Electrical Cortical Stimulation for Language Mapping in Epilepsy Surgery—A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Search Strategy
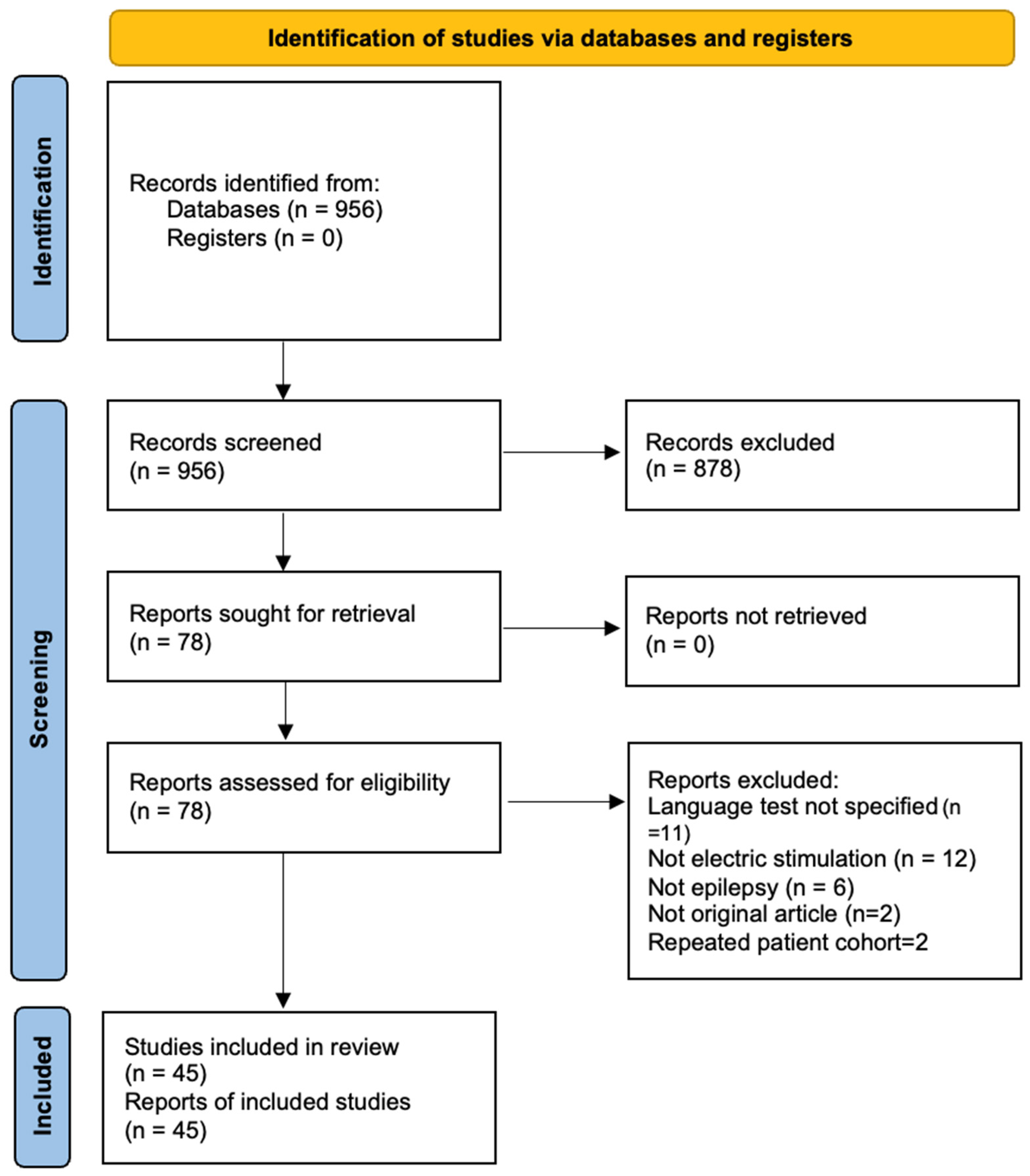
2.4. Study Selection and Data Extraction
3. Results
4. Discussion
4.1. Methodological Heterogeneity and Clinical Implications
4.2. Stimulation Parameters and Protocols
4.3. Surgical Margins and Resection Planning
4.4. Age-Specific Considerations: Paediatric Versus Adult Language Mapping
4.5. Cross-Linguistic Considerations
4.6. Developmental Language Reorganisation
4.7. Strengths and Limitations of This Review
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Ojemann, G.A. Individual variability in cortical localization of language. J. Neurosurg. 1979, 50, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Rolston, J.D.; Chang, E.F. Critical language areas show increased functional connectivity in human cortex. Cereb. Cortex 2018, 28, 4161–4168. [Google Scholar] [CrossRef]
- Ojemann, G.; Ojemann, J.; Lettich, E.; Berger, M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J. Neurosurg. 1989, 71, 316–326. [Google Scholar] [CrossRef]
- Hickok, G.; Venezia, J.; Teghipco, A. Beyond Broca: Neural architecture and evolution of a dual motor speech coordination system. Brain 2023, 146, 1775–1790. [Google Scholar] [CrossRef]
- Binding, L.P.; Dasgupta, D.; Giampiccolo, D.; Duncan, J.S.; Vos, S.B. Structure and function of language networks in temporal lobe epilepsy. Epilepsia 2022, 63, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Marcelle, M.; You, X.; Fanto, E.J.; Sepeta, L.N.; Gaillard, W.D.; Berl, M.M. Impact of development and recent-onset epilepsy on language dominance. Epilepsia 2022, 63, 2637–2649. [Google Scholar] [CrossRef]
- Doss, D.J.; Johnson, G.W.; Englot, D.J. Imaging and Stereotactic Electroencephalography Functional Networks to Guide Epilepsy Surgery. Neurosurg. Clin. N. Am. 2024, 35, 61–72. [Google Scholar] [CrossRef]
- Reecher, H.M.; Bearden, D.J.; Koop, J.I.; Berl, M.M.; Patrick, K.E.; Ailion, A.S. The changing landscape of electrical stimulation language mapping with subdural electrodes and stereoelectroencephalography for pediatric epilepsy: A literature review and commentary. Epilepsia 2024, 65, 1879–1898. [Google Scholar] [CrossRef]
- Abarrategui, B.; Mariani, V.; Rizzi, M.; Berta, L.; Scarpa, P.; Zauli, F.M.; Squarza, S.; Banfi, P.; d’Orio, P.; Cardinale, F.; et al. Language lateralization mapping (reversibly) masked by non-dominant focal epilepsy: A case report. Front. Hum. Neurosci. 2023, 17, 1254779. [Google Scholar] [CrossRef]
- Janecek, J.K.; Swanson, S.J.; Sabsevitz, D.S.; Hammeke, T.A.; Raghavan, M.; Rozman, E.M.; Binder, J.R. Language lateralization by fMRI and Wada testing in 229 patients with epilepsy: Rates and predictors of discordance. Epilepsia 2013, 54, 314–322. [Google Scholar] [CrossRef]
- Borchers, S.; Himmelbach, M.; Logothetis, N.; Karnath, H.O. Direct electrical stimulation of human cortex-the gold standard for mapping brain functions? Nat. Rev. Neurosci. 2012, 13, 63–70. [Google Scholar] [CrossRef]
- Mandonnet, E.; Winkler, P.A.; Duffau, H. Direct electrical stimulation as an input gate into brain functional networks: Principles, advantages and limitations. Acta Neurochir. 2010, 152, 185–193. [Google Scholar] [CrossRef]
- Jahangiri, F.R.; Chima, G.S.; Pearson, M.; Jackson, J.; Siddiqui, A.A. Mapping of the Language Cortex. Cureus 2021, 13, e14960. [Google Scholar] [CrossRef]
- Alarcón, G.; Bird Pedersen, M.; Juárez-Torrejón, N.; Martín-López, D.; Ughratdar, I.; Selway, R.P.; Valentín, A. The Single Word Auditory Comprehension (SWAC) test: A simple method to identify receptive language areas with electrical stimulation. Epilepsy Behav. 2019, 90, 266–272. [Google Scholar] [CrossRef]
- Hamberger, M.J.; Williams, A.C.; Schevon, C.A. Extraoperative neurostimulation mapping: Results from an international survey of epilepsy surgery programs. Epilepsia 2014, 55, 933–939. [Google Scholar] [CrossRef]
- Sakpichaisakul, K.; Byars, A.W.; Horn, P.S.; Aungaroon, G.; Greiner, H.M.; Mangano, F.T.; Holland, K.D.; Arya, R. Neuropsychological outcomes after pediatric epilepsy surgery: Role of electrical stimulation language mapping. Seizure 2020, 80, 183–191. [Google Scholar] [CrossRef]
- Hamberger, M.J. Cortical language mapping in epilepsy: A critical review. Neuropsychol. Rev. 2007, 17, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Aron, O.; Mezjan, I.; Krieg, J.; Ferrand, M.; Colnat-Coulbois, S.; Maillard, L. Mapping the basal temporal language network: A SEEG functional connectivity study. Brain Lang. 2024, 258, 105486. [Google Scholar] [CrossRef] [PubMed]
- De Witte, E.; Mariën, P. The neurolinguistic approach to awake surgery reviewed. Clin. Neurol. Neurosurg. 2013, 115, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Hamberger, M.J.; Cole, J. Language organization and reorganization in epilepsy. Neuropsychol. Rev. 2011, 21, 240–251. [Google Scholar] [CrossRef]
- Ruis, C. Monitoring cognition during awake brain surgery in adults: A systematic review. J. Clin. Exp. Neuropsychol. 2018, 40, 1081–1104. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Z.; Zhang, J.; Wu, B.; Zhu, Y.; Chang, E.F.; Wu, J.; Duffau, H.; Berger, M.S. Functional maps of direct electrical stimulation-induced speech arrest and anomia: A multicentre retrospective study. Brain 2021, 144, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Korostenskaja, M.; Wilson, A.J.; Rose, D.F.; Brunner, P.; Schalk, G.; Leach, J.; Mangano, F.T.; Fujiwara, H.; Rozhkov, L.; Harris, E.; et al. Real-time functional mapping with electrocorticography in pediatric epilepsy: Comparison with FMRI and ESM findings. Clin. EEG Neurosci. 2014, 45, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Aron, O.; Jonas, J.; Colnat-Coulbois, S.; Maillard, L. Language Mapping Using Stereo Electroencephalography: A Review and Expert Opinion. Front. Hum. Neurosci. 2021, 15, 619521. [Google Scholar] [CrossRef] [PubMed]
- Enatsu, R.; Kanno, A.; Ookawa, S.; Ochi, S.; Ishiai, S.; Nagamine, T.; Mikuni, N. Distribution and Network of Basal Temporal Language Areas: A Study of the Combination of Electric Cortical Stimulation and Diffusion Tensor Imaging. World Neurosurg. 2017, 106, 1–8. [Google Scholar] [CrossRef]
- Aron, O.; Krieg, J.; Brissart, H.; Abdallah, C.; Colnat-Coulbois, S.; Jonas, J.; Maillard, L. Naming impairments evoked by focal cortical electrical stimulation in the ventral temporal cortex correlate with increased functional connectivity. Neurophysiol. Clin. 2022, 52, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Wilson, J.A.; Vannest, J.; Byars, A.W.; Greiner, H.M.; Buroker, J.; Fujiwara, H.; Mangano, F.T.; Holland, K.D.; Horn, P.S.; et al. Electrocorticographic language mapping in children by high-gamma synchronization during spontaneous conversation: Comparison with conventional electrical cortical stimulation. Epilepsy Res. 2015, 110, 78–87. [Google Scholar] [CrossRef]
- Arya, R.; Ervin, B.; Dudley, J.; Buroker, J.; Rozhkov, L.; Scholle, C.; Horn, P.S.; Vannest, J.; Byars, A.W.; Leach, J.L.; et al. Electrical stimulation mapping of language with stereo-EEG. Epilepsy Behav. 2019, 99, 106395. [Google Scholar] [CrossRef]
- Arya, R.; Frink, C.; Kargol, C.; Byars, A.W.; Huddleston, D.; Diedenhofer, D.B.; Aungaroon, G.; Ervin, B.; Horn, P.S.; Ihnen, S.K.Z.; et al. Neuropsychological outcomes after epilepsy surgery: A comparison of stereo electroencephalography and subdural electrodes. Eur. J. Neurol. 2023, 30, 2986–2998. [Google Scholar] [CrossRef]
- Arya, R.; Wilson, J.A.; Fujiwara, H.; Rozhkov, L.; Leach, J.L.; Byars, A.W.; Greiner, H.M.; Vannest, J.; Buroker, J.; Milsap, G.; et al. Presurgical language localization with visual naming associated ECoG high- gamma modulation in pediatric drug-resistant epilepsy. Epilepsia 2017, 58, 663–673. [Google Scholar] [CrossRef]
- Asano, E. Contribution of research on ‘Epilepsy & Behavior’ to the refinement of functional brain atlas in four dimensions. Epilepsy Behav. 2014, 40, 86–88. [Google Scholar] [CrossRef][Green Version]
- Babajani-Feremi, A.; Holder, C.M.; Narayana, S.; Fulton, S.P.; Choudhri, A.F.; Boop, F.A.; Wheless, J.W. Predicting postoperative language outcome using presurgical fMRI, MEG, TMS, and high gamma ECoG. Clin. Neurophysiol. 2018, 129, 560–571. [Google Scholar] [CrossRef]
- Balogun, J.A.; Khan, O.H.; Taylor, M.; Dirks, P.; Der, T.; Snead, O.C.; Weiss, S.; Ochi, A.; Drake, J.; Rutka, J.T. Pediatric awake craniotomy and intra-operative stimulation mapping. J. Clin. Neurosci. 2014, 21, 1891–1894. [Google Scholar] [CrossRef]
- Bauer, P.R.; Vansteensel, M.J.; Bleichner, M.G.; Hermes, D.; Ferrier, C.H.; Aarnoutse, E.J.; Ramsey, N.F. Mismatch between electrocortical stimulation and electrocorticography frequency mapping of language. Brain Stimul. 2013, 6, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Hamberger, M.J.; Miozzo, M.; Schevon, C.A.; Morrison, C.; Carlson, C.; Mehta, A.D.; Klein, G.E.; McKhann, G.M.; Williams, A.C. Functional differences among stimulation-identified cortical naming sites in the temporal region. Epilepsy Behav. 2016, 60, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Hamberger, M.J.; Schevon, C.A.; Seidel, W.T.; McKhann, G.M.; Morrison, C. Cortical naming sites and increasing age in adults with refractory epilepsy: More might be less. Epilepsia 2019, 60, 1619–1626. [Google Scholar] [CrossRef]
- Rolinski, R.; Austermuehle, A.; Wiggs, E.; Agrawal, S.; Sepeta, L.N.; Gaillard, W.D.; Zaghloul, K.A.; Inati, S.K.; Theodore, W.H. Functional MRI and direct cortical stimulation: Prediction of postoperative language decline. Epilepsia 2019, 60, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Shimotake, A.; Matsumoto, R.; Ueno, T.; Kunieda, T.; Saito, S.; Hoffman, P.; Kikuchi, T.; Fukuyama, H.; Miyamoto, S.; Takahashi, R.; et al. Direct Exploration of the Role of the Ventral Anterior Temporal Lobe in Semantic Memory: Cortical Stimulation and Local Field Potential Evidence From Subdural Grid Electrodes. Cereb. Cortex 2015, 25, 3802–3817. [Google Scholar] [CrossRef]
- Aungaroon, G.; Vedala, K.; Byars, A.W.; Ervin, B.; Rozhkov, L.; Horn, P.S.; Ihnen, S.K.Z.; Holland, K.D.; Tenney, J.R.; Kremer, K.; et al. Comparing electrical stimulation functional mapping with subdural electrodes and stereoelectroencephalography. Epilepsia 2023, 64, 1527–1540. [Google Scholar] [CrossRef] [PubMed]
- Austermuehle, A.; Cocjin, J.; Reynolds, R.; Agrawal, S.; Sepeta, L.; Gaillard, W.D.; Zaghloul, K.A.; Inati, S.; Theodore, W.H. Language functional MRI and direct cortical stimulation in epilepsy preoperative planning. Ann. Neurol. 2017, 81, 526–537. [Google Scholar] [CrossRef]
- Oane, I.; Barborica, A.; Chetan, F.; Donos, C.; Maliia, M.D.; Arbune, A.A.; Daneasa, A.; Pistol, C.; Nica, A.E.; Bajenaru, O.A.; et al. Cingulate cortex function and multi-modal connectivity mapped using intracranial stimulation. NeuroImage 2020, 220, 117059. [Google Scholar] [CrossRef]
- Sabsevitz, D.S.; Middlebrooks, E.H.; Tatum, W.; Grewal, S.S.; Wharen, R.; Ritaccio, A.L. Examining the function of the visual word form area with stereo EEG electrical stimulation: A case report of pure alexia. Cortex 2020, 129, 112–118. [Google Scholar] [CrossRef]
- Serafini, S.; Clyde, M.; Tolson, M.; Haglund, M.M. Multimodality word-finding distinctions in cortical stimulation mapping. Neurosurgery 2013, 73, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Enatsu, R.; Kanno, A.; Imataka, S.; Komura, S.; Tamada, T.; Sakashita, K.; Chiba, R.; Saito, T.; Mikuni, N. Comparison of Thresholds between Bipolar and Monopolar Electrical Cortical Stimulation. Neurol. Med.-Chir. 2022, 62, 294–299. [Google Scholar] [CrossRef]
- Wen, J.; Yu, T.; Li, Y.; Li, X. Using electrocorticography for presurgical language mapping in epilepsy patients. J. Clin. Neurosci. 2017, 44, 320–322. [Google Scholar] [CrossRef]
- Yu, K.; Yu, T.; Qiao, L.; Liu, C.; Wang, X.; Zhou, X.; Ni, D.; Zhang, G.; Li, Y. Electrical stimulation of the insulo-opercular region: Visual phenomena and altered body-ownership symptoms. Epilepsy Res. 2018, 148, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, G.; Yu, T.; Ni, D.; Cai, L.; Qiao, L.; Du, W.; Li, Y. Surgical treatment for epilepsy involving language cortices: A combined process of electrical cortical stimulation mapping and intra-operative continuous language assessment. Seizure 2013, 22, 780–786. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Zhang, G.; Yu, T.; Xu, C.; Yan, X.; Ma, K.; Du, W.; Gao, R.; Li, Y. Multitask preoperative language mapping in epilepsy surgery: A combination of navigated transcranial magnetic stimulation and extra-operative electrical cortical stimulation. J. Clin. Neurosci. 2020, 79, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yu, T.; Li, Z.; Zhou, X.; Wen, J.; Li, X. Functional mapping of language-related areas from natural, narrative speech during awake craniotomy surgery. NeuroImage 2021, 245, 118720. [Google Scholar] [CrossRef]
- Cockle, E.; Malpas, C.B.; Coleman, H.; McIlroy, A.; Laing, J.; Kwan, P.; Hunn, M.; Gutman, M.; Harb, C.; Meade, C.; et al. Cortical stimulation predicts language decline following SEEG radiofrequency thermocoagulation. Brain 2025, 148, 3314–3324. [Google Scholar] [CrossRef] [PubMed]
- Ervin, B.; Buroker, J.; Rozhkov, L.; Holloway, T.; Horn, P.S.; Scholle, C.; Byars, A.W.; Mangano, F.T.; Leach, J.L.; Greiner, H.M.; et al. High-gamma modulation language mapping with stereo-EEG: A novel analytic approach and diagnostic validation. Clin. Neurophysiol. 2020, 131, 2851–2860. [Google Scholar] [CrossRef]
- Matoba, K.; Matsumoto, R.; Shimotake, A.; Nakae, T.; Imamura, H.; Togo, M.; Yamao, Y.; Usami, K.; Kikuchi, T.; Yoshida, K.; et al. Basal temporal language area revisited in Japanese language with a language function density map. Cereb. Cortex 2024, 34, bhae218. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; O’Hara, N.; Sonoda, M.; Kuroda, N.; Juhasz, C.; Asano, E.; Dong, M.; Jeong, J.W. Novel Deep Learning Network Analysis of Electrical Stimulation Mapping-Driven Diffusion MRI Tractography to Improve Preoperative Evaluation of Pediatric Epilepsy. IEEE Trans. Biomed. Eng. 2020, 67, 3151–3162. [Google Scholar] [CrossRef]
- Bearden, D.J.; Ehrenberg, A.; Selawski, R.; Ono, K.E.; Drane, D.L.; Pedersen, N.P.; Cernokova, I.; Marcus, D.J.; Luongo-Zink, C.; Chern, J.J.; et al. Four-Way Wada: SEEG-based mapping with electrical stimulation, high frequency activity, and phase amplitude coupling to complement traditional Wada and functional MRI prior to epilepsy surgery. Epilepsy Res. 2023, 192, 107129. [Google Scholar] [CrossRef] [PubMed]
- Young, J.J.; Coulehan, K.; Fields, M.C.; Yoo, J.Y.; Marcuse, L.V.; Jette, N.; Panov, F.; Ghatan, S.; Bender, H.A. Language mapping using electrocorticography versus stereoelectroencephalography: A case series. Epilepsy Behav. 2018, 84, 148–151. [Google Scholar] [CrossRef]
- Perrone-Bertolotti, M.; Alexandre, S.; Jobb, A.S.; De Palma, L.; Baciu, M.; Mairesse, M.P.; Hoffmann, D.; Minotti, L.; Kahane, P.; David, O. Probabilistic mapping of language networks from high frequency activity induced by direct electrical stimulation. Hum. Brain Mapp. 2020, 41, 4113–4126. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Sweet, J.; Miller, J. Speech mapping using depth electrodes: The “electric Wada”. Clin. Neurol. Neurosurg. 2016, 144, 88–90. [Google Scholar] [CrossRef]
- Nakai, Y.; Jeong, J.W.; Brown, E.C.; Rothermel, R.; Kojima, K.; Kambara, T.; Shah, A.; Mittal, S.; Sood, S.; Asano, E. Three- and four-dimensional mapping of speech and language in patients with epilepsy. Brain 2017, 140, 1351–1370. [Google Scholar] [CrossRef]
- Bohm, P.; McKay, J.; Lucas, J.; Sabsevitz, D.; Feyissa, A.M.; Ritaccio, T.; Grewal, S.S.; Wharen, R.E.; Gupta, V.; Tatum, W.O. Wada testing and fMRI in a polyglot evaluated for epilepsy surgery. Epileptic Disord. 2020, 22, 207–213. [Google Scholar] [CrossRef]
- Hirano, T.; Enatsu, R.; Sasagawa, A.; Arihara, M.; Kuribara, T.; Yokoyama, R.; Suzuki, H.; Ochi, S.; Mikuni, N. Anatomical and functional distribution of functional MRI language mapping. J. Clin. Neurosci. 2020, 77, 116–122. [Google Scholar] [CrossRef]
- Pearce, D.; Picone, J. Aurora working group: DSR front end LVCSR evaluation AU/384/02. Inst. Signal Inform. Process. Miss. State Univ. Tech. Rep. 2002. Available online: https://isip.piconepress.com/publications/reports/2002/aurora/frontend/report_012202_v20.pdf (accessed on 1 January 2025).
- Ohlerth, A.K.; Valentin, A.; Vergani, F.; Ashkan, K.; Bastiaanse, R. The verb and noun test for peri-operative testing (VAN-POP): Standardized language tests for navigated transcranial magnetic stimulation and direct electrical stimulation. Acta Neurochir. 2020, 162, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, J. Atlas and Text-Book of Human Anatomy, Vol. 3: Vascular System, Lymphatic System, Nervous System and Sense Organs; W.B. Saunders Company: Philadelphia, PA, USA, 1907; p. 138. [Google Scholar]
- Arya, R.; Babajani-Feremi, A.; Byars, A.W.; Vannest, J.; Greiner, H.M.; Wheless, J.W.; Mangano, F.T.; Holland, K.D. A model for visual naming based on spatiotemporal dynamics of ECoG high-gamma modulation. Epilepsy Behav. 2019, 99, 106455. [Google Scholar] [CrossRef] [PubMed]
- Babajani-Feremi, A.; Narayana, S.; Rezaie, R.; Choudhri, A.F.; Fulton, S.P.; Boop, F.A.; Wheless, J.W.; Papanicolaou, A.C. Language mapping using high gamma electrocorticography, fMRI, and TMS versus electrocortical stimulation. Clin. Neurophysiol. 2016, 127, 1822–1836. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Muragaki, Y.; Maruyama, T.; Saito, T.; Nitta, M.; Tamura, M.; Kawamata, T. Localization and symptoms associated with removal of negative motor area during awake surgery. Br. J. Neurosurg. 2025, 39, 440–448. [Google Scholar] [CrossRef]
- Zhou, X.; Wen, J.; Yu, T.; Qiao, L.; Zhang, X.; Ni, D.; Zhou, T.; Wang, X.; Zhang, G.; Ren, L.; et al. Clinical application of intraoperative trial-free online-based language mapping for patients with refractory epilepsy. Epilepsy Behav. 2021, 116, 107496. [Google Scholar] [CrossRef]
- Labudda, K.; Mertens, M.; Kalbhenn, T.; Schulz, R.; Woermann, F.G. Partial resection of presurgical fMRI activation is associated with a postsurgical loss of language function after frontal lobe epilepsy surgery. Neurocase 2017, 23, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Lioumis, P.; Autti, S.; Wilenius, J.; Vaalto, S.; Lehtinen, H.; Laakso, A.; Kirveskari, E.; Mäkelä, J.P.; Liljeström, M.; Renvall, H. Study Design for Navigated Repetitive Transcranial Magnetic Stimulation for Speech Cortical Mapping. J. Vis. Exp. 2023, 193, e64492. [Google Scholar] [CrossRef]
- Trébuchon, A.; Chauvel, P. Electrical Stimulation for Seizure Induction and Functional Mapping in Stereoelectroencephalography (review). J. Clin. Neurophysiol. 2016, 33, 511–521. [Google Scholar] [CrossRef]
- Schäffler, L.; Lüders, H.O.; Beck, G.J. Quantitative comparison of language deficits produced by extraoperative electrical stimulation of Broca’s, Wernicke’s, and basal temporal language areas. Epilepsia 1996, 37, 463–475. [Google Scholar] [CrossRef]
- Lüders, H.; Lesser, R.P.; Hahn, J.; Dinner, D.S.; Morris, H.H.; Wyllie, E.; Godoy, J. Basal Temporal Language Area. Brain 1991, 114, 743–754. [Google Scholar] [CrossRef]
- Benjamin, C.F.; Walshaw, P.D.; Hale, K.; Gaillard, W.D.; Baxter, L.C.; Berl, M.M.; Polczynska, M.; Noble, S.; Alkawadri, R.; Hirsch, L.J.; et al. Presurgical language fMRI: Mapping of six critical regions. Hum. Brain Mapp. 2017, 38, 4239–4255. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Basu, A.; Kumaran, S.S.; Khushu, S. Functional mapping of language networks in the normal brain using a word-association task. Indian. J. Radiol. Imaging 2010, 20, 182–187. [Google Scholar] [CrossRef]
- Bargalló, N.; Cano-López, I.; Rosazza, C.; Vernooij, M.W.; Smits, M.; Vitali, P.; Alvarez-Linera, J.; Urbach, H.; Mancini, L.; Ramos, A.; et al. Clinical practice of language fMRI in epilepsy centers: A European survey and conclusions by the ESNR Epilepsy Working Group. Neuroradiology 2020, 62, 549–562. [Google Scholar] [CrossRef]
- St-Denis, A.; Hooker, M.; L’Abbée Lacas, K.; Corriveau, I.; Pirmoradi, M.; Simard-Tremblay, E.; Atkinson, J.; Myers, K.A. Awake Craniotomy Language Mapping in Children With Drug-Resistant Epilepsy due to Focal Cortical Dysplasia. Pediatr. Neurol. 2023, 144, 39–43. [Google Scholar] [CrossRef]
- Schwartz, T.H.; Devinsky, O.; Doyle, W.; Perrine, K. Function-Specific High-Probability “Nodes” Identified in Posterior Language Cortex. Epilepsia 1999, 40, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, M.C.; Corines, J.; Boatman-Reich, D.F.; Eloyan, A.; Sheng, X.; Franaszczuk, P.J.; Crone, N.E. Electrocorticographic functional mapping identifies human cortex critical for auditory and visual naming. Neuroimage 2013, 69, 267–276. [Google Scholar] [CrossRef]
- Cossu, M.; Cardinale, F.; Castana, L.; Citterio, A.; Francione, S.; Tassi, L.; Benabid, A.L.; Lo Russo, G. Stereoelectroencephalography in the presurgical evaluation of focal epilepsy: A retrospective analysis of 215 procedures. Neurosurgery 2005, 57, 706–718, discussion 706–718. [Google Scholar] [CrossRef] [PubMed]
- Corina, D.P.; Loudermilk, B.C.; Detwiler, L.; Martin, R.F.; Brinkley, J.F.; Ojemann, G. Analysis of naming errors during cortical stimulation mapping: Implications for models of language representation. Brain Lang. 2010, 115, 101–112. [Google Scholar] [CrossRef]
- Ilmberger, J.; Ruge, M.; Kreth, F.-W.; Briegel, J.; Reulen, H.-J.; Tonn, J.-C. Intraoperative mapping of language functions: A longitudinal neurolinguistic analysis. J. Neurosurg. 2008, 109, 583–592. [Google Scholar] [CrossRef]
- Brunner, P.; Ritaccio, A.L.; Lynch, T.M.; Emrich, J.F.; Wilson, J.A.; Williams, J.C.; Aarnoutse, E.J.; Ramsey, N.F.; Leuthardt, E.C.; Bischof, H.; et al. A practical procedure for real-time functional mapping of eloquent cortex using electrocorticographic signals in humans. Epilepsy Behav. 2009, 15, 278–286. [Google Scholar] [CrossRef]
- Swift, J.R.; Coon, W.G.; Guger, C.; Brunner, P.; Bunch, M.; Lynch, T.; Frawley, B.; Ritaccio, A.L.; Schalk, G. Passive functional mapping of receptive language areas using electrocorticographic signals. Clin. Neurophysiol. 2018, 129, 2517–2524. [Google Scholar] [CrossRef]
- Ojemann, S.G.; Berger, M.S.; Lettich, E.; Ojemann, G.A. Localization of language function in children: Results of electrical stimulation mapping. J. Neurosurg. 2003, 98, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, S.B.; Thompson, P.J.; Yogarajah, M.; Vollmar, C.; Powell, R.H.W.; Symms, M.R.; McEvoy, A.W.; Micallef, C.; Koepp, M.J.; Duncan, J.S. Imaging language networks before and after anterior temporal lobe resection: Results of a longitudinal fMRI study. Epilepsia 2012, 53, 639–650. [Google Scholar] [CrossRef]
- Schevon, C.A.; Carlson, C.; Zaroff, C.M.; Weiner, H.J.; Doyle, W.K.; Miles, D.; Lajoie, J.; Kuzniecky, R.; Pacia, S.; Vazquez, B.; et al. Pediatric language mapping: Sensitivity of neurostimulation and Wada testing in epilepsy surgery. Epilepsia 2007, 48, 539–545. [Google Scholar] [CrossRef]
- Zea Vera, A.; Aungaroon, G.; Horn, P.S.; Byars, A.W.; Greiner, H.M.; Tenney, J.R.; Arthur, T.M.; Crone, N.E.; Holland, K.D.; Mangano, F.T.; et al. Language and motor function thresholds during pediatric extra-operative electrical cortical stimulation brain mapping. Clin. Neurophysiol. 2017, 128, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Y.; Zhao, Z.; Liu, Y.; Zhu, Y.; Mao, Y.; Wu, J.; Chang, E.F. Neural control of lexical tone production in human laryngeal motor cortex. Nat. Commun. 2023, 14, 6917. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.H.; Laird, A.R.; Li, K.; Fox, P.T. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta-analysis. Hum. Brain Mapp. 2005, 25, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, J.; Kang, C.K.; Park, C.A.; Lim, M.R.; Kim, Y.B.; Bak, B.G. Human Brain Mapping of Visual Script Familiarity between Phonological and Logographic Language: 3 T Functional MRI Study. Biomed. Res. Int. 2017, 2017, 5732642. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.H.; Devinsky, O.; Doyle, W.; Perrine, K. Preoperative predictors of anterior temporal language areas. J. Neurosurg. 1998, 89, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Wellmer, J.; Weber, C.; Mende, M.; Groeben, F.V.D.; Urbach, H.; Clusmann, H.; Elger, C.E.; Helmstaedter, C. Multitask electrical stimulation for cortical language mapping: Hints for necessity and economic mode of application. Epilepsia 2009, 50, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
| Linguistic Task | Description of the Test and Time of Delivering the Test | Error Appreciation/Comments | Literature Reference |
|---|---|---|---|
| Visual object naming | Patients were shown pictures of common items (e.g., umbrella) and instructed to say, “This is a…,” Pictures were either line drawings or coloured photographs In most studies, patients were trained to name each picture (control trials) to ensure task feasibility and picture familiarity (baseline level). One study involved semantic odd picture naming where patient identified one picture out of four that did not fit and explained why ([25]) Examples in other languages include the French visual naming task (DO80) [23,26], visual naming task for Kanji and Kana nouns [27] Covert (silent) naming was asked in one centre examining paediatric patients | Content of errors In most studies, anomia or paraphasia was considered abnormal. In two studies, errors were subcategorised as semantic, phonological, or mixed. In some, dysarthria (sensorimotor component) was considered abnormal. Speed reduction was considered abnormal in two studies and hypophonia in one. Memory (amnestic anomia) declines were considered in one study. More types of errors noted in one study: apraxia, neologisms, perseverations. Quantity of errors Sometimes an error in more than one trial per task would be assumed abnormal. If results were ambiguous, additional trials were administered. In some, “reproducible changes” were considered abnormal. | [9,11,12,18,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] |
| Auditory description naming | Descriptive cue for the target word delivered aloud. The cue is read and named by the patient within the stimulation period. Descriptions of objects using <10 words: e.g., “a household pet that purrs” Stimulation occurs immediately after the item is presented, and before the oral response of the carrier phrase by the patient [49] Used both intra- and extraoperatively | Inaccuracy. Sites were deemed critical if the patient failed to name items during stimulation but gave correct responses upon cessation of stimulation. | [11,35,37,38,46,47,49,52] |
| Auditory comprehension | Examples of questions asked: “Do flamingos stand on one leg?” Or assessed by asking the patient to follow one or two-step verbal commands, e.g., finger tipping, hand or tongue movement, simple arithmetic calculations | Yes/No answer. Unable to follow commands. | [36,37,42,43,44,46,50,51] |
| Comprehension (auditory + semantics) | Pt was asked to answer brief auditory questions: e.g., ‘What flies in the sky? All questions, beginning with either ‘what’, ‘where’, ‘when’ or ‘who’, were designed to elicit one- or two-word answers with nouns | Patients were instructed to answer, “I don’t know” when they did not know the answer to or did not understand a question. In case they failed to verbalise a relevant answer when asked for the reason. Neuropsychologists present to assess. | [55] |
| Single-word auditory comprehension (SWAC) test | Patients are asked to listen to a target word and describe what it means without saying the target word Stimulation is delivered as the patient listens to the target word | Error if the patient mentioned that the target word was not understood, a word was not spoken or asked if the target word could be repeated. | [15] |
| Token test | Patients pointed at objects given the experimenter’s verbal description Ex: “Point to the small, red circle” | Right/wrong. | [40,41,56] |
| Semantic orientated testing | Patients were presented with pictured objects (1 per stimulation trial) and instructed to indicate whether objects had one of the following semantic features: e.g., “found indoors”, “something people typically eat”, “a musical instrument”, “found in a garden” | Yes/No answer. | [34] |
| Sentence completion | “A bow is used to...” “You blow air into...” Stimulation occurs immediately after the item was presented, and before the oral response of the carrier phrase by the patient [49] Used intraoperatively | [25,49] | |
| Phonological test | Patients were instructed to indicate whether the pictured objects begin with a particular sound (first phoneme): e.g., “Does this begin with the sound “t” as in toy?” Used extraoperatively | Yes/No answer. | [11,34] |
| Spontaneous conversation | Three conditions were identified: speaking, listening, and rest Having a dialogue on an everyday topic [57] Duration was 3 min Used intraoperatively | Speech arrest. Detection of slight changes in language performance (decreased fluency and inarticulacy) Not described thoroughly in most studies. | [23,25,28,36,42,48,51,58] |
| Numerical and/or Alphabet recitation | It is identified as part of spontaneous conversation in one study Word list recall in one study [56] Sequence naming of months of the year in one study [40] Reciting poetry in two studies on Mandarin language testing [57,58] Used intraoperatively | A reproducible functional change, such as a pause or dysarthria. | [36,47,48,55,56,58,59,60] |
| Reading | Patients were instructed to read short sentences aloud In studies on the Japanese language, morphograms (Kanji) reading, and syllabograms (Kana) reading tasks were employed as well [27,40] | Speech arrest [59]. In one study [46], error types were categorised as follows: slow/effortful reading (apraxia), syntactic errors, sentence stem additions, sentence stem omissions, mixed sentence stem errors (additions + omissions). | [30,31,36,37,40,41,42,43,44,45,46,48,49,50,51,56,60] |
| Verb generation | Auditory presentation of a series of nouns and instructed to covertly generate as many associated verbs as possible | Arrest or interruption of speech. | [28,61] |
| Repetition | Sentence, word or consonant–vowel syllable repetition (e.g., /da/) | Speech arrest. | [25,39,44,46,47,51,55,58,62] |
| Story processing | Yes/No answer. | [61] | |
| Sensorimotor Component testing | Humming | [55] | |
| Automatic speech recognition | Passive listening to a corpus of short sentences (Texas Instruments/Massachusetts Institute of Technology (TIMIT) | [63] | |
| Written word–picture matching | Japanese version adapted from the Cambridge 64-item semantic battery Performed extraoperatively | [40] | |
| Text question response task | Overtly provide answers to questions presented in text Performed extraoperatively | [25] | |
| Writing | Used a validated Chinese version of the Western Aphasia Battery (C-WAB) | [49,50] | |
| Tone production monitoring | Tone of speech in Mandarin was monitored as patients were undertaking other tests, e.g., spontaneous speech or picture naming | [50] | |
| Animal sound recognition and naming | Patients are asked to listen to typical sounds produced by animals and name them, e.g., barking sound produced by a dog Performed extraoperatively | [47] | |
| Verb and noun test for peri-operative testing (VAN-POP) | Black-and-white drawings of person/animal performing action with lead-in phrase. Patient completes with verbs inflected for person, number, tense. Used for nTMS and DES | Anomias, paraphasias, speech arrest, grammatical errors (inflection failures), light verb paraphrasing. | [64] |
| Spoken word–picture matching | Six pictures shown. Patients indicate target after hearing spoken name. Intraoperative DES. | Arrest, slowing, incorrect matching. Quantity of errors: Highest impairment rate. | [54] |
| Spoken verbal command | Make gestures following simple spoken instruction. Auditory only. Intraoperative DES. | Slowing, unable to follow commands. Not impaired in PHG. | [54] |
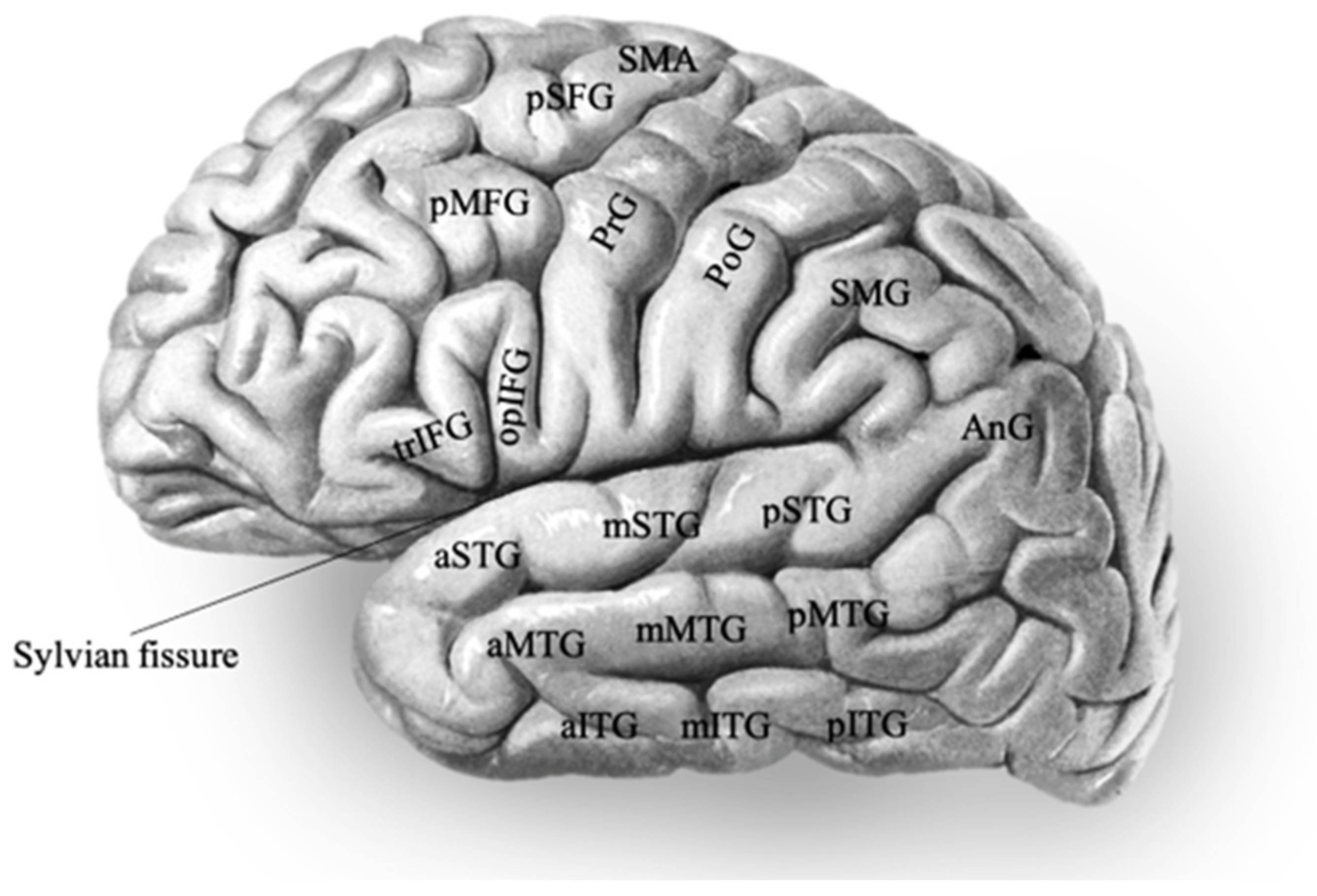
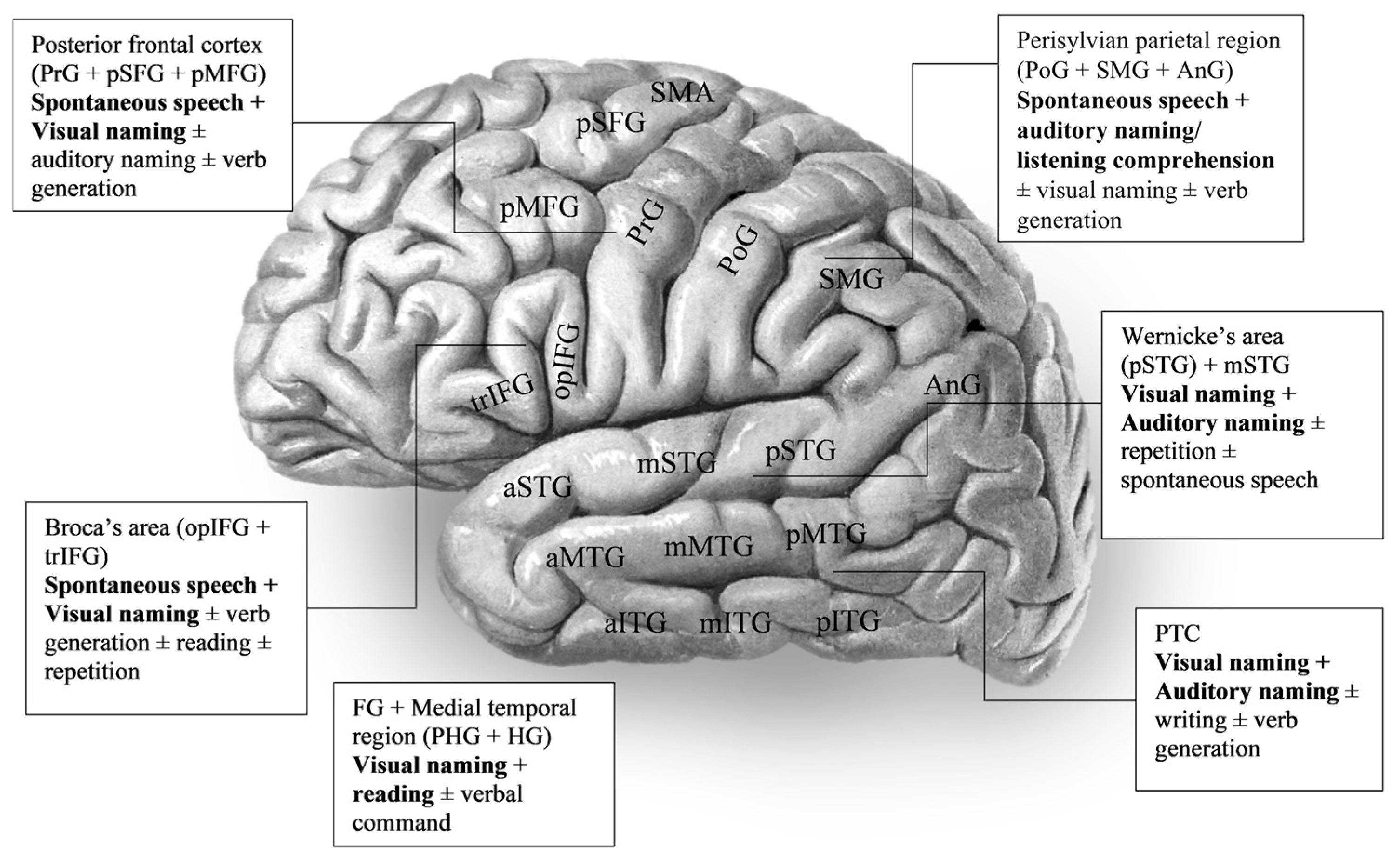
| Studies | Stimulation Modalities | Language Tests | Task-Specific Language Response Sites |
| Alarcón et al., 2019 [15] | eoECS + ioECS | Single-word auditory comprehension (SAWC) test | L-PTC especially pSTG, AnG |
| Alonso et al., 2016 [59] | SEEG | Reading | Dominant IFG |
| Aron et al., 2022 [28] | SEEG | French visual naming (DO80) | Ventral temporal cortex, including PHG, FG, ITG |
| Aron et al., 2024 [19] | SEEG | French visual naming (DO80) | Anterior/posterior parahippocampal gyrus (PHG), fusiform gyrus (FG), and inferior temporal gyrus (ITG) |
| Arya et al., 2015 [29] | eoECS + eoECoG | ECS—visual naming ECoG—spontaneous speech (dialogue) | Picture naming—L/R-pSTG, L/R inferior PoG, L-pIFG Spontaneous speech (listening)—L-pSTG, L-mSTG, adjacent perisylvian parietal cortex Spontaneous (speaking)—L-inferior PrG, L-pIFG, AnG, SMG |
| Arya et al., 2017 [32] | eoECS + eoECoG | ECS—visual naming ECoG—covert visual naming | L-pMFG, L-pIFG |
| Arya et al., 2019 [30] | SEEG | Picture naming | Picture naming—L/R-STG, L-MTG, L-HG, L-IFG Dysarthria—STG, HG, planum temporale |
| Austermuehle et al., 2017 [42] | eoECS | Counting/alphabet recitation, visual naming, reading, token test | Reading—temporal receptive regions |
| Babajani-Feremi et al., 2018 [34] | eoECS + eoECoG | ECS—sentence reading, comprehension, token task ECoG—overt object naming | L-IFG, STG, MTG, dorsal premotor region, inferior-Rolandic region |
| Bauer et al., 2013 [36] | eoECS + eoECoG | ECS—object naming ECoG—spontaneous conversation, verb generation, picture naming | Spontaneous speech (listening)—inferior Sylvian fissure Spontaneous speech (speaking)—superior Sylvian fissure Verb generation—PTC, AnG, SMG, PrG, PoG, IFG Naming—perisylvian area |
| Bearden et al., 2023 [56] | SEEG | Confrontation naming, word list recall, word list encoding | L-trIFG, hippocampus, anterior temporal pole |
| Bohm et al., 2020 [61] | eoECS | Reading, comprehension, naming in three languages | Naming—PTC |
| Cockle et al., 2025 [52] | SEEG | Visual and auditory naming, reading, spontaneous speech, and counting | Fusiform gyrus, ITG, MTG, STG, temporal pole, entorhinal cortex, pre-SMA |
| Enatsu et al., 2017 [27] | ECS | Reading (paragraph and words) | Paragraph reading—L-FG, L-PHG, L- ITG |
| Ervin et al., 2020 [53] | SEEG | Visual naming (Snodgrass picture set) | Posterior temporal and temporoparietal cortices (bilateral); posterior quadrant HGM sites, often ESM linked to declines in working memory, naming, and verbal learning; no ESM+ sites resected |
| Hamberger et al., 2014 [16] | eoECS | Language task in different centres: speech production (76%), comprehension (68%), naming (89%), reading (75%) | Anterolateral temporal region, basal temporal region, insular cortex |
| Hamberger et al., 2016 [37] | eoECS | Visual naming, auditory naming, semantic task (yes/no to auditory questions regarding pictures), phonological task (yes/no to auditory questions regarding first phoneme of object names) | Phonological tasks—L-pSTG, L-MTG, SMG Semantic tasks—L-pMTG, L-pITG |
| Hamberger et al., 2019 [38] | eoECS + ioECS | Visual naming, auditory naming | Visual naming—mSTG (younger patients), STG and MTG (older patients) Auditory naming—STG (younger patients), STG, MTG, and SMG (older patients) |
| Hirano et al., 2020 [62] | eoECS | Reading sentences, spontaneous speech (counting/ speaking), object naming, auditory comprehension (following verbal commands) | L-pO/pT, p STS, p MTG, and SMG |
| Korostenskaja et al., 2014 [25] | eoECS + eoECoG | ECS—picture naming ECoG—story processing, picture naming (overt/ covert), verb generation | ECS (picture naming)—no area found ECoG—story processing and picture naming in right lateral and basal temporal regions; verb generation in L-frontal and R-temporal lobes |
| Labudda et al., 2017 [70] | eoECS | Object naming, reading, following verbal commands | L IFG + PTG small area in R MFG |
| Lee et al., 2020 [55] | eoECS | Visual naming, auditory naming | Expressive aphasia (auditory naming)—pIFG, PrG, PTC Expressive aphasia (visual naming)—mTG, ITG Receptive aphasia (auditory + naming)—pSTG, pMTG |
| Lioumis et al., 2023 [71] | eoECS | Naming, sentence repetition | Naming—trIFG, opIFG Sentence repetition—pSTG |
| Matoba et al., 2024 [54] | ECS | Picture naming, spoken word–picture matching, Kanji word reading, paragraph reading, spoken verbal command, Kana word reading | Anterior FG and ITG (visual + auditory) semantic impairments; middle FG mainly unimodal (visual) processing; PHG least impaired |
| Nakai et al., 2017 [60] | eoECS + eoECoG | Auditory naming, syllable repetition, humming, counting, reciting alphabet | Auditory naming (receptive aphasia)—L-pSTG, L-pMTG Auditory naming (expressive aphasia)—L-PTC, L-FG Auditory naming (speech arrest)—bilateral inferior PrG, L-pSFG |
| Oane et al., 2020 [43] | SEEG | Reading, counting | Cingulate cortex |
| Perrone-Bertolotti et al., 2020 [58] | ECS + SEEG | Picture naming, reading, etc. | Picture naming (speech arrest)—left frontal region (PrG, opIFG, trIFG, SMA, insula), left temporal region (TG, FG), PoG Picture naming (speech paraphasia)—L-insula, L-TG Picture naming (phonological paraphasia)—opIFG, MFG, TG |
| Rolinski et al., 2019 [39] | eoECS | Continuous recitation task, visual naming, reading, token test | Basal temporal cortex |
| Rolston et al., 2018 [3] | eoECoG | Sentence listening, consonant-vowel syllables repetition (e.g., /da/) | Frontal operculum, precentral gyrus, ITG, MTG, STG, postcentral gyrus, SMG |
| Sabsevitz et al., 2020 [44] | SEEG | Picture naming, auditory naming, famous face naming, syllable repetition, single word reading, writing to dictation | Impaired reading without agraphia—lateral L-FG |
| Serafini et al., 2013 [45] | eoECS | Visual naming, auditory naming, reading, sentence completion | Auditory naming—STG, AnG Visual naming—mMTG, SMG, AnG Sentence completion—STG, MTG, AnG |
| Shimotake et al., 2015 [40] | eoECS + eoECoG | ECS—reading, picture naming, spoken verbal command, spoken and written word–picture matching ECoG—picture naming | All tasks in ITG, anterior FG |
| Takahashi et al., 2022 [46] | eoECS | Sentence reading, spontaneous speech, object naming, verbal command | STG, ITG, SMG, IFG |
| Wen et al., 2017 [47] | eoECoG | Picture naming, animal sound recognition and naming, text question response, auditory question response, word reading, word repetition | Language production—opIFG, pMFG, inferior precentral and postcentral gyrus, SMG Language comprehension—PTC |
| Zhou et al., 2021 [69] | ioECS + ioECoG | ECS—spontaneous speech, comprehension task ECoG—counting, reciting traditional Chinese poems, having dialogues | STG and IFG |
| Young et al., 2018 [57] | SEEG + eoECoG | Verbal fluency (counting, reciting months), visual naming, auditory naming, repetition | ECoG—IFG, STG, MTG, ITG SEEG—STG, pMTG, MFG |
| Yu et al., 2018 [48] | SEEG | Reading/counting | L-PSG (posterior short gyrus) close to the precentral operculum and L-precentral operculum at pIFG |
| Zhang et al., 2013 [49] | eoECS + ioECS | io + eoECS—spontaneous speech, comprehension, visual naming eoECS only—repetition, reading, writing | Writing—L-PTC Repetition—L-IFG, L-pSTG Tone—L-IFG Reading—L-STG, L-pIFG Visual naming—L-PTC |
| Zhang et al., 2020 [50] | eoECS | Picture naming, spontaneous speech, listening comprehension, tone monitor | Tone—R-pMFG in left-handed patient Naming—L-pSTG, L-vPrG, L-pMTG Comprehension—L-STG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Korona, E.; Shirani, S.; Samadian, F.; Alarcon, G.; Valentin, A.; Stavropoulos, I. Electrical Cortical Stimulation for Language Mapping in Epilepsy Surgery—A Systematic Review. Brain Sci. 2025, 15, 1267. https://doi.org/10.3390/brainsci15121267
Zhu H, Korona E, Shirani S, Samadian F, Alarcon G, Valentin A, Stavropoulos I. Electrical Cortical Stimulation for Language Mapping in Epilepsy Surgery—A Systematic Review. Brain Sciences. 2025; 15(12):1267. https://doi.org/10.3390/brainsci15121267
Chicago/Turabian StyleZhu, Honglin, Efthymia Korona, Sepehr Shirani, Fatemeh Samadian, Gonzalo Alarcon, Antonio Valentin, and Ioannis Stavropoulos. 2025. "Electrical Cortical Stimulation for Language Mapping in Epilepsy Surgery—A Systematic Review" Brain Sciences 15, no. 12: 1267. https://doi.org/10.3390/brainsci15121267
APA StyleZhu, H., Korona, E., Shirani, S., Samadian, F., Alarcon, G., Valentin, A., & Stavropoulos, I. (2025). Electrical Cortical Stimulation for Language Mapping in Epilepsy Surgery—A Systematic Review. Brain Sciences, 15(12), 1267. https://doi.org/10.3390/brainsci15121267






