Abstract
Background/Aim: Currently, there are limited evidence-based protocols for improving upper extremity (UE) motor function after stroke. The Keys protocol, a distributed form of constraint-induced movement therapy (CIMT), delivers CIMT components in fewer hours per day over an extended period, fitting outpatient rehabilitation schedules and third-party payor models. This pilot study aimed to assess the effectiveness of the Keys protocol in enhancing UE capacity and performance poststroke. Methods: Ten adults with chronic stroke (>6 months) participated in an 8-week intervention. The protocol included 22 supervised training sessions (1.5 h each): 4 days/week for 4 weeks, 2 days/week for weeks 5–6, and 1 day/week for weeks 7–8. Participants wore a restraint mitt on the less-affected UE during waking hours and used an adapted transfer package. Outcome measures included the Motor Activity Log (MAL), Wolf Motor Function Test (WMFT), Stroke Impact Scale (SIS), and Zung Depression Scale, assessed pre-treatment, mid-treatment (4 weeks), and posttreatment. Results: Significant improvements were observed in SIS Strength, ADLs/IADLs, Mobility, and Hand Function domains, exceeding MCID thresholds. Memory and Communication domains improved significantly at the 3-month follow-up. WMFT performance times improved, with fewer incomplete tasks. MAL scores for Amount of Use and Quality of Movement increased across all time points. Depressive symptoms significantly decreased posttreatment. Conclusions: The Keys protocol effectively improves UE use, motor function, mood, and quality of life, with the greatest gains observed mid-intervention. These findings support its feasibility and potential for outpatient stroke rehabilitation (ClinicalTrials.gov Registration: NCT05311384).
1. Introduction
Stroke is the leading cause of disability in the U.S., resulting in reduced mobility, impaired activities of daily living, social isolation, depression, and unemployment for those with stroke and their caregivers [1]. Consequently, stroke can significantly diminish capacity and performance, two key constructs within the Activities and Participation components of the International Classification of Functioning, Disability, and Health (ICF). Capacity refers to an individual’s ability to do tasks in a controlled, standardized environment (e.g., a laboratory setting), whereas performance reflects what a person can accomplish in real-world contexts [2]. Understanding these constructs is essential for designing effective rehabilitation strategies that address both clinical and everyday challenges.
To combat the high and increasing incidence of stroke, particularly among young adults [3], and the resulting disability coming from stroke worldwide, a few intervention protocols have been developed and recommended by stroke rehabilitation guidelines for improving the affected UE motor function, including constraint-induced movement therapy (CIMT) [4,5]. The signature CIMT protocol systematically delivers intensive treatment sessions in person over 10 consecutive weekdays and behavioral components over one weekend, with a structured approach to maximize outcomes. Each treatment day includes a total of 3.5 h of therapist interaction with 3 h of supervised motor training using shaping and task practice, followed by 30 min dedicated to behavioral strategies known as the Transfer Package, designed to enhance the use of the more-affected upper extremity (UE) in real-life situations. Additionally, the protocol incorporates strategies to encourage consistent use of the more-affected UE, including the restraint of the less-affected UE [6].
Although CIMT is known for its strong evidence, the implementation of the protocol in clinical settings is still challenging and therefore seldom used with all components with fidelity [7]. Multiple barriers for the availability of the CIMT protocol in the clinic have been identified, including its incompatibility with insurance payment policies and the demand for increased therapist time for supervised training, resulting in high costs for implementation [8,9,10,11,12,13,14,15]. Various modified (mCIMT) protocols have been developed in order to overcome these barriers; however, only one or two components of the signature CIMT protocol are usually administered [7,16], with the use of the restraint device and the intensive motor training being the most commonly used in these protocols [16]. The Transfer Package is often omitted, even though it is known to amplify the results of the motor training itself [17,18].
Research suggests that alternative delivery models of CIMT enable the application of all the elements of the CIMT protocol, optimizing therapist time and resources while aligning with the reimbursement policies of many U.S. health insurance providers. Evidence indicates that the original 6 h supervised daily training schedule can be shortened to as little as 2 h/daily without compromising treatment outcomes [19]. Furthermore, additional evidence comes from a study investigating potential changes in the effectiveness of the protocol when adding and omitting elements of the signature protocol. Findings indicated that omitting the transfer package led to a 50% reduction in functional outcomes [18]. This line of evidence was confirmed by brain imaging data that showed reduced brain remodeling in participants with stroke who did not receive the Transfer Package. These results underscore the critical role of the Transfer Package in promoting neuroplasticity and supporting continued movement practice outside the clinical setting, ultimately contributing to more sustainable functional improvements. Therefore, an extended interaction with the Transfer Package might amplify the results observed on the signature protocol.
A distributed form of the signature CIMT, the Keys treatment protocol, consists of all the components of CIMT delivered in fewer hours per day over a greater number of weeks. The name of Keys indicates “Keys to unlock real-world function”. This distributed version of the CIMT treatment protocol is not considered to be a “modified CIMT” because it includes all the components of the signature protocol. Most importantly, in the Keys protocol, the participant interaction with the Transfer Package (TP) is extended to 8 weeks, providing more opportunities for participants to use the affected UE in their own environment. We hypothesize that use of the Keys protocol, including prolonged exposure to the TP, may lead to improvements in UE capacity, performance, and participation that are as significant or more significant than that obtained with the signature CIMT protocol. The purpose of this pilot study was to investigate the effects of the Keys treatment protocol in more-affected UE capacity, performance, and participation and to compare with changes observed with the signature CIMT used in previous studies. This investigation is important because, in contrast to the signature CIMT protocol, the Keys treatment protocol (1) includes an extended interaction with the TP, (2) is more consistent with reimbursement policies in the U.S., and (3) is compatible with implementation in traditional rehabilitation settings.
2. Materials and Methods
2.1. Study Design
This pilot clinical trial investigated the effect of the Keys treatment protocol on real-world use and motor function of the affected UE of individuals with chronic stroke. Changes in quality of life and occupational performance were also explored in this study. This study was approved by the UAB Institutional Review Board (IRB-300005407), and all participants signed an informed consent.
2.2. Participants
In this study, ten individuals were included if they (1) were age 18 years or older; (2) had a stroke at least 6 months prior to enrollment; (3) were able to demonstrate minimum movement criteria of the more-affected UE, including 10 degrees of wrist extension (starting from a fully flexed position), 10 degrees of thumb abduction, and 10 degrees of extension of two additional fingers at all joints; (4) scored less than 2.5 on the Motor Activity Log (MAL); (5) achieved score of 24 or higher on the Mini Mental State Examination (MMSE); (6) demonstrated the ability to comprehend and answer the MAL questions; and (7) had not received a botulin toxin injection or adjustments in anti-spasticity drug regimens within 3 months of treatment. Sensory deficits were not an exclusion. Individuals who were not fluent in English were excluded from the study.
2.3. Intervention
All participants received the Keys intervention protocol over an 8-week period with delivery at four times per week for 4 weeks, then tapered to two times a week for 2 weeks, and then one time a week for two weeks (Figure 1). Specific CIMT elements were delivered as previously described [6] except for the following adaptations: (1) 1.5 h session, including supervised movement training carried out for 1 h in the form of shaping [20] and task practice (i.e., 2 shaping tasks), with 30 min allocated for the administration of the Transfer Package [18]; (2) a total of 22 sessions distributed as follows: 4 days/week for the first 4 weeks, 2 days/week for weeks 5 and 6, and 1 day/week for weeks 7 and 8 (Figure 1) [21]; (3) participants used the restraint mitt on their less-affected UE for most of their waking hours for an 8 week period for a target of 90% of waking hours; and (4) interaction with the elements of the Transfer Package throughout the 8 weeks (Table 1). The detailed descriptions and objectives of each element of the treatment protocol as well as the differences between the Keys treatment and the CIMT signature protocol are shown in Table 1.
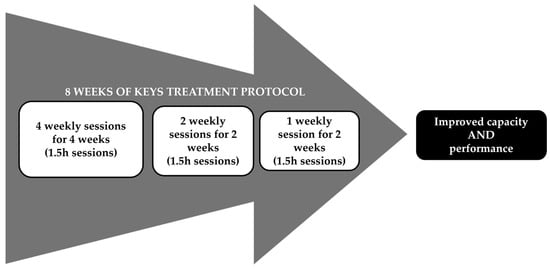
Figure 1.
Keys Treatment Protocol Schedule.

Table 1.
Parameters and Components of the CIMT and their Application in the Signature and the Keys Treatment Protocols.
Another important element of the CIMT protocol is patient education. Although not always explicitly included as part of the treatment, it plays a pivotal role in enhancing patients’ engagement and adherence. Patient education content consists of oral explanations and printed resources that describe how and why CIMT promotes adaptive neuroplasticity, including neuroimagery figures depicting structural gray mater changes following CIMT available in the literature [17]. Information for stroke recovery and health literacy is also covered during educational procedures.
2.4. Outcome Measures
The primary outcome measures of this study are the changes in the more-affected UE as it relates to self-reported performance (i.e., real-world use) measured by the [22,23,24], and in motor capacity (i.e., motor function) as assessed by the Wolf Motor Function Test (WMFT) [25,26,27,28]. Secondary outcome measures include the Stroke Impact Scale (SIS) [29,30,31] and the Zung Self-Rating Depression Scale (ZSDS) [32,33]. All measures are reliable and valid to be used with the stroke population.
All measures, except the WMFT, were administered before the treatment (pre-treatment), at the 4-week point in the intervention (during treatment), immediately after the treatment (posttreatment), 1 month after the end of the treatment (follow-up 1), and at 3 months after the end of treatment (follow-up 2). The WMFT was administered by a tester therapist who did not perform the intervention with participants at the pre-treatment, during treatment, and post-treatment time points, and all administrations were recorded and coded. The Functional Ability Scale (FAS) of the WMFT was scored by blinded assessors using videos of the testing sessions. Data were reported in means and changes in scores at pre-treatment, during treatment (after 4 weeks of treatment), post-treatment, and both follow-up assessments.
3. Results
Potential participants were recruited from July 2022 to August 2023, and the last participant completed the follow-up assessment visit in January 2024. Overall, 56 individuals were screened, and from those, 46 were excluded (Figure 2). A total of 10 participants were included in this pilot trial; however, three individuals were withdrawn from the study due to reasons unrelated to the treatment protocol. Two participants were withdrawn because of uncontrolled health conditions and one due to family emergency. The seven participants who completed the study attended all treatment sessions and assessment visits.
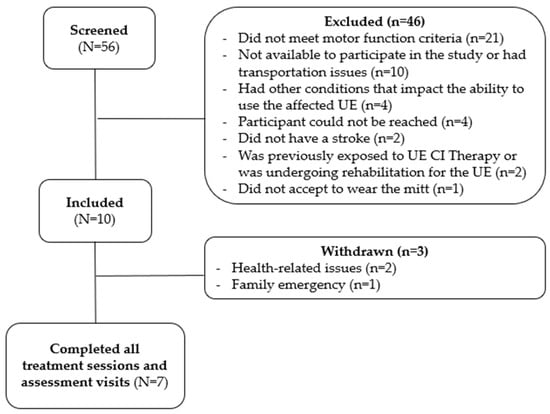
Figure 2.
Recruitment flowchart.
As shown in Table 2, participants were, on average, 61.7 years old and had a stroke 26.9 months prior to their enrollment in the study. Most participants were White (n = 6), had ischemic stroke (n = 5), and were right-handed (n = 8). Half of the participants had left hemiparesis due to their stroke.

Table 2.
Demographic characteristics of the participants.
Overall, participants showed improvements in all outcome measures at the 4-week (during treatment) assessment (Table 3). These changes were also observed on the post-treatment assessments, except on the SIS Memory and Communication scores. Although the average of the scores on these two SIS domains was not improved on the post-treatment assessment, the scores had substantially improved on the 3-month follow-up assessment visit.

Table 3.
Average scores on outcome measures on each time point.
Participants showed changes in the SIS scores above the minimal clinically important difference (MCID) on the Strength, ADLs and IADLs, Mobility, and Hand Function domains [34]. Substantially improved scores on the SIS were also observed on the Strength and the Mobility domains at the post-treatment and the follow-up assessments. Further notable improvements were observed on the SIS for the ADLs/IADLs and the Hand Function domains during and at the post-treatment assessment and the 3-month follow-up scores. The substantial improvement in depressive symptoms, indicated by the Zung Depression Scale, further underscores the protocol’s benefits.
The only capacity measure administered in this study was the WMFT. The changes observed in the assessment follow the same pattern as the other outcome measures. The difference on the overall performance time from the pre-treatment assessment was higher than the MCID [27] at both during (−1.5 s) and post-treatment (−2.09 s) time points. Quality of movement during the performance of the WMFT tasks was assessed using the Functional Ability Scale. As seen in Table 3, there was no change in the scores obtained on this scale, but the number of tasks that the participants were unable to complete decreased during and after the treatment.
Participants showed improvement in the use of the more-affected upper extremity assessed by the MAL in both the amount of use and quality of movement (Figure 3). The difference in the scores obtained at the pre- and during treatment time points (4-week assessment visit—AOU: 2.5, and QOM: 2.2) were, on average, higher than the 0.5-point MCID previously reported in the literature [23,24]. The same was observed at both the post-treatment time point and 3-month follow-up visit where the differences in both scales of the MAL (AOU: 2.9 and QOM: 3.0; AOU: 2.3 and QOM: 2.6, respectively) were higher than the MCID previously reported.
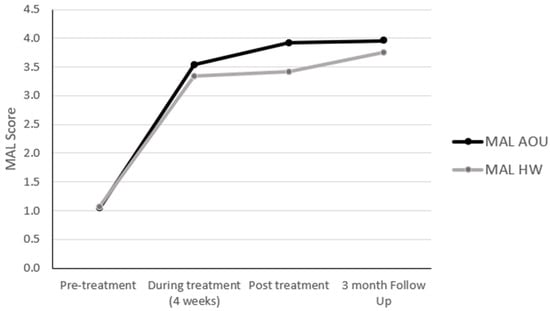
Figure 3.
Changes in the MAL scores.
Of the ten participants initially included in the trial, seven completed the treatment. Three participants withdrew due to reasons unrelated to the clinical trial. All participants who completed the trial had 100% attendance in both treatment and assessment visits. No adverse events or falls were reported.
4. Discussion
This study protocol delivered all the elements of the signature CIMT protocol [4] in a distributed schedule, in shorter supervised sessions, to make it potentially reimbursable and time-efficient. Specifically, adjustments were made to the treatment duration, session length, and weekly frequency. The purpose of this pilot trial was to preliminarily assess the effectiveness of a distributed CIMT, i.e., the Keys treatment protocol, addressing barriers that limit access to traditional CIMT in clinical practice. Although the Keys protocol retained all the core elements of CIMT, adaptations were made to the session duration and frequency, with use of the mitt 90% of the waking hours daily throughout the 8 weeks of treatment. These changes aimed to address common barriers to implementing traditional CIMT, such as the time-intensive nature of the intervention for the therapist, patient adherence to wearing the mitt, and consistent engagement in transfer package activities.
This pilot trial evaluated the impact of the Keys protocol on motor capacity, upper extremity (UE) use, depression, activities of daily living (ADL), and overall quality of life in individuals with chronic stroke. Notably, participants showed improvements in the Hand Function domain of the SIS, surpassing results reported in the literature protocol [35]. Important changes in UE use and quality of life were observed during and after treatment, with these improvements maintained three months after the intervention. The results indicate that the distributed model may enhance outcomes, as evidenced by superior improvements in the MAL measures compared to previous studies [18,35,36,37,38]. Importantly, the positive effects persisted or were even amplified at follow-up. A substantial improvement on performance time measures by the WMFT was observed; however, this change was not reflected in the Functional Ability Score of the WMFT.
The variability in CIMT protocols across the literature makes direct comparisons challenging [16]. One critical element often neglected in other protocols is the behavioral strategy component known as the “Transfer Package”. In the Keys protocol, participants engaged with these strategies for eight weeks, compared to the typical two-week period in the signature CIMT [6]. The extended exposure to the Transfer Package likely contributed to sustained improvements in UE use, reinforcing motor gains through continuous practice in real-world contexts.
The Transfer Package is essential for integrating the more-affected limb into daily activities, promoting motor retention by encouraging functional use outside therapy. This component involves problem-solving tasks, home-based practice, and self-monitoring [18]. Research indicates that including the Transfer Package in CIMT significantly enhances long-term motor outcomes, helping patients maintain improvements and avoid reverting to compensatory behaviors [18,39,40,41].
Despite strong evidence supporting CIMT and its endorsement in stroke rehabilitation guidelines [4,5], real-world implementation remains challenging. Barriers include the higher number of hours required from patients and clinicians to engage in the supervised sessions, maintaining patient adherence, the logistical administration of Transfer Package elements, lack of reimbursement from third-party payers, and lack of therapists training on the application of CIMT [8,9,10,11,12,13,14,15,42]. These challenges highlight the need for innovative adaptations of CIMT to align with the current healthcare scenario [43]. The Keys treatment protocol addresses some of these issues, and more efforts to reduce barriers to delivering this evidence-based intervention should be considered. Additionally, the Keys protocol presents a feasible and potentially reimbursable delivery option for implementing CIMT in the clinical settings while achieving outcomes comparable to the signature protocol.
4.1. Limitations
The primary limitation of this study is the small sample size, which restricts the ability to generalize the findings to a broader population. Furthermore, with a small sample, individual variability among participants can skew the findings and limit their reliability. Although the incidence of stroke has increased [44,45,46], there are multiple barriers to recruitment, including challenges caused by the COVID-19 pandemic, transportation limitations, and difficulties in controlling health conditions (e.g., arterial hypertension).
While the results are promising, larger studies with comparison groups are required to better understand the potential of the Keys treatment protocol and identify what results are associated specifically with this protocol. Although the MAL has shown a high correlation with objective measures (e.g., accelerometry and neuroimaging data) [17,23], another potential limitation is the use of self-reported (perceived) performance outcomes, as participants may provide inaccurate information due to memory issues or personal biases. Despite these limitations, the MAL is an appropriate outcome measure for this study because of its strong psychometric properties, and it has been used extensively in CIMT and other stroke rehabilitation studies. Also, even with recent technological advances, there are still challenges in using activity monitors with the specificity needed to accurately measure actual performance improvements.
4.2. Future Directions
To overcome the logistical challenges and barriers posed by signature CIMT, future research should explore the feasibility of delivering adapted protocols remotely. Telehealth approaches could increase access to CIMT, especially in rural or underserved areas, and reduce the burden of frequent clinic visits. Since the finding of this study showed such robust changes after just 4 weeks of treatment, the intervention could be studied for 4 weeks of treatment. Further investigation of the effectiveness of the Keys treatment protocol should include larger sample sizes as well as comparison groups such as the signature CIMT protocol and interventions commonly delivered in the clinical settings. Additionally, future studies could investigate changes in motor performance using wearable sensors and accelerometers to provide objective measures of improvement [47,48,49,50]. These technologies can capture real-time data on movement patterns and intensity, offering deeper insights into patients’ progress both during and after treatment.
5. Conclusions
The results of this study highlight the positive impact of the adapted Keys treatment protocol on motor capacity, upper extremity performance, ADLs, and overall well-being in individuals with chronic stroke. Significant improvements were observed across multiple domains of the SIS, including Hand Function, Mood, Mobility, and Participation, with most gains maintained or amplified at the three-month post-treatment assessment.
This distributed version of CIMT is promising for addressing barriers associated with reimbursement and therapist scheduling in rehabilitation settings by distributing the treatment delivery and applying behavioral strategies over a longer period. While the small sample size limits the generalizability of these findings, the results point to the potential for this approach to enhance functional recovery and quality of life. Future research should focus on larger trials, potentially integrating telehealth solutions, reducing the overall number sessions, and utilizing objective measures (e.g., wearable sensors) to validate these findings and explore sustainable delivery models for CIMT in clinical practice.
Author Contributions
Conceptualization, S.d.A., M.B. and D.M.; data curation, S.d.A. and M.B.; formal analysis, S.d.A.; funding acquisition, S.d.A.; investigation, M.B. and D.M.; methodology, S.d.A., M.B. and D.M.; project administration, S.d.A. and M.B.; resources, M.B.; supervision, S.d.A.; visualization, M.B.; writing—original draft, S.d.A.; writing—review and editing, S.d.A., M.B. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by UAB Center for Engagement in Disability Health and Rehabilitation Sciences (CEDHARS) Pilot Funding Grant 2021.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the UAB Institutional Review Board (IRB 300008977, approved on 31 March 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Due to Institutional restrictions, results will be available at https://clinicaltrials.gov/study/NCT05311384. For additional information or inquiries, please contact the corresponding author.
Acknowledgments
The authors wish to acknowledge Edward Taub, Jean Crago, Danna Kay King, Jamie Wade, and Mary Bowman, who developed and named (Taub) the original Keys treatment protocol at Spain Rehabilitation Center at UAB Medicine over the period of 2018–2020.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report from the American Heart Association. Circulation 2023, 147, E93–E621. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. How to Use the ICF A Practical Manual for Using the International Classification of Functioning, Disability and Health (ICF) How to Use the ICF A Practical Manual for Using the International Classification of Functioning, Disability and Health (ICF) Exposure Draft for Comment; WHO: Geneva, Switzerland, 2013; Available online: https://cdn.who.int/media/docs/default-source/classification/icf/drafticfpracticalmanual2.pdf?sfvrsn=8a214b01_4&download=true (accessed on 26 November 2024).
- Scott, C.A.; Li, L.; Rothwell, P.M. Diverging Temporal Trends in Stroke Incidence in Younger vs Older People: A Systematic Review and Meta-Analysis. JAMA Neurol. 2022, 79, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Hildebrand, M.W.; Geller, D.; Proffitt, R. Occupational Therapy Practice Guidelines for Adults with Stroke. Am. J. Occup. Ther. 2023, 77, 7705397010. [Google Scholar] [CrossRef]
- Morris, D.M.; Taub, E.; Mark, V.W. Constraint-Induced Movement Therapy: Characterizing the Intervention Protocol. Eur. Medicophysica 2006, 42, 257–268. [Google Scholar]
- Kwakkel, G.; Veerbeek, J.M.; van Wegen, E.E.H.; Wolf, S.L. Constraint-Induced Movement Therapy after Stroke. Lancet Neurol. 2015, 14, 224–234. [Google Scholar] [CrossRef]
- Pedlow, K.; Lennon, S.; Wilson, C. Application of Constraint-Induced Movement Therapy in Clinical Practice: An Online Survey. Arch. Phys. Med. Rehabil. 2014, 95, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.; Teasell, R. Barriers to the Implementation of Constraint-Induced Movement Therapy into Practice. Top. Stroke Rehabil. 2012, 19, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Daniel, L.; Howard, W.; Braun, D.; Page, S.J. Opinions of Constraint-Induced Movement Therapy Among Therapists in Southwestern Ohio. Top. Stroke Rehabil. 2012, 19, 268–275. [Google Scholar] [CrossRef]
- Cahill, L.S.; Carey, L.M.; Mak-Yuen, Y.; McCluskey, A.; Neilson, C.; O’Connor, D.A.; Lannin, N.A. Factors Influencing Allied Health Professionals’ Implementation of Upper Limb Sensory Rehabilitation for Stroke Survivors: A Qualitative Study to Inform Knowledge Translation. BMJ Open 2021, 11, e042879. [Google Scholar] [CrossRef]
- Christie, L.J.; McCluskey, A.; Lovarini, M. Constraint-Induced Movement Therapy for Upper Limb Recovery in Adult Neurorehabilitation: An International Survey of Current Knowledge and Experience. Aust. Occup. Ther. J. 2019, 66, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Weerakkody, A.; White, J.; Hill, C.; Godecke, E.; Singer, B. Delivering Constraint-Induced Movement Therapy in Stroke Rehabilitation Requires Informed Stakeholders, Sufficient Resources and Organisational Buy-in: A Mixed-Methods Systematic Review. J. Physiother. 2023, 69, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.P.; Wolf, S.L.; Hammel, E.A.; McLeod, E.L.; Williams, E.A. Constraint-Induced Movement Therapy (CIMT): Current Perspectives and Future Directions. Stroke Res. Treat. 2012, 2012, 159391. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Pink, M.J. Occupational Therapists and the Use of Constraint-Induced Movement Therapy in Neurological Practice. Aust. Occup. Ther. J. 2009, 56, 436–437. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, Y.; Long, J.; Pan, M.; Wang, J.; Yang, F. Effect of Different Constraint-Induced Movement Therapy Protocols on Recovery of Stroke Survivors with Upper Extremity Dysfunction: A Systematic Review and Network Meta-Analysis. Int. J. Rehabil. Res. 2023, 46, 133–150. [Google Scholar] [CrossRef]
- Gauthier, L.V.; Taub, E.; Perkins, C.; Ortmann, M.; Mark, V.W.; Uswatte, G. Remodeling the Brain: Plastic Structural Brain Changes Produced by Different Motor Therapies after Stroke. Stroke 2008, 39, 1520–1525. [Google Scholar] [CrossRef]
- Taub, E.; Uswatte, G.; Mark, V.W.; Morris, D.M.; Barman, J.; Bowman, M.H.; Bryson, C.; Delgado, A.; Bishop-Mckay, S. Method for Enhancing Real-World Use of a More Affected Arm in Chronic Stroke: Transfer Package of Constraint-Induced Movement Therapy. Stroke 2013, 44, 1383–1388. [Google Scholar] [CrossRef]
- Sterr, A.; Elbert, T.; Berthold, I.; Koölbel, S.; Rockstroh, B.; Taub, E. Longer versus Shorter Daily Constraint-Induced Movement Therapy of Chronic Hemiparesis: An Exploratory Study. Arch. Phys. Med. Rehabil. 2002, 83, 1374–1377. [Google Scholar] [CrossRef]
- Uswatte, G.; Taub, E.; Morris, D.; Barman, J.; Crago, J. Contribution of the Shaping and Restraint Components of Constraint-Induced Movement Therapy to Treatment Outcome. NeuroRehabilitation 2006, 21, 147–156. [Google Scholar] [CrossRef]
- dos Anjos, S.; Bowman, M.; Morris, D. Preliminary Results of a Pilot Clinical Trial Using a Reimbursable Form of Constraint-Induced Movement Therapy: The Keys Treatment Protocol. Arch. Phys. Med. Rehabil. 2024, 105, e37. [Google Scholar] [CrossRef]
- Uswatte, G.; Taub, E.; Morris, D.; Light, K.; Thompson, P.A. The Motor Activity Log-28: Assessing Daily Use of the Hemiparetic Arm after Stroke. Neurology 2006, 67, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Uswatte, G.; Taub, E.; Morris, D.; Vignolo, M.; McCulloch, K. Reliability and Validity of the Upper-Extremity Motor Activity Log-14 for Measuring Real-World Arm Use. Stroke 2005, 36, 2493–2496. [Google Scholar] [CrossRef] [PubMed]
- van der Lee, J.H.; Beckerman, H.; Knol, D.L.; de Vet, H.C.W.; Bouter, L.M. Clinimetric Properties of the Motor Activity Log for the Assessment of Arm Use in Hemiparetic Patients. Stroke 2004, 35, 1410–1414. [Google Scholar] [CrossRef]
- Wolf, S.L.; Thompson, P.A.; Morris, D.M.; Rose, D.K.; Winstein, C.J.; Taub, E.; Giuliani, C.; Pearson, S.L. The EXCITE Trial: Attributes of the Wolf Motor Function Test in Patients with Subacute Stroke. Neurorehabil. Neural Repair 2005, 19, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Duff, S.V.; He, J.; Nelsen, M.A.; Lane, C.J.; Rowe, V.T.; Wolf, S.L.; Dromerick, A.W.; Winstein, C.J. Interrater Reliability of the Wolf Motor Function Test-Functional Ability Scale: Why It Matters. Neurorehabil. Neural Repair 2015, 29, 436–443. [Google Scholar] [CrossRef]
- Fritz, S.L.; Blanton, S.; Uswatte, G.; Taub, E.; Wolf, S.L. Minimal Detectable Change Scores for the Wolf Motor Function Test. Neurorehabil. Neural Repair 2009, 23, 662–667. [Google Scholar] [CrossRef]
- Morris, D.M.; Uswatte, G.; Crago, J.E.; Cook, E.W.; Taub, E. The Reliability of the Wolf Motor Function Test for Assessing Upper Extremity Function after Stroke. Arch. Phys. Med. Rehabil. 2001, 82, 750–755. [Google Scholar] [CrossRef]
- Duncan, P.W.; Lai, S.M.; Bode, R.K.; Perera, S.; DeRosa, J. Stroke Impact Scale-16: A Brief Assessment of Physical Function. Neurology 2003, 60, 291–296. [Google Scholar] [CrossRef]
- Duncan, P.W.; Bode, R.K.; Min Lai, S.; Perera, S. Rasch Analysis of a New Stroke-Specific Outcome Scale: The Stroke Impact Scale. Arch. Phys. Med. Rehabil. 2003, 84, 950–963. [Google Scholar] [CrossRef]
- Duncan, P.W.; Wallace, D.; Lai, S.M.; Johnson, D.; Embretson, S.; Laster, L.J. The Stroke Impact Scale Version 2.0 Evaluation of Reliability, Validity, and Sensitivity to Change. Stroke 1999, 30, 2131–2140. [Google Scholar]
- Biggs, J.T.; Wylie, L.T.; Ziegler, V.E. Validity of the Zung Self Rating Depression Scale. Br. J. Psychiatry 1978, 132, 381–385. [Google Scholar] [CrossRef]
- Dunstan, D.A.; Scott, N. Clarification of the Cut-off Score for Zung’s Self-Rating Depression Scale. BMC Psychiatry 2019, 19, 177. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.C.; Fu, T.; Wu, C.Y.; Wang, Y.H.; Liu, J.S.; Hsieh, C.J.; Lin, S.F. Minimal Detectable Change and Clinically Important Difference of the Stroke Impact Scale in Stroke Patients. Neurorehabil. Neural Repair 2010, 24, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.L.; Winstein, C.J.; Miller, J.P.; Morris, D. Effect of Constraint-Induced Movement on Upper Extremity Function 3 to 9 Months after Stroke: The EXCITE Randomized Cinical Trial. Jama 2006, 296, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.; Viana, R.; Janzen, S.; Mehta, S.; Pereira, S.; Teasell, R. Systematic Review and Meta-Analysis of Constraint-Induced Movement Therapy in the Hemiparetic Upper Extremity More than Six Months Post Stroke. Top. Stroke Rehabil. 2012, 19, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Page, S.J.; Sisto, S.A.; Levine, P.; McGrath, R.E. Efficacy of Modified Constraint-Induced Movement Therapy in Chronic Stroke: A Single-Blinded Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2004, 85, 14–18. [Google Scholar] [CrossRef]
- Page, S.J.; Levine, P.; Leonard, A.; Szaflarski, J.P.; Kissela, B.M. Modified Constraint-Induced Therapy in Chronic Stroke: Results of a Single-Blinded Randomized Controlled Trial Background and Purpose. Phys. Ther. 2008, 88, 333–340. [Google Scholar] [CrossRef]
- Taub, E. Harnessing Brain Plasticity through Behavioral Techniques to Produce New Treatments in Neurorehabilitation. Am. Psychol. 2004, 59, 692–704. [Google Scholar] [CrossRef]
- Uswatte, G.; Taub, E. Constraint-Induced Movement Therapy: A Method for Harnessing Neuroplasticity to Treat Motor Disorders. Prog. Brain Res. 2013, 207, 379–401. [Google Scholar] [CrossRef]
- de Azevedo, J.A.; Barbosa, F.D.S.; Seixas, V.M.; da Silva Scipioni, K.R.D.; Sampaio, P.Y.S.; da Cruz, D.M.C.; Piscitelli, D.; Chui, K.K.; de Freitas Zanona, A. Effects of Constraint-Induced Movement Therapy on Activity and Participation after a Stroke: Systematic Review and Meta-Analysis. Front. Hum. Neurosci. 2022, 16, 987061. [Google Scholar] [CrossRef]
- Sterr, A.; Saunders, A. CI Therapy Distribution: Theory, Evidence and Practice. NeuroRehabilitation 2006, 21, 97–105. [Google Scholar] [CrossRef]
- Sterr, A. Training-Based Interventions in Motor Rehabilitation After Stroke: Theoretical and Clinical Considerations. Behav. Neurol. 2004, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Imoisili, O.E.; Chung, A.; Tong, X.; Hayes, D.K.; Loustalot, F. Prevalence of Stroke—Behavioral Risk Factor. Surveillance System, United States, 2011–2022. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.S.; Pinto, C.B.; Saleh Velez, F.G.; Leffa, D.T.; Vulcano de Toledo Piza, P.; Fregni, F. Recruitment Challenges in Stroke Neurorecovery Clinical Trials. Contemp. Clin. Trials Commun. 2019, 15, 100404. [Google Scholar] [CrossRef] [PubMed]
- McGill, K.; Sackley, C.M.; Godwin, J.; McGarry, J.; Brady, M.C. A Systematic Review of the Efficiency of Recruitment to Stroke Rehabilitation Randomised Controlled Trials. Trials 2020, 21, 68. [Google Scholar] [CrossRef]
- Heye, A.L.; Kersting, C.; Kneer, M.; Barzel, A. Suitability of Accelerometry as an Objective Measure for Upper Extremity Use in Stroke Patients. BMC Neurol. 2022, 22, 220. [Google Scholar] [CrossRef]
- Kim, G.J.; Parnandi, A.; Eva, S.; Schambra, H. The Use of Wearable Sensors to Assess and Treat the Upper Extremity after Stroke: A Scoping Review. Disabil. Rehabil. 2022, 44, 6119–6138. [Google Scholar] [CrossRef]
- Wolff, A.L.; Kwasnicki, R.M.; Farnebo, S.; Horwitz, M.D. Dynamic Assessment of the Upper Extremity: A Review of Available and Emerging Technologies. J. Hand Surg. Eur. Vol. 2023, 48, 404–411. [Google Scholar] [CrossRef]
- Uswatte, G.; Foo, W.L.; Olmstead, H.; Lopez, K.; Holand, A.; Simms, L.B. Ambulatory Monitoring of Arm Movement Using Accelerometry: An Objective Measure of Upper-Extremity Rehabilitation in Persons with Chronic Stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1498–1501. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).