriorly projected aneurysms associated with increased risk of infarction and neurological complications. Accordingly, aneurysm projection should be considered when selecting the optimal treatment strategy, whether endovascular or microsurgical. Intraoperative ICG angiography proved beneficial in reducing ischemic events and may be recommended as an adjunct intraoperative tool.
Despite the increasing availability of endovascular techniques, microsurgical clipping remains a valuable option, particularly in cases with complex anatomy, wide necks, or prior endovascular failure. These findings support the continued role of microsurgery in the interdisciplinary management of AComA aneurysms and highlight the importance of individualized treatment planning based on anatomical and radiological characteristics.
Future prospective studies are needed to validate these findings and further refine interdisciplinary treatment strategies for AComA aneurysms.
Author Contributions
Conceptualization, N.S.-H. and M.G. (Matthias Gmeiner); methodology, N.S.-H.; formal analysis, P.H., H.W.; investigation, S.A., P.R., W.S., M.G. (Maria Gollwitzer); resources, N.S.-H.; data curation, N.S.-H., M.S.V., B.L., W.S.; writing—original draft preparation, N.S.-H., M.S.V., M.G. (Matthias Gmeiner); writing—review and editing, N.S.-H., A.G., M.G. (Matthias Gmeiner); visualization, M.S., A.G.; supervision, M.G. (Matthias Gmeiner); project administration, N.S.-H., M.G. (Matthias Gmeiner). All authors have read and agreed to the published version of the manuscript.
Funding
The authors did not receive financial support for the research, authorship, and/or publication of this article. This research received no external funding.
Institutional Review Board Statement
The retrospective study obtained ethical approval from the local Ethics Committee of the Federal State Upper Austria (EK-No.: 1318/2020, ethics approval date: 13 January 2021).
Informed Consent Statement
Patient consent was waived due to the retrospective study design and in accordance with local laws and regulations, which do not require individual consent for retrospective data analysis. Written informed consent has been obtained from the patients whose images are shown in
Figure 1 to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
During the preparation of this manuscript, the authors used Microsoft 365 Copilot (Version 2023), an AI-assisted technology, to support language editing and enhance readability. All content generated or modified with the assistance of this tool was subsequently reviewed and edited by the authors. The authors take full responsibility for the content of the final manuscript. Supported by Johannes Kepler University Open Access Publishing Fund.
Conflicts of Interest
Andreas Gruber, MD, PhD, co-author of this manuscript, serves as head of the Department of Neurosurgery (Kepler University Hospital, Johannes Kepler University Linz, Austria) and as Guest Editor of the Special Issue “Advances in Cerebral Aneurysm Surgery: The Latest Technologies and Techniques” in Brain Sciences.
Abbreviations
The following abbreviations are used in this manuscript:
| AComA | Anterior communicating artery |
| ADPKD | Autosomal dominant polycystic kidney disease |
| ASA | American Society of Anesthesiologists |
| cSDH | chronic subdural hematoma |
| CTA | Computed Tomography Angiography |
| DSA | Digital Subtraction Angiography |
| FD | Flow Diverter |
| ICG | Indocyanine green angiography |
| ICH | Intracerebral hemorrhage |
| MEP | Motor evoked potential |
| MRA | Magnetic Resonance Angiography |
| NND | New neurological deficit |
| pNND | Permanent new neurological deficit |
| RROC | Raymond–Roy occlusion classification |
| SAH | Subarachnoid hemorrhage |
| SD | Standard deviation (SD) |
| SSEP | Somatosensory evoked potential |
| SSI | Surgical site infection |
| tNND | Transient new neurological deficit |
| UIA | Unruptured intracranial aneurysm |
| WEB | Woven Endo Bridge |
References
- Vlak, M.H.; Algra, A.; Brandenburg, R.; Rinkel, G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef]
- Yasargil, M.G. Microneurosurgery: Clinical Considerations, Surgery of the Intracranial Aneurysms and Results. Vol. II; Thieme: New York, NY, USA, 1984. [Google Scholar]
- Ujiie, H.; Liepsch, D.W.; Goetz, M.; Yamaguchi, R.; Yonetani, H.; Takakura, K. Hemodynamic study of the anterior communicating artery. Stroke 1996, 27, 2086–2093, discussion 2094. [Google Scholar] [CrossRef]
- Brilstra, E.H.; Rinkel, G.J.; van der Graaf, Y.; van Rooij, W.J.; Algra, A. Treatment of intracranial aneurysms by embolization with coils: A systematic review. Stroke 1999, 30, 470–476. [Google Scholar] [CrossRef]
- Zhang, X.J.; Gao, B.L.; Hao, W.L.; Wu, S.S.; Zhang, D.H. Presence of Anterior Communicating Artery Aneurysm Is Associated With Age, Bifurcation Angle, and Vessel Diameter. Stroke 2018, 49, 341–347. [Google Scholar] [CrossRef]
- Wiebers, D.O.; Whisnant, J.P.; Huston, J., 3rd; Meissner, I.; Brown, R.D., Jr.; Piepgras, D.G.; Forbes, G.S.; Thielen, K.; Nichols, D.; O’Fallon, W.M.; et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003, 362, 103–110. [Google Scholar] [CrossRef]
- Matsukawa, H.; Uemura, A.; Fujii, M.; Kamo, M.; Takahashi, O.; Sumiyoshi, S. Morphological and clinical risk factors for the rupture of anterior communicating artery aneurysms. J. Neurosurg. 2013, 118, 978–983. [Google Scholar] [CrossRef]
- Morita, A.; Kirino, T.; Hashi, K.; Aoki, N.; Fukuhara, S.; Hashimoto, N.; Nakayama, T.; Sakai, M.; Teramoto, A.; Tominari, S.; et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N. Engl. J. Med. 2012, 366, 2474–2482. [Google Scholar] [CrossRef] [PubMed]
- Etminan, N.; Brown, R.D., Jr.; Beseoglu, K.; Juvela, S.; Raymond, J.; Morita, A.; Torner, J.C.; Derdeyn, C.P.; Raabe, A.; Mocco, J.; et al. The unruptured intracranial aneurysm treatment score: A multidisciplinary consensus. Neurology 2015, 85, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Etminan, N.; Beseoglu, K.; Barrow, D.L.; Bederson, J.; Brown, R.D., Jr.; Connolly, E.S., Jr.; Derdeyn, C.P.; Hänggi, D.; Hasan, D.; Juvela, S.; et al. Multidisciplinary consensus on assessment of unruptured intracranial aneurysms: Proposal of an international research group. Stroke 2014, 45, 1523–1530. [Google Scholar] [CrossRef]
- Greving, J.P.; Wermer, M.J.; Brown, R.D., Jr.; Morita, A.; Juvela, S.; Yonekura, M.; Ishibashi, T.; Torner, J.C.; Nakayama, T.; Rinkel, G.J.; et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014, 13, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Nieuwkamp, D.J.; Setz, L.E.; Algra, A.; Linn, F.H.; de Rooij, N.K.; Rinkel, G.J. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: A meta-analysis. Lancet Neurol. 2009, 8, 635–642. [Google Scholar] [CrossRef]
- Etminan, N.; de Sousa, D.A.; Tiseo, C.; Bourcier, R.; Desal, H.; Lindgren, A.; Koivisto, T.; Netuka, D.; Peschillo, S.; Lémeret, S.; et al. European Stroke Organisation (ESO) guidelines on management of unruptured intracranial aneurysms. Eur. Stroke J. 2022, 7, 81–106. [Google Scholar] [CrossRef]
- Backes, D.; Rinkel, G.J.; Laban, K.G.; Algra, A.; Vergouwen, M.D. Patient- and Aneurysm-Specific Risk Factors for Intracranial Aneurysm Growth: A Systematic Review and Meta-Analysis. Stroke 2016, 47, 951–957. [Google Scholar] [CrossRef]
- Korja, M.; Lehto, H.; Juvela, S. Lifelong rupture risk of intracranial aneurysms depends on risk factors: A prospective Finnish cohort study. Stroke 2014, 45, 1958–1963. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Wang, J.; Zhang, L.; Li, C. The Biological Effects of Smoking on the Formation and Rupture of Intracranial Aneurysms: A Systematic Review and Meta-Analysis. Front. Neurol. 2022, 13, 862916. [Google Scholar] [CrossRef]
- Vlak, M.H.; Rinkel, G.J.; Greebe, P.; van der Bom, J.G.; Algra, A. Trigger factors and their attributable risk for rupture of intracranial aneurysms: A case-crossover study. Stroke 2011, 42, 1878–1882. [Google Scholar] [CrossRef]
- Lall, R.R.; Eddleman, C.S.; Bendok, B.R.; Batjer, H.H. Unruptured intracranial aneurysms and the assessment of rupture risk based on anatomical and morphological factors: Sifting through the sands of data. Neurosurg. Focus 2009, 26, E2. [Google Scholar] [CrossRef]
- Waqas, M.; Chin, F.; Rajabzadeh-Oghaz, H.; Gong, A.D.; Rai, H.H.; Mokin, M.; Vakharia, K.; Dossani, R.H.; Meng, H.; Snyder, K.V.; et al. Size of ruptured intracranial aneurysms: A systematic review and meta-analysis. Acta Neurochir. 2020, 162, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, F.; Sela, S.; Gesualdo, L.; Chevrel, S.; Tollet, F.; Pailler-Mattei, C.; Tacconi, L.; Turjman, F.; Vacca, A.; Schul, D.B. Hemodynamic Stress, Inflammation, and Intracranial Aneurysm Development and Rupture: A Systematic Review. World Neurosurg. 2018, 115, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, X. Risk factors and predictive indicators of rupture in cerebral aneurysms. Front. Physiol. 2024, 15, 1454016. [Google Scholar] [CrossRef] [PubMed]
- Unruptured intracranial aneurysms--risk of rupture and risks of surgical intervention. N. Engl. J. Med. 1998, 339, 1725–1733. [CrossRef]
- Sattari, S.A.; Shahbandi, A.; Lee, R.P.; Feghali, J.; Rincon-Torroella, J.; Yang, W.; Abdulrahim, M.; Ahmadi, S.; So, R.J.; Hung, A.; et al. Surgery or Endovascular Treatment in Patients with Anterior Communicating Artery Aneurysm: A Systematic Review and Meta-Analysis. World Neurosurg. 2023, 175, 31–44. [Google Scholar] [CrossRef]
- Moret, J.; Pierot, L.; Boulin, A.; Castaings, L.; Rey, A. Endovascular treatment of anterior communicating artery aneurysms using Guglielmi detachable coils. Neuroradiology 1996, 38, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, K.; Yu, J. Current state of endovascular treatment of anterior cerebral artery aneurysms. Front. Neurol. 2024, 15, 1396701. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, P.; Martinez Moreno, R.; Ganslandt, O.; Bäzner, H.; Henkes, H.; Perez, M.A. Use of flow diverters in the treatment of unruptured saccular aneurysms of the anterior cerebral artery. J. Neurointerv. Surg. 2017, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Behme, D.; Berlis, A.; Weber, W. Woven EndoBridge Intrasaccular Flow Disrupter for the Treatment of Ruptured and Unruptured Wide-Neck Cerebral Aneurysms: Report of 55 Cases. AJNR Am. J. Neuroradiol. 2015, 36, 1501–1506. [Google Scholar] [CrossRef]
- Etminan, N.; Rinkel, G.J. Unruptured intracranial aneurysms: Development, rupture and preventive management. Nat. Rev. Neurol. 2016, 12, 699–713. [Google Scholar] [CrossRef]
- Wolman, D.N.; Moraff, A.M.; Heit, J.J. Anatomy of the Intracranial Arteries: The Anterior Intracranial and Vertebrobasilar Circulations. Neuroimaging Clin. 2022, 32, 617–636. [Google Scholar] [CrossRef]
- Hernesniemi, J.; Dashti, R.; Lehecka, M.; Niemelä, M.; Rinne, J.; Lehto, H.; Ronkainen, A.; Koivisto, T.; Jääskeläinen, J.E. Microneurosurgical management of anterior communicating artery aneurysms. Surg. Neurol. 2008, 70, 8–28, discussion 29. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, S.; Zang, B.; Lv, X. Classification of anterior communicating aneurysms on a basis of endovascular treatments. Neuroradiol. J. 2024, 37, 68–73. [Google Scholar] [CrossRef]
- Proust, F.; Debono, B.; Hannequin, D.; Gerardin, E.; Clavier, E.; Langlois, O.; Fréger, P. Treatment of anterior communicating artery aneurysms: Complementary aspects of microsurgical and endovascular procedures. J. Neurosurg. 2003, 99, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, M.; Zhu, X.; Chen, Y.; Zhang, C.; Shi, W.; Chen, Q.; Wang, Y. Anterior Communicating Artery Aneurysms: Anatomical Considerations and Microsurgical Strategies. Front. Neurol. 2020, 11, 1020. [Google Scholar] [CrossRef]
- Nussbaum, E.S.; Touchette, J.C.; Madison, M.T.; Goddard, J.K.; Lassig, J.P.; Nussbaum, L.A. Microsurgical Treatment of Unruptured Anterior Communicating Artery Aneurysms: Approaches and Outcomes in a Large Contemporary Series and Review of the Literature. Oper. Neurosurg. 2020, 19, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jeon, H.J.; Ihm, E.H.; Park, K.Y.; Lee, J.W.; Huh, S.K. Microsurgical efficacy and safety of a right-hemispheric approach for unruptured anterior communicating artery aneurysms. Clin. Neurol. Neurosurg. 2015, 137, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.T.; Gragnaniello, C.; Morgan, M.K. Outcomes for a case series of unruptured anterior communicating artery aneurysm surgery. J. Clin. Neurosci. 2013, 20, 1688–1692. [Google Scholar] [CrossRef]
- Mori, K.; Wada, K.; Otani, N.; Tomiyama, A.; Toyooka, T.; Tomura, S.; Takeuchi, S.; Yamamoto, T.; Nakao, Y.; Arai, H. Long-Term Neurological and Radiological Results of Consecutive 63 Unruptured Anterior Communicating Artery Aneurysms Clipped via Lateral Supraorbital Keyhole Minicraniotomy. Oper. Neurosurg. 2018, 14, 95–103. [Google Scholar] [CrossRef]
- Nanda, A.; Patra, D.P.; Bir, S.C.; Maiti, T.K.; Kalakoti, P.; Bollam, P. Microsurgical Clipping of Unruptured Intracranial Aneurysms: A Single Surgeon’s Experience over 16 Years. World Neurosurg. 2017, 100, 85–99. [Google Scholar] [CrossRef]
- Moon, J.S.; Choi, C.H.; Lee, T.H.; Ko, J.K. Result of coiling versus clipping of unruptured anterior communicating artery aneurysms treated by a hybrid vascular neurosurgeon. J. Cerebrovasc. Endovasc. Neurosurg. 2020, 22, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Catapano, J.S.; Karahalios, K.; Rumalla, K.; Srinivasan, V.M.; Rutledge, C.; Baranoski, J.F.; Cole, T.S.; Jadhav, A.P.; Ducruet, A.F.; Albuquerque, F.C. Endovascular treatment of ruptured anterior communicating aneurysms: A 17-year institutional experience with coil embolization. J. Neurointerv Surg. 2022, 14, 1018–1021. [Google Scholar] [CrossRef]
- Adeeb, N.; Dibas, M.; Diestro, J.D.B.; Phan, K.; Cuellar-Saenz, H.H.; Sweid, A.; Lay, S.V.; Guenego, A.; Aslan, A.; Renieri, L.; et al. Comparing treatment outcomes of various intracranial bifurcation aneurysms locations using the Woven EndoBridge (WEB) device. J. Neurointerv. Surg. 2023, 15, 558–565. [Google Scholar] [CrossRef]
- Pagiola, I.; Mihalea, C.; Caroff, J.; Ikka, L.; Chalumeau, V.; Yasuda, T.; Marenco de la Torre, J.; Iacobucci, M.; Ozanne, A.; Gallas, S.; et al. Flow diversion treatment of aneurysms of the complex region of the anterior communicating artery: Which stent placement strategy should ‘I’ use? A single center experience. J. Neurointerv. Surg. 2019, 11, 1118–1122. [Google Scholar] [CrossRef]
- Kasinathan, S.; Yamada, Y.; Cheikh, A.; Teranishi, T.; Kawase, T.; Kato, Y. Prognostic Factors Influencing Outcome in Unruptured Anterior Communicating Artery Aneurysm after Microsurgical Clipping. Asian J. Neurosurg. 2019, 14, 28–34. [Google Scholar] [CrossRef]
- Navrátil, O.; Ďuriš, K.; Juráň, V.; Svoboda, K.; Hustý, J.; Hovorka, E.; Neuman, E.; Mrlian, A.; Smrčka, M. Current Treatment of Anterior Communicating Artery Aneurysms: Single Center Study. Brain Sci. 2020, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Tajsic, T.; Cullen, J.; Guilfoyle, M.; Helmy, A.; Kirollos, R.; Kirkpatrick, P.; Trivedi, R. Indocyanine green fluorescence video angiography reduces vascular injury-related morbidity during micro-neurosurgical clipping of ruptured cerebral aneurysms: A retrospective observational study. Acta Neurochir. 2019, 161, 2397–2401. [Google Scholar] [CrossRef]
- Gruber, A.; Dorfer, C.; Standhardt, H.; Bavinzski, G.; Knosp, E. Prospective comparison of intraoperative vascular monitoring technologies during cerebral aneurysm surgery. Neurosurgery 2011, 68, 657–673, discussion 673. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Kim, B.H.; Lee, S.E.; Jeong, E.; Cho, K.; Park, J.K.; Choi, Y.J.; Jin, S.; Hong, D.; Kim, M.C. Usefulness of Intraoperative Neurophysiological Monitoring During the Clipping of Unruptured Intracranial Aneurysm: Diagnostic Efficacy and Detailed Protocol. Front. Surg. 2021, 8, 631053. [Google Scholar] [CrossRef]
- Drexler, R.; Sauvigny, T.; Pantel, T.F.; Ricklefs, F.L.; Catapano, J.S.; Wanebo, J.E.; Lawton, M.T.; Sanchin, A.; Hecht, N.; Vajkoczy, P.; et al. Global Outcomes for Microsurgical Clipping of Unruptured Intracranial Aneurysms: A Benchmark Analysis of 2245 Cases. Neurosurgery 2024, 94, 369–378. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Illustration of representative cases of the cohort—Anterior communicating artery aneurysms and the four aneurysm projections: anterior, posterior, superior and inferior.
Figure 1.
Illustration of representative cases of the cohort—Anterior communicating artery aneurysms and the four aneurysm projections: anterior, posterior, superior and inferior.
Table 1.
Descriptive statistics of preoperative patient- and aneurysm-specific parameters. For categorial variables, absolute frequencies (percent) are reported without missing values. ADPKD = autosomal dominant polycystic kidney disease, SAH = subarachnoidal haemorrhage, max. = maximal, mm = millimeter, prev. = previously. ASA = American Society of Anesthesiologists. a = data missing for 4 patients, b = data missing for 26 patients, c = data missing for 13 patients, d = data missing for 10 patients, e = data missing for 2 patients, f = data missing for 8 patients.
Table 1.
Descriptive statistics of preoperative patient- and aneurysm-specific parameters. For categorial variables, absolute frequencies (percent) are reported without missing values. ADPKD = autosomal dominant polycystic kidney disease, SAH = subarachnoidal haemorrhage, max. = maximal, mm = millimeter, prev. = previously. ASA = American Society of Anesthesiologists. a = data missing for 4 patients, b = data missing for 26 patients, c = data missing for 13 patients, d = data missing for 10 patients, e = data missing for 2 patients, f = data missing for 8 patients.
| Preoperative Parameters | Value |
|---|
| Number of cases (n) | 106 |
| Patient-specific parameters |
| Mean Age in years (standard deviation) | 56.42 (10.27) |
| ADPKD a | 3 (2.94%) |
| Mean stay in hospital in days (standard deviation) | 18.48 (16.33) |
| SAH in anamnesis | 12 (11.3%) |
| Distribution of ASA Score b | |
| 1 | 12 (15.00%) |
| 2 | 47 (58.75%) |
| 3 | 20 (25.00%) |
| 4 | 0 (0%) |
| 5 | 1 (1.25%) |
| Arterial hypertension c | 59 (63.44%) |
| Smoking d | 41(42.71%) |
| Diabetes Mellitus e | 4 (3.85%) |
| Alcohol f | 16 (16.33%) |
| Aneurysm-specific parameters |
| Fundus Projection | anterior | 50 (47.17%) |
| superior | 32 (30.19%) |
| inferior | 14 (13.21%) |
| posterior | 10 (9.43%) |
| Max. Diameter mean in mm (range) | 5.92 (1.00–19.00) |
| Prev. Coiled | yes | 7 (6.60%) |
| Thrombosed | yes | 1 (0.94%) |
| Blebs | yes | 60 (56.60%) |
| Calcifications | yes | 5 (4.72%) |
| Number of other aneurysms | 0 | 68 (64.15%) |
| 1 | 30 (28.30%) |
| 2 | 7 (6.60%) |
| 3 | 1 (0.94%) |
Table 2.
Intraoperative parameters. For categorical variables, absolute frequencies (percent of non-missing values) are reported. MEP = motor evoked potentials, SSEP = somatosensory evoked potentials, ICG = indocyanine green angiography.
Table 2.
Intraoperative parameters. For categorical variables, absolute frequencies (percent of non-missing values) are reported. MEP = motor evoked potentials, SSEP = somatosensory evoked potentials, ICG = indocyanine green angiography.
| Intraoperative Parameters | Value (%) |
|---|
| Reposition of clips | 21 (19.81%) |
| Average number of clips | 1.35 (0–3) |
| Number of Clips | 1 | 71 (66.98%) |
| 2 | 30 (28.30%) |
| 3 | 4 (3.77%) |
| Intraoperative Rupture of aneurysm | 3 (2.83%) |
| Temporary Clipping | 12 (11.32%) |
| Intraoperative Neuromonitoring (MEP, SSEP) | 31 (29.25%) |
| Intraoperative ICG angiography | 41(38.68%) |
| Side of approach | right | 69 (65.09%) |
| left | 36 (33.96%) |
| median | 1 (0.94%) |
| Wrapping | 3 (2.83%) |
| Main Target Criteria |
| | Complete occlusion (surgeon) | 101 (97.12%) |
| | Complete occlusion (angiographic) | 94 (92.16%) |
Table 3.
Postoperative complications. For categorical variables, absolute frequencies (percent of non-missing values) are reported. cSDH = chronic subdural hematoma.
Table 3.
Postoperative complications. For categorical variables, absolute frequencies (percent of non-missing values) are reported. cSDH = chronic subdural hematoma.
| Postoperative Complications | Value (%) |
|---|
| Revision surgery | 4 (3.77%) |
| Postoperative hemorrhage | 6 (5.66%) |
| Remote bleeding | 2 (1.89%) |
| cSDH | 0 (0%) |
| Infarction | 9 (8.49%) |
| Neurological deficit | 18 (16.98%) |
| Permanent deficit | 6 (5.66%) |
| Transient deficit | 12 (11.32%) |
| Postoperative seizure | 7 (6.60%) |
| Surgical site infection | 13 (12.26%) |
| Perioperative death | 3 (2.83%) |
| Recurrence | 1 (0.94%) |
| Retreatment rate | 1 (0.94%) |
Table 4.
This table reports the results of the logistic regression model for the outcome parameter tNND. tNND = transient new neurological deficit. Stepwise variable selection was performed using the Akaike Information Criterion (AIC); ADPKD = autosomal dominant polycystic kidney disease, ICG = indocyanine green angiography, SAH = subarachnoidal hemorrhage, IONM = intraoperative neuromonitoring, AcomA = Arteria communicans anterior aneurysm, mASA = American Society of Anesthesiologists classification.
Table 4.
This table reports the results of the logistic regression model for the outcome parameter tNND. tNND = transient new neurological deficit. Stepwise variable selection was performed using the Akaike Information Criterion (AIC); ADPKD = autosomal dominant polycystic kidney disease, ICG = indocyanine green angiography, SAH = subarachnoidal hemorrhage, IONM = intraoperative neuromonitoring, AcomA = Arteria communicans anterior aneurysm, mASA = American Society of Anesthesiologists classification.
| | Estimate | ci-Lower | ci-Upper | p-Value |
|---|
| Penalized likelihood logistic regression model für tNND |
| Intercept | −0.455 | −1.951 | 0.939 | 0.523 |
| Superior Projection | 0.266 | −1.265 | 1.778 | 0.726 |
| Posterior Projection | −0.396 | −5.332 | 2.112 | 0.792 |
| Inferior Projection | 1.009 | −0.726 | 2.732 | 0.243 |
| Diabetes | 0.023 | −0.168 | 0.198 | 0.798 |
| Calcification | 0.379 | −4.657 | 3.116 | 0.823 |
| Blebs | −0.939 | −2.265 | 0.297 | 0.136 |
| Intraoperative Neuromonitoring | 0.383 | 2.394 | 5.410 | 0.809 |
| Intraoperative ICG angiography | −1.413 | −6.414 | 1.005 | 0.290 |
| Side of approach (right) | −1.692 | −3.161 | −0.411 | 0.009 |
| Clip Repositioning | 0.839 | −0.789 | 2.456 | 0.300 |
| Penalized likelihood logistic regression model for transient deficit after variable selection |
| Intercept | −0.066 | −1.201 | 1.049 | 0.906 |
| Diabetes | 0.039 | −0.134 | 0.193 | 0.634 |
| Blebs | −1.171 | −2.532 | 0.066 | 0.064 |
| Intraoperative ICG angiography | −1.418 | −3.161 | −0.038 | 0.044 |
| Right-sided approach | −1.663 | −3.032 | −0.424 | 0.008 |
Table 5.
This table reports the results of the logistic regression model for the outcome parameter pNND. pNND = permanent new neurological deficit. Stepwise variable selection was performed using the Akaike Information Criterion (AIC); ADPKD = autosomal dominant polycystic kidney disease, ICG = indocyanine green angiography, SAH = subarachnoidal hemorrhage, IONM = intraoperative monitoring, AcomA = Arteria communicans anterior aneurysm, mASA = American Society of Anesthesiologists classification.
Table 5.
This table reports the results of the logistic regression model for the outcome parameter pNND. pNND = permanent new neurological deficit. Stepwise variable selection was performed using the Akaike Information Criterion (AIC); ADPKD = autosomal dominant polycystic kidney disease, ICG = indocyanine green angiography, SAH = subarachnoidal hemorrhage, IONM = intraoperative monitoring, AcomA = Arteria communicans anterior aneurysm, mASA = American Society of Anesthesiologists classification.
| | Estimate | ci-Lower | ci-Upper | p-Value |
|---|
| Penalized likelihood regression model for pNND |
| Intercept | −1.926 | −4.362 | −0.182 | 0.029 |
| Superior Projection | 0.961 | −1.184 | 3.446 | 0.374 |
| Posterior Projection | 2.611 | 0.226 | 5.604 | 0.032 |
| Inferior Projection | 1.311 | −1.291 | 3.905 | 0.292 |
| Diabetes | −0.117 | −0.509 | 0.132 | 0.389 |
| Calcification | 1.586 | −3.448 | 4.815 | 0.416 |
| Blebs | −0.343 | −2.115 | 1.336 | 0.681 |
| IONM | 0.935 | −2.282 | 6.079 | 0.587 |
| Intraoperative ICG angiography | −1.933 | −7.137 | 0.943 | 0.222 |
| Side of surgical approach (right) | −1.873 | −4.069 | −0.118 | 0.036 |
| Clip Repositioning | 1.348 | −1.114 | 3.702 | 0.252 |
| Penalized likelihood regression model for pNND after manual backward selection |
| Intercept | −2.168 | −4.438 | −0.670 | 0.003 |
| Superior Projection | 1.011 | −1.108 | 3.482 | 0.344 |
| Posterior Projection | 2.481 | 0.257 | 5.111 | 0.030 |
| Inferior Projection | 1.394 | −1.229 | 4.041 | 0.270 |
| Intraoperative ICG angiography | −1.272 | −3.724 | 0.528 | 0.177 |
| Side of surgical approach (right) | −1.763 | −3.735 | −0.104 | 0.037 |
Table 6.
This table reports the results of the logistic regression model for the outcome parameter postoperative Infarction. Stepwise variable selection was performed using the Akaike Information Criterion (AIC); ADPKD = autosomal dominant polycystic kidney disease, ICG = indocyanine green angiography, SAH = subarachnoidal hemorrhage, IONM = intraoperative monitoring, AcomA = Arteria communicans anterior aneurysm, mASA = American Society of Anesthesiologists classification.
Table 6.
This table reports the results of the logistic regression model for the outcome parameter postoperative Infarction. Stepwise variable selection was performed using the Akaike Information Criterion (AIC); ADPKD = autosomal dominant polycystic kidney disease, ICG = indocyanine green angiography, SAH = subarachnoidal hemorrhage, IONM = intraoperative monitoring, AcomA = Arteria communicans anterior aneurysm, mASA = American Society of Anesthesiologists classification.
| | Estimate | ci-Lower | ci-Upper | p-Value |
|---|
| Penalized-likelihood logistic regression model for infarct |
| Intercept | −1.784 | −4.135 | −0.104 | 0.036 |
| Superior Projection | 1.660 | −0.247 | 4.133 | 0.090 |
| Posterior Projection | 3.055 | 0.606 | 6.139 | 0.015 |
| Inferior Projection | 1.859 | −0.337 | 4.400 | 0.095 |
| Diabetes | −0.076 | −0.336 | 0.136 | 0.494 |
| Calcification | 1.560 | −3.476 | 4.740 | 0.421 |
| blebs | −1.173 | −2.957 | 0.295 | 0.120 |
| IONM | 0.898 | −2.367 | 6.209 | 0.613 |
| Intraoperative ICG angiography | −2.341 | −7.666 | 0.432 | 0.112 |
| Side of approach (right) | −1.589 | −3.356 | −0.044 | 0.044 |
| Clip Repositioning | 1.519 | −0.484 | 3.533 | 0.130 |
| Penalized-likelihood logistic regression model for infarct after manual backward selection |
| Intercept | −1.477 | −3.776 | 0.136 | 0.075 |
| Superior Projection | 1.360 | −0.488 | 3.753 | 0.154 |
| Posterior Projection | 2.534 | 0.283 | 5.204 | 0.028 |
| Inferior Projection | 1.726 | −0.491 | 4.290 | 0.124 |
| Diabetes | −0.052 | −0.291 | 0.150 | 0.628 |
| Blebs | −1.127 | −2.908 | 0.353 | 0.139 |
| Intraop ICG Angiography | −1.882 | −4.452 | −0.129 | 0.034 |
| Side of approach (right) | −1.264 | −2.773 | 0.157 | 0.081 |
Table 7.
This table reports the results of the logistic regression model for the outcome parameter postoperative bleeding. Stepwise variable selection was performed using the Akaike Information Criterion (AIC); ADPKD = autosomal dominant polycystic kidney disease, ICG = indocyanine green angiography, SAH = subarachnoid hemorrhage, IONM = intraoperative monitoring, AcomA = Arteria communicans anterior aneurysm, ASA = American Society of Anesthesiologists classification.
Table 7.
This table reports the results of the logistic regression model for the outcome parameter postoperative bleeding. Stepwise variable selection was performed using the Akaike Information Criterion (AIC); ADPKD = autosomal dominant polycystic kidney disease, ICG = indocyanine green angiography, SAH = subarachnoid hemorrhage, IONM = intraoperative monitoring, AcomA = Arteria communicans anterior aneurysm, ASA = American Society of Anesthesiologists classification.
| | Estimate | ci-Lower | ci-Upper | p-Value |
|---|
| Penalized likelihood regression model for postoperative bleeding |
| Intercept | −2.784 | −13.476 | 1.716 | 0.224 |
| mASA | 1.088 | −0.465 | 4.455 | 0.181 |
| Arterial Hypertension | 1.192 | −1.046 | 6.158 | 0.293 |
| ADPKD | 2.095 | −4.531 | 10.269 | 0.429 |
| Diabetes | −0.015 | −5.508 | 6.567 | 0.995 |
| Nicotine Abuse | −0.127 | −2.113 | 3.446 | 0.899 |
| Alcohol consumption | 0.986 | −2.509 | 4.387 | 0.451 |
| Previously Coiled AcomA | −0.588 | −5.751 | 4.994 | 0.765 |
| SAH | 0.395 | −5.656 | 4.508 | 0.835 |
| Other Aneurysms | −1.579 | −16.298 | 6.749 | 0.665 |
| Number of other aneurysms | 1.670 | −5.952 | 12.755 | 0.571 |
| factor preoperative imaging | −0.190 | −8.892 | 3.703 | 0.918 |
| factor superior projection | −0.176 | −3.231 | 3.079 | 0.882 |
| factor posterior projection | −0.536 | −6.048 | 3.937 | 0.766 |
| factor inferior projection | −2.996 | −12.021 | 2.582 | 0.319 |
| Diabetes | 0.196 | −0.196 | 0.738 | 0.242 |
| Calcification | 1.227 | −5.031 | 8.502 | 0.661 |
| Blebs | −0.162 | −2.449 | 2.985 | 0.883 |
| IONM | −2.533 | −9.761 | 3.017 | 0.307 |
| Intraoperative ICG angiography | 2.622 | −3.281 | 10.481 | 0.315 |
| Side of approach right | −1.062 | −3.708 | 0.889 | 0.288 |
| Number of Clips used | 0.543 | −1.251 | 3.874 | 0.51 |
| Clip Repositioning | −0.677 | −4.192 | 1.784 | 0.6 |
| Temporal Clipping | −2.337 | −7.685 | 0.723 | 0.148 |
| Penalized likelihood regression model for postoperative bleeding after manual backward selection |
| Intercept | −3.325 | −7.050 | −1.540 | <0.001 |
| mASA | 2.181 | 0.338 | 5.591 | 0.016 |
| Alcohol consumption | 3.032 | 0.193 | 7.917 | 0.036 |
| Diabetes | 0.458 | 0.052 | 1.234 | 0.026 |
| Side of approach (right) | −2.180 | −6.510 | 0.440 | 0.107 |
| Temporal Clipping | −2.956 | −9.295 | 0.475 | 0.104 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).