Abstract
Patients with major depressive disorder (MDD) exhibit an abnormal physiological arousal pattern known as hyperarousal, which may contribute to their depressive symptoms. However, the neurobiological mechanisms linking this abnormal arousal to depressive symptoms are not yet fully understood. In this review, we summarize the physiological and neural features of arousal, and review the literature indicating abnormal arousal in depressed patients. Evidence suggests that a hyperarousal state in depression is characterized by abnormalities in sleep behavior, physiological (e.g., heart rate, skin conductance, pupil diameter) and electroencephalography (EEG) features, and altered activity in subcortical (e.g., hypothalamus and locus coeruleus) and cortical regions. While recent studies highlight the importance of subcortical–cortical interactions in arousal, few have explored the relationship between subcortical–cortical interactions and hyperarousal in depressed patients. This gap limits our understanding of the neural mechanism through which hyperarousal affects depressive symptoms, which involves various cognitive processes and the cerebral cortex. Based on the current literature, we propose that the hyperconnectivity in the thalamocortical circuit may contribute to both the hyperarousal pattern and depressive symptoms. Future research should investigate the relationship between thalamocortical connections and abnormal arousal in depression, and explore its implications for non-invasive treatments for depression.
1. Introduction
Major depressive disorder (MDD), a prevalent neuropsychiatric disorder, is emerging as a serious public health concern. Despite decades of research, the neurobiological underpinnings of depression remain elusive. This may partly be due to incomprehensive investigations regarding the neural basis of arousal—key features of the brain states—in depression [1]. A theory in affective disorders suggests that hyperstable arousal regulation may lead to depressive symptoms such as social withdrawal and sensation avoidance [2,3,4]. In fact, recent evidence supports this abnormal arousal pattern in depression. Specifically, a higher and hyperstable arousal state has been observed in depressed patients compared with healthy, non-depressed individuals [4,5,6]. Moreover, this abnormal arousal pattern is associated with the severity of depressive symptoms and prolonged sleep onset latency in depression [3,7,8]. Therefore, neural mechanisms of physiological arousal appear to play a critical role in depressed patients, affecting their depressive symptoms.
Physiological arousal is considered as a key component of consciousness [9,10], and is supported by complex cooperation between the subcortex and cortex, particularly thalamocortical circuitry [11,12]. Previous studies have demonstrated that arousal affects perception [13], decision making [14], spatial memory [15], and attention [16], and is also a critical characteristic in mental disorders [3,17,18,19,20,21], including depression [3]. For now, altered activities of several arousal neural correlates have been identified in depression, such as the hypothalamic–pituitary–adrenal (HPA) axis [22,23] and locus coeruleus (LC) [3,24]. However, these subcortical findings did not reveal the subcortical–cortical interactions, and could not explicitly explain dysfunctions in high-order cognition or symptoms in depression. Currently, alterations in thalamocortical interactions that underlie the aberrations in physiological arousal in depression remain largely unclear.
In this review, we first present an overview of the behavioral and physiological characteristics of arousal. Next, we provide a comprehensive review of abnormal physiological arousal in depression, including both behavior and psychophysiological evidence. Subsequently, upon overviewing the role of thalamocortical circuits in arousal and MDD, we propose that abnormalities of these circuits could be a key neural mechanism underlying both hyperarousal and depressive symptoms. Finally, we discuss the future outlook for investigating the abnormal arousal in depression, fostering future research on the theoretical understanding of the pathology of MDD and its treatment approaches, including repetitive transcranial magnetic stimulation (rTMS), music interventions, and pharmacotherapy.
2. Overview of Physiological Arousal
Arousal is closely linked to consciousness, cognition, and mental disorders [9,25]. The level of arousal influences the performances of various cognitive tasks. For instance, a brief increase in arousal can shorten reaction time in decision making [14], while the restoration of arousal after light sleep improves detection in visuomotor tasks [26]. Additionally, the level of arousal before stimulus can predict perceptual task performance [27,28]. In the context of mental disorders, abnormal arousal is commonly found. Depression, for example, often co-occurs with hyperarousal and sustained tension [3,4,5,29]. Individuals with autism spectrum disorder (ASD) demonstrate abnormal arousal, but arguments exist regarding whether hypoarousal or hyperarousal accounts for the attentional and social skills in autism [21,30]. Therefore, understanding the neural basis of arousal is essential for disclosing the neural mechanisms of consciousness, cognition, and mental disorders.
It should be noticed that, although physiological arousal and emotional arousal have overlapping neural underpinnings, they are distinct concepts [31]. Emotional arousal is related to an individual’s brain and bodily responses to arousing stimuli, focusing on emotional reactivity [32]. On the other hand, physiological arousal measures the degree of wakefulness of the individuals. In this review, we focus on physiological arousal, hereafter referred to simply as arousal.
2.1. Physiological Correlates of Arousal
Physiological indices provide a comprehensive evaluation of arousal, providing objective and quantifiable measurements of arousal levels. These indices include behavior, electrodermal activity (EDA), heart rate variability (HRV), and pupil diameter, each of which provides unique insights into arousal states [11,33,34,35,36]. Behavior, as the most intuitive among these indices, visibly changes with shifts in arousal levels, such as when an individual awakens from sleep, performs limb movements, or speaks [11,33]; EDA reflects autonomic nervous system activity, which typically increases during states of high arousal [36]; HRV measures the natural variability in time intervals between heartbeats, and shows an inverse relationship with arousal levels [34]. Additionally, pupil diameter, influenced by the sympatho-vagal balance within the autonomic nervous system, expands with increased physiological arousal [35,37]. Together, these indices provide a solid foundation for assessing physiological arousal levels as well as exploring their neurophysiological underpinnings.
2.2. Neural Correlates of Physiological Arousal
The complex interplay of subcortical brain regions is essential for the neural mechanisms of arousal. These regions, primarily comprising the brainstem, hypothalamus, basal forebrain, and thalamus, work in concert to regulate various aspects of arousal, ranging from hormonal responses to neuronal activity [11,12]. This intricate system coordinates the physiological processes underlying our ability to wake and remain alert, influencing cognitive functions and mood states. The brainstem, particularly critical for physiological arousal regulation, contains structures like the reticular formation and various nuclei that are integral to the sleep–wake cycle [38,39,40]. The reticular formation enhances arousal by releasing acetylcholine, while brainstem nuclei, including the locus coeruleus (LC) with its norepinephrine-producing neurons, regulate cortical activities affecting mood and cognitive functions [41]. The ascending reticular activating system (ARAS) from the brainstem, projecting through the thalamus to the cerebral cortex, plays a crucial role in cortical activation and maintaining alertness [42,43,44]. The hypothalamus, which governs the hypothalamic–pituitary–adrenal (HPA) axis, significantly influences arousal [45,46]. Hyperactivity in the HPA axis can lead to increased arousal and stress responses, potentially resulting in anxiety and depression [47]. Within the hypothalamus, the lateral hypothalamus (LH) is critical for wakefulness, with neurons like hypocretin/orexin (Hcrt), glutamatergic, and GABAergic types modulating sleep stages [48,49,50,51]. The basal forebrain (BF) also contributes to arousal, with its cholinergic neurons enhancing cortical activity and wakefulness, and GABAergic neurons promoting arousal by modulating cortical inhibitory interneurons [52,53]. The intricate neuronal dynamics within the BF have significant impacts on cognitive functions and the sleep–wake cycle [52,54,55,56]. Additionally, the thalamus has long been involved in the regulation of sleep–wake cycle and arousal, serving a critical role in the transmission and integration of information [57,58,59]. It primarily relays sensory inputs to the cortex, which is a process essential for perception, emotion, and consciousness [57,60,61], and influences brain activation and consciousness states, primarily through neurotransmitters such as glutamate [61].
Numerous studies have focused on how subcortical brain regions regulate arousal. However, recent research has also revealed that the cortex is also involved in arousal alteration. For instance, the amplitude of global signals (GS), predominantly constituted by activities in primary sensory areas such as the sensorimotor cortex [62,63], negatively correlates with arousal levels [64,65], and increases during light sleep and mild anesthesia [66,67]. This indicates a broader engagement of cortical areas in arousal regulation. Notably, decreased physiological arousal is associated with increased thalamic activity and reduced activity in cortical areas, especially the default mode network (DMN) [68]. During anesthesia-induced unconsciousness, while cortico-cortical functional connectivity is preserved, thalamocortical connectivity is disrupted, with consciousness recovery linked to its restoration [69]. Additionally, the sensorimotor cortex drives dynamic functional connectivity of spontaneous signals across the cortex, and is closely related to arousal regulation (Figure 1A) [70,71,72]. Transient increases in GS, co-occurring with decreased activity in the dorsal midline thalamus, nucleus basalis and midbrain, suggest brief decreases in arousal [63]. Moreover, electrical stimulation of central thalamic regions, such as the central lateral nucleus of the thalamus, can induce widespread cortical activity and elevate arousal levels in animals under anesthesia and sleep states [59,73]. Our previous studies exploring altered arousal from eyes-open to eyes-closed states revealed that interactions between cortical networks are closely linked to arousal, underscoring the cortical contribution to arousal regulation (Figure 1B) [74,75]. These studies demonstrate that arousal regulation is governed by not only subcortical mechanisms, but also involves significant cortical contributions, particularly from areas like the DMN and sensorimotor cortex.
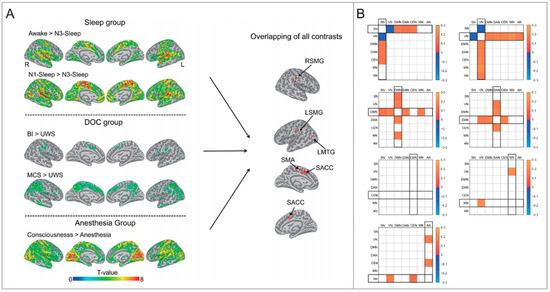
Figure 1.
Evidence of altered cortical connectivity in different arousal levels. (A) The brain regions with decreased degree centrality during unconsciousness. Adapted from [69]. SMA = supplementary motor area; LSMG = left supramarginal gyrus; RSMG = right supramarginal gyrus; LMTG = left middle temporal gyrus; SACC = supragenual anterior cingulate cortex. (B) The FC differential patterns (EC–EO) between networks. Adapted from [73]. FC = functional connectivity; EC = eye closed; EO = eye open; SN = salience network; VN = visual network; DMN = default mode network; DAN = dorsal attention network; CEN = central executive network; MN = motor network; AN = auditory network.
Taken together, physiological arousal is a complex and dynamic neurological process that engages multiple brain functional regions, including the brainstem, hypothalamus, basal forebrain, thalamus, and cerebral cortex. While initial studies of arousal focused extensively on the role of subcortical regions and hormonal interactions in arousal regulation, recent findings have shed light on the impact of cortical activities and their interaction with the subcortex. These findings indicate that arousal is not solely governed by subcortical mechanisms, but also involves critical contributions from cortical areas, especially through the integration and processing of information relayed by the thalamus. Therefore, the connectivity between cortical and subcortical regions may be a critical mechanism for the abnormal arousal in mental disorders, such as depression.
3. Abnormal Arousal in Depression
In this study, we mainly focus on patients (adults) diagnosed with MDD to illustrate the abnormal arousal in depression. We conducted a comprehensive search in the Web of Science for articles published up to November 2023 with the following terms: “MDD AND (“heart rate” OR “heart rate variability” OR “pupil” OR “skin conductance” OR “electrodermal activity”); “MDD AND HPA”; “MDD AND locus coeruleus AND norepinephrine”; and “MDD AND EEG vigilance”. Articles resulting from these searches and relevant references cited in those articles were thoroughly reviewed for this research, and this selection was also completed by searches in the authors’ personal files, where articles published in English were included.
3.1. Behavior Characteristics
Abnormal arousal in depression is manifested in sleep behavior. The DSM-5 has indicated insomnia as a primary symptoms of MDD [7,76]. Numerous studies have revealed that MDD patients suffer from various sleep disturbances. These disturbances include permanently increased inner tension [77], difficulties to relax or to initiate sleep, prolonged sleep onset latency, early morning awakenings with an inability to return to sleep, decreased sleep efficiency, and overall reduced sleep duration [78]. Additionally, electroencephalography (EEG) recordings also revealed irregular sleep architectures in depression that are characterized by a decrease in slow wave sleep and alterations in rapid eye movement (REM) sleep patterns. Specifically, the first REM stage occurs earlier and lasts 3–4 times longer in MDD patients compared to healthy individuals. There is also an increased proportion of REM sleep in the early part of sleep and higher REM density (i.e., more ocular movements during REM sleep) [8].
3.2. Physiological Evidence
Physiological evidence further confirms a hyperarousal state in depression that is characterized by several distinct markers. These include elevated heart rate (HR), decreased heart rate variability (HRV), increased skin conductance, larger pupil diameters, hyperactivity of the HPA axis and locus coeruleus–norepinephrine (LC–NE) system, and hyperstable arousal regulation as indexed by EEG vigilance.
3.2.1. Autonomic Function Indices
Abnormal arousal in depression is reflected by autonomic function markers such as HR, HRV, electrodermal activity (EDA), and pupil diameters. Studies have demonstrated elevated HR and decreased HRV in MDD patients at rest [79,80,81,82,83,84,85,86,87]. Specifically, studies controlling for factors such as age, gender, smoking habits, and education levels found that unmedicated MDD patients displayed a higher average HR than healthy individuals [84,86]. Additionally, reviews have consistently shown lower HRV in MDD than healthy individuals [88,89], despite some inconsistent results [87]. A comprehensive study using several autonomic function indices revealed that unmedicated MDD patients in a resting state (20 min in a supine position) exhibited higher HR, increased skin conductance levels and fluctuations, and larger pupil diameters than healthy individuals [87]. These findings indicate an increased level of arousal in depression.
3.2.2. Hyperactivity of HPA Axis
Hyperactivity of the HPA axis in MDD has been broadly reported [22,23,90]. The hypothalamus secretes corticotrophin-releasing factor (CRF) and vasopressin, which in turn activate the pituitary to release adrenocorticotropin hormone (ACTH). This finally stimulates the release of cortisol from the adrenal cortex [23]. Typically, hyperactivity of the HPA axis is always indirectly detected in humans by measuring hormone levels such as cortisol and ACTH. Many studies have reported elevated cortisol levels in the plasma [91,92,93,94,95,96] and urine [97,98] of depression patients. For instance, the mean plasma cortisol level of depressed patients before treatment was observed to be elevated by 10 μg (per 100 mL) above normal levels [91]. An analysis of plasma cortisol every 20 min over a 24-h period revealed higher cortisol levels and more frequent secretory episodes in depressed patients compared to normal individuals [92]. The mean urinary free cortisol level in depressed patients was significantly elevated (90.1 μg), compared to the normal level (48 μg) [97]. It has also been observed that cortisol levels can return to their normal levels following treatments like electroconvulsive therapy or antidepressive drugs (imipramine) [91,98]. Notably, cortisol levels in MDD patients, both during depressed and recovery states, are higher than in healthy individuals [99]. A meta-analysis further supports these findings, indicating a robust elevated ACTH level in depression [22]. The increase in the ACTH level in MDD after administration of CRF is slower than in healthy individuals [99,100]. Additionally, research indicates that the volume of the adrenal gland increases during depressive episodes in MDD and returns to normal size during remission [101].
3.2.3. Hyperactivity of Noradrenergic System (LC)
In MDD, hyperactivity of the central noradrenergic system, particularly the LC, is evident. Specifically, there is a notably higher level of norepinephrine (NE) and its metabolites. NE levels in the cerebrospinal fluid of MDD patients during 30-h recordings are consistently higher compared to healthy individuals [102]. Additionally, the appearance of NE in both extravascular and vascular compartments is elevated in MDD compared to healthy individuals [103]. Furthermore, the concentration of noradrenaline metabolites is also higher in the saliva of MDD compared to healthy individuals [104].
Hyperactivity of the LC–NE system in MDD is also reflected in the abnormal activities of neurotransmitters, including glutamate and tyrosine hydroxylase. It has been reported that the LC primarily receives excitatory input from glutamate and increased glutamatergic activity in MDD. Specifically, increased gene expression of glutamate receptors and a deficiency in astrocyte glutamate transporter gene expression have been observed in MDD post-mortem studies [105,106]. Furthermore, levels of tyrosine hydroxylase, which reflect the neuronal activity of the LC, have been found to be elevated in the LC of post-mortem MDD brains [107].
Moreover, the effectiveness of some antidepressants is associated with down-regulation of the LC’s activity. For instance, the selective NE reuptake inhibitor (reboxetine) has been shown to reduce the firing activity of NE neurons in the LC of rats [41,108]. This effect is also observed following a series of electroconvulsive shocks [109]. These findings suggest a close link between the pathogenesis of depression and the hyperactivity of the central noradrenergic system, potentially contributing to the hyperarousal pattern in depression.
3.2.4. Hyperstable Arousal Regulation as Indexed by EEG Vigilance
Electrophysiological evidence, characterized by EEG vigilance markers such as alpha, theta, and delta activity, demonstrates a typical hyperstable and higher arousal state in MDD patients than healthy individuals [110]. During resting states without external interruptions, most healthy individuals exhibit a progressive decline to a lower vigilance stage, suggesting a decrease in arousal levels [3]. In contrast, MDD patients often exhibit a hyperstable pattern of arousal regulation [3,4,5].
For instance, in a 15-minute EEG recording with closed eyes, unmedicated MDD patients spend more time in the highest EEG vigilance stages and exhibit a delayed decline to lower EEG vigilance stages compared to healthy individuals [4]. Subsequent studies have consistently confirmed the hyperstable arousal regulation pattern in depressed patients, especially in MDD [6,110,111,112,113,114,115]. Notably, even during two-minute EEG recordings, this pattern is observed [6]. It has also been found that more sleep disturbances before the day of recording correlate with a higher score of arousal stability in depression patients, but not in healthy individuals [114]. Similarly, symptom severity measured using the Beck Depression Inventory (BDI) was found to correspond with higher arousal levels and slower declines in arousal as indexed by EEG vigilance [113]. And patients with bipolar disorder in a depressive episode also exhibit higher mean vigilance levels as measured by EEG vigilance [116].
Moreover, studies have revealed that the arousal regulation pattern was related with responses to antidepressant treatments [111,115]. Specifically, during 15-minute EEG recordings, individuals who responded to antidepressants (like escitalopram or/and mirtazapine) exhibited a reduction in time spent in high vigilance states and an increase in time spent in low vigilance states two weeks after beginning treatment, compared to those who did not respond [111]. Remitters demonstrate a stronger tendency to decline to lower arousal levels compared to non-remitters [115].
In summary, evidence for abnormal hyperarousal in depressed patients is supported by a range of behavioral and physiological findings. These include sleep disturbances, increased heart rate, enlarged pupil diameters, heightened skin conductance, and hyperactivity in the subcortical areas, particularly in the hypothalamus and LC (Figure 2). Recent EEG studies suggest a hyperstable arousal regulation in depressed individuals that is linked to the severity of depressive symptoms.
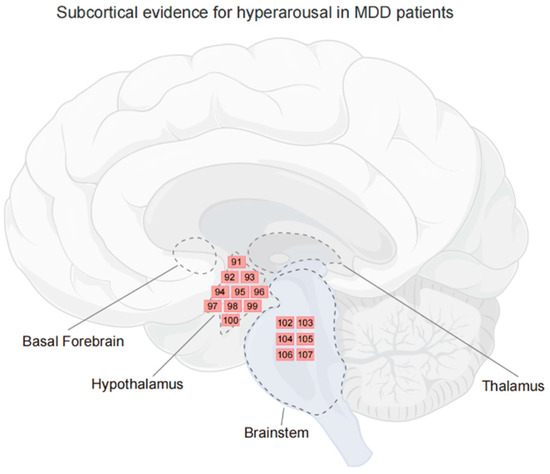
Figure 2.
Subcortical evidence for hyperarousal in MDD patients. Regions where activity is related to the hyperarousal in MDD. Each number corresponds to a reference, and indicates the contribution of the region, not to an exact location. The subcortical areas related to arousal are delineated by dashed lines. Figure created using FigDraw.
Despite the comprehensive results these studies provide, several limitations must be acknowledged. Firstly, although the studies suggest correlations between arousal regulation patterns and antidepressant treatments, the causality cannot be firmly established due to their observational nature. Additionally, the distinction between neurobiological underpinnings of hyperarousal in MDD and in other mood disorders, such as the depression phase in bipolar disorder and post-traumatic stress disorder [76,116,117,118,119], require further investigation. More importantly, previous studies did not thoroughly explore the association between subcortical–cortical connections, particularly thalamocortical interactions, and abnormal arousal in depression. The neural mechanism underlying various depressive symptoms—phenomena that involve multiple cognitive processes and cortical activities [120]—as responses to abnormal hyperarousal in depression remains poorly understood.
4. Thalamocortical Circuits Possibly Account for Abnormal Arousal in Depression
Thalamocortical circuits have been implicated in the regulation of physiological arousal [121,122]. The central thalamus is specialized to maintain the thalamocortical and cortico-cortical connections, prompting brief shifts in arousal, and injury to the central thalamus induces impairment of arousal regulation [123]. The firing of centromedial thalamus neurons has been proposed to implement dual control over sleep–wake states by modulating brain-wide cortical activities [124]. During non-rapid eye movement (NREM) sleep, the thalamus has also been found to modulate the slow waves that predominate in the neocortex [125]. Recent studies in macaques have shown that deep brain stimulation of the central or central lateral thalamus can facilitate their interactions with the brain-wide cortex, thereby restoring arousal from an anesthetized state [59,73]. Additionally, human research using fast fMRI has revealed a temporal sequence of activity across thalamic nuclei and the brain-wide cortex during the transition in arousal, suggesting thalamocortical dynamics that support arousal [126]. These studies provide empirical evidence for the critical role of thalamocortical circuits in the arousal system.
In fact, the thalamus and its cortical connections exhibit functional aberrance in patients with depression. Previous research has identified a positive correlation between increased thalamic metabolism and the severity of depressive symptoms [127,128], while a decrease in thalamic metabolism has been observed during remission in depressed patients [129]. Furthermore, several studies have revealed altered thalamic connectivities in depression, including increased thalamic connectivities with the default-mode network [130,131], somatosensory cortex [132,133], temporal cortex [133], insula [134], and dorsolateral prefrontal cortex [135], as well as decreased thalamic connectivities with the anterior cingulate [130,131]. Importantly, thalamocortical interactions appear to play a critical role in the pathology of depression. A recent review demonstrated converging evidence of enhanced effective connections from the thalamus to various cortical regions, and reduced effective connections from other regions to the thalamus, suggesting that the thalamus is the key casual hub region for MDD [136]. Moreover, a study utilizing machine learning and advanced deep learning methods to distinguish MDD patients and healthy individuals in large resting-state fMRI datasets, identified thalamocortical hyperconnectivity as a specialized and critical neurophysiological signature in MDD [137]. Given the importance of thalamocortical circuits in arousal regulation, as previously discussed, it is proposed that hyperconnectivity of this circuit could be the subcortical–cortical neural mechanism underlying abnormal arousal regulation in depressed patients.
Except for the thalamus, several subcortical regions are related to arousal, such as the brainstem, basal forebrain, and hypothalamus, but few of them have been found to show altered connectivity in the cortex of depressed patients. Based on various evidence regarding aberrant thalamocortical connections in depression, it can be inferred that hyperconnectivity of thalamocortical circuits may play a significant role in hyperarousal and depressive symptoms (Figure 3).
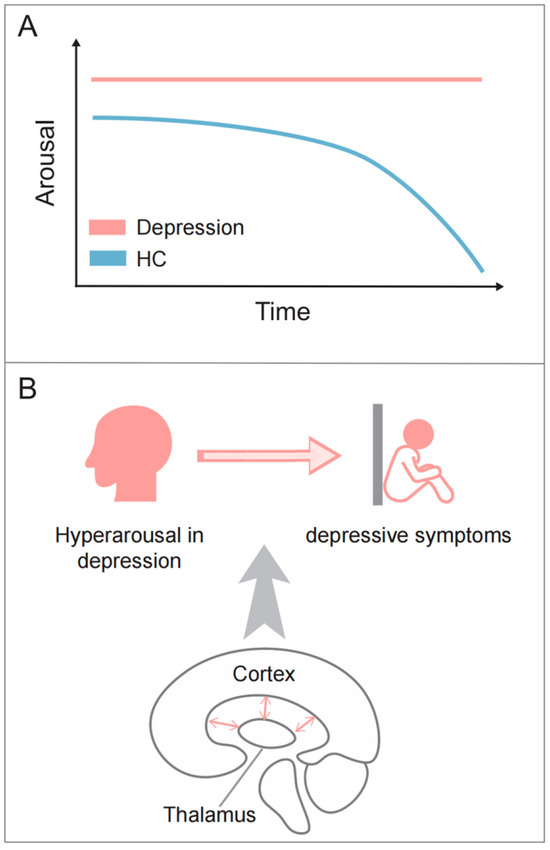
Figure 3.
The abnormal arousal pattern in depression. (A) During resting state, healthy individuals exhibit a progressive decline to lower arousal, while MDD patients often exhibit a hyperstable pattern of arousal regulation; (B) thalamocortical circuits may contribute to both the hyperarousal pattern and depressive symptoms.
5. Future Directions
Currently, converging findings have revealed hyperarousal in depression, as indicated by sleep difficulties [8,77,78], increase in HR [79,80,81,82,83,84,85,86,87], decrease in HRV [88,89], pupil diameters and skin conductance [87], and hyperactivity of the HPA axis [22,23,90,91,92,93,94,95,96,97,98,99,100,101] and locus coeruleus [102,103,104,105,106,107] in depressed individuals. Given the intimate involvement of thalamocortical circuits in physiological arousal, it is proposed that thalamocortical circuits could account for abnormal arousal regulation in depression. However, existing research has not yet elucidated how these abnormalities in thalamocortical circuits lead to hyperarousal regulation in depressed individuals. To further elucidate this relationship, advanced techniques such as simultaneous fMRI–EEG or fMRI–pupillometry could be further utilized to investigate the dynamics of thalamocortical interactions and their impact on arousal regulation in depression.
The thalamus possesses a complex neuroanatomy, comprising a variety of nuclei and including both excitatory and inhibitory neurons. Its connections to other brain areas are diverse, contributing to a wide range of cognitive and behavioral functions [138]. For instance, based on histological criteria, the thalamus is divided into distinctive nuclei, such as centromedial, ventral posterolateral, pulvinar, etc. [139]. Moreover, according to the patterns of afferent connection, excitatory thalamic nuclei could be divided into first-order nuclei (e.g., lateral geniculate nucleus), which receive driver input from subcortical regions, and higher-order nuclei (e.g., mediodorsal and pulvinar nuclei), which receive modulatory inputs from the cortex [138,140]. This classification, however, varies depending on the criteria used [139], suggesting the complex anatomy of thalamus and its cortical connections. A recent study found that, compared to normal controls, first-episode, drug-naïve MDD patients exhibit increased gray matter volume in specific thalamic nuclei, but not in the whole thalamus, suggesting heterogeneous alterations across thalamic nuclei [141]. Therefore, it is crucial to further investigate the interactions between various thalamic nuclei and the cortex in depressed individuals [142], in order to determine which of these interactions is related to abnormal arousal in depression.
Using rTMS that targets the dorsolateral prefrontal cortex (DLPFC) is a common non-pharmacological clinical treatment for MDD. However, recent studies indicate its limited effectiveness, with less than half of treatment-resistant MDD patients responding to DLPFC-rTMS [143]. Additionally, rTMS at other alternative targets near the DLPFC, such as the dorsomedial prefrontal cortex, did not shown improvements in depressive symptoms for treatment-refractory depression [144]. This underscores the urgent need to identify alternative rTMS targets, particularly for those with treatment-resistant depression. Intriguingly, rTMS at the left motor cortex, which has been proposed to be intimately associated with arousal systems [72], has recently been found to have comparable efficacy to DLPFC stimulation in MDD patients who show psychomotor retardation [145]. Given this context, we propose that the cortical regions connected to the thalamus are related to hyperarousal regulation in depression, and may be potential targets for rTMS in treating MDD patients.
Moreover, music intervention is increasingly used in depressed symptoms alleviation. Previous study has shown the impact that music has on stress levels in healthy individuals [146]. A recent meta-analysis investigating the effects of music interventions on depression confirmed their efficacy, both in terms of music medicine or music therapy [147]. Future research should explore whether the mechanisms underlying the effectiveness of music intervention are linked to arousal modulation, and can thereby contribute to the alleviation of depressive symptoms. This exploration could guide the development of more effective, non-invasive treatments.
In addition to non-invasive approaches, pharmacotherapy is another effective means of depression treatment. Recent studies have revealed ketamine’s effectiveness as an antidepressant in patients with treatment-resistant depression [148,149,150,151]. It should be noticed that, ketamine, commonly used as an anesthetic, is known to reduce arousal levels [152,153,154,155,156]. However, it remains unclear whether its antidepressant effects are linked to a reduction in thalamocortical connectivity in MDD. Future research is needed to determine whether ketamine alters thalamocortical interactions, and how such changes may contribute to its antidepressant effects. Should a link between thalamocortical connectivity and antidepressant effects be established, it could shed light on the development of targeted drug treatments in the future by focusing on their impact on thalamocortical connectivity.
Recent findings suggest that sex differences [157] should be considered in the neural mechanisms underlying the drug treatment. Specifically, hyperarousal in MDD exhibits sex differences [157], with women often experiencing more hyperarousal symptoms compared to men, a condition linked to excessive secretion of CRF [158,159]. Females show increased sensitivity to CRF in the LC due to sex differences in the CRF1 receptor, leading to increased cyclic adenosine monophosphate–protein kinase A (cyclic AMP–PKA) pathway signaling [160,161,162]. This increased response may contribute to increased hyperarousal symptoms in females [163,164,165]. In contrast, the CRF1 receptor in males preferentially binds to β-arrestin, leading to different signaling pathways and potentially mitigating hyperarousal in the context of CRF hypersecretion [160,166,167,168]. These findings indicate that sex-specific responses to stress and CRF regulation could promote the development of targeted drug treatments for MDD that take into account these sex differences.
6. Conclusions
In conclusion, converging evidence indicates a hyperarousal pattern in depressed patients, characterized by sleep disturbance, increased arousal-related biological markers, hyperactivity in the HPA axis and LC, and hyperstable EEG vigilance. Notably, there have been limited studies investigating the contribution of thalamocortical circuits to abnormal arousal in depression. This knowledge gap hinders our understanding of the neurobiological underpinnings of how abnormal arousal contributes to various depressive symptoms, which are phenomena involving complex cognitive functions and multiple cortical regions. By examining the critical role of thalamocortical connections in arousal regulation and MDD, we propose that hyperconnectivity of thalamocortical circuits could account for both the hyperarousal pattern and the related social difficulties in depressed patients. Future investigations should adopt advanced techniques, such as simultaneous fMRI–EEG or fMRI–pupillometry, to elucidate the relationship between thalamocortical interactions and hyperarousal in depression, specifically identifying which nuclei and their cortical interactions contribute to abnormal arousal. This could provide potential targets for rTMS treatment. As for other non-invasive treatments, music intervention shows promise; however, its mechanisms and the potential links with arousal need further investigation. In pharmacotherapy, the development of targeted drugs in treatment-resistant depression could consider modulating thalamocortical connectivity. Additionally, observed sex differences in the neurobiological underpinnings of hyperarousal in MDD should be considered in future hyperarousal and treatment research.
Author Contributions
Conceptualization, J.H. and P.Q.; investigation, M.X., Y.H., W.C., B.Z., H.H. and J.H.; writing—original draft preparation, M.X., Y.H., W.C., B.Z., H.H. and J.H.; writing—review and editing, M.X., Y.H., Q.L., P.Q. and J.H.; visualization, M.X. and Y.H.; supervision, J.H. and P.Q.; project administration, J.H. and P.Q.; funding acquisition, P.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32371098), the Major Program of the National Social Science Fund of China (18ZDA293), and Neuroeconomics Laboratory of Guangzhou Huashang College (2021WSYS002).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McCormick, D.A.; Nestvogel, D.B.; He, B.J. Neuromodulation of Brain State and Behavior. Annu. Rev. Neurosci. 2020, 43, 391–415. [Google Scholar] [CrossRef] [PubMed]
- Hegerl, U.; Sander, C.; Olbrich, S.; Schoenknecht, P. Are Psychostimulants a Treatment Option in Mania? Pharmacopsychiatry 2009, 42, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Hegerl, U.; Hensch, T. The Vigilance Regulation Model of Affective Disorders and ADHD. Neurosci. Biobehav. Rev. 2014, 44, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Hegerl, U.; Wilk, K.; Olbrich, S.; Schoenknecht, P.; Sander, C. Hyperstable Regulation of Vigilance in Patients with Major Depressive Disorder. World J. Biol. Psychiatry 2012, 13, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, S.; Sander, C.; Minkwitz, J.; Chittka, T.; Mergl, R.; Hegerl, U.; Himmerich, H. EEG Vigilance Regulation Patterns and Their Discriminative Power to Separate Patients with Major Depression from Healthy Controls. Neuropsychobiology 2012, 65, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Ulke, C.; Tenke, C.E.; Kayser, J.; Sander, C.; Böttger, D.; Wong, L.Y.X.; Alvarenga, J.E.; Fava, M.; McGrath, P.J.; Deldin, P.J.; et al. Resting EEG Measures of Brain Arousal in a Multisite Study of Major Depression. Clin. EEG Neurosci. 2019, 50, 3–12. [Google Scholar] [CrossRef]
- Armitage, R. Sleep and Circadian Rhythms in Mood Disorders. Acta Psychiatr. Scand. Suppl. 2007, 115, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Tsuno, N.; Besset, A.; Ritchie, K. Sleep and Depression. J. Clin. Psychiatry 2005, 66, 1254–1269. [Google Scholar] [CrossRef]
- Laureys, S. The Neural Correlate of (Un)Awareness: Lessons from the Vegetative State. Trends Cogn. Sci. 2005, 9, 556–559. [Google Scholar] [CrossRef]
- Owen, A.M. Improving Diagnosis and Prognosis in Disorders of Consciousness. Brain 2020, 143, 1050–1053. [Google Scholar] [CrossRef]
- Sulaman, B.A.; Wang, S.; Tyan, J.; Eban-Rothschild, A. Neuro-Orchestration of Sleep and Wakefulness. Nat. Neurosci. 2023, 26, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Scammell, T.E.; Arrigoni, E.; Lipton, J.O. Neural Circuitry of Wakefulness and Sleep. Neuron 2017, 93, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Goodale, S.E.; Ahmed, N.; Zhao, C.; de Zwart, J.A.; Özbay, P.S.; Picchioni, D.; Duyn, J.; Englot, D.J.; Morgan, V.L.; Chang, C. fMRI-Based Detection of Alertness Predicts Behavioral Response Variability. eLife 2021, 10, e62376. [Google Scholar] [CrossRef] [PubMed]
- van Kempen, J.; Loughnane, G.M.; Newman, D.P.; Kelly, S.P.; Thiele, A.; O’Connell, R.G.; Bellgrove, M.A. Behavioural and Neural Signatures of Perceptual Decision-Making Are Modulated by Pupil-Linked Arousal. eLife 2019, 8, e42541. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wen, R.; Fu, S.; Cheng, X.; Ren, S.; Lu, M.; Qian, L.; Luo, F.; Wang, Y.; Xiao, Q.; et al. Spatial Memory Requires Hypocretins to Elevate Medial Entorhinal Gamma Oscillations. Neuron, 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Foucher, J.R.; Otzenberger, H.; Gounot, D. Where Arousal Meets Attention: A Simultaneous fMRI and EEG Recording Study. Neuroimage 2004, 22, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Motelow, J.E.; Li, W.; Zhan, Q.; Mishra, A.M.; Sachdev, R.N.S.; Liu, G.; Gummadavelli, A.; Zayyad, Z.; Lee, H.S.; Chu, V.; et al. Decreased Subcortical Cholinergic Arousal in Focal Seizures. Neuron 2015, 85, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.; Bleich-Cohen, M.; Hahamy-Dubossarsky, A.; Dinstien, I.; Weizman, R.; Poyurovsky, M.; Kupchik, M.; Kotler, M.; Hendler, T.; Malach, R. Global Functional Connectivity Deficits in Schizophrenia Depend on Behavioral State. J. Neurosci. 2011, 31, 12972–12981. [Google Scholar] [CrossRef]
- Bonnet, M.H.; Arand, D.L. Hyperarousal and Insomnia: State of the Science. Sleep Med. Rev. 2010, 14, 9–15. [Google Scholar] [CrossRef]
- Riemann, D.; Spiegelhalder, K.; Feige, B.; Voderholzer, U.; Berger, M.; Perlis, M.; Nissen, C. The Hyperarousal Model of Insomnia: A Review of the Concept and Its Evidence. Sleep Med. Rev. 2010, 14, 19–31. [Google Scholar] [CrossRef]
- Arora, I.; Bellato, A.; Ropar, D.; Hollis, C.; Groom, M.J. Is Autonomic Function during Resting-State Atypical in Autism: A Systematic Review of Evidence. Neurosci. Biobehav. Rev. 2021, 125, 417–441. [Google Scholar] [CrossRef]
- Stetler, C.; Miller, G.E. Depression and Hypothalamic-Pituitary-Adrenal Activation: A Quantitative Summary of Four Decades of Research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Lightman, S.L. The HPA Axis in Major Depression: Classical Theories and New Developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Yoshida, S.; Jaiswal, M.K. Molecular Mechanism of Noradrenaline during the Stress-Induced Major Depressive Disorder. Neural Regen. Res. 2018, 13, 1159–1169. [Google Scholar] [PubMed]
- Bayne, T.; Hohwy, J.; Owen, A.M. Are There Levels of Consciousness? Trends Cogn. Sci. 2016, 20, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Poudel, G.R.; Innes, C.R.H.; Jones, R.D. Temporal Evolution of Neural Activity and Connectivity during Microsleeps When Rested and Following Sleep Restriction. Neuroimage 2018, 174, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Waschke, L.; Tune, S.; Obleser, J. Local Cortical Desynchronization and Pupil-Linked Arousal Differentially Shape Brain States for Optimal Sensory Performance. eLife 2019, 8, e51501. [Google Scholar] [CrossRef]
- Podvalny, E.; King, L.E.; He, B.J. Spectral Signature and Behavioral Consequence of Spontaneous Shifts of Pupil-Linked Arousal in Human. eLife 2021, 10, e68265. [Google Scholar] [CrossRef]
- Huang, J.; Ulke, C.; Strauss, M. Brain Arousal Regulation and Depressive Symptomatology in Adults with Attention-Deficit/Hyperactivity Disorder (ADHD). BMC Neurosci. 2019, 20, 43. [Google Scholar] [CrossRef]
- Yi, L.; Wang, Q.; Song, C.; Han, Z.R. Hypo- or Hyperarousal? The Mechanisms Underlying Social Information Processing in Autism. Child Dev. Perspect. 2022, 16, 215–222. [Google Scholar] [CrossRef]
- Satpute, A.B.; Kragel, P.A.; Barrett, L.F.; Wager, T.D.; Bianciardi, M. Deconstructing Arousal into Wakeful, Autonomic and Affective Varieties. Neurosci. Lett. 2019, 693, 19–28. [Google Scholar] [CrossRef]
- Meneguzzo, P.; Tsakiris, M.; Schioth, H.B.; Stein, D.J.; Brooks, S.J. Subliminal versus Supraliminal Stimuli Activate Neural Responses in Anterior Cingulate Cortex, Fusiform Gyrus and Insula: A Meta-Analysis of fMRI Studies. BMC Psychol. 2014, 2, 52. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, W.J.; Klerman, E.B. Circadian Neurobiology and the Physiologic Regulation of Sleep and Wakefulness. Neurol. Clin. 2019, 37, 475–486. [Google Scholar] [CrossRef] [PubMed]
- da Estrela, C.; McGrath, J.; Booij, L.; Gouin, J.-P. Heart Rate Variability, Sleep Quality, and Depression in the Context of Chronic Stress. Ann. Behav. Med. 2021, 55, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Gold, J.I. Pupil Size as a Window on Neural Substrates of Cognition. Trends Cogn. Sci. 2020, 24, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Posada-Quintero, H.F.; Bolkhovsky, J.B.; Qin, M.; Chon, K.H. Human Performance Deterioration Due to Prolonged Wakefulness Can Be Accurately Detected Using Time-Varying Spectral Analysis of Electrodermal Activity. Hum. Factors 2018, 60, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Meissner, S.N.; Bächinger, M.; Kikkert, S.; Imhof, J.; Missura, S.; Carro Dominguez, M.; Wenderoth, N. Self-Regulating Arousal via Pupil-Based Biofeedback. Nat. Hum. Behav. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Saper, C.B.; Fuller, P.M.; Pedersen, N.P.; Lu, J.; Scammell, T.E. Sleep State Switching. Neuron 2010, 68, 1023–1042. [Google Scholar] [CrossRef]
- Saper, C.B.; Chou, T.C.; Scammell, T.E. The Sleep Switch: Hypothalamic Control of Sleep and Wakefulness. Trends Neurosci. 2001, 24, 726–731. [Google Scholar] [CrossRef]
- Parvizi, J.; Damasio, A. Consciousness and the Brainstem. Cognition 2001, 79, 135–160. [Google Scholar] [CrossRef]
- West, C.H.K.; Ritchie, J.C.; Boss-Williams, K.A.; Weiss, J.M. Antidepressant Drugs with Differing Pharmacological Actions Decrease Activity of Locus Coeruleus Neurons. Int. J. Neuropsychopharmacol. 2009, 12, 627–641. [Google Scholar] [CrossRef]
- Grady, F.S.; Boes, A.D.; Geerling, J.C. A Century Searching for the Neurons Necessary for Wakefulness. Front. Neurosci. 2022, 16, 930514. [Google Scholar] [CrossRef]
- Neylan, T.C. Physiology of Arousal: Moruzzi and Magoun’s Ascending Reticular Activating System. J. Neuropsychiatry Clin. Neurosci. 1995, 7, 250. [Google Scholar] [PubMed]
- Wijdicks, E.F.M. The Ascending Reticular Activating System. Neurocrit. Care 2019, 31, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Lightman, S. The Human Stress Response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Tsigos, C.; Chrousos, G.P. Hypothalamic-Pituitary-Adrenal Axis, Neuroendocrine Factors and Stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Leistner, C.; Menke, A. Hypothalamic-pituitary-adrenal axis and stress. Handb. Clin. Neurol. 2020, 175, 55–64. [Google Scholar] [PubMed]
- Herrera, C.G.; Cadavieco, M.C.; Jego, S.; Ponomarenko, A.; Korotkova, T.; Adamantidis, A. Hypothalamic Feedforward Inhibition of Thalamocortical Network Controls Arousal and Consciousness. Nat. Neurosci. 2016, 19, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Venner, A.; Anaclet, C.; Broadhurst, R.Y.; Saper, C.B.; Fuller, P.M. A Novel Population of Wake-Promoting GABAergic Neurons in the Ventral Lateral Hypothalamus. Curr. Biol. 2016, 26, 2137–2143. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Hassani, O.K.; Jones, B.E. Discharge of Identified Orexin/Hypocretin Neurons across the Sleep-Waking Cycle. J. Neurosci. 2005, 25, 6716–6720. [Google Scholar] [CrossRef]
- Li, S.-B.; Borniger, J.C.; Yamaguchi, H.; Hédou, J.; Gaudilliere, B.; de Lecea, L. Hypothalamic Circuitry Underlying Stress-Induced Insomnia and Peripheral Immunosuppression. Sci. Adv. 2020, 6, eabc2590. [Google Scholar] [CrossRef]
- Xu, M.; Chung, S.; Zhang, S.; Zhong, P.; Ma, C.; Chang, W.-C.; Weissbourd, B.; Sakai, N.; Luo, L.; Nishino, S.; et al. Basal Forebrain Circuit for Sleep-Wake Control. Nat. Neurosci. 2015, 18, 1641–1647. [Google Scholar] [CrossRef]
- Zant, J.C.; Kim, T.; Prokai, L.; Szarka, S.; McNally, J.; McKenna, J.T.; Shukla, C.; Yang, C.; Kalinchuk, A.V.; McCarley, R.W.; et al. Cholinergic Neurons in the Basal Forebrain Promote Wakefulness by Actions on Neighboring Non-Cholinergic Neurons: An Opto-Dialysis Study. J. Neurosci. 2016, 36, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Boucetta, S.; Cissé, Y.; Mainville, L.; Morales, M.; Jones, B.E. Discharge Profiles across the Sleep-Waking Cycle of Identified Cholinergic, GABAergic, and Glutamatergic Neurons in the Pontomesencephalic Tegmentum of the Rat. J. Neurosci. 2014, 34, 4708–4727. [Google Scholar] [CrossRef] [PubMed]
- Anaclet, C.; Pedersen, N.P.; Ferrari, L.L.; Venner, A.; Bass, C.E.; Arrigoni, E.; Fuller, P.M. Basal Forebrain Control of Wakefulness and Cortical Rhythms. Nat. Commun. 2015, 6, 8744. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yin, D.; Wang, T.-X.; Guo, W.; Dong, H.; Xu, Q.; Luo, Y.-J.; Cherasse, Y.; Lazarus, M.; Qiu, Z.-L.; et al. Basal Forebrain Cholinergic Neurons Primarily Contribute to Inhibition of Electroencephalogram Delta Activity, Rather Than Inducing Behavioral Wakefulness in Mice. Neuropsychopharmacology 2016, 41, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Gent, T.C.; Bassetti, C.L.; Adamantidis, A.R. Sleep-Wake Control and the Thalamus. Curr. Opin. Neurobiol. 2018, 52, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, Y.; Yue, F.; Cheng, X.; Dang, R.; Qiao, Q.; Sun, X.; Li, X.; Jiang, Q.; Yao, J.; et al. The Paraventricular Thalamus Is a Critical Thalamic Area for Wakefulness. Science 2018, 362, 429–434. [Google Scholar] [CrossRef]
- Tasserie, J.; Uhrig, L.; Sitt, J.D.; Manasova, D.; Dupont, M.; Dehaene, S.; Jarraya, B. Deep Brain Stimulation of the Thalamus Restores Signatures of Consciousness in a Nonhuman Primate Model. Sci. Adv. 2022, 8, eabl5547. [Google Scholar] [CrossRef]
- Penzo, M.A.; Gao, C. The Paraventricular Nucleus of the Thalamus: An Integrative Node Underlying Homeostatic Behavior. Trends Neurosci. 2021, 44, 538–549. [Google Scholar] [CrossRef]
- Bordes, S.; Werner, C.; Mathkour, M.; McCormack, E.; Iwanaga, J.; Loukas, M.; Lammle, M.; Dumont, A.S.; Tubbs, R.S. Arterial Supply of the Thalamus: A Comprehensive Review. World Neurosurg. 2020, 137, 310–318. [Google Scholar] [CrossRef]
- Zhang, J.; Northoff, G. Beyond Noise to Function: Reframing the Global Brain Activity and Its Dynamic Topography. Commun. Biol. 2022, 5, 1350. [Google Scholar] [CrossRef]
- Liu, X.; de Zwart, J.A.; Schölvinck, M.L.; Chang, C.; Ye, F.Q.; Leopold, D.A.; Duyn, J.H. Subcortical Evidence for a Contribution of Arousal to fMRI Studies of Brain Activity. Nat. Commun. 2018, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Olafsson, V.; Tal, O.; Liu, T.T. The Amplitude of the Resting-State fMRI Global Signal Is Related to EEG Vigilance Measures. Neuroimage 2013, 83, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; DeYoung, P.N.; Liu, T.T. Differences in the Resting-State fMRI Global Signal Amplitude between the Eyes Open and Eyes Closed States Are Related to Changes in EEG Vigilance. Neuroimage 2016, 124, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, V.J.; Haanpää, H.; Kantola, J.-H.; Jauhiainen, J.; Vainionpää, V.; Alahuhta, S.; Tervonen, O. Midazolam Sedation Increases Fluctuation and Synchrony of the Resting Brain BOLD Signal. Magn. Reson. Imaging 2005, 23, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M.; Horovitz, S.G.; van Gelderen, P.; de Zwart, J.A.; Jansma, J.M.; Ikonomidou, V.N.; Chu, R.; Deckers, R.H.R.; Leopold, D.A.; Duyn, J.H. Large-Amplitude, Spatially Correlated Fluctuations in BOLD fMRI Signals during Extended Rest and Early Sleep Stages. Magn. Reson. Imaging 2006, 24, 979–992. [Google Scholar] [CrossRef]
- Chang, C.; Leopold, D.A.; Schölvinck, M.L.; Mandelkow, H.; Picchioni, D.; Liu, X.; Ye, F.Q.; Turchi, J.N.; Duyn, J.H. Tracking Brain Arousal Fluctuations with fMRI. Proc. Natl. Acad. Sci. USA 2016, 113, 4518–4523. [Google Scholar] [CrossRef]
- Akeju, O.; Loggia, M.L.; Catana, C.; Pavone, K.J.; Vazquez, R.; Rhee, J.; Contreras Ramirez, V.; Chonde, D.B.; Izquierdo-Garcia, D.; Arabasz, G.; et al. Disruption of Thalamic Functional Connectivity Is a Neural Correlate of Dexmedetomidine-Induced Unconsciousness. eLife 2014, 3, e04499. [Google Scholar] [CrossRef]
- Qin, P.; Wu, X.; Wu, C.; Wu, H.; Zhang, J.; Huang, Z.; Weng, X.; Zang, D.; Qi, Z.; Tang, W.; et al. Higher-Order Sensorimotor Circuit of the Brain’s Global Network Supports Human Consciousness. Neuroimage 2021, 231, 117850. [Google Scholar] [CrossRef]
- Kong, X.; Kong, R.; Orban, C.; Wang, P.; Zhang, S.; Anderson, K.; Holmes, A.; Murray, J.D.; Deco, G.; van den Heuvel, M.; et al. Sensory-Motor Cortices Shape Functional Connectivity Dynamics in the Human Brain. Nat. Commun. 2021, 12, 6373. [Google Scholar] [CrossRef]
- Liu, D.; Dan, Y. A Motor Theory of Sleep-Wake Control: Arousal-Action Circuit. Annu. Rev. Neurosci. 2019, 42, 27–46. [Google Scholar] [CrossRef]
- Redinbaugh, M.J.; Phillips, J.M.; Kambi, N.A.; Mohanta, S.; Andryk, S.; Dooley, G.L.; Afrasiabi, M.; Raz, A.; Saalmann, Y.B. Thalamus Modulates Consciousness via Layer-Specific Control of Cortex. Neuron 2020, 106, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhou, L.; Wu, H.; Huang, Y.; Qiu, M.; Huang, L.; Lee, C.; Lane, T.J.; Qin, P. Eyes-Open and Eyes-Closed Resting State Network Connectivity Differences. Brain Sci. 2023, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wu, X.; Wu, H.; Wang, D.; She, X.; Xie, M.; Zhang, F.; Zhang, D.; Zhang, X.; Qin, P. Eye-Opening Alters the Interaction Between the Salience Network and the Default-Mode Network. Neurosci. Bull. 2020, 36, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Montgomery, S.A.; Åsberg, M. A New Depression Scale Designed to Be Sensitive to Change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Mendlewicz, J. Sleep Disturbances: Core Symptoms of Major Depressive Disorder Rather than Associated or Comorbid Disorders. World J. Biol. Psychiatry 2009, 10, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Herbsleb, M.; Schumann, A.; Lehmann, L.; Gabriel, H.H.W.; Bär, K.-J. Cardio-Respiratory Fitness and Autonomic Function in Patients with Major Depressive Disorder. Front. Psychiatry 2020, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Volkers, A. Motor Activity and Autonomic Cardiac Functioning in Major Depressive Disorder. J. Affect. Disord. 2003, 76, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Koschke, M.; Boettger, M.K.; Schulz, S.; Berger, S.; Terhaar, J.; Voss, A.; Yeragani, V.K.; Bär, K.-J. Autonomy of Autonomic Dysfunction in Major Depression. Psychosom. Med. 2009, 71, 852–860. [Google Scholar] [CrossRef]
- Chang, C.-C.; Tzeng, N.-S.; Yeh, C.-B.; Kuo, T.B.J.; Huang, S.-Y.; Chang, H.-A. Effects of Depression and Melatonergic Antidepressant Treatment Alone and in Combination with Sedative–Hypnotics on Heart Rate Variability: Implications for Cardiovascular Risk. World J. Biol. Psychiatry 2018, 19, 368–378. [Google Scholar] [CrossRef]
- Chang, H.-A.; Chang, C.-C.; Chen, C.-L.; Kuo, T.B.J.; Lu, R.-B.; Huang, S.-Y. Major Depression Is Associated with Cardiac Autonomic Dysregulation. Acta Neuropsychiatr. 2012, 24, 318–327. [Google Scholar] [CrossRef]
- Berger, S.; Kliem, A.; Yeragani, V.; Bär, K.-J. Cardio-Respiratory Coupling in Untreated Patients with Major Depression. J. Affect. Disord. 2012, 139, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Schulz, S.; Kletta, C.; Voss, A.; Bär, K.-J. Autonomic Modulation in Healthy First-Degree Relatives of Patients with Major Depressive Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1723–1728. [Google Scholar] [CrossRef]
- Agelink, M.W.; Boz, C.; Ullrich, H.; Andrich, J. Relationship between Major Depression and Heart Rate Variability. Clinical Consequences and Implications for Antidepressive Treatment. Psychiatry Res. 2002, 113, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Schumann, A.; Andrack, C.; Bär, K.-J. Differences of Sympathetic and Parasympathetic Modulation in Major Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.H.; Quintana, D.S.; Gray, M.A.; Felmingham, K.L.; Brown, K.; Gatt, J.M. Impact of Depression and Antidepressant Treatment on Heart Rate Variability: A Review and Meta-Analysis. Biol. Psychiatry 2010, 67, 1067–1074. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, Y.; Zhang, Y.; Chen, L.; Zou, Y.; Xiao, J.; Min, W.; Yuan, C.; Ye, Y.; Li, M.; et al. Heart Rate Variability in Generalized Anxiety Disorder, Major Depressive Disorder and Panic Disorder: A Network Meta-Analysis and Systematic Review. J. Affect. Disord. 2023, 330, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Plotsky, P.M.; Owens, M.J.; Nemeroff, C.B. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr. Clin. N. Am. 1998, 21, 293–307. [Google Scholar]
- Gibbons, J.L.; McHugh, P.R. Plasma Cortisol in Depressive Illness. J. Psychiatr. Res. 1962, 1, 162–171. [Google Scholar] [CrossRef]
- Sachar, E.J. Disrupted 24-Hour Patterns of Cortisol Secretion in Psychotic Depression. Arch. Gen. Psychiatry 1973, 28, 19. [Google Scholar] [CrossRef]
- Linkowski, P.; Mendlewicz, J.; Leclercq, R.; Brasseur, M.; Hubain, P.; Golstein, J.; Copinschi, G.; Cauter, E.V. The 24-Hour Profile of Adrenocorticotropin and Cortisol in Major Depressive Illness. J. Clin. Endocrinol. Metab. 1985, 61, 429–438. [Google Scholar] [CrossRef]
- Carpenter, W.T., Jr.; Bunney, W.E., Jr. Adrenal Cortical Activity in Depressive Illness. Am. J. Psychiatry 1971, 128, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Paslakis, G.; Krumm, B.; Gilles, M.; Schweiger, U.; Heuser, I.; Richter, I.; Deuschle, M. Discrimination between Patients with Melancholic Depression and Healthy Controls: Comparison between 24-h Cortisol Profiles, the DST and the Dex/CRH Test. Psychoneuroendocrinology 2011, 36, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso, S.; Duval, F.; Mokrani, M.-C.; Schaltenbrand, N.; Castro, J.O.; Crocq, M.-A.; Macher, J.-P. Growth Hormone Response to Clonidine and the Cortisol Response to Dexamethasone in Depressive Patients. Psychiatry Res. 1996, 60, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.J.; Curtis, G.C.; Davies, B.M.; Mendels, J.; Sugerman, A.A. Urinary Free Cortisol Excretion in Depression. Psychol. Med. 1976, 6, 43–50. [Google Scholar] [CrossRef]
- Sachar, E.J. Cortisol Production in Depressive Illness: A Clinical and Biochemical Clarification. Arch. Gen. Psychiatry 1970, 23, 289. [Google Scholar] [CrossRef] [PubMed]
- Pintor, L.; Torres, X.; Navarro, V.; Martinez De Osaba, M.J.; Matrai, S.; Gastó, C. Corticotropin-Releasing Factor Test in Melancholic Patients in Depressed State versus Recovery: A Comparative Study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 1027–1033. [Google Scholar] [CrossRef]
- Maes, M.; Claes, M.; Vandewoude, M.; Schotte, C.; Martin, M.; Blockx, P.; Cosyns, P. Adrenocorticotropin Hormone, β-Endorphin and Cortisol Responses to oCRF in Melancholic Patients. Psychol. Med. 1992, 22, 317–329. [Google Scholar] [CrossRef]
- Rubin, R.T. Adrenal Gland Volume in Major Depression: Increase during the Depressive Episode and Decrease with Successful Treatment. Arch. Gen. Psychiatry 1999, 52, 213–218. [Google Scholar] [CrossRef]
- Wong, M.-L.; Kling, M.A.; Munson, P.J.; Listwak, S.; Licinio, J.; Prolo, P.; Karp, B.; McCutcheon, I.E.; Geracioti, T.D.; DeBellis, M.D.; et al. Pronounced and Sustained Central Hypernoradrenergic Function in Major Depression with Melancholic Features: Relation to Hypercortisolism and Corticotropin-Releasing Hormone. Proc. Natl. Acad. Sci. USA 2000, 97, 325–330. [Google Scholar] [CrossRef]
- Veith, R.C. Sympathetic Nervous System Activity in Major Depression: Basal and Desipramine-Induced Alterations in Plasma Norepinephrine Kinetics. Arch. Gen. Psychiatry 1994, 51, 411. [Google Scholar] [CrossRef]
- Egami, M.; Imamura, Y.; Nabeta, H.; Mizoguchi, Y.; Yamada, S. Saliva Levels of 3-methoxy-4-hydroxyphenylglycol and Clinical Efficacy of Mirtazapine or Selective Serotonin Reuptake Inhibitors in Patients with Major Depression. Hum. Psychopharmacol. 2013, 28, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Chandley, M.; Szebeni, K.; Szebeni, A.; Crawford, J.; Stockmeier, A.; Turecki, G.; Miguel-Hidalgo, J.; Ordway, G. Gene Expression Deficits in Pontine Locus Coeruleus Astrocytes in Men with Major Depressive Disorder. J. Psychiatry Neurosci. 2013, 38, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Chandley, M.J.; Szebeni, A.; Szebeni, K.; Crawford, J.D.; Stockmeier, C.A.; Turecki, G.; Kostrzewa, R.M.; Ordway, G.A. Elevated Gene Expression of Glutamate Receptors in Noradrenergic Neurons from the Locus Coeruleus in Major Depression. Int. J. Neuropsychopharmacol. 2014, 17, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-Y.; Klimek, V.; Dilley, G.E.; Haycock, J.W.; Stockmeier, C.; Overholser, J.C.; Meltzer, H.Y.; Ordway, G.A. Elevated Levels of Tyrosine Hydroxylase in the Locus Coeruleus in Major Depression. Biol. Psychiatry 1999, 46, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.T.; Blier, P. Effect of the Selective Noradrenergic Reuptake Inhibitor Reboxetine on the Fring Activity of Noradrenaline and Serotonin Neurons. Eur. J. Neurosci. 2001, 13, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.M.; Weiss, J.M. Effects of Chronic Antidepressant Drug Administration and Electroconvulsive Shock on Locus Coeruleus Electrophysiologic Activity. Biol. Psychiatry 2001, 49, 117–129. [Google Scholar] [CrossRef]
- Surova, G.; Ulke, C.; Schmidt, F.M.; Hensch, T.; Sander, C.; Hegerl, U. Fatigue and Brain Arousal in Patients with Major Depressive Disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 527–536. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Sander, C.; Dietz, M.-E.; Nowak, C.; Schröder, T.; Mergl, R.; Schönknecht, P.; Himmerich, H.; Hegerl, U. Brain Arousal Regulation as Response Predictor for Antidepressant Therapy in Major Depression. Sci. Rep. 2017, 7, 45187. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Pschiebl, A.; Sander, C.; Kirkby, K.C.; Thormann, J.; Minkwitz, J.; Chittka, T.; Weschenfelder, J.; Holdt, L.M.; Teupser, D.; et al. Impact of Serum Cytokine Levels on EEG-Measured Arousal Regulation in Patients with Major Depressive Disorder and Healthy Controls. Neuropsychobiology 2016, 73, 1–9. [Google Scholar] [CrossRef]
- Ulke, C.; Wittekind, D.A.; Spada, J.; Franik, K.; Jawinski, P.; Hensch, T.; Hegerl, U. Brain Arousal Regulation in SSRI-Medicated Patients with Major Depression. J. Psychiatr. Res. 2019, 108, 34–39. [Google Scholar] [CrossRef]
- Ulke, C.; Sander, C.; Jawinski, P.; Mauche, N.; Huang, J.; Spada, J.; Wittekind, D.; Mergl, R.; Luck, T.; Riedel-Heller, S.; et al. Sleep Disturbances and Upregulation of Brain Arousal during Daytime in Depressed versus Non-Depressed Elderly Subjects. World J. Biol. Psychiatry 2017, 18, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Ip, C.-T.; Ganz, M.; Dam, V.H.; Ozenne, B.; Rüesch, A.; Köhler-Forsberg, K.; Jørgensen, M.B.; Frokjaer, V.G.; Søgaard, B.; Christensen, S.R.; et al. NeuroPharm Study: EEG Wakefulness Regulation as a Biomarker in MDD. J. Psychiatr. Res. 2021, 141, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wittekind, D.A.; Spada, J.; Gross, A.; Hensch, T.; Jawinski, P.; Ulke, C.; Sander, C.; Hegerl, U. Early Report on Brain Arousal Regulation in Manic vs Depressive Episodes in Bipolar Disorder. Bipolar Disord. 2016, 18, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Elklit, A.; Chen, Y.Y.; Ghazali, S.R.; Shevlin, M. Sex Differences in PTSD Symptoms: A Differential Item Functioning Approach. Psychol. Trauma 2019, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Van Sweden, B. Disturbed Vigilance in Mania. Biol. Psychiatry 1986, 21, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, I.; Mellman, T.A. Gender Differences in Sleep during the Aftermath of Trauma and the Development of Posttraumatic Stress Disorder. Behav. Sleep Med. 2012, 10, 180–190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suffel, A.; Nagels, A.; Steines, M.; Kircher, T.; Straube, B. Feeling Addressed! The Neural Processing of Social Communicative Cues in Patients with Major Depression. Hum. Brain Mapp. 2020, 41, 3541–3554. [Google Scholar] [CrossRef]
- Steriade, M.; McCormick, D.A.; Sejnowski, T.J. Thalamocortical Oscillations in the Sleeping and Aroused Brain. Science 1993, 262, 679–685. [Google Scholar] [CrossRef]
- Steriade, M. Arousal--Revisiting the Reticular Activating System. Science 1996, 272, 225. [Google Scholar] [CrossRef]
- Schiff, N.D. Central Thalamic Contributions to Arousal Regulation and Neurological Disorders of Consciousness. Ann. N. Y. Acad. Sci. 2008, 1129, 105–118. [Google Scholar] [CrossRef]
- Gent, T.C.; Bandarabadi, M.; Herrera, C.G.; Adamantidis, A.R. Thalamic Dual Control of Sleep and Wakefulness. Nat. Neurosci. 2018, 21, 974–984. [Google Scholar] [CrossRef] [PubMed]
- David, F.; Schmiedt, J.T.; Taylor, H.L.; Orban, G.; Di Giovanni, G.; Uebele, V.N.; Renger, J.J.; Lambert, R.C.; Leresche, N.; Crunelli, V. Essential Thalamic Contribution to Slow Waves of Natural Sleep. J. Neurosci. 2013, 33, 19599–19610. [Google Scholar] [CrossRef] [PubMed]
- Setzer, B.; Fultz, N.E.; Gomez, D.E.; Williams, S.D.; Bonmassar, G.; Polimeni, J.R.; Lewis, L.D. A Temporal Sequence of Thalamic Activity Unfolds at Transitions in Behavioral Arousal State. Nat. Commun. 2022, 13, 5442. [Google Scholar] [CrossRef] [PubMed]
- Neumeister, A.; Nugent, A.C.; Waldeck, T.; Geraci, M.; Schwarz, M.; Bonne, O.; Bain, E.E.; Luckenbaugh, D.A.; Herscovitch, P.; Charney, D.S. Neural and Behavioral Responses to Tryptophan Depletion in Unmedicatedpatients with Remitted Major Depressive Disorder and Controls. Arch. Gen. Psychiatry 2004, 61, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, D.D.; Weiss, A.P.; Cosgrove, G.R.; Alpert, N.M.; Cassem, E.H.; Nierenberg, A.A.; Price, B.H.; Mayberg, H.S.; Fischman, A.J.; Rauch, S.L. Cerebral Metabolic Correlates as Potential Predictors of Response to Anterior Cingulotomy for Treatment of Major Depression. J. Neurosurg. 2003, 99, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Holthoff, V.A.; Beuthien-Baumann, B.; Zündorf, G.; Triemer, A.; Lüdecke, S.; Winiecki, P.; Koch, R.; Füchtner, F.; Herholz, K. Changes in Brain Metabolism Associated with Remission in Unipolar Major Depression. Acta Psychiatr. Scand. 2004, 110, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Flores, B.H.; Menon, V.; Glover, G.H.; Solvason, H.B.; Kenna, H.; Reiss, A.L.; Schatzberg, A.F. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol. Psychiatry 2007, 62, 429–437. [Google Scholar] [CrossRef]
- Anand, A.; Li, Y.; Wang, Y.; Wu, J.; Gao, S.; Bukhari, L.; Mathews, V.P.; Kalnin, A.; Lowe, M.J. Activity and Connectivity of Brain Mood Regulating Circuit in Depression: A Functional Magnetic Resonance Study. Biol. Psychiatry 2005, 57, 1079–1088. [Google Scholar] [CrossRef]
- Kang, L.; Zhang, A.; Sun, N.; Liu, P.; Yang, C.; Li, G.; Liu, Z.; Wang, Y.; Zhang, K. Functional Connectivity between the Thalamus and the Primary Somatosensory Cortex in Major Depressive Disorder: A Resting-State fMRI Study. BMC Psychiatry 2018, 18, 339. [Google Scholar] [CrossRef]
- Brown, E.C.; Clark, D.L.; Hassel, S.; MacQueen, G.; Ramasubbu, R. Thalamocortical Connectivity in Major Depressive Disorder. J. Affect. Disord. 2017, 217, 125–131. [Google Scholar] [CrossRef]
- Wang, C.; Wu, H.; Chen, F.; Xu, J.; Li, H.; Li, H.; Wang, J. Disrupted Functional Connectivity Patterns of the Insula Subregions in Drug-Free Major Depressive Disorder. J. Affect. Disord. 2018, 234, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, R.; Lv, L.; Qi, X.; Shi, J.; Xie, S. Correlation between Cognitive Deficits and Dorsolateral Prefrontal Cortex Functional Connectivity in First-Episode Depression. J. Affect. Disord. 2022, 312, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xiao, K.; Ao, Y.; Cui, Q.; Jing, X.; Wang, Y. The Thalamus Is the Causal Hub of Intervention in Patients with Major Depressive Disorder: Evidence from the Granger Causality Analysis. NeuroImage Clin. 2022, 37, 103295. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; El-Gazzar, A.; Zhutovsky, P.; Thomas, R.M.; Javaheripour, N.; Li, M.; Bartova, L.; Bathula, D.; Dannlowski, U.; Davey, C. Functional Connectivity Signatures of Major Depressive Disorder: Machine Learning Analysis of Two Multicenter Neuroimaging Studies. Mol. Psychiatry 2023, 28, 3013–3022. [Google Scholar] [CrossRef] [PubMed]
- Shine, J.M.; Lewis, L.D.; Garrett, D.D.; Hwang, K. The Impact of the Human Thalamus on Brain-Wide Information Processing. Nat. Rev. Neurosci. 2023, 24, 416–430. [Google Scholar] [CrossRef]
- Halassa, M.M.; Sherman, S.M. Thalamocortical Circuit Motifs: A General Framework. Neuron 2019, 103, 762–770. [Google Scholar] [CrossRef]
- Sherman, S.M.; Guillery, R.W. The Role of the Thalamus in the Flow of Information to the Cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Li, M.; Liu, Z.; Li, X.; Huai, H.; Jia, D.; Jin, W.; Zhao, Z.; Liu, L.; Li, J. Heterogeneous Alterations in Thalamic Subfields in Major Depression Disorder. J. Affect. Disord. 2021, 295, 1079–1086. [Google Scholar] [CrossRef]
- Kong, Q.-M.; Qiao, H.; Liu, C.-Z.; Zhang, P.; Li, K.; Wang, L.; Li, J.-T.; Su, Y.; Li, K.-Q.; Yan, C.-G. Aberrant Intrinsic Functional Connectivity in Thalamo-cortical Networks in Major Depressive Disorder. CNS Neurosci. Ther. 2018, 24, 1063–1072. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.E.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J. Effectiveness of Theta Burst versus High-Frequency Repetitive Transcranial Magnetic Stimulation in Patients with Depression (THREE-D): A Randomised Non-Inferiority Trial. Lancet 2018, 391, 1683–1692. [Google Scholar] [CrossRef]
- Dunlop, K.; Sheen, J.; Schulze, L.; Fettes, P.; Mansouri, F.; Feffer, K.; Blumberger, D.M.; Daskalakis, Z.J.; Kennedy, S.H.; Giacobbe, P. Dorsomedial Prefrontal Cortex Repetitive Transcranial Magnetic Stimulation for Treatment-Refractory Major Depressive Disorder: A Three-Arm, Blinded, Randomized Controlled Trial. Brain Stimul. 2020, 13, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-T.; Hu, X.-W.; Han, J.-F.; Zhang, J.-F.; Wang, Y.-Y.; Wolff, A.; Tremblay, S.; Hirjak, D.; Tan, Z.-L.; Northoff, G. Motor Cortex Repetitive Transcranial Magnetic Stimulation in Major Depressive Disorder-A Preliminary Randomized Controlled Clinical Trial. J. Affect. Disord. 2024, 344, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Paszkiel, S.; Dobrakowski, P.; Łysiak, A. The Impact of Different Sounds on Stress Level in the Context of EEG, Cardiac Measures and Subjective Stress Level: A Pilot Study. Brain Sci. 2020, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Huang, Z.; Zhou, H.; Ye, P. Effects of Music Therapy on Depression: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2020, 15, e0240862. [Google Scholar] [CrossRef] [PubMed]
- Moda-Sava, R.N.; Murdock, M.H.; Parekh, P.K.; Fetcho, R.N.; Huang, B.S.; Huynh, T.N.; Witztum, J.; Shaver, D.C.; Rosenthal, D.L.; Alway, E.J. Sustained Rescue of Prefrontal Circuit Dysfunction by Antidepressant-Induced Spine Formation. Science 2019, 364, eaat8078. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Shinohara, R.; Fogaça, M.V.; Hare, B. Neurobiology of Rapid-Acting Antidepressants: Convergent Effects on GluA1-Synaptic Function. Mol. Psychiatry 2019, 24, 1816–1832. [Google Scholar] [CrossRef] [PubMed]
- Smith-Apeldoorn, S.Y.; Veraart, J.K.; Spijker, J.; Kamphuis, J.; Schoevers, R.A. Maintenance Ketamine Treatment for Depres-sion: A Systematic Review of Efficacy, Safety, and Tolerability. Lancet Psychiatry 2022, 9, 907–921. [Google Scholar] [CrossRef]
- Zanos, P.; Gould, T. Mechanisms of Ketamine Action as an Antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar] [CrossRef]
- Huang, Z.; Mashour, G.A.; Hudetz, A.G. Functional Geometry of the Cortex Encodes Dimensions of Consciousness. Nat. Commun. 2023, 14, 72. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Wu, J.; Mashour, G.A.; Hudetz, A.G. Temporal Circuit of Macroscale Dynamic Brain Activity Supports Human Consciousness. Sci. Adv. 2020, 6, eaaz0087. [Google Scholar] [CrossRef]
- Bonhomme, V.; Vanhaudenhuyse, A.; Demertzi, A.; Bruno, M.-A.; Jaquet, O.; Bahri, M.A.; Plenevaux, A.; Boly, M.; Boveroux, P.; Soddu, A.; et al. Resting-State Network-Specific Breakdown of Functional Connectivity during Ketamine Alteration of Consciousness in Volunteers. Anesthesiology 2016, 125, 873–888. [Google Scholar] [CrossRef]
- Sarasso, S.; Boly, M.; Napolitani, M.; Gosseries, O.; Charland-Verville, V.; Casarotto, S.; Rosanova, M.; Casali, A.G.; Brichant, J.-F.; Boveroux, P.; et al. Consciousness and Complexity during Unresponsiveness Induced by Propofol, Xenon, and Ketamine. Curr. Biol. 2015, 25, 3099–3105. [Google Scholar] [CrossRef]
- Mashour, G.A.; Hudetz, A.G. Neural Correlates of Unconsciousness in Large-Scale Brain Networks. Trends Neurosci. 2018, 41, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Bangasser, D.A.; Cuarenta, A. Sex Differences in Anxiety and Depression: Circuits and Mechanisms. Nat. Rev. Neurosci. 2021, 22, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Plante, D.T.; Landsness, E.C.; Peterson, M.J.; Goldstein, M.R.; Riedner, B.A.; Wanger, T.; Guokas, J.J.; Tononi, G.; Benca, R.M. Sex-Related Differences in Sleep Slow Wave Activity in Major Depressive Disorder: A High-Density EEG Investigation. BMC Psychiatry 2012, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Nolen-Hoeksema, S.; Larson, J.; Grayson, C. Explaining the Gender Difference in Depressive Symptoms. J. Pers. Soc. Psychol. 1999, 77, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Bangasser, D.A.; Curtis, A.; Reyes, B.A.S.; Bethea, T.T.; Parastatidis, I.; Ischiropoulos, H.; Van Bockstaele, E.J.; Valentino, R.J. Sex Differences in Corticotropin-Releasing Factor Receptor Signaling and Trafficking: Potential Role in Female Vulnerability to Stress-Related Psychopathology. Mol. Psychiatry 2010, 15, 877, 896–904. [Google Scholar] [CrossRef]
- Curtis, A.L.; Grigoriadis, D.E.; Page, M.E.; Rivier, J.; Valentino, R.J. Pharmacological Comparison of Two Corticotro-pin-Releasing Factor Antagonists: In Vivo and In Vitro Studies. J. Pharmacol. Exp. Ther. 1994, 268, 359–365. [Google Scholar]
- Valentino, R.J.; Page, M.E.; Curtis, A.L. Activation of Noradrenergic Locus Coeruleus Neurons by Hemodynamic Stress Is Due to Local Release of Corticotropin-Releasing Factor. Brain. Res. 1991, 555, 25–34. [Google Scholar] [CrossRef]
- Valentino, R.J.; Bangasser, D.A. Sex-Biased Cellular Signaling: Molecular Basis for Sex Differences in Neuropsychiatric Diseases. Dialogues Clin. Neurosci. 2016, 18, 385–393. [Google Scholar] [CrossRef]
- Valentino, R.J.; Bangasser, D.; Van Bockstaele, E.J. Sex-Biased Stress Signaling: The Corticotropin-Releasing Factor Receptor as a Model. Mol. Pharmacol. 2013, 83, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Bangasser, D.A.; Eck, S.R.; Telenson, A.M.; Salvatore, M. Sex Differences in Stress Regulation of Arousal and Cognition. Physiol. Behav. 2018, 187, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Bangasser, D.A.; Dong, H.; Carroll, J.; Plona, Z.; Ding, H.; Rodriguez, L.; McKennan, C.; Csernansky, J.G.; Seeholzer, S.H.; Valentino, R.J. Corticotropin-Releasing Factor Overexpression Gives Rise to Sex Differences in Alzheimer’s Disease-Related Signaling. Mol. Psychiatry 2017, 22, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Violin, J.D.; Lefkowitz, R.J. Beta-Arrestin-Biased Ligands at Seven-Transmembrane Receptors. Trends Pharmacol. Sci. 2007, 28, 416–422. [Google Scholar] [CrossRef]
- Lefkowitz, R.J.; Shenoy, S.K. Transduction of Receptor Signals by Beta-Arrestins. Science 2005, 308, 512–517. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).