Correlation and Interchangeability of Amyloid, Tau, and Glucose Metabolism PET in Mild Cognitive Impairment and Alzheimer: A Review
Abstract
1. Introduction
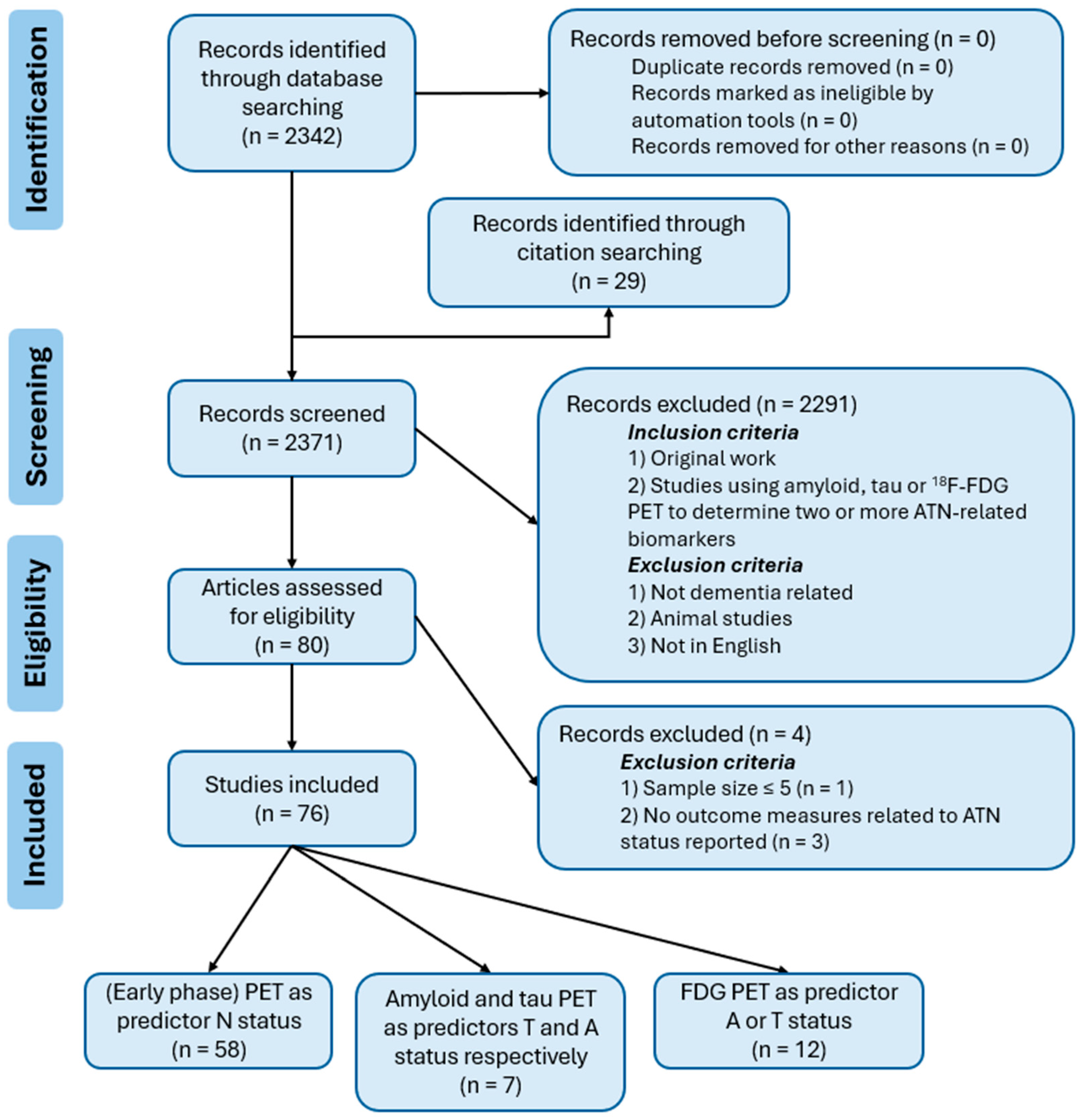
2. Materials and Methods
3. Results
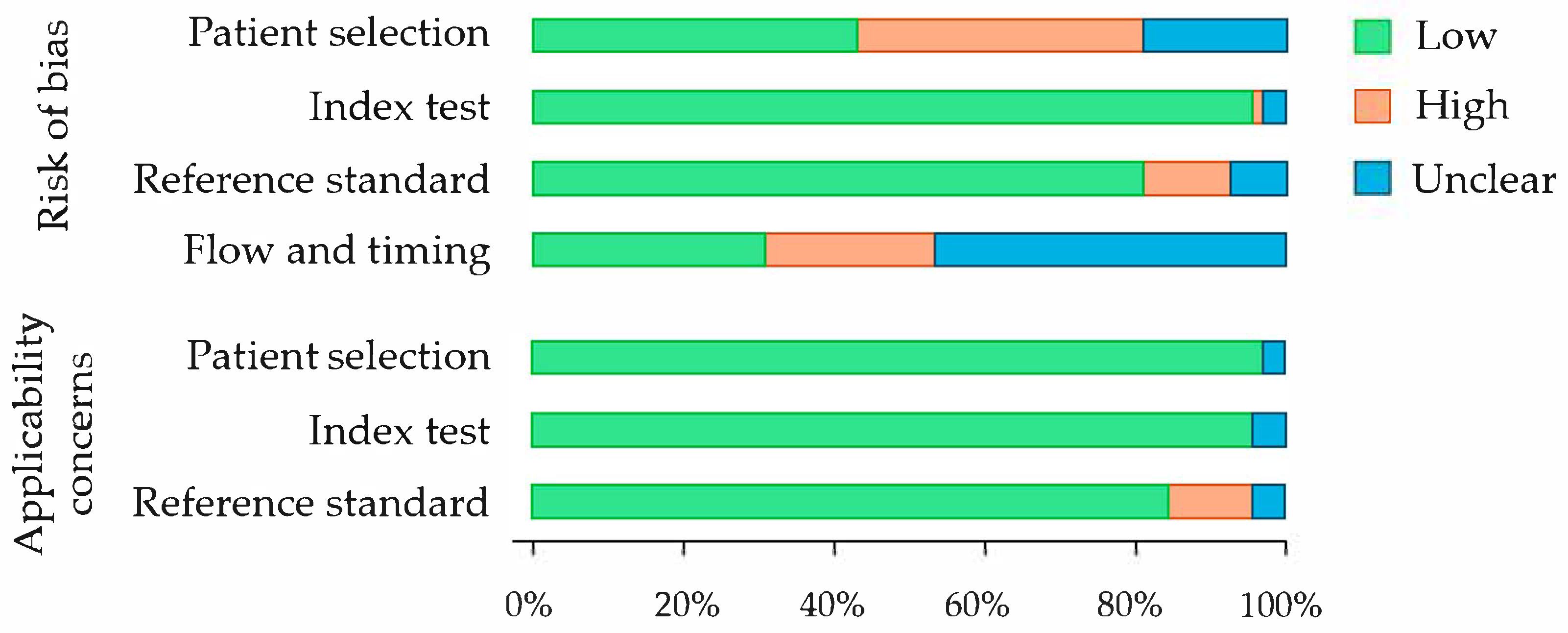
3.1. Quality Assessment
3.2. Amyloid and Tau PET as Predictors of N Status
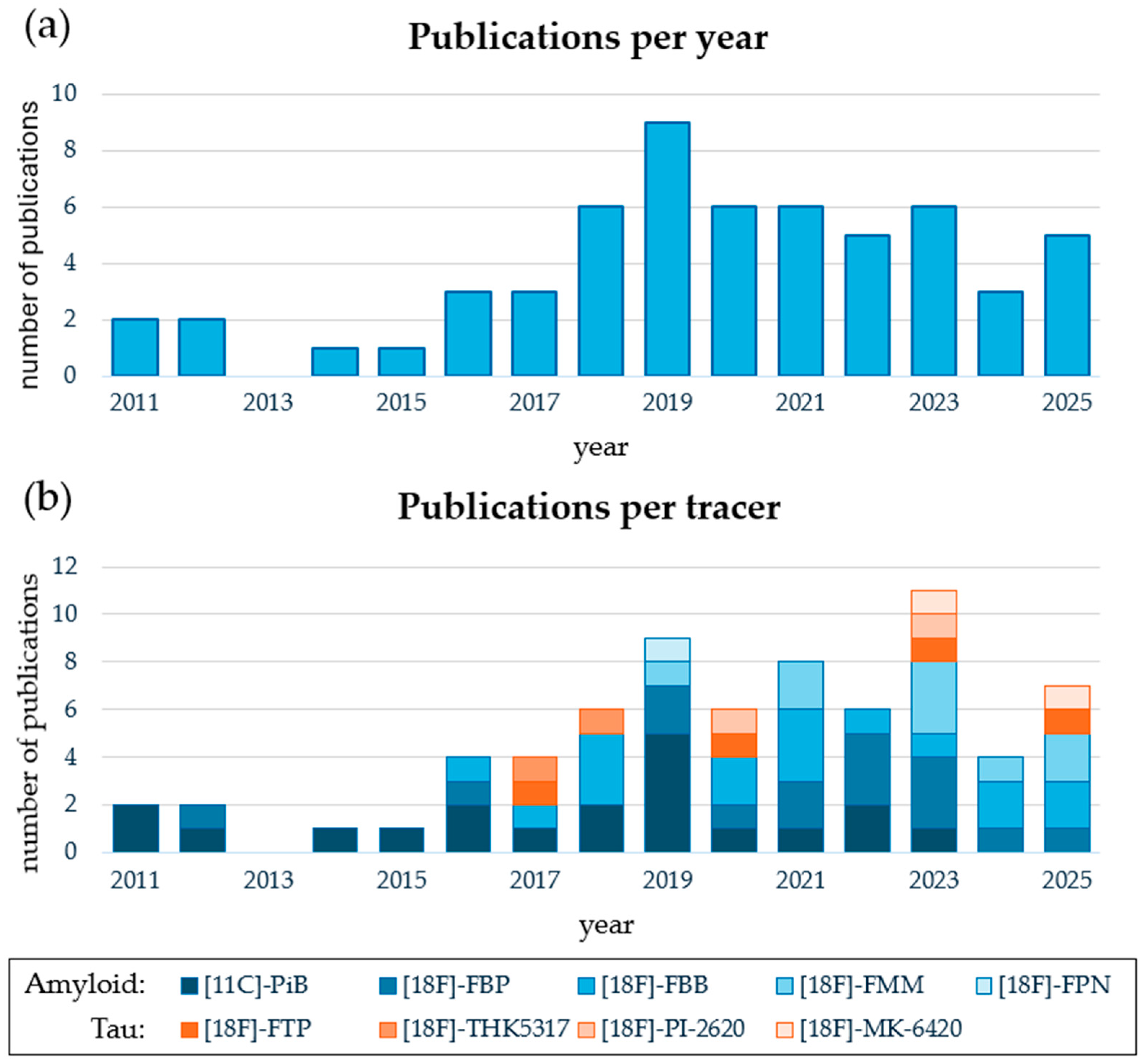
3.2.1. General Characteristics
3.2.2. Analytical Approaches and Findings
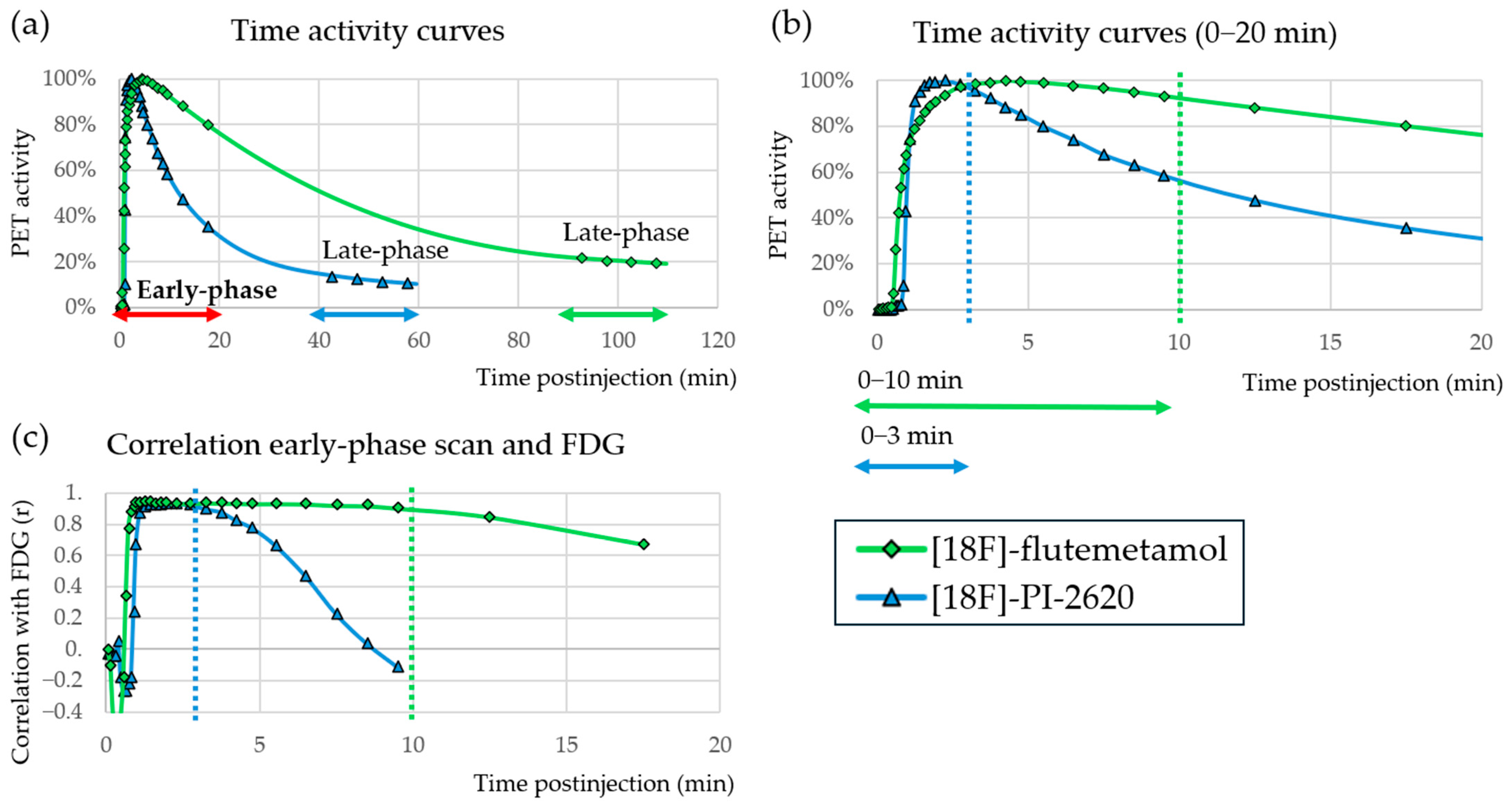
3.2.3. Early Phase PET
3.2.4. Kinetic Modelling Parametric Images
3.2.5. Artificial Intelligence Techniques
3.3. Amyloid and Tau PET as Predictors of Both A and T Status
3.3.1. General Characteristics
3.3.2. Tau PET to Predict A Status
3.3.3. Amyloid PET to Predict T Status
3.4. Neurodegeneration Scans as Predictor A or T Status
3.4.1. General Characteristics
3.4.2. FDG PET as Predictor A or T Status
3.4.3. Early Phase PET to Predict the Late-Phase PET Status
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Ref. | Author, Year | Risk of Bias | Applicability Concerns | |||||
|---|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | ||
| [21] | Albano et al., 2022 | Low | Low | Low | Low | Low | Low | Low |
| [22] | Asghar et al., 2019 | High | Low | Low | High | Low | Low | Low |
| [23] | Aye et al., 2024 | High | Low | Unclear | Low | Low | Low | Unclear |
| [24] | Beyer et al., 2020 | Low | High | Low | High | Low | Low | Low |
| [25] | Bilgel et al., 2020 | High | Low | Low | Low | Unclear | Low | Low |
| [26] | Boccalini et al., 2023 | Low | Low | Low | High | Low | Low | Low |
| [27] | Boccalini et al., 2025 | Low | Low | Low | High | Low | Low | Low |
| [28] | Bunai et al., 2019 | Low | Low | Low | Low | Low | Low | Low |
| [29] | Carneiro et al., 2022 | Low | Low | Low | Low | Low | Low | Low |
| [30] | Chen et al., 2015 | Low | Low | Low | Unclear | Low | Low | Low |
| [31] | Choi et al., 2023 | High | Unclear | Unclear | Unclear | Low | Unclear | Low |
| [32] | Daerr et al., 2017 | Low | Low | Low | High | Low | Low | Low |
| [33] | Dghoughi et al., 2019 | High | Low | Low | Unclear | Low | Low | Low |
| [34] | Fettahoglu et al., 2024 | Low | Low | Low | Unclear | Low | Low | Low |
| [35] | Florek et al., 2018 | High | Low | High | Unclear | Low | Low | High |
| [36] | Forsberg et al., 2012 | Low | Low | Low | Unclear | Low | Low | Low |
| [37] | Fu J. et al., 2025 | Low | Low | Low | High | Low | Low | Low |
| [38] | Fu L. et al., 2014 | Low | Low | Low | Low | Low | Low | Low |
| [39] | Gómez-Grande et al., 2023 | High | Low | Low | Unclear | Low | Low | Low |
| [40] | Guehl et al., 2023 | Low | Low | High | Unclear | Low | Low | High |
| [41] | Hammes et al., 2017 | Low | Low | Low | Low | Low | Low | Low |
| [42] | Hsiao et al., 2012 | Unclear | Low | Low | Unclear | Low | Low | Low |
| [43] | Jeong et al., 2019 | High | Low | Low | Low | Low | Low | Low |
| [44] | Joseph-Mathurin et al., 2018 | Low | Low | Low | High | Low | Low | Low |
| [45] | Kwon et al., 2021 | High | Low | Unclear | Low | Low | Low | Low |
| [46] | Leuzy et al., 2018 | Low | Low | Low | Unclear | Low | Low | Low |
| [47] | Lin et al., 2016 | Low | Low | High | Unclear | Low | Low | High |
| [48] | Lojo-Ramírez et al., 2025 | High | Low | Low | High | Low | Low | Low |
| [49] | Matthews et al., 2022 | High | Low | Low | High | Low | Low | Low |
| [50] | Meyer et al., 2011 | Low | Low | Low | Low | Low | Low | Low |
| [51] | Myoraku et al., 2022 | High | Low | Low | High | Low | Low | Low |
| [52] | Oliveira et al., 2018 | Low | Low | Low | Low | Low | Low | Low |
| [53] | Ottoy et al., 2019 | Low | Low | Low | High | Low | Low | Low |
| [54] | Peretti et al., 2019 | Unclear | Low | Low | Low | Low | Low | Low |
| [55] | Peretti et al., 2019 | Unclear | Low | Low | Low | Low | Low | Low |
| [56] | Peretti et al., 2021 | Unclear | Low | Low | Low | Low | Low | Low |
| [57] | Peretti et al., 2022 | Unclear | Low | Low | Low | Low | Low | Low |
| [58] | Ponto et al., 2019 | Low | Low | Low | Unclear | Low | Low | Low |
| [59] | Ribaldi et al., 2025 | High | Low | Unclear | Unclear | Low | Low | Unclear |
| [60] | Rodriguez-Vieitez et al., 2016 | Unclear | Low | Low | Unclear | Low | Low | Low |
| [61] | Rodriguez-Vieitez et al., 2017 | Unclear | Low | Low | Unclear | Low | Low | Low |
| [62] | Rostomian et al., 2011 | Low | Low | Low | Unclear | Low | Low | Low |
| [63] | Sanaat et al., 2024 | Low | Low | Low | High | Low | Low | Low |
| [64] | Schmitt et al., 2021 | Low | Low | Low | High | Low | Low | Low |
| [65] | Segovia et al., 2018 | Low | Low | Low | Low | Low | Low | Low |
| [66] | Segovia et al., 2018 | Low | Unclear | Low | Unclear | Low | Unclear | Low |
| [67] | Segovia et al., 2020 | Low | Low | Low | Low | Low | Low | Low |
| [69] | Seiffert et al., 2021 | High | Low | Low | Unclear | Low | Low | Low |
| [68] | Seiffert et al., 2020 | High | Low | Low | Unclear | Low | Low | Low |
| [70] | Son et al., 2020 | Low | Low | Low | Low | Low | Low | Low |
| [71] | Tiepolt et al., 2016 | High | Low | Low | Unclear | Low | Low | Low |
| [72] | Tiepolt et al., 2019 | High | Low | High | Unclear | Low | Low | High |
| [73] | Tuncel et al., 2023 | Low | Low | High | Low | Unclear | Low | High |
| [74] | Vanhoutte et al., 2021 | High | Low | Low | Low | Low | Low | Low |
| [75] | Völter et al., 2023 | Low | Low | High | Low | Low | Low | High |
| [76] | Völter et al., 2025 | High | Low | High | Low | Low | Low | High |
| [77] | Wolters et al., 2020 | High | Low | Low | High | Low | Low | Low |
| [78] | Yoon et al., 2021 | High | Low | High | Unclear | Low | Low | High |
| [82] | Gnörich et al., 2025 * | Low | Low | Low | Unclear | Low | Low | Low |
| [83] | Hammes et al., 2021 | Low | Low | Low | Unclear | Low | Low | Low |
| [84] | Lee et al., 2024 | High | Low | Low | Unclear | Low | Low | Low |
| [85] | Naseri et al., 2023 ** | High | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| [86] | Raman et al., 2022 | Unclear | Low | Low | High | Low | Low | Low |
| [87] | Ruwanpathirana et al., 2022 | Unclear | Low | Low | Unclear | Low | Low | Low |
| [88] | Shcherbinin et al., 2023 | High | Low | Low | Unclear | Low | Low | Low |
| [90] | Alongi et al., 2022 | Low | Low | Low | High | Low | Low | Low |
| [91] | Ardakani et al., 2025 | Unclear | Low | Low | Low | Low | Low | Low |
| [92] | Choi et al., 2025 | High | Low | Low | Unclear | Low | Low | Low |
| [15] | Kim et al., 2021 | High | Low | Low | Unclear | Low | Low | Low |
| [93] | Komori et al., 2022 | High | Low | Low | Low | Low | Low | Low |
| [94] | Park et al., 2025 *** | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| [95] | Parmera et al., 2021 | Low | Low | Low | High | Low | Low | Low |
| [96] | Rasi et al., 2024 | High | Low | Low | High | Low | Low | Low |
| [97] | Wang et al., 2021 | Unclear | Low | Low | Unclear | Low | Low | Low |
| [98] | Yamada et al., 2025 | High | Low | Low | Unclear | Low | Low | Low |
| [99] | Zhou et al., 2021 | Unclear | Low | Low | Unclear | Low | Low | Low |
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s Disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Alzheimer Europe. Dementia in Europe Yearbook 2019: Estimating the Prevalence of Dementia in Europe; Alzheimer Europe: Bologna, Italy, 2019; ISBN 978-99959-995-9-9. [Google Scholar]
- Alzheimer’s Disease International. World Alzheimer Report 2018—The State of the Art of Dementia Research: New Frontiers; Alzheimer’s Disease International: London, UK, 2018. [Google Scholar]
- Jack, C.R.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised Criteria for Diagnosis and Staging of Alzheimer’s Disease: Alzheimer’s Association Workgroup. Alzheimer’s Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An Unbiased Descriptive Classification Scheme for Alzheimer Disease Biomarkers. Neurology 2016, 87, 539. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Leqembi. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/leqembi (accessed on 17 July 2025).
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Kisunla. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kisunla (accessed on 6 October 2025).
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.D.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Jack, C.R.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking Pathophysiological Processes in Alzheimer’s Disease: An Updated Hypothetical Model of Dynamic Biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Jueptner, M.; Weiller, C. Review: Does Measurement of Regional Cerebral Blood Flow Reflect Synaptic Activity?—Implications for PET and FMRI. Neuroimage 1995, 2, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Gur, R.C.; Ragland, J.D.; Reivich, M.; Greenberg, J.H.; Alavi, A.; Gur, R.E. Regional Differences in the Coupling between Resting Cerebral Blood Flow and Metabolism May Indicate Action Preparedness as a Default State. Cereb. Cortex 2009, 19, 375–382. [Google Scholar] [CrossRef]
- Kim, S.; Lee, P.; Oh, K.T.; Byun, M.S.; Yi, D.; Lee, J.H.; Kim, Y.K.; Ye, B.S.; Yun, M.J.; Lee, D.Y.; et al. Deep Learning-Based Amyloid PET Positivity Classification Model in the Alzheimer’s Disease Continuum by Using 2-[18F]FDG PET. EJNMMI Res. 2021, 11, 56. [Google Scholar] [CrossRef]
- Li, Q.; Cui, L.; Guan, Y.; Li, Y.; Xie, F.; Guo, Q. Prediction Model and Nomogram for Amyloid Positivity Using Clinical and MRI Features in Individuals with Subjective Cognitive Decline. Hum. Brain Mapp. 2025, 46, e70238. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Kim, J.Y.; Kim, J.; Whitlow, C.T. Synthesizing Beta-Amyloid PET Images from T1-Weighted Structural MRI: A Preliminary Study. arXiv 2024, arXiv:2409.18282. [Google Scholar]
- Moon, J.; Kim, S.; Chung, H.; Jang, I. Cyclic 2.5D Perceptual Loss for Cross-Modal 3D Medical Image Synthesis: T1w MRI to Tau PET. arXiv 2025, arXiv:2406.12632. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Albano, D.; Premi, E.; Peli, A.; Camoni, L.; Bertagna, F.; Turrone, R.; Borroni, B.; Calhoun, V.D.; Rodella, C.; Magoni, M.; et al. Correlation between Brain Glucose Metabolism (18F-FDG) and Cerebral Blood Flow with Amyloid Tracers (18F-Florbetapir) in Clinical Routine: Preliminary Evidences. Rev. Española Med. Nucl. Imagen Mol. (Engl. Ed.) 2022, 41, 146–152. [Google Scholar] [CrossRef]
- Asghar, M.; Hinz, R.; Herholz, K.; Carter, S.F. Dual-Phase [18F]Florbetapir in Frontotemporal Dementia. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 304–311. [Google Scholar] [CrossRef]
- Aye, W.W.T.; Stark, M.R.; Horne, K.L.; Livingston, L.; Grenfell, S.; Myall, D.J.; Pitcher, T.L.; Almuqbel, M.M.; Keenan, R.J.; Meissner, W.G.; et al. Early-Phase Amyloid PET Reproduces Metabolic Signatures of Cognitive Decline in Parkinson’s Disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2024, 16, e12601. [Google Scholar] [CrossRef]
- Beyer, L.; Nitschmann, A.; Barthel, H.; van Eimeren, T.; Unterrainer, M.; Sauerbeck, J.; Marek, K.; Song, M.; Palleis, C.; Respondek, G.; et al. Early-Phase [18F]PI-2620 Tau-PET Imaging as a Surrogate Marker of Neuronal Injury. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2911–2922. [Google Scholar] [CrossRef]
- Bilgel, M.; Beason-Held, L.; An, Y.; Zhou, Y.; Wong, D.F.; Resnick, S.M. Longitudinal Evaluation of Surrogates of Regional Cerebral Blood Flow Computed from Dynamic Amyloid PET Imaging. J. Cereb. Blood Flow Metab. 2020, 40, 288–297. [Google Scholar] [CrossRef]
- Boccalini, C.; Peretti, D.E.; Ribaldi, F.; Scheffler, M.; Stampacchia, S.; Tomczyk, S.; Rodriguez, C.; Montandon, M.L.; Haller, S.; Giannakopoulos, P.; et al. Early-Phase 18F-Florbetapir and 18F-Flutemetamol Images as Proxies of Brain Metabolism in a Memory Clinic Setting. J. Nucl. Med. 2023, 64, 266–273. [Google Scholar] [CrossRef]
- Boccalini, C.; Peretti, D.E.; Mathoux, G.; Iaccarino, L.; Ribaldi, F.; Scheffler, M.; Perani, D.; Frisoni, G.B.; Garibotto, V. Early-Phase 18F-Flortaucipir Tau-PET as a Proxy of Brain Metabolism in Alzheimer’s Disease: A Comparison with 18F-FDG-PET and Early-Phase Amyloid-PET. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 1958–1969. [Google Scholar] [CrossRef]
- Bunai, T.; Kakimoto, A.; Yoshikawa, E.; Terada, T.; Ouchi, Y. Biopathological Significance of Early-Phase Amyloid Imaging in the Spectrum of Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 69, 529–538. [Google Scholar] [CrossRef]
- de Carneiro, C.G.; de Faria, D.P.; Coutinho, A.M.; Ono, C.R.; Duran, F.L.d.S.; da Costa, N.A.; Garcez, A.T.; da Silveira, P.S.; Forlenza, O.V.; Brucki, S.M.D.; et al. Evaluation of 10-Minute Post-Injection11C-PiB PET and Its Correlation with 18F-FDG PET in Older Adults Who Are Cognitively Healthy, Mildly Impaired, or with Probable Alzheimer’s Disease. Braz. J. Psychiatry 2022, 44, 495–506. [Google Scholar] [CrossRef]
- Chen, Y.J.; Rosario, B.L.; Mowrey, W.; Laymon, C.M.; Lu, X.; Lopez, O.L.; Klunk, W.E.; Lopresti, B.J.; Mathis, C.A.; Price, J.C. Relative 11C-PiB Delivery as a Proxy of Relative CBF: Quantitative Evaluation Using Single-Session 15O-Water and 11C-PiB PET. J. Nucl. Med. 2015, 56, 1199–1205. [Google Scholar] [CrossRef]
- Choi, H.J.; Seo, M.; Kim, A.; Park, S.H. Generation of Conventional 18F-FDG PET Images from 18F-Florbetaben PET Images Using Generative Adversarial Network: A Preliminary Study Using ADNI Dataset. Medicina 2023, 59, 1281. [Google Scholar] [CrossRef] [PubMed]
- Daerr, S.; Brendel, M.; Zach, C.; Mille, E.; Schilling, D.; Zacherl, M.J.; Bürger, K.; Danek, A.; Pogarell, O.; Schildan, A.; et al. Evaluation of Early-Phase [18F]-Florbetaben PET Acquisition in Clinical Routine Cases. Neuroimage Clin. 2017, 14, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Dghoughi, W.; Seiffert, A.P.; Gómez-Grande, A.; Villarejo-Galende, A.; Bueno, H.; Gómez, E.J.; Sánchez-González, P. Quantitative analysis of early-phase 18F-flutemetamol PET brain images. In Proceedings of the Actas Del XXXVII Congreso Anual de La Sociedad Española de Ingeniería Biomédica, Santander, Spain, 27–29 November 2019; ISBN 9788409167074. [Google Scholar]
- Fettahoglu, A.; Zhao, M.; Khalighi, M.; Vossler, H.; Jovin, M.; Davidzon, G.; Zeineh, M.; Boada, F.; Mormino, E.; Henderson, V.W.; et al. Early-Frame [18F]Florbetaben PET/MRI for Cerebral Blood Flow Quantification in Patients with Cognitive Impairment: Comparison to an [15O]Water Gold Standard. J. Nucl. Med. 2024, 65, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Florek, L.; Tiepolt, S.; Schroeter, M.L.; Berrouschot, J.; Saur, D.; Hesse, S.; Jochimsen, T.; Luthardt, J.; Sattler, B.; Patt, M.; et al. Dual Time-Point [18F]Florbetaben PET Delivers Dual Biomarker Information in Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 66, 1105–1116. [Google Scholar] [CrossRef]
- Forsberg, A.; Engler, H.; Blomquist, G.; Långström, B.; Nordberg, A. The Use of PIB-PET as a Dual Pathological and Functional Biomarker in AD. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2012, 1822, 380–385. [Google Scholar] [CrossRef]
- Fu, J.F.; Juttukonda, M.R.; Garimella, A.; Salvatore, A.N.; Lois, C.; Ranasinghe, A.; Efthimiou, N.; Sari, H.; Aye, W.; Guehl, N.J.; et al. [18F]MK-6240 Radioligand Delivery Indices as Surrogates of Cerebral Perfusion: Bias and Correlation Against [15O]Water. J. Nucl. Med. 2025, 66, 410–417. [Google Scholar] [CrossRef]
- Fu, L.; Liu, L.; Zhang, J.; Xu, B.; Fan, Y.; Tian, J. Comparison of Dual-Biomarker PIB-PET and Dual-Tracer PET in AD Diagnosis. Eur. Radiol. 2014, 24, 2800–2809. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Grande, A.; Seiffert, A.P.; Villarejo-Galende, A.; González-Sánchez, M.; Llamas-Velasco, S.; Bueno, H.; Gómez, E.J.; Tabuenca, M.J.; Sánchez-González, P. Static First-Minute-Frame (FMF) PET Imaging after 18F-Labeled Amyloid Tracer Injection Is Correlated to [18F]FDG PET in Patients with Primary Progressive Aphasia. Rev. Española Med. Nucl. Imagen Mol. (Engl. Ed.) 2023, 42, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Guehl, N.J.; Dhaynaut, M.; Hanseeuw, B.J.; Moon, S.H.; Lois, C.; Thibault, E.; Fu, J.F.; Price, J.C.; Johnson, K.A.; Fakhri, G.E.; et al. Measurement of Cerebral Perfusion Indices from the Early Phase of [18F]MK6240 Dynamic Tau PET Imaging. J. Nucl. Med. 2023, 64, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Hammes, J.; Leuwer, I.; Bischof, G.N.; Drzezga, A.; Van Eimeren, T. Multimodal Correlation of Dynamic [18F]-AV-1451 Perfusion PET and Neuronal Hypometabolism in [18F]-FDG PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2249–2256. [Google Scholar] [CrossRef]
- Hsiao, I.T.; Huang, C.C.; Hsieh, C.J.; Hsu, W.C.; Wey, S.P.; Yen, T.C.; Kung, M.P.; Lin, K.J. Correlation of Early-Phase 18F-Florbetapir (AV-45/Amyvid) PET Images to FDG Images: Preliminary Studies. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 613–620. [Google Scholar] [CrossRef]
- Jeong, J.; Jeong, Y.J.; Park, K.W.; Kang, D.Y. Correlation of Early-Phase F-18 Florapronal PET with F-18 FDG PET in Alzheimer’s Disease and Normal Brain. Nucl. Med. Mol. Imaging 2019, 53, 328–333. [Google Scholar] [CrossRef]
- Joseph-Mathurin, N.; Su, Y.; Blazey, T.M.; Jasielec, M.; Vlassenko, A.; Friedrichsen, K.; Gordon, B.A.; Hornbeck, R.C.; Cash, L.; Ances, B.M.; et al. Utility of Perfusion PET Measures to Assess Neuronal Injury in Alzheimer’s Disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2018, 10, 669–677. [Google Scholar] [CrossRef]
- Kwon, S.J.; Ha, S.; Yoo, S.W.; Shin, N.Y.; O, J.H.; Yoo, I.R.; Kim, J.S. Comparison of Early F-18 Florbetaben PET/CT to Tc-99m ECD SPECT Using Voxel, Regional, and Network Analysis. Sci. Rep. 2021, 11, 16738. [Google Scholar] [CrossRef]
- Leuzy, A.; Rodriguez-Vieitez, E.; Saint-Aubert, L.; Chiotis, K.; Almkvist, O.; Savitcheva, I.; Jonasson, M.; Lubberink, M.; Wall, A.; Antoni, G.; et al. Longitudinal Uncoupling of Cerebral Perfusion, Glucose Metabolism, and Tau Deposition in Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.J.; Hsiao, I.T.; Hsu, J.L.; Huang, C.C.; Huang, K.L.; Hsieh, C.J.; Wey, S.P.; Yen, T.C. Imaging Characteristic of Dual-Phase 18F-Florbetapir (AV-45/Amyvid) PET for the Concomitant Detection of Perfusion Deficits and Beta-Amyloid Deposition in Alzheimer’s Disease and Mild Cognitive Impairment. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1304–1314. [Google Scholar] [CrossRef]
- Lojo-Ramírez, J.A.; Fernández-Rodríguez, P.; Guerra-Gómez, M.; Marín-Cabañas, A.M.; Franco-Macías, E.; Jiménez-Hoyuela-García, J.M.; García-Solís, D. Evaluation of Early-Phase 18 F-Florbetaben PET as a Surrogate Biomarker of Neurodegeneration: In-Depth Comparison with 18 F-FDG PET at Group and Single Patient Level. J. Alzheimer’s Dis. 2025, 106, 304–316. [Google Scholar] [CrossRef]
- Matthews, D.C.; Lukic, A.S.; Andrews, R.D.; Wernick, M.N.; Strother, S.C.; Schmidt, M.E. Measurement of Neurodegeneration Using a Multivariate Early Frame Amyloid PET Classifier. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12325. [Google Scholar] [CrossRef]
- Meyer, P.T.; Hellwig, S.; Amtage, F.; Rottenburger, C.; Sahm, U.; Reuland, P.; Weber, W.A.; Hüll, M. Dual-Biomarker Imaging of Regional Cerebral Amyloid Load and Neuronal Activity in Dementia with PET and 11C-Labeled Pittsburgh Compound B. J. Nucl. Med. 2011, 52, 393–400. [Google Scholar] [CrossRef]
- Myoraku, A.; Klein, G.; Landau, S.; Tosun, D. Regional Uptakes from Early-Frame Amyloid PET and 18F-FDG PET Scans Are Comparable Independent of Disease State. Eur. J. Hybrid Imaging 2022, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.P.M.; Moreira, A.P.; De Mendonça, A.; Verdelho, A.; Xavier, C.; Barroca, D.; Rio, J.; Cardoso, E.; Cruz, Â.; Abrunhosa, A.; et al. Can 11 C-PiB-PET Relative Delivery R 1 or 11 C-PiB-PET Perfusion Replace 18 F-FDG-PET in the Assessment of Brain Neurodegeneration? J. Alzheimer’s Dis. 2018, 65, 89–97. [Google Scholar] [CrossRef]
- Ottoy, J.; Verhaeghe, J.; Niemantsverdriet, E.; De Roeck, E.; Wyffels, L.; Ceyssens, S.; Van Broeckhoven, C.; Engelborghs, S.; Stroobants, S.; Staelens, S. 18F-FDG PET, the Early Phases and the Delivery Rate of 18F-AV45 PET as Proxies of Cerebral Blood Flow in Alzheimer’s Disease: Validation against 15O-H2O PET. Alzheimer’s Dement. 2019, 15, 1172–1182. [Google Scholar] [CrossRef]
- Peretti, D.E.; García, D.V.; Reesink, F.E.; Van der Goot, T.; De Deyn, P.P.; De Jong, B.M.; Dierckx, R.A.J.O.; Boellaard, R. Relative Cerebral Flow from Dynamic PIB Scans as an Alternative for FDG Scans in Alzheimer’s Disease PET Studies. PLoS ONE 2019, 14, e0211000. [Google Scholar] [CrossRef]
- Peretti, D.E.; Vállez García, D.; Reesink, F.E.; Doorduin, J.; de Jong, B.M.; De Deyn, P.P.; Dierckx, R.A.J.O.; Boellaard, R. Diagnostic Performance of Regional Cerebral Blood Flow Images Derived from Dynamic PIB Scans in Alzheimer’s Disease. EJNMMI Res. 2019, 9, 59. [Google Scholar] [CrossRef]
- Peretti, D.E.; Renken, R.J.; Reesink, F.E.; de Jong, B.M.; De Deyn, P.P.; Dierckx, R.A.J.O.; Doorduin, J.; Boellaard, R.; Vállez García, D. Feasibility of Pharmacokinetic Parametric PET Images in Scaled Subprofile Modelling Using Principal Component Analysis. Neuroimage Clin. 2021, 30, 102625. [Google Scholar] [CrossRef] [PubMed]
- Peretti, D.E.; Vállez García, D.; Renken, R.J.; Reesink, F.E.; Doorduin, J.; de Jong, B.M.; De Deyn, P.P.; Dierckx, R.A.J.O.; Boellaard, R. Alzheimer’s Disease Pattern Derived from Relative Cerebral Flow as an Alternative for the Metabolic Pattern Using SSM/PCA. EJNMMI Res. 2022, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Ponto, L.L.B.; Moser, D.J.; Menda, Y.; Harlynn, E.L.; DeVries, S.D.; Oleson, J.J.; Magnotta, V.A.; Schultz, S.K. Early Phase PIB-PET as a Surrogate for Global and Regional Cerebral Blood Flow Measures. J. Neuroimaging 2019, 29, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Ribaldi, F.; Mendes, A.J.; Galazzo, I.B.; Natale, V.; Mathoux, G.; Pievani, M.; Lovblad, K.O.; Scheffler, M.; Frisoni, G.B.; Garibotto, V.; et al. Agreement between Early-Phase Amyloid-PET and Pulsed Arterial Spin Labeling in a Memory Clinic Cohort. J. Mol. Med. 2025, 103, 809–819. [Google Scholar] [CrossRef]
- Rodriguez-Vieitez, E.; Carter, S.F.; Chiotis, K.; Saint-Aubert, L.; Leuzy, A.; Schöll, M.; Almkvist, O.; Wall, A.; Långström, B.; Nordberg, A. Comparison of Early-Phase 11C-Deuterium-l-Deprenyl and 11C-Pittsburgh Compound B PET for Assessing Brain Perfusion in Alzheimer Disease. J. Nucl. Med. 2016, 57, 1071–1077. [Google Scholar] [CrossRef]
- Rodriguez-Vieitez, E.; Leuzy, A.; Chiotis, K.; Saint-Aubert, L.; Wall, A.; Nordberg, A. Comparability of [18F]THK5317 and [11C]PIB Blood Flow Proxy Images with [18F]FDG Positron Emission Tomography in Alzheimer’s Disease. J. Cereb. Blood Flow Metab. 2017, 37, 740–749. [Google Scholar] [CrossRef]
- Rostomian, A.H.; Madison, C.; Rabinovici, G.D.; Jagust, W.J. Early 11C-PIB Frames and 18F-FDG PET Measures Are Comparable: A Study Validated in a Cohort of AD and FTLD Patients. J. Nucl. Med. 2011, 52, 173–179. [Google Scholar] [CrossRef]
- Sanaat, A.; Boccalini, C.; Mathoux, G.; Perani, D.; Frisoni, G.B.; Haller, S.; Montandon, M.L.; Rodriguez, C.; Giannakopoulos, P.; Garibotto, V.; et al. A Deep Learning Model for Generating [18F]FDG PET Images from Early-Phase [18F]Florbetapir and [18F]Flutemetamol PET Images. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3518–3531. [Google Scholar] [CrossRef]
- Schmitt, J.; Palleis, C.; Sauerbeck, J.; Unterrainer, M.; Harris, S.; Prix, C.; Weidinger, E.; Katzdobler, S.; Wagemann, O.; Danek, A.; et al. Dual-Phase β-Amyloid PET Captures Neuronal Injury and Amyloidosis in Corticobasal Syndrome. Front. Aging Neurosci. 2021, 13, 661284. [Google Scholar] [CrossRef]
- Segovia, F.; Gómez-Río, M.; Sánchez-Vañó, R.; Górriz, J.M.; Ramírez, J.; Triviño-Ibáñez, E.; Carnero-Pardo, C.; Martínez-Lozano, M.D.; Sopena-Novales, P. Usefulness of Dual-Point Amyloid PET Scans in Appropriate Use Criteria: A Multicenter Study. J. Alzheimer’s Dis. 2018, 65, 765–779. [Google Scholar] [CrossRef]
- Segovia, F.; Gorriz, J.M.; Ramirez, J.; Martinez-Murcia, F.J.; Castillo-Barnes, D.; Sanchez-Vano, R.; Sopena-Novales, P.; Gomez-Rio, M. Using Early Acquisitions of Amyloid-PET as a Surrogate of FDG-PET: A Machine Learning Based Approach. In Proceedings of the 2018 International Workshop on Pattern Recognition in Neuroimaging, PRNI 2018, Singapore, 12–14 June 2018. [Google Scholar] [CrossRef]
- Segovia, F.; Ramírez, J.; Castillo-Barnes, D.; Salas-Gonzalez, D.; Gómez-Río, M.; Sopena-Novales, P.; Phillips, C.; Zhang, Y.; Górriz, J.M. Multivariate Analysis of Dual-Point Amyloid PET Intended to Assist the Diagnosis of Alzheimer’s Disease. Neurocomputing 2020, 417, 1–9. [Google Scholar] [CrossRef]
- Seiffert, A.P.; Gómez-Grande, A.; Sánchez-González, P.; Dghoughi, W.; Villarejo-Galende, A.; Bueno, H.; Gómez, E.J. Quantitative Analysis of Brain 18F-Fluordesoxyglucose and Early-Phase 18F-Florbetapir Positron Emission Tomography. In IFMBE Proceedings, Proceedings of the XV Mediterranean Conference on Medical and Biological Engineering and Computing—MEDICON 2019, Coimbra, Portugal, 26–28 September 2019; Springer: Berlin/Heidelberg, Germany, 2020; Volume 76, pp. 427–436. [Google Scholar]
- Seiffert, A.P.; Gómez-Grande, A.; Villarejo-Galende, A.; González-Sánchez, M.; Bueno, H.; Gómez, E.J.; Sánchez-González, P. High Correlation of Static First-Minute-Frame (Fmf) Pet Imaging After18f-Labeled Amyloid Tracer Injection with [18f]Fdg Pet Imaging. Sensors 2021, 21, 5182. [Google Scholar] [CrossRef]
- Son, S.H.; Kang, K.; Ko, P.W.; Lee, H.W.; Lee, S.W.; Ahn, B.C.; Lee, J.; Yoon, U.; Jeong, S.Y. Early-Phase 18F-Florbetaben PET as an Alternative Modality for 18F-FDG PET. Clin. Nucl. Med. 2020, 45, E8–E14. [Google Scholar] [CrossRef]
- Tiepolt, S.; Hesse, S.; Patt, M.; Luthardt, J.; Schroeter, M.L.; Hoffmann, K.T.; Weise, D.; Gertz, H.J.; Sabri, O.; Barthel, H. Early [18F]Florbetaben and [11C]PiB PET Images Are a Surrogate Biomarker of Neuronal Injury in Alzheimer’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1700–1709. [Google Scholar] [CrossRef]
- Tiepolt, S.; Luthardt, J.; Patt, M.; Hesse, S.; Hoffmann, K.T.; Weise, D.; Gertz, H.J.; Sabri, O.; Barthel, H. Early after Administration [11 C]PiB PET Images Correlate with Cognitive Dysfunction Measured by the CERAD Test Battery. J. Alzheimer’s Dis. 2019, 68, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, H.; Visser, D.; Timmers, T.; Wolters, E.E.; Ossenkoppele, R.; van der Flier, W.M.; van Berckel, B.N.M.; Boellaard, R.; Golla, S.S.V. Head-to-Head Comparison of Relative Cerebral Blood Flow Derived from Dynamic [18F]Florbetapir and [18F]Flortaucipir PET in Subjects with Subjective Cognitive Decline. EJNMMI Res. 2023, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, M.; Landeau, B.; Sherif, S.; de la Sayette, V.; Dautricourt, S.; Abbas, A.; Manrique, A.; Chocat, A.; Chételat, G. Evaluation of the Early-Phase [18F]AV45 PET as an Optimal Surrogate of [18F]FDG PET in Ageing and Alzheimer’s Clinical Syndrome. Neuroimage Clin. 2021, 31, 102750. [Google Scholar] [CrossRef] [PubMed]
- Völter, F.; Beyer, L.; Eckenweber, F.; Scheifele, M.; Bui, N.; Patt, M.; Barthel, H.; Katzdobler, S.; Palleis, C.; Franzmeier, N.; et al. Assessment of Perfusion Deficit with Early Phases of [18F]PI-2620 Tau-PET versus [18F]Flutemetamol-Amyloid-PET Recordings. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1384–1394. [Google Scholar] [CrossRef]
- Völter, F.; Eckenweber, S.; Scheifele, M.; Eckenweber, F.; Hirsch, F.; Franzmeier, N.; Kreuzer, A.; Griessl, M.; Steward, A.; Janowitz, D.; et al. Correlation of Early-Phase β-Amyloid Positron-Emission-Tomography and Neuropsychological Testing in Patients with Alzheimer’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 2918–2928. [Google Scholar] [CrossRef]
- Wolters, E.E.; van de Beek, M.; Ossenkoppele, R.; Golla, S.S.V.; Verfaillie, S.C.J.; Coomans, E.M.; Timmers, T.; Visser, D.; Tuncel, H.; Barkhof, F.; et al. Tau PET and Relative Cerebral Blood Flow in Dementia with Lewy Bodies: A PET Study. Neuroimage Clin. 2020, 28, 102504. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, B.S.; Jeong, J.H.; Kim, G.H.; Park, H.K.; Chun, M.Y.; Ha, S. Dual-Phase 18F-Florbetaben PET Provides Cerebral Perfusion Proxy along with Beta-Amyloid Burden in Alzheimer’s Disease. Neuroimage Clin. 2021, 31, 102773. [Google Scholar] [CrossRef]
- Minoshima, S.; Drzezga, A.E.; Barthel, H.; Bohnen, N.; Djekidel, M.; Lewis, D.H.; Mathis, C.A.; McConathy, J.; Nordberg, A.; Sabri, O.; et al. SNMMI Procedure Standard/EANM Practice Guideline for Amyloid PET Imaging of the Brain 1.0. J. Nucl. Med. 2016, 57, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Bullich, S.; Barret, O.; Constantinescu, C.; Sandiego, C.; Mueller, A.; Berndt, M.; Papin, C.; Perrotin, A.; Koglin, N.; Kroth, H.; et al. Evaluation of Dosimetry, Quantitative Methods, and Test–Retest Variability of 18F-PI-2620 PET for the Assessment of Tau Deposits in the Human Brain. J. Nucl. Med. 2020, 61, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Morbelli, S.; Van Weehaeghe, D.; Verger, A.; Tolboom, N.; Fernandez, P.A.; Brendel, M.; Guedj, E.; Garibotto, V.; Cecchin, D.; Yakushev, I.; et al. Perspectives of the European Association of Nuclear Medicine (EANM) on Early Perfusion Imaging in the Context of Amyloid PET Imaging Protocols. EANM J. 2025, 1, 100005. [Google Scholar] [CrossRef]
- Gnörich, J.; Kusche-Palenga, J.; Kling, A.; Dehsarvi, A.; Bronte, A.; Frontzkowski, L.; Zatcepin, A.; Zaganjori, M.; Schöberl, F.; Roemer, S.N.; et al. Assessment and Staging of A/T/N with a Single Dynamic [18F]PI-2620 Recording. medRxiv 2025. [Google Scholar] [CrossRef]
- Hammes, J.; Bischof, G.N.; Bohn, K.P.; Onur, Ö.; Schneider, A.; Fliessbach, K.; Hönig, M.C.; Jessen, F.; Neumaier, B.; Drzezga, A.; et al. One-Stop Shop: 18F-Flortaucipir PET Differentiates Amyloid-Positive and -Negative Forms of Neurodegenerative Diseases. J. Nucl. Med. 2021, 62, 240–246. [Google Scholar] [CrossRef]
- Lee, J.; Burkett, B.J.; Min, H.K.; Senjem, M.L.; Dicks, E.; Corriveau-Lecavalier, N.; Mester, C.T.; Wiste, H.J.; Lundt, E.S.; Murray, M.E.; et al. Synthesizing Images of Tau Pathology from Cross-Modal Neuroimaging Using Deep Learning. Brain 2024, 147, 980–995. [Google Scholar] [CrossRef]
- Naseri, M.; Carmichael, O.T. Deep Learning Based Estimation of Synthetic Tau PET from Amyloid PET. Alzheimer’s Dement. 2023, 19, e076790. [Google Scholar] [CrossRef]
- Raman, F.; Fang, Y.H.D.; Grandhi, S.; Murchison, C.F.; Kennedy, R.E.; Morris, J.C.; Massoumzadeh, P.; Benzinger, T.; Roberson, E.D.; McConathy, J. Dynamic Amyloid PET: Relationships to 18F-Flortaucipir Tau PET Measures. J. Nucl. Med. 2022, 63, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ruwanpathirana, G.P.; Williams, R.C.; Masters, C.L.; Rowe, C.C.; Johnston, L.A.; Davey, C.E. Mapping the Association between Tau-PET and Aβ-Amyloid-PET Using Deep Learning. Sci. Rep. 2022, 12, 14797. [Google Scholar] [CrossRef]
- Shcherbinin, S.; Morris, A.; Higgins, I.A.; Tunali, I.; Lu, M.; Deveau, C.; Southekal, S.; Kotari, V.; Evans, C.D.; Arora, A.K.; et al. Tau as a Diagnostic Instrument in Clinical Trials to Predict Amyloid in Alzheimer’s Disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2023, 9, e12415. [Google Scholar] [CrossRef] [PubMed]
- Klunk, W.E.; Koeppe, R.A.; Price, J.C.; Benzinger, T.L.; Devous, M.D.; Jagust, W.J.; Johnson, K.A.; Mathis, C.A.; Minhas, D.; Pontecorvo, M.J.; et al. The Centiloid Project: Standardizing Quantitative Amyloid Plaque Estimation by PET. Alzheimer’s Dement. 2015, 11, 1–15.e4. [Google Scholar] [CrossRef]
- Alongi, P.; Laudicella, R.; Panasiti, F.; Stefano, A.; Comelli, A.; Giaccone, P.; Arnone, A.; Minutoli, F.; Quartuccio, N.; Cupidi, C.; et al. Radiomics Analysis of Brain [18 F]FDG PET/CT to Predict Alzheimer’s Disease in Patients with Amyloid PET Positivity: A Preliminary Report on the Application of SPM Cortical Segmentation, Pyradiomics and Machine-Learning Analysis. Diagnostics 2022, 12, 933. [Google Scholar] [CrossRef]
- Ardakani, I.; Yamada, T.; Iwano, S.; Kumar Maurya, S.; Ishii, K. A Robust Residual Three-Dimensional Convolutional Neural Networks Model for Prediction of Amyloid-β Positivity by Using FDG-PET. Clin. Nucl. Med. 2025, 50, 707–713. [Google Scholar] [CrossRef]
- Choi, D.H.; Ahn, S.H.; Chung, Y.; Kim, J.S.; Jeong, J.H.; Yoon, H.J. Machine Learning Model for Predicting Amyloid-β Positivity and Cognitive Status Using Early-Phase 18F-Florbetaben PET and Clinical Features. Sci. Rep. 2025, 15, 21987. [Google Scholar] [CrossRef]
- Komori, S.; Cross, D.J.; Mills, M.; Ouchi, Y.; Nishizawa, S.; Okada, H.; Norikane, T.; Thientunyakit, T.; Anzai, Y.; Minoshima, S. Deep-Learning Prediction of Amyloid Deposition from Early-Phase Amyloid Positron Emission Tomography Imaging. Ann. Nucl. Med. 2022, 36, 913–921. [Google Scholar] [CrossRef]
- Park, Y.-J.; Seo, S.W.; Choi, S.H.; Moon, S.Y.; Son, S.J.; Hong, C.H.; An, Y.-S. Machine Learning-Based Prediction of Amyloid Positivity Using Early-Phase F-18 Flutemetamol PET. J. Alzheimer’s Dis. 2025, 106, 1198–1211. [Google Scholar] [CrossRef]
- Parmera, J.B.; Coutinho, A.M.; Aranha, M.R.; Studart-Neto, A.; de Godoi Carneiro, C.; de Almeida, I.J.; Fontoura Solla, D.J.; Ono, C.R.; Barbosa, E.R.; Nitrini, R.; et al. FDG-PET Patterns Predict Amyloid Deposition and Clinical Profile in Corticobasal Syndrome. Mov. Disord. 2021, 36, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Rasi, R.; Guvenis, A. Predicting Amyloid Positivity from FDG-PET Images Using Radiomics: A Parsimonious Model. Comput. Methods Programs Biomed. 2024, 247, 108098. [Google Scholar] [CrossRef]
- Wang, R.; Liu, H.; Toyonaga, T.; Shi, L.; Wu, J.; Onofrey, J.A.; Tsai, Y.J.; Naganawa, M.; Ma, T.; Liu, Y.; et al. Generation of Synthetic PET Images of Synaptic Density and Amyloid from 18F-FDG Images Using Deep Learning. Med. Phys. 2021, 48, 5115–5129. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Kimura, Y.; Watanabe, S.; Watanabe, A.; Honda, M.; Nagaoka, T.; Nemoto, M.; Hanaoka, K.; Kaida, H.; Kojita, Y.; et al. Evaluation of Amyloid PET Positivity Using Machine Learning on 18F-FDG PET Images. Jpn. J. Radiol. 2025, 43, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, R.; Chen, M.-K.; Mecca, A.P.; O’dell, R.S.; Dyck, C.H.V.; Carson, R.E.; Duncan, J.S.; Liu, C. Synthesizing Multi-Tracer PET Images for Alzheimer’s Disease Patients Using a 3D Unified Anatomy-Aware Cyclic Adversarial Network. arXiv 2021, arXiv:2107.05491. [Google Scholar]
- Guedj, E.; Varrone, A.; Boellaard, R.; Albert, N.L.; Barthel, H.; van Berckel, B.; Brendel, M.; Cecchin, D.; Ekmekcioglu, O.; Garibotto, V.; et al. EANM Procedure Guidelines for Brain PET Imaging Using [18F]FDG, Version 3. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 632–651. [Google Scholar] [CrossRef]
- Scheinin, N.M.; Tolvanen, T.K.; Wilson, I.A.; Arponen, E.M.; Någren, K.Å.; Rinne, J.O. Biodistribution and Radiation Dosimetry of the Amyloid Imaging Agent 11C-PIB in Humans. J. Nucl. Med. 2007, 48, 128–133. [Google Scholar]
- Lin, K.J.; Hsu, W.C.; Hsiao, I.T.; Wey, S.P.; Jin, L.W.; Skovronsky, D.; Wai, Y.Y.; Chang, H.P.; Lo, C.W.; Yao, C.H.; et al. Whole-Body Biodistribution and Brain PET Imaging with [18F]AV-45, a Novel Amyloid Imaging Agent—A Pilot Study. Nucl. Med. Biol. 2010, 37, 497–508. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lyoo, C.H.; Lee, J.H.; Cho, H.; Kim, K.M.; Kim, J.S.; Ryu, Y.H. Human Radiation Dosimetry of [18F]AV-1451(T807) to Detect Tau Pathology. Mol. Imaging Biol. 2016, 18, 479–482. [Google Scholar] [CrossRef]
- Soret, M.; Maisonobe, J.A.; Desarnaud, S.; Bergeret, S.; Causse-Lemercier, V.; Berenbaum, A.; Rozenblum, L.; Habert, M.O.; Kas, A. Ultra-Low-Dose in Brain 18F-FDG PET/MRI in Clinical Settings. Sci. Rep. 2022, 12, 15341. [Google Scholar] [CrossRef] [PubMed]
- Catana, C. The Dawn of a New Era in Low-Dose PET Imaging. Radiology 2018, 290, 657–658. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.; Gong, E.; de Carvalho Macruz, F.B.; Xu, J.; Boumis, A.; Khalighi, M.; Poston, K.L.; Sha, S.J.; Greicius, M.D.; Mormino, E.; et al. Ultra–Low-Dose 18F-Florbetaben Amyloid PET Imaging Using Deep Learning with Multi-Contrast MRI Inputs. Radiology 2018, 290, 649–656. [Google Scholar] [CrossRef]
| Ref. | Author, Year | PET Radiotracer | Methodology vs. Comparator | Sample Size (n) | Outcome Measures |
|---|---|---|---|---|---|
| [21] | Albano et al., 2022 | [18F]-FBP | 1–6 min vs. FDG | 12 | r = 0.89 |
| [22] | Asghar et al., 2019 | [18F]-FBP | 2–5 min vs. FDG | 28 | r = 0.79 |
| [23] | Aye et al., 2024 | [18F]-FBB | 0–10 min vs. ASL MRI | 115 | r = 0.15–0.49 and ROC AUC = 0.83 |
| [24] | Beyer et al., 2020 | [18F]-PI-2620 | 0.5–2.5 min, R1 vs. FDG | 26 | r = 0.76 (0.5–2.5 min), r = 0.77 (R1) |
| [25] | Bilgel et al., 2020 | [11C]-PiB | 0.75–2.5 min, R1 vs. H2O | 149 | r = 0.79 (0.75–2.5 min), r = 0.76 (R1) |
| [26] | Boccalini et al., 2023 | [18F]-FBP, [18F]-FMM | 0–5 min vs. FDG, 0–10 min vs. FDG | 166 | r = 0.79 (FBP), r = 0.81 (FMM) and ROC AUC = 0.80–0.89 |
| [27] | Boccalini et al., 2025 | [18F]-FTP | 0–10 min vs. FDG | 58 | r = 0.84 and ROC AUC = 0.60 |
| [28] | Bunai et al., 2019 | [11C]-PiB | 1–8 min vs. FDG | 95 | r = 0.63–0.94 |
| [29] | Carneiro et al., 2022 | [11C]-PiB | 0–10 min vs. FDG | 90 | r = ~0.70–0.95 |
| [30] | Chen et al., 2015 | [11C]-PiB | R1 vs. H2O | 19 | ρ = ~0.80–0.90 |
| [31] | Choi et al., 2023 | [18F]-FBB | DL (90–110 min) vs. FDG | 110 | / |
| [32] | Daerr et al., 2017 | [18F]-FBB | 0–5 min, 0–10 min vs. FDG | 33 | r = 0.86 |
| [33] | Dghoughi et al., 2019 | [18F]-FMM | 0–1 min vs. FDG | 19 | r = 0.76 |
| [34] | Fettahoglu et al., 2024 | [18F]-FBB | 0–2 min vs. H2O | 20 | r = 0.90 |
| [35] | Florek et al., 2018 | [18F]-FBB | 0–10 min | 112 | / |
| [36] | Forsberg et al., 2012 | [11C]-PiB | 0–6 min vs. FDG | 64 | r = ~0.39–0.74 |
| [37] | Fu J. et al., 2025 | [18F]-MK-6420 | 0–3 min, R1 vs. H2O | 17 | r = 0.84, r = 0.88 |
| [38] | Fu L. et al., 2014 | [11C]-PiB | 1.33–8 min vs. FDG | 40 | r = 0.87 |
| [39] | Gómez-Grande et al., 2023 | [18F]-FBP, [18F]-FMM | 0–1 min vs. FDG, 0–1 min vs. FDG | 17 | r = 0.92 |
| [40] | Guehl et al., 2023 | [18F]-MK-6420, [11C]-PiB | R1 (MK-6420) vs. R1 (PiB) | 49 | r = 0.95 |
| [41] | Hammes et al., 2017 | [18F]-FTP | 1–6 min vs. FDG | 20 | r = ~0.82–0.95 |
| [42] | Hsiao et al., 2012 | [18F]-FBP | 0–2 min, 1–6 min, R1 vs. FDG | 14 | r = 0.78 (0–2 min), r = 0.87 (1–6 min), r = 0.78 (R1) |
| [43] | Jeong et al., 2019 | [18F]-FPN | 0–10 min vs. FDG | 33 | r = 0.83 |
| [44] | Joseph-Mathurin et al., 2018 | [11C]-PiB | 1–9 min, R1 vs. H2O | 110 | / |
| [45] | Kwon et al., 2021 | [18F]-FBB | 0–10 min vs. ECD SPECT | 27 | r = 0.90 and ROC AUC = 0.91 |
| [46] | Leuzy et al., 2018 | [18F]-THK5317 | 0–3 min, R1 vs. FDG | 16 | r = 0.83 (0–3 min), r = 0.85 (R1) |
| [47] | Lin et al., 2016 | [18F]-FBP | 1–6 min | 82 | / |
| [48] | Lojo-Ramírez et al., 2025 | [18F]-FBB | 0–5 min vs. FDG | 103 | ρ = 0.88 and ROC AUC = 0.86 |
| [49] | Matthews et al., 2022 | [18F]-FBP | ML (0–6 min) vs. FDG | 111 | / |
| [50] | Meyer et al., 2011 | [11C]-PiB | R1 vs. FDG | 22 | r = 0.79 |
| [51] | Myoraku et al., 2022 | [18F]-FBP, [18F]-FBB | 0.75–6 min vs. FDG, 0.75–6 min vs. FDG | 100 | r = 0.74 |
| [52] | Oliveira et al., 2018 | [11C]-PiB | 0–6 min, 1–8 min, R1 vs. FDG | 52 | / |
| [53] | Ottoy et al., 2019 | [18F]-FBP | 0–2 min, R1 vs. H2O | 39 | r = 0.70–0.94 (0–2 min), r = 0.65–0.92 (R1) and ROC AUC = 0.87–0.95 (0–2 min), 0.86–0.95 (R1) |
| [54] | Peretti et al., 2019 | [11C]-PiB | 20–130 s, R1 vs. FDG | 30 | r = 0.76 (20–130 s), r = 0.85 (R1) |
| [55] | Peretti et al., 2019 | [11C]-PiB | 20–130 s, 1–8 min, R1 vs. FDG | 52 | ROC AUC = 0.94 (20–130 s), 0.89 (1–8 min), 0.92 (R1) |
| [56] | Peretti et al., 2021 | [11C]-PiB | R1 vs. FDG | 79 | ROC AUC = 0.81 |
| [57] | Peretti et al., 2022 | [11C]-PiB | 20–130 s, 1–8 min, R1 vs. FDG | 52 | r = 0.59 (20–130 s), r = 0.49 (1–8 min), r = 0.79 (R1) and ROC AUC = 0.69 (20–130 s), 0.85 (1–8 min), 0.83 (R1) |
| [58] | Ponto et al., 2019 | [11C]-PiB | 3.5–4 min, 0–6 min, R1 vs. H2O | 24 | r = 0.61 (3.5–4 min), r = 0.52 (0–6 min), r = 0.62 (R1) |
| [59] | Ribaldi et al., 2025 | [18F]-FBP, [18F]-FMM | 0–5 min vs. ASL MRI, 0–10 min vs. ASL MRI | 46 | / |
| [60] | Rodriguez-Vieitez et al., 2016 | [11C]-PiB | 1–4 min vs. FDG | 41 | r = 0.61 and ROC AUC = 0.84–0.90 |
| [61] | Rodriguez-Vieitez et al., 2017 | [18F]-THK5317, [11C]-PiB | 0–3 min, R1 vs. FDG, 1–8 min, R1 vs. FDG | 20 | r = 0.86 (THK), r = 0.88 (PiB), r = 0.86 (R1 THK), r = 0.90 (R1 PiB) and ROC AUC = 0.82 (THK), 0.78 (PiB), 0.84 (R1 THK), 0.79 (R1 PiB) |
| [62] | Rostomian et al., 2011 | [11C]-PiB | 1–8 min vs. FDG | 83 | r = 0.91 |
| [63] | Sanaat et al., 2024 | [18F]-FBP, [18F]-FMM | DL (0–5 min) vs. FDG, DL (0–10 min) vs. FDG | 166 | r = 0.82 (FBP), r = 0.85 (FMM) |
| [64] | Schmitt et al., 2021 | [18F]-FMM | 0–10 min vs. FDG | 20 | r = 0.86 |
| [65] | Segovia et al., 2018 | [18F]-FBB | 0–10 min vs. FDG | 47 | r = ~0.5 |
| [66] | Segovia et al., 2018 | [18F]-FBB | ML vs. FDG | 47 | / |
| [67] | Segovia et al., 2020 | [18F]-FBB | ML (0–20 min) vs. FDG | 43 | ROC AUC > 0.8 |
| [68] | Seiffert et al., 2020 | [18F]-FBP | 0–10 min vs. FDG | 19 | r = 0.72 |
| [69] | Seiffert et al., 2021 | [18F]-FBP, [18F]-FBB, [18F]-FMM | 0–1 min vs. FDG, 0–1 min vs. FDG, 0–1 min vs. FDG | 60 | r = 0.86 (FBP), r = 0.77 (FBB), r = 0.78 (FMM) |
| [70] | Son et al., 2020 | [18F]-FBB | 0–5 min vs. FDG | 40 | r = ~0.77 |
| [71] | Tiepolt et al., 2016 | [11C]-PiB, [18F]-FBB | 1–9 min vs. FDG, 1–9 min vs. FDG | 22 | r = 0.73 (PiB), r = 0.81 (FBB) |
| [72] | Tiepolt et al., 2019 | [11C]-PiB | 1–9 min | 31 | / |
| [73] | Tuncel et al., 2023 | [18F]-FBP, [18F]-FTP | R1 (FBP) vs. R1 (FTP) | 50 | r = 0.89–0.93 |
| [74] | Vanhoutte et al., 2021 | [18F]-FBP | 0–4 min vs. FDG | 191 | / |
| [75] | Völter et al., 2023 | [18F]-PI-2620, [18F]-FMM | 0.5–2.5 min (PI-2620) vs. 0–10 min (FMM) | 64 | r = 0.82 |
| [76] | Völter et al., 2025 | [18F]-FBB, [18F]-FMM | 0–10 min (FBB), 0–10 min (FMM) | 82 | / |
| [77] | Wolters et al., 2020 | [18F]-FTP | R1 vs. FDG | 133 | AUC = 0.94 |
| [78] | Yoon et al., 2021 | [18F]-FBB | 0–10 min vs. R1 | 60 | r = 0.75–0.91 |
| Ref. | Author, Year | PET Radiotracer | Methodology (Specified Model) | Sample Size (n) | Outcome Measures |
|---|---|---|---|---|---|
| [82] | Gnörich et al., 2025 * | [18F]-PI-2620 | K2a using kinetic modelling (SRTM2) | 146 | prediction of A status ROC AUC = 0.99, PPV = 0.915, NPV = 0.951 |
| [83] | Hammes et al., 2021 | [18F]-FTP | SSM/PCA + ML (SVM) | 54 | prediction of A status ROC AUC = 0.95, SS = 0.94, SP = 0.83 |
| [84] | Lee et al., 2024 | [11C]-PiB | DL (CNN) | 1480 | generation of tau PET correlation r = 0.41–0.76, ROC AUC > 0.9 |
| [85] | Naseri et al., 2023 ** | [18F]-FBP | DL (cGAN) | 475 | generation of tau PET ROC AUC = 0.84, SSIM = 0.917 |
| [86] | Raman et al., 2022 | [18F]-FBP | early phase | 410 | prediction of T status ROC AUC = 0.86, SS = 0.71, SP = 0.93 |
| [87] | Ruwanpathirana et al., 2022 | [18F]-MK6240 | DL (CNN) | 134 | prediction of centiloid score RMSE = 29.93, R2 = 0.79 |
| [88] | Shcherbinin et al., 2023 | [18F]-FTP | late-phase | 1781 | prediction of A status PPV ≥ 93%, NPV = 60–77%, ROC AUC = 0.88 |
| Ref. | Author, Year | PET Radiotracer | Methodology (Specified Model) | Sample Size (n) | Outcome Measures |
|---|---|---|---|---|---|
| [90] | Alongi et al., 2022 | [18F]-FDG | ML (DA) | 43 | prediction of A status SS = 84.92%, SP = 75.13%, PR = 73.75% and ACC = 79.56% |
| [91] | Ardakani et al., 2025 | [18F]-FDG | DL (CNN) | 286 | prediction of A status ROC AUC = 0.815–0.844 and F1 Score = 0.770–0.809 |
| [92] | Choi et al., 2025 | eFBB | ML (DT, RF, GB, and more) | 176 | prediction of A status ROC AUC = 0.83 and F1 Score = 0.80 |
| [15] | Kim et al., 2021 | [18F]-FDG | DL (CNN) | 1533 | prediction of A status ROC AUC = 0.798–0.811 and F1 Score = 0.709–0.712 |
| [93] | Komori et al., 2022 | ePiB | DL (CNN) | 253 | generation of delayed PET image intra-reader agreement κ = 0.59–0.60 and inter-reader agreement κ = 0.79 SSIM = 0.45 and PSNR = 21.8 |
| [84] | Lee et al., 2024 | [18F]-FDG | DL (CNN) | 1480 | generation of tau PET correlation r > 0.8, ROC AUC > 0.9 |
| [94] | Park et al., 2025 * | eFMM | ML (LR, DA) | 454 | prediction of A status ROC AUC = 0.779–0.791 |
| [95] | Parmera et al., 2021 | [18F]-FDG | \ | 45 | prediction of A status SS = 76.92%, SP = 100%, PPV = 100%, ACC = 88.5% |
| [96] | Rasi et al., 2024 | [18F]-FDG | ML (RF, GNB, and more) | 301 | prediction of A status ROC AUC = 0.924 |
| [97] | Wang et al., 2021 | [18F]-FDG | DL (CNN) | 54 | generation of amyloid PET exploratory, visual analysis |
| [98] | Yamada et al., 2025 | [18F]-FDG | ML (SVM) | 194 | prediction of A status ROC AUC = 0.918 |
| [99] | Zhou et al., 2021 | [18F]-FDG | DL (GAN) | 35 | generation of amyloid PET SSIM = 0.764 and NMSE = 14.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balot, E.; Vandenberghe, S.; Van Langenhove, T.; De Meulenaere, V.; D’Asseler, Y.; Van Weehaeghe, D. Correlation and Interchangeability of Amyloid, Tau, and Glucose Metabolism PET in Mild Cognitive Impairment and Alzheimer: A Review. Brain Sci. 2025, 15, 1271. https://doi.org/10.3390/brainsci15121271
Balot E, Vandenberghe S, Van Langenhove T, De Meulenaere V, D’Asseler Y, Van Weehaeghe D. Correlation and Interchangeability of Amyloid, Tau, and Glucose Metabolism PET in Mild Cognitive Impairment and Alzheimer: A Review. Brain Sciences. 2025; 15(12):1271. https://doi.org/10.3390/brainsci15121271
Chicago/Turabian StyleBalot, Emile, Stefaan Vandenberghe, Tim Van Langenhove, Valerie De Meulenaere, Yves D’Asseler, and Donatienne Van Weehaeghe. 2025. "Correlation and Interchangeability of Amyloid, Tau, and Glucose Metabolism PET in Mild Cognitive Impairment and Alzheimer: A Review" Brain Sciences 15, no. 12: 1271. https://doi.org/10.3390/brainsci15121271
APA StyleBalot, E., Vandenberghe, S., Van Langenhove, T., De Meulenaere, V., D’Asseler, Y., & Van Weehaeghe, D. (2025). Correlation and Interchangeability of Amyloid, Tau, and Glucose Metabolism PET in Mild Cognitive Impairment and Alzheimer: A Review. Brain Sciences, 15(12), 1271. https://doi.org/10.3390/brainsci15121271







