A Tragedy of Errors: The State of Psychedelic Research in the Treatment of Alcohol Use Disorder
Abstract
1. Introduction
2. Psychedelics and Alcohol Use Disorder: The Historical Concept
3. Psychedelics and Alcohol Use Disorder: The Evidence
3.1. Clinical Outcomes
| Study | Design | n | Intervention | Comparator | Outcome | Key Results |
|---|---|---|---|---|---|---|
| Krebs & Johansen, 2012 [41] | Meta-analysis of 6 RCTs | 536 | LSD 200–800 mcg | Varied | Alcohol misuse | OR 1.96; 95% CI; 1.36–2.84 |
| Bogenschutz et al., 2015 [12] | Open-label pilot | 10 | Psilocybin 0.3–0.4 mg/kg | None | PHDD | Reduction in PHDD (MD 22.4; 95% CI; 8.7–43.2) |
| Bogenschutz et al., 2022 [11] | RCT | 95 | Psilocybin 20–45 mg/kg | Diphenhydramine 50–100 mg | PHDD | Reduction in PHDD (MD 13.9%; 95% CI; 3.0–24.7) |
| Rieser et al., 2025 [48] | RCT | 60 | Psilocybin 25 mg | Mannitol | Abstinence (4 weeks) | No difference |
3.2. Functional Unblinding
3.3. Abstinence as a Primary Outcome
3.4. Considering AA in Future Study Designs
4. Whither Neuroimaging?
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Esser, M.B.; Sherk, A.; Liu, Y.; Naimi, T.S. Deaths from Excessive Alcohol Use—United States, 2016–2021. Morb. Mortal. Wkly. Rep. 2024, 73, 154–161. [Google Scholar] [CrossRef] [PubMed]
- White, A.M.; Castle, I.-J.P.; Powell, P.A.; Hingson, R.W.; Koob, G.F. Alcohol-Related Deaths During the COVID-19 Pandemic. JAMA 2022, 327, 1704–1706. [Google Scholar] [CrossRef] [PubMed]
- Sacks, J.J.; Gonzales, K.R.; Bouchery, E.E.; Tomedi, L.E.; Brewer, R.D. 2010 National and State Costs of Excessive Alcohol Consumption. Am. J. Prev. Med. 2015, 49, e73–e79. [Google Scholar] [CrossRef] [PubMed]
- Reus, V.I.; Fochtmann, L.J.; Bukstein, O.; Eyler, A.E.; Hilty, D.M.; Horvitz-Lennon, M.; Mahoney, J.; Pasic, J.; Weaver, M.; Wills, C.D.; et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder. Am. J. Psychiatry 2018, 175, 86–90. [Google Scholar] [CrossRef]
- Walker, J.R.; Korte, J.E.; McRae-Clark, A.L.; Hartwell, K.J. Adherence Across FDA-Approved Medications for Alcohol Use Disorder in a Veterans Administration Population. J. Stud. Alcohol Drugs 2019, 80, 572–577. [Google Scholar] [CrossRef]
- McPheeters, M.; O’Connor, E.A.; Riley, S.; Kennedy, S.M.; Voisin, C.; Kuznacic, K.; Coffey, C.P.; Edlund, M.D.; Bobashev, G.; Jonas, D.E. Pharmacotherapy for Alcohol Use Disorder: A Systematic Review and Meta-Analysis. JAMA 2023, 330, 1653. [Google Scholar] [CrossRef]
- Heifets, B.D.; Olson, D.E. Therapeutic Mechanisms of Psychedelics and Entactogens. Neuropsychopharmacology 2024, 49, 104–118. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Anderson, B.T. Psychedelic Therapies Reconsidered: Compounds, Clinical Indications, and Cautious Optimism. Neuropsychopharmacology 2024, 49, 96–103. [Google Scholar] [CrossRef]
- Reiff, C.M.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M.; the Work Group on Biomarkers and Novel Treatments, a Division of the American Psychiatric Association Council of Research. Psychedelics and Psychedelic-Assisted Psychotherapy. Am. J. Psychiatry 2020, 177, 391–410. [Google Scholar] [CrossRef]
- Bogenschutz, M.P. It’s Time to Take Psilocybin Seriously as a Possible Treatment for Substance Use Disorders. Am. J. Drug Alcohol Abus. 2017, 43, 4–6. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Ross, S.; Bhatt, S.; Baron, T.; Forcehimes, A.A.; Laska, E.; Mennenga, S.E.; O’Donnell, K.; Owens, L.T.; Podrebarac, S.; et al. Percentage of Heavy Drinking Days Following Psilocybin-Assisted Psychotherapy vs Placebo in the Treatment of Adult Patients with Alcohol Use Disorder. JAMA Psychiatry 2022, 79, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Bogenschutz, M.P.; Forcehimes, A.A.; Pommy, J.A.; Wilcox, C.E.; Barbosa, P.; Strassman, R.J. Psilocybin-Assisted Treatment for Alcohol Dependence: A Proof-of-Concept Study. J. Psychopharmacol. 2015, 29, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, A.S.; Fonzo, G.A.; Gray, J.C.; Krystal, J.H.; Grzenda, A.; Widge, A.S.; Kraguljac, N.V.; McDonald, W.M.; Rodriguez, C.I.; Nemeroff, C.B. MDMA and MDMA-Assisted Therapy. Am. J. Psychiatry 2025, 182, 79–103. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Ot’alora G., M.; van der Kolk, B.; Shannon, S.; Bogenschutz, M.; Gelfand, Y.; Paleos, C.; Nicholas, C.R.; Quevedo, S.; Balliett, B.; et al. MDMA-Assisted Therapy for Moderate to Severe PTSD: A Randomized, Placebo-Controlled Phase 3 Trial. Nat. Med. 2023, 29, 2473–2480. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’alora G., M.; Garas, W.; Paleos, C.; Gorman, I.; et al. MDMA-Assisted Therapy for Severe PTSD: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Study. Nat. Med. 2021, 27, 1025–1033. [Google Scholar] [CrossRef]
- Sloshower, J.; Skosnik, P.D.; Safi-Aghdam, H.; Pathania, S.; Syed, S.; Pittman, B.; D’Souza, D.C. Psilocybin-Assisted Therapy for Major Depressive Disorder: An Exploratory Placebo-Controlled, Fixed-Order Trial. J. Psychopharmacol. 2023, 37, 698–706. [Google Scholar] [CrossRef]
- Szigeti, B.; Heifets, B.D. Expectancy Effects in Psychedelic Trials. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2024, 9, 512–521. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Malievskaia, E.; Fonzo, G.A.; Nemeroff, C.B. Must Psilocybin Always “Assist Psychotherapy”? Am. J. Psychiatry 2024, 181, 20–25. [Google Scholar] [CrossRef]
- Cristea, I.A.; Cuijpers, P.; Halvorsen, J.Ø. The Psychotherapy in MDMA-Assisted Psychotherapy. JAMA Psychiatry 2024, 81, 1053–1054. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Sanacora, G. Issues in Clinical Trial Design—Lessons From the FDA’s Rejection of MDMA. JAMA Psychiatry 2025, 82, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.D.; McKee, S.A.; Cosgrove, K.P. Why Language Matters in Alcohol Research: Reducing Stigma. Alcohol Clin. Exp. Res. 2022, 46, 1103–1109. [Google Scholar] [CrossRef]
- Kelly, J.F. Is Alcoholics Anonymous Religious, Spiritual, Neither? Findings from 25 Years of Mechanisms of Behavior Change Research. Addiction 2017, 112, 929–936. [Google Scholar] [CrossRef]
- Galanter, M. Alcoholics Anonymous and Twelve-step Recovery: A Model Based on Social and Cognitive Neuroscience. Am. J. Addict. 2014, 23, 300–307. [Google Scholar] [CrossRef]
- Alcoholics Anonymous World Services. Alcoholics Anonymous, 4th ed.; Alcoholics Anonymous World Services: New York, NY, USA, 2002. [Google Scholar]
- Alcoholics Anonymous World Services. Twelve Steps and Twelve Traditions; Alcoholics Anonymous World Services: New York, NY, USA, 1989. [Google Scholar]
- Wilson, B. Alcoholics Anonymous Comes of Age; Harper: New York, NY, USA, 1957. [Google Scholar]
- Kurtz, E. Not-God: A History of Alcoholics Anonymous; Hazelden: Center City, MN, USA, 1991. [Google Scholar]
- McDonnell, R.; Moriarty, J.; Cabe, I.M.; Higgins, E. AA, Bill Wilson, Carl Jung and LSD. J. Anal. Psychol. 2024, 69, 550–580. [Google Scholar] [CrossRef]
- Lackovic, Z. “Bunanje”: XX Century Abuse of Atropa Belladonna Halucinogenic Berries in Continental Croatia. Psychiatr. Danub. 2017, 29, 379–382. [Google Scholar] [CrossRef]
- James, W. The Varieties of Religious Experience; Touchstone; Simon and Schuster, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Tiebout, H.M. Surrender versus Compliance in Therapy, with Special Reference to Alcoholism. Q. J. Stud. Alcohol 1953, 14, 58–68. [Google Scholar] [CrossRef]
- Galanter, M.; Dermatis, H.; Sampson, C. Spiritual Awakening in Alcoholics Anonymous: Empirical Findings. Alcohol. Treat. Q. 2014, 32, 319–334. [Google Scholar] [CrossRef]
- Abramson, H.A. (Ed.) The Use of LSD in Psychotherapy and Alcoholism; The Bobbs-Merrill Company, Inc.: Indianapolis, IN, USA, 1967. [Google Scholar]
- Osmond, H. A review of the clinical effects of psychotomimetic agents. Ann. N. Y. Acad. Sci. 1957, 66, 418–434. [Google Scholar] [CrossRef]
- Hoffer, A.; Osmond, H. The Hallucinogens; Academic Press: New York, NY, USA, 1967. [Google Scholar]
- Geyer, M.A. A Brief Historical Overview of Psychedelic Research. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2024, 9, 464–471. [Google Scholar] [CrossRef]
- Kurtz, E. Drugs and the Spiritual: Bill W. Takes LSD. In The Collected Ernie Kurtz; The Bishop of Books: Wheeling, WV, USA, 1989. [Google Scholar]
- Ko, K.; Knight, G.; Rucker, J.J.; Cleare, A.J. Psychedelics, Mystical Experience, and Therapeutic Efficacy: A Systematic Review. Front. Psychiatry 2022, 13, 917199. [Google Scholar] [CrossRef]
- Nutt, D.J.; King, L.A.; Nichols, D.E. Effects of Schedule I Drug Laws on Neuroscience Research and Treatment Innovation. Nat. Rev. Neurosci. 2013, 14, 577–585. [Google Scholar] [CrossRef]
- Krebs, T.S.; Johansen, P.-Ø. Lysergic Acid Diethylamide (LSD) for Alcoholism: Meta-Analysis of Randomized Controlled Trials. J. Psychopharmacol. 2012, 26, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Hagman, B.T.; Falk, D.; Litten, R.; Koob, G.F. Defining Recovery From Alcohol Use Disorder: Development of an NIAAA Research Definition. Am. J. Psychiatry 2022, 179, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Falk, D.E.; O’Malley, S.S.; Witkiewitz, K.; Anton, R.F.; Litten, R.Z.; Slater, M.; Kranzler, H.R.; Mann, K.F.; Hasin, D.S.; Johnson, B.; et al. Evaluation of Drinking Risk Levels as Outcomes in Alcohol Pharmacotherapy Trials. JAMA Psychiatry 2019, 76, 374–381. [Google Scholar] [CrossRef]
- O’Donnell, K.C.; Mennenga, S.E.; Owens, L.T.; Podrebarac, S.K.; Baron, T.; Rotrosen, J.; Ross, S.; Forcehimes, A.A.; Bogenschutz, M.P. Psilocybin for Alcohol Use Disorder: Rationale and Design Considerations for a Randomized Controlled Trial. Contemp. Clin. Trials 2022, 123, 106976. [Google Scholar] [CrossRef]
- Rosenbaum, J.F. Functional Unblinding in Pivotal Studies and the Future of Psychedelic Medicine. J. Clin. Psychiatry 2024, 85, 24com15504. [Google Scholar] [CrossRef]
- Kranzler, H.R.; Hartwell, E.E. Treating Alcohol Use Disorder with Hallucinogens—Renewed Interest After a 50-Year Hiatus. JAMA Psychiatry 2022, 79, 945–946. [Google Scholar] [CrossRef]
- Wittenkeller, L.; Gudelsky, G.; Winhusen, T.J.; Amato, D. Psychedelics as Pharmacotherapeutics for Substance Use Disorders: A Scoping Review on Clinical Trials and Perspectives on Underlying Neurobiology. Br. J. Pharmacol. 2025, 1–26. [Google Scholar] [CrossRef]
- Rieser, N.M.; Bitar, R.; Halm, S.; Rossgoderer, C.; Gubser, L.P.; Thévenaz, M.; Kreis, Y.; von Rotz, R.; Nordt, C.; Visentini, M.; et al. Psilocybin-Assisted Therapy for Relapse Prevention in Alcohol Use Disorder: A Phase 2 Randomized Clinical Trial. eClinicalMedicine 2025, 82, 103149. [Google Scholar] [CrossRef]
- Aday, J.S.; Simonsson, O.; Schindler, E.A.D.; D’Souza, D.C. Addressing Blinding in Classic Psychedelic Studies with Innovative Active Placebos. Int. J. Neuropsychopharmacol. 2025, 28, pyaf023. [Google Scholar] [CrossRef]
- Muthukumaraswamy, S.D.; Forsyth, A.; Lumley, T. Blinding and Expectancy Confounds in Psychedelic Randomized Controlled Trials. Expert Rev. Clin. Pharmacol. 2021, 14, 1133–1152. [Google Scholar] [CrossRef]
- Colloca, L.; Fava, M. What Should Constitute a Control Condition in Psychedelic Drug Trials? Nat. Ment. Health 2024, 2, 1152–1160. [Google Scholar] [CrossRef]
- Guyatt, G.; Rennie, D.; Meade, M.O.; Cook, D.J. Users’ Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice, 3rd ed.; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Brewer, C.; Streel, E.; Skinner, M. Supervised Disulfiram’s Superior Effectiveness in Alcoholism Treatment: Ethical, Methodological, and Psychological Aspects. Alcohol Alcohol. 2017, 52, 213–219. [Google Scholar] [CrossRef]
- Skinner, M.D.; Lahmek, P.; Pham, H.; Aubin, H.-J. Disulfiram Efficacy in the Treatment of Alcohol Dependence: A Meta-Analysis. PLoS ONE 2014, 9, e87366. [Google Scholar] [CrossRef] [PubMed]
- Lehrner, A.; Hildebrandt, T.B.; Yehuda, R. Rethinking Placebo-Controlled Clinical Trials in Psychedelic Therapies for Psychiatric Illness. Br. J. Psychiatry 2025, 227, 721–722. [Google Scholar] [CrossRef]
- Ray, L.A.; Meredith, L.R.; Kiluk, B.D.; Walthers, J.; Carroll, K.M.; Magill, M. Combined Pharmacotherapy and Cognitive Behavioral Therapy for Adults with Alcohol or Substance Use Disorders. JAMA Netw. Open 2020, 3, e208279. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Forcehimes, A.A. Development of a Psychotherapeutic Model for Psilocybin-Assisted Treatment of Alcoholism. J. Humanist. Psychol. 2017, 57, 389–414. [Google Scholar] [CrossRef]
- Witkiewitz, K.; Montes, K.S.; Schwebel, F.J.; Tucker, J.A. What Is Recovery? Alcohol Res. 2020, 40, 01. [Google Scholar] [CrossRef]
- Witkiewitz, K.; Heather, N.; Falk, D.E.; Litten, R.Z.; Hasin, D.S.; Kranzler, H.R.; Mann, K.F.; O’Malley, S.S.; Anton, R.F. World Health Organization Risk Drinking Level Reductions Are Associated with Improved Functioning and Are Sustained among Patients with Mild, Moderate and Severe Alcohol Dependence in Clinical Trials in the United States and United Kingdom. Addiction 2020, 115, 1668–1680. [Google Scholar] [CrossRef]
- Srivastava, B.; Gold, M.S. Naltrexone: A History and Future Directions. Cerebrum Dana Forum Brain Sci. 2018, 2018, cer-13-18. [Google Scholar]
- Srivastava, A.B. Medications for OUD: Extended-Release Naltrexone. Curr. Addict. Rep. 2025, 12, 13. [Google Scholar] [CrossRef]
- Fan, A.Z.; Chou, S.P.; Zhang, H.; Jung, J.; Grant, B.F. Prevalence and Correlates of Past-Year Recovery From DSM-5 Alcohol Use Disorder: Results From National Epidemiologic Survey on Alcohol and Related Conditions-III. Alcohol Clin. Exp. Res. 2019, 43, 2406–2420. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, G.E. A 60-year Follow-up of Alcoholic Men. Addiction 2003, 98, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, M.S.; Witbrodt, J. Differences between Abstinent and Non-Abstinent Individuals in Recovery from Alcohol Use Disorders. Addict. Behav. 2014, 39, 1730–1735. [Google Scholar] [CrossRef]
- Eddie, D.; Bergman, B.G.; Hoffman, L.A.; Kelly, J.F. Abstinence versus Moderation Recovery Pathways Following Resolution of a Substance Use Problem: Prevalence, Predictors, and Relationship to Psychosocial Well-being in a U.S. National Sample. Alcohol. Clin. Exp. Res. 2022, 46, 312–325. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5®); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Kaskutas, L.A. Alcoholics Anonymous Effectiveness: Faith Meets Science. J. Addict. Dis. 2009, 28, 145–157. [Google Scholar] [CrossRef]
- Magura, S.; McKean, J.; Kosten, S.; Tonigan, J.S. A Novel Application of Propensity Score Matching to Estimate Alcoholics Anonymous’ Effect on Drinking Outcomes. Drug Alcohol Depend. 2013, 129, 54–59. [Google Scholar] [CrossRef]
- Humphreys, K. Professional Interventions That Facilitate 12-Step Self-Help Group Involvement. Alcohol Res. Health 1999, 23, 93–98. [Google Scholar]
- Kelly, J.F.; Abry, A.; Ferri, M.; Humphreys, K. Alcoholics Anonymous and 12-Step Facilitation Treatments for Alcohol Use Disorder: A Distillation of a 2020 Cochrane Review for Clinicians and Policy Makers. Alcohol Alcohol. 2020, 55, 667–673. [Google Scholar] [CrossRef]
- Kelly, J.F.; Humphreys, K.; Ferri, M. Alcoholics Anonymous and Other 12-step Programs for Alcohol Use Disorder. Cochrane Database Syst. Rev. 2020, 3, CD012880. [Google Scholar] [CrossRef]
- Walitzer, K.S.; Dermen, K.H.; Barrick, C. Facilitating Involvement in Alcoholics Anonymous during Out-patient Treatment: A Randomized Clinical Trial. Addiction 2009, 104, 391–401. [Google Scholar] [CrossRef]
- Alcoholics Anonymous World Services. Living Sober; Alcoholics Anonymous World Services, Inc.: New York, NY, USA, 1975. [Google Scholar]
- Kelly, J.F.; Hoeppner, B.; Stout, R.L.; Pagano, M. Determining the Relative Importance of the Mechanisms of Behavior Change within Alcoholics Anonymous: A Multiple Mediator Analysis. Addiction 2012, 107, 289–299. [Google Scholar] [CrossRef]
- Litt, M.D.; Kadden, R.M.; Kabela-Cormier, E.; Petry, N. Changing Network Support for Drinking: Initial Findings From the Network Support Project. J. Consult. Clin. Psychol. 2007, 75, 542–555. [Google Scholar] [CrossRef]
- Project MATCH Research Group. Matching Alcoholism Treatments to Client Heterogeneity: Project Match Posttreatment Drinking Outcomes. J. Stud. Alcohol 1997, 58, 7–29. [Google Scholar] [CrossRef]
- MacLean, K.A.; Leoutsakos, J.S.; Johnson, M.W.; Griffiths, R.R. Factor Analysis of the Mystical Experience Questionnaire: A Study of Experiences Occasioned by the Hallucinogen Psilocybin. J. Sci. Study Relig. 2012, 51, 721–737. [Google Scholar] [CrossRef]
- Barrett, F.S.; Johnson, M.W.; Griffiths, R.R. Validation of the Revised Mystical Experience Questionnaire in Experimental Sessions with Psilocybin. J. Psychopharmacol. 2015, 29, 1182–1190. [Google Scholar] [CrossRef]
- Costa, P.T.; McCrae, R.R. The SAGE Handbook of Personality Theory and Assessment: Volume 2—Personality Measurement and Testing; SAGE Publications Ltd.: London, UK, 2008; pp. 179–198. [Google Scholar] [CrossRef]
- Schwartz, S.H. An Overview of the Schwartz Theory of Basic Values. Online Read. Psychol. Cult. 2012, 2, 11. [Google Scholar] [CrossRef]
- Schwartz, S.H. Universals in the Content and Structure of Values: Theoretical Advances and Empirical Tests in 20 Countries. Adv. Exp. Soc. Psychol. 1992, 25, 1–65. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Johnson, M.W.; Richards, W.A.; Richards, B.D.; Jesse, R.; MacLean, K.A.; Barrett, F.S.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin-Occasioned Mystical-Type Experience in Combination with Meditation and Other Spiritual Practices Produces Enduring Positive Changes in Psychological Functioning and in Trait Measures of Prosocial Attitudes and Behaviors. J. Psychopharmacol. 2018, 32, 49–69. [Google Scholar] [CrossRef]
- Danioni, F.; Villani, D.; Ranieri, S. Personal Values and Substance Use in Adolescence and Young Adulthood: Risk or Protective Factors? Subst. Use Misuse 2023, 58, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Morell-Gomis, R.; Irles, D.L.; Moriano, J.A.; Edú-Valsania, S.; González, A.L. Predicting Cannabis Use among Adolescents in Four European Countries: Combining Personal Values and the Theory of Planned Behaviour. Addict. Res. Theory 2018, 26, 498–506. [Google Scholar] [CrossRef]
- Begotti, T.; Borca, G.; Rabaglietti, E.; Ciairano, S. Fattori Associati All’interruzione Del Consumo Di Sostanze Psicoattive in Adolescenza: Uno Studio Sui Valori, l’uso Del Tempo Libero, i Modelli e Gli Atteggiamenti Dei Genitori. Psicol. Clin. Dello Svilupp. 2011, 2, 427–447. [Google Scholar]
- Gold, N.D.; Pagni, B.A.; Petridis, P.D.; Bogenschutz, M.P. Psilocybin-Assisted Therapy May Enhance Conservation Values in Patients with Alcohol Use Disorder. Psychedelic Med. 2025, 3, 31–40. [Google Scholar] [CrossRef]
- Pagni, B.A.; Zeifman, R.J.; Mennenga, S.E.; Carrithers, B.M.; Goldway, N.; Bhatt, S.; O’Donnell, K.C.; Ross, S.; Bogenschutz, M.P. Multidimensional Personality Changes Following Psilocybin-Assisted Therapy in Patients with Alcohol Use Disorder: Results From a Double-Blind, Placebo-Controlled Clinical Trial. Am. J. Psychiatry 2025, 182, 114–125. [Google Scholar] [CrossRef]
- Nowinski, J.; Baker, S.; Carroll, K. Twelve Step Facilitation Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence; National Institute on Alcohol Abuse and Alcoholism Project MATCH Monograph Series; U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism: Rockville, MD, USA, 1992. [Google Scholar]
- Connors, G.J.; Tonigan, J.S.; Miller, W.R. A Measure of Religious Background and Behavior for Use in Behavior Change Research. Psychol. Addict. Behav. 1996, 10, 90–96. [Google Scholar] [CrossRef]
- Tonigan, J.S.; Rynes, K.N.; McCrady, B.S. Spirituality as a Change Mechanism in 12-Step Programs: A Replication, Extension, and Refinement. Subst. Use Misuse 2013, 48, 1161–1173. [Google Scholar] [CrossRef]
- Tonigan, J.S.; Connors, G.J.; Miller, W.R. Alcoholics Anonymous Involvement (AAI) Scale: Reliability and Norms. Psychol. Addict. Behav. 1996, 10, 75–80. [Google Scholar] [CrossRef]
- Lashgari, N.-A.; Khalaji, M.; Rana, P.; Badrabadi, F.; Rahnama, M.; Nasoori, H.; Roudsari, N.M.; Nia, M.M.K.; Shafaroodi, H. Psychedelics in the Treatment of Neurologic and Psychiatric Disorders: Coincidence or a New Point of View. Mol. Neurobiol. 2025, 62, 15070–15092. [Google Scholar] [CrossRef]
- Pagni, B.A.; Petridis, P.D.; Podrebarac, S.K.; Grinband, J.; Claus, E.D.; Bogenschutz, M.P. Psilocybin-Induced Changes in Neural Reactivity to Alcohol and Emotional Cues in Patients with Alcohol Use Disorder: An fMRI Pilot Study. Sci. Rep. 2024, 14, 3159. [Google Scholar] [CrossRef]
- Friedman, N.P.; Robbins, T.W. The Role of Prefrontal Cortex in Cognitive Control and Executive Function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.E.; Dekonenko, C.J.; Mayer, A.R.; Bogenschutz, M.P.; Turner, J.A. Cognitive Control in Alcohol Use Disorder: Deficits and Clinical Relevance. Rev. Neurosci. 2014, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zilverstand, A.; Huang, A.S.; Alia-Klein, N.; Goldstein, R.Z. Neuroimaging Impaired Response Inhibition and Salience Attribution in Human Drug Addiction: A Systematic Review. Neuron 2018, 98, 886–903. [Google Scholar] [CrossRef]
- Grady, C.L.; Rieck, J.R.; Nichol, D.; Rodrigue, K.M.; Kennedy, K.M. Influence of Sample Size and Analytic Approach on Stability and Interpretation of Brain-behavior Correlations in Task-related fMRI Data. Hum. Brain Mapp. 2021, 42, 204–219. [Google Scholar] [CrossRef]
- Turner, B.O.; Paul, E.J.; Miller, M.B.; Barbey, A.K. Small Sample Sizes Reduce the Replicability of Task-Based fMRI Studies. Commun. Biol. 2018, 1, 62. [Google Scholar] [CrossRef]
- Marek, S.; Tervo-Clemmens, B.; Calabro, F.J.; Montez, D.F.; Kay, B.P.; Hatoum, A.S.; Donohue, M.R.; Foran, W.; Miller, R.L.; Hendrickson, T.J.; et al. Reproducible Brain-Wide Association Studies Require Thousands of Individuals. Nature 2022, 603, 654–660. [Google Scholar] [CrossRef]
- Lee, J.K.; Drysdale, A.T.; Srivastava, A.B.; Shi, T.C.; Patel, G.H. Methods for and Use of Functional Magnetic Resonance Imaging in Psychiatry. In Neurophysiologic Biomarkers in Neuropsychiatric Disorders; Springer: Cham, Switzerland, 2024; Volume 40. [Google Scholar] [CrossRef]
- Siegel, J.S.; Subramanian, S.; Perry, D.; Kay, B.P.; Gordon, E.M.; Laumann, T.O.; Reneau, T.R.; Metcalf, N.V.; Chacko, R.V.; Gratton, C.; et al. Psilocybin Desynchronizes the Human Brain. Nature 2024, 632, 131–138. [Google Scholar] [CrossRef]
- Laumann, T.O.; Zorumski, C.F.; Dosenbach, N.U.F. Precision Neuroimaging for Localization-Related Psychiatry. JAMA Psychiatry 2023, 80, 763–764. [Google Scholar] [CrossRef]
- Gordon, E.M.; Laumann, T.O.; Gilmore, A.W.; Newbold, D.J.; Greene, D.J.; Berg, J.J.; Ortega, M.; Hoyt-Drazen, C.; Gratton, C.; Sun, H.; et al. Precision Functional Mapping of Individual Human Brains. Neuron 2017, 95, 791–807.e7. [Google Scholar] [CrossRef]
- Laumann, T.O.; Snyder, A.Z. Brain Activity Is Not Only for Thinking. Curr. Opin. Behav. Sci. 2021, 40, 130–136. [Google Scholar] [CrossRef]
- Christoff, K.; Irving, Z.C.; Fox, K.C.; Spreng, N.R.; Andrews-Hanna, J.R. Mind-Wandering as Spontaneous Thought: A Dynamic Framework. Nat. Rev. Neurosci. 2016, 17, 718–731. [Google Scholar] [CrossRef]
- Raichle, M.; MacLeod, A.; Snyder, A.; Powers, W.; Gusnard, D.; Shulman, G. A Default Mode of Brain Function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Raichle, M.E. Spontaneous Fluctuations in Brain Activity Observed with Functional Magnetic Resonance Imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Essen, D.C.; Raichle, M.E. The Human Brain Is Intrinsically Organized into Dynamic, Anticorrelated Functional Networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef]
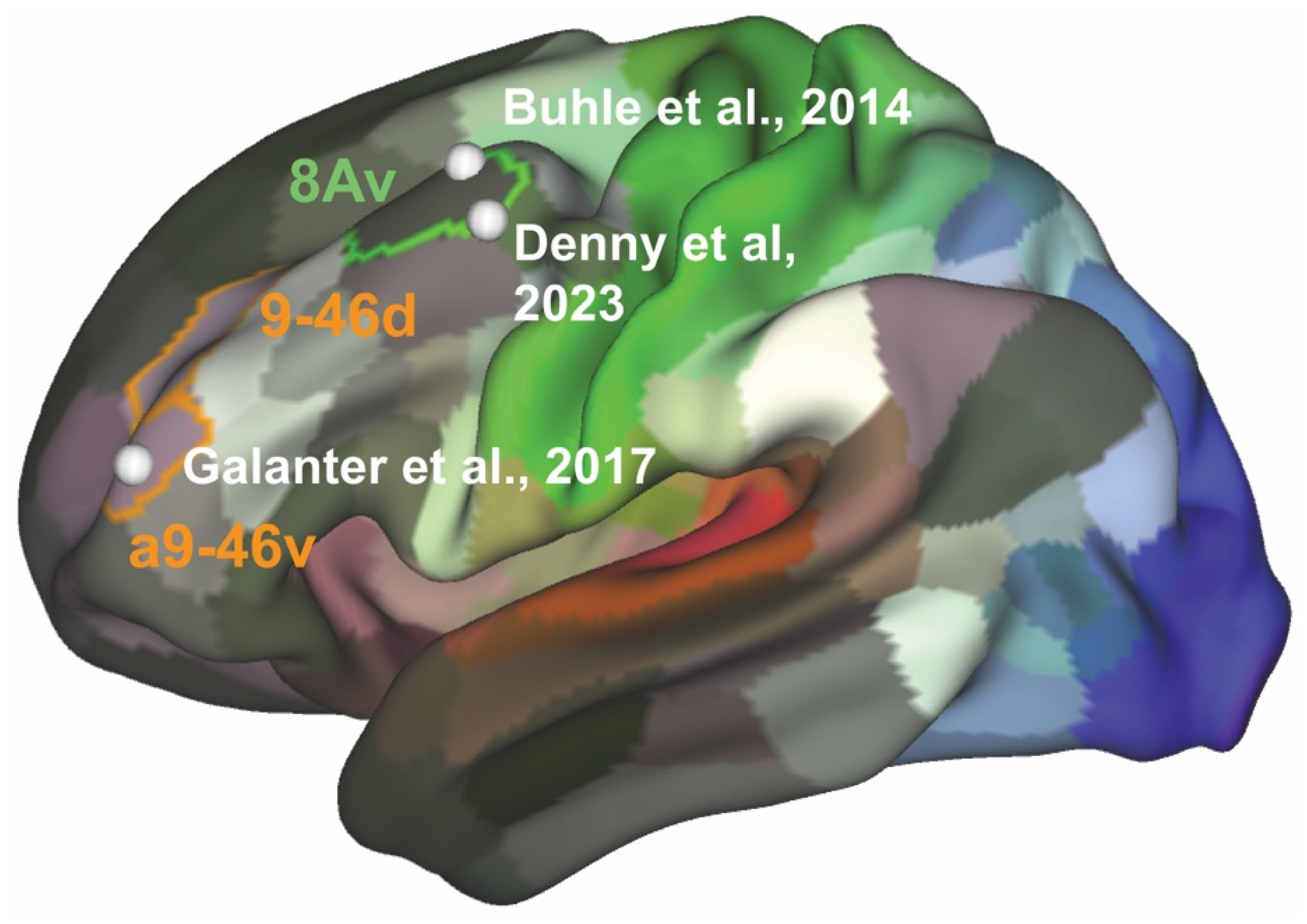
- Galanter, M.; Josipovic, Z.; Dermatis, H.; Weber, J.; Millard, M.A. An Initial fMRI Study on Neural Correlates of Prayer in Members of Alcoholics Anonymous. Am. J. Drug Alcohol Abus. 2017, 43, 44–54. [Google Scholar] [CrossRef]
- Petrides, M.; Pandya, D.N. Dorsolateral Prefrontal Cortex: Comparative Cytoarchitectonic Analysis in the Human and the Macaque Brain and Corticocortical Connection Patterns. Eur. J. Neurosci. 1999, 11, 1011–1036. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Gross, J.J. The Cognitive Control of Emotion. Trends Cogn. Sci. 2005, 9, 242–249. [Google Scholar] [CrossRef]
- Olsson, A.; Ochsner, K.N. The Role of Social Cognition in Emotion. Trends Cogn. Sci. 2008, 12, 65–71. [Google Scholar] [CrossRef]
- Suzuki, S.; Mell, M.M.; O’Malley, S.S.; Krystal, J.H.; Anticevic, A.; Kober, H. Regulation of Craving and Negative Emotion in Alcohol Use Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 5, 239–250. [Google Scholar] [CrossRef]
- Kober, H.; Mende-Siedlecki, P.; Kross, E.F.; Weber, J.; Mischel, W.; Hart, C.L.; Ochsner, K.N. Prefrontal–Striatal Pathway Underlies Cognitive Regulation of Craving. Proc. Natl. Acad. Sci. USA 2010, 107, 14811–14816. [Google Scholar] [CrossRef]
- Kober, H.; Kross, E.F.; Mischel, W.; Hart, C.L.; Ochsner, K.N. Regulation of Craving by Cognitive Strategies in Cigarette Smokers. Drug. Alcohol. Depend. 2010, 106, 52–55. [Google Scholar] [CrossRef]
- Koban, L.; Wager, T.D.; Kober, H. A Neuromarker for Drug and Food Craving Distinguishes Drug Users from Non-Users. Nat. Neurosci. 2022, 26, 316–325. [Google Scholar] [CrossRef]
- Naqvi, N.H.; Ochsner, K.N.; Kober, H.; Kuerbis, A.; Feng, T.; Wall, M.; Morgenstern, J. Cognitive Regulation of Craving in Alcohol-Dependent and Social Drinkers. Alcohol Clin. Exp. Res. 2015, 39, 343–349. [Google Scholar] [CrossRef]
- Naqvi, N.H.; Srivastava, A.B.; Sanchez--Peña, J.; Lee, J.K.; Drysdale, A.T.; Mariani, J.J.; Ochsner, K.N.; Morgenstern, J.; Patel, G.H.; Levin, F.R. Neural Correlates of Drinking Reduction during a Clinical Trial of Cognitive Behavioral Therapy for Alcohol Use Disorder. Alcohol Clin. Exp. Res. 2024, 48, 260–272. [Google Scholar] [CrossRef]
- Buhle, J.T.; Silvers, J.A.; Wager, T.D.; Lopez, R.; Onyemekwu, C.; Kober, H.; Weber, J.; Ochsner, K.N. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cereb. Cortex 2014, 24, 2981–2990. [Google Scholar] [CrossRef]
- Denny, B.T.; Jungles, M.L.; Goodson, P.N.; Dicker, E.E.; Chavez, J.; Jones, J.S.; Lopez, R.B. Unpacking Reappraisal: A Systematic Review of fMRI Studies of Distancing and Reinterpretation. Soc. Cogn. Affect. Neurosci. 2023, 18, nsad050. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.S.; Harms, M.P.; Snyder, A.Z.; Jenkinson, M.; Wilson, J.A.; Glasser, M.F.; Barch, D.M.; Archie, K.A.; Burgess, G.C.; Ramaratnam, M.; et al. Human Connectome Project Informatics: Quality Control, Database Services, and Data Visualization. Neuroimage 2013, 80, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.F.; Coalson, T.S.; Robinson, E.C.; Hacker, C.D.; Harwell, J.; Yacoub, E.; Ugurbil, K.; Andersson, J.; Beckmann, C.F.; Jenkinson, M.; et al. A Multi-Modal Parcellation of Human Cerebral Cortex. Nature 2016, 536, 171–178. [Google Scholar] [CrossRef]
- Kadden, R.; Carroll, K.; Donovan, D.; Cooney, N.; Monti, P.; Abrams, D.; Litt, M.; Hester, R. Cognitive-Behavioral Coping Skills Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence; National Institute on Alcohol Abuse and Alcoholism Project MATCH Monograph Series; U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism: Rockville, MD, USA, 2003. [Google Scholar]
- Fedorenko, E.; Hsieh, P.-J.; Nieto-Castañón, A.; Whitfield-Gabrieli, S.; Kanwisher, N. New Method for fMRI Investigations of Language: Defining ROIs Functionally in Individual Subjects. J. Neurophysiol. 2010, 104, 1177–1194. [Google Scholar] [CrossRef]
- Fedorenko, E.; Behr, M.K.; Kanwisher, N. Functional Specificity for High-Level Linguistic Processing in the Human Brain. Proc. Natl. Acad. Sci. USA 2011, 108, 16428–16433. [Google Scholar] [CrossRef]
- Marek, S.; Dosenbach, N.U. The Frontoparietal Network: Function, Electrophysiology, and Importance of Individual Precision Mapping. Dialogues Clin. Neurosci. 2018, 20, 133–140. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Fair, D.A.; Cohen, A.L.; Schlaggar, B.L.; Petersen, S.E. A Dual-Networks Architecture of Top-down Control. Trends Cogn. Sci. 2008, 12, 99–105. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Visscher, K.M.; Palmer, E.D.; Miezin, F.M.; Wenger, K.K.; Kang, H.C.; Burgund, E.D.; Grimes, A.L.; Schlaggar, B.L.; Petersen, S.E. A Core System for the Implementation of Task Sets. Neuron 2006, 50, 799–812. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Fair, D.A.; Miezin, F.M.; Cohen, A.L.; Wenger, K.K.; Dosenbach, R.A.T.; Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E.; et al. Distinct Brain Networks for Adaptive and Stable Task Control in Humans. Proc. Natl. Acad. Sci. USA 2007, 104, 11073–11078. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Raichle, M.E.; Gordon, E.M. The Brain’s Action-Mode Network. Nat. Rev. Neurosci. 2025, 26, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, N.H.; Bechara, A. The Hidden Island of Addiction: The Insula. Trends Neurosci. 2009, 32, 56–67. [Google Scholar] [CrossRef]
- Naqvi, N.H.; Gaznick, N.; Tranel, D.; Bechara, A. The Insula: A Critical Neural Substrate for Craving and Drug Seeking under Conflict and Risk. Ann. N. Y. Acad. Sci. 2014, 1316, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.B.; Sanchez-Peña, J.; Levin, F.R.; Mariani, J.J.; Patel, G.H.; Naqvi, N.H. Drinking Reduction during Cognitive Behavioral Therapy for Alcohol Use Disorder Is Associated with a Reduction in Anterior Insula-bed Nucleus of the Stria Terminalis Resting-state Functional Connectivity. Alcohol Clin. Exp. Res. 2021, 45, 1596–1606. [Google Scholar] [CrossRef]
- Fox, M.D. Mapping Symptoms to Brain Networks with the Human Connectome. N. Engl. J. Med. 2018, 379, 2237–2245. [Google Scholar] [CrossRef]
- Joutsa, J.; Moussawi, K.; Siddiqi, S.H.; Abdolahi, A.; Drew, W.; Cohen, A.L.; Ross, T.J.; Deshpande, H.U.; Wang, H.Z.; Bruss, J.; et al. Brain Lesions Disrupting Addiction Map to a Common Human Brain Circuit. Nat. Med. 2022, 28, 1249–1255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, A.B.; Gold, M.S. A Tragedy of Errors: The State of Psychedelic Research in the Treatment of Alcohol Use Disorder. Brain Sci. 2025, 15, 1190. https://doi.org/10.3390/brainsci15111190
Srivastava AB, Gold MS. A Tragedy of Errors: The State of Psychedelic Research in the Treatment of Alcohol Use Disorder. Brain Sciences. 2025; 15(11):1190. https://doi.org/10.3390/brainsci15111190
Chicago/Turabian StyleSrivastava, A. Benjamin, and Mark S. Gold. 2025. "A Tragedy of Errors: The State of Psychedelic Research in the Treatment of Alcohol Use Disorder" Brain Sciences 15, no. 11: 1190. https://doi.org/10.3390/brainsci15111190
APA StyleSrivastava, A. B., & Gold, M. S. (2025). A Tragedy of Errors: The State of Psychedelic Research in the Treatment of Alcohol Use Disorder. Brain Sciences, 15(11), 1190. https://doi.org/10.3390/brainsci15111190






