Abstract
In recent years, repetitive transcranial magnetic stimulation (rTMS) has received much attention as a non-invasive, effective treatment modality for mild cognitive impairment (MCI). Although several meta-analyses have reported that rTMS can improve cognitive abilities, improvements in individual memory domains (speech, language, concentration, and memory) are poorly understood. In addition, stimulation parameters may be flawed in studies of global populations because of ethnic differences between Caucasians and Asians. This meta-analysis aimed to systematically characterize the efficacy of different combinations of rTMS parameters on different cognitive domains in Caucasian patients with MCI. We conducted a systematic literature search in Medline PubMed, Pubpsych, and Embase on the use of rTMS in MCI patients through November 2022. Randomized, double-blind, and sham-controlled trials (RCTs) from the Caucasian patient population were included. The studies reported outcome measures for different domains of cognition, such as language, concentration, or memory. Possible effects of covariates were examined using meta-regressions. The search yielded five publications. The analyses found that rTMS improved cognitive functions, memory, concentration, and language in patients with MCI and treatment with rTMS compared with the sham stimulation group. The statistical analysis results of the studies showed that rTMS could improve various cognitive functions, such as memory and concentration, in Caucasian MCI patients. A particular effect was found at a frequency of 10 Hz and stimulation of the LDLPFC. However, further studies are needed to validate these findings and explore more effective stimulation protocols and targets.
1. Introduction
Mild cognitive impairment (MCI) is an intermediate stage between normal aging and dementia [1,2]. The prevalence of MCI is between 16 and 20% for people over 65 years [1,2,3]. In about 20% of all patients, the mild impairment progresses to manifest dementia within one year [1,2,3]. Various studies show that currently used medications are ineffective in alleviating MCI symptoms [2,4]. Therefore, MCI is a prevalent, disabling, and challenging illness for which new treatment options are needed [2,4]. In recent years, repetitive transcranial magnetic stimulation (rTMS) has proven to be a promising new treatment approach. Current research suggests that alternating between high and low-frequency rTMS can significantly improve memory function over the long term. However, the efficacy of rTMS in patients with MCI has not been fully elucidated due to the low statistical power and heterogeneity of previous studies [1,2]. Several recent meta-analyses have examined various rTMS effects on cognition in patients with Alzheimer’s disease. The studies mainly included patients with prodromal Alzheimer’s disease, i.e., MCI or amnestic MCI (aMCI), and Alzheimer’s [5,6,7]. A recent meta-analysis reported on the effect of rTMS on cognition in patients with MCI, although the publication did not refer exclusively to randomized controlled trials (RCTs) [8]. From our knowledge, only one meta-analysis has exclusively investigated patients worldwide with MCI [9]. Although various studies have frequently reported that treatment with rTMS can improve cognition in MCI patients, the optimal stimulation protocols and application parameters are poorly understood [1,2,3,4,5,6,7,8,9]. Here, the rTMS frequency is one of the main factors affecting cortical activity [10]. However, there is no consensus on the optimal number of rTMS pulses required to achieve cortical excitability.
In addition, evidence points to inherent differences in cortical plasticity between Caucasians and Asians. Also, there is evidence that there appear to be differences between rTMS measures and, respectively, outcomes of different ethnic groups [11,12,13]. Likewise, studies are often so heterogeneous that various tests are used to assess overall cognition. This heterogeneity often hampers the assessment of performance in different cognitive domains. Although several meta-analyses have reported that rTMS can improve cognition in MCI patients [14,15,16], the improvements in specific cognitive domains are poorly understood. In addition, stimulation parameters in studies of unselected samples may be flawed because of ethnic differences between Caucasians and Asians. Due to the heterogeneity of the different studies, this meta-analysis is based on strict inclusion criteria depending on published RCTs in the European population. The pooled effects of rTMS were analyzed to assess the impact and safety of rTMS on depression and various cognitive functions in memory, concentration, speech, and language in Caucasian patients with MCI.
2. Materials and Methods
The present study conducted a systematic literature search on rTMS in MCI patients in November 2022. The study was registered in the internal study center of Paracelsus Medical University (registration number: FMS_FP_051.23-XI-1). The databases used were Medline PubMed, Pubpsych, and Embase, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17]. Following search terms for PubMed: (“MCI” OR “mild cognitive impairment” OR “cognition” OR “cogn”) AND (“rTMS” OR “transcranial magnet stimulation” OR “TBS” OR “theta burst stimulation”). An equivalent search was performed in Pubpsych using the following search terms: (“MCI” OR “mild cognitive impairment” OR “cognition” OR “cogn”) AND (“rTMS” OR “transcranial magnet stimulation” OR “TBS” OR “theta burst stimulation”). In Embase, we used the following search terms: (“MCI” OR “mild cognitive impairment” OR “cognition” OR “cogn”) AND (“rTMS” OR “transcranial magnet stimulation” OR “TBS” OR “theta burst stimulation”). Studies on humans were included if published between 1 January 2000 and 31 December 2022. All references found were downloaded into Zotero, and duplicates were deleted. The reference lists of the identified articles were screened for additional publications, which were added if applicable.
Inclusion Criteria: (1) randomized controlled studies investigating the effects of rTMS on cognitive function in Caucasian patients with MCI; (2) participants diagnosed with MCI based on any diagnostic criteria, such as the Mini-Mental Status Test or the Petersen criteria [18,19]; (3) the experimental group received rTMS treatment; (4) the control group received sham rTMS stimulation; and (5) outcomes included the global cognitive ability and specific cognitive domains as determined by neuropsychological tests in which a mean and a standard deviation or confidence interval was given for each test result.
Titles and abstracts, followed by full texts, were reviewed by authors, and hits were scored according to the PICO scheme and PRISMA checklist for studies [17,20]. Inconclusive judgments were resolved by consensus. Articles were classified for the first author, year of publication, number of participants, mean age, standard deviation, using cognitive tests, rTMS site, rTMS frequency, rTMS intensity, number of given pulses, and days of treatment. If test results were reported at different time points, the data were labeled for each time point: T1 = test results before any rTMS, T2 = test results immediately after finishing the whole rTMS session. Because the interval between cognitive testing differed in the various studies, additional testing time points were designated later as T3 and T4 (Table 1). To further investigate the factors influencing the effects of rTMS on overall cognitive outcomes, the following four subgroup analyses were conducted: effects on (1) depression, (2) memory, (3) concentration, and (4) speech and language.

Table 1.
Description of the studies included in the final sample. The studies were chosen as specified in the method section. Abbreviations: SD = standard deviation, P = parallel study design, C = cross-over study design, LDLPFC = left dorsolateral prefrontal cortex, RIFG = right inferior frontal gyrus, # = no testing.
The effect of rTMS on cognitive function in patients with MCI has been defined based on Hedges’ [21] standardized mean difference (SMD). Based on this, multivariate random-effect models were computed [22,23]. As the mean difference in the change in cognitive outcome measures depends on different influencing variables, we considered three SMD: (a) rTMS—control group (treatment), (b) final—baseline (time point), and (c) T2—baseline (time point). Given the various cognitive tests used in the included studies, a categorization according to (1)–(4) was used to summarize the pooled effect sizes of the eligible studies. All subtests described in different studies were distributed as follows: Beck’s depression inventory II, geriatric depression scale, and brief neuropsychological test battery = (1) depression. Rivermead behavioral memory test, logical memory test, story recall test, list recall test, rey auditory–verbal learning test, list-learning test, story memory test, figure-copy test, digit-span memory test, and mini-mental state test = (2) memory. Letter-number-sequencing test, trail-making test A and B, line orientation test, attentive matrices test, and Stroop effect test = (3) concentration. Verbal-fluency animal-naming test, picture-naming test, semantic fluency test, phonemic verbal fluency test, and semantic verbal fluency test = (4) speech and language. Other tests that could not be assigned to a category are described individually. For the calculation of the models, the direction of action of individual outcomes was adjusted so that all outcomes indicate a healthier status at higher values. This was necessary because not all tests indicate improvement at higher values. Depending on the measurement instrument, lower values can also show an improvement. Pre-, post-, and follow-up stimulus scale scores, if published, were quoted for each group. In addition, we performed funnel plots to evaluate publication bias. We used the software R (R Core Team R version 4.3.1) [24] and the meta-analysis package metafor [25] for all calculations. In this analysis, the p-value was considered significant at p < 0.05. Thus, the significance level was 0.05.
3. Results
3.1. Findings of the Literature Search
The number of publications found and selected during the process is indicated in Figure 1. We retrieved a total number of 1069 studies from the selected databases and removed 85 duplicates. In addition, 818 articles were excluded because MCI was not the topic of the studies. The remaining 166 were further assessed for eligibility. A total of 32 studies were excluded after reading their titles and abstracts because no RCT was conducted. In addition, we excluded 127 studies after full-text reading because MCI was not the main topic. Finally, seven studies [26,27,28,29,30,31,32] were included in the analysis. The mean values and standard deviations of the test results were missing in the two included studies. The meta-analysis was, therefore, performed based on the remaining five studies [26,27,28,29,32], fulfilling the criteria for data extraction and analysis (Figure 1). The baseline characteristics of the included studies are presented in Table 1.
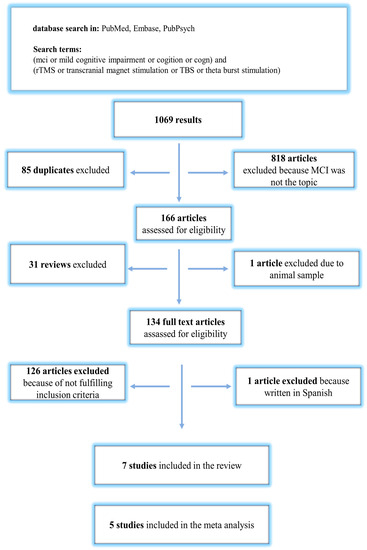
Figure 1.
Flow chart of publication search and selection process.
Published data were available for five studies included in the present meta-analysis. In total, 60 participants were randomly assigned to the active rTMS group, while the number of participants randomly assigned to the sham rTMS group was 64. Only one study stimulated the right hemisphere [31]. The remaining studies used a stimulation above the left dorsolateral prefrontal cortex (LDLPFC). The used frequencies varied between 5 [25,28] and 10 Hz [26,27,31]. The stimulation intensity was between 80 and 120% of the resting motor threshold. The number of pulses ranged between 500 and 3000 from 1 to 30 days of treatment. Follow-up tests were performed between 0.5 and 190 days after the baseline measurements before the first rTMS (T1). Similar stimulation parameters were applied in the control group. The coils were placed vertically for sham stimulation, or a special sham stimulation coil was used instead.
3.2. Subgroup Analysis
3.2.1. Comparison between the rTMS and Control Group
The results are shown in Table 2. There is no evidence of bias in the funnel plot generated (Figure 2). The meta-analyzed studies showed no significant improvement or worsening in depressive symptoms, concentration, or speech and language compared to the control group at any time points T1–T4/T3. In the subgroup memory, there was a tendency for significant improvement in the rTMS group compared with the control group at time point T2 (estimate = 0.237, 95% CI = [−0.059, 0.533]). In the further course, a significant improvement in the rTMS group at time T3 became apparent (estimate = 0.422, 95% CI = [0.065, 0.780]). At time point T4, there was a positive estimate, but it was no longer significant with a p-value of 0.178.

Table 2.
Comparison between the rTMS and control group in the categories (1)–(4) at any time point T1–T4. SE = standard error, 95% CI = 95% confidence interval.
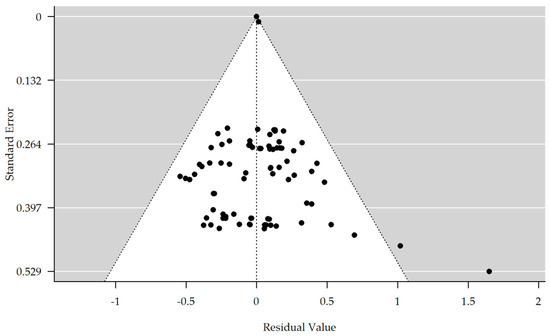
Figure 2.
Funnel plot showing no significant publication bias (rank correlation test for funnel plot asymmetry: Kendall’s tau = 0.071, p-value = 0.346).
3.2.2. Comparison between Different Time Points T1 to T4/T3 in the rTMS and Control Group
The results are shown in Table 3. There is no evidence of bias in the funnel plot generated (Figure 3). The meta-analyzed studies showed no significant improvement or worsening between the T1 and T4/T3 time points in depressive symptoms, concentration, or speech and language. However, an interesting result was obtained concerning apathy. Here, a significant improvement of the symptomatology could be achieved in comparison from time T1 to time T4/T3 in the rTMS group (estimate = 1.981, 95% CI = [0.064, 3.898]). Also, in memory, there was a significant improvement in the rTMS group compared to time points T1 to T4 (estimate = 0.596, 95% CI = [0.138, 1.055]). The meta-analyzed studies for the control group showed no significant improvement or worsening between the time points T1 and T4 in apathy, memory, or concentration. However, a significant improvement in the categories of depression (estimate = 0.723, 95% CI = [−0.045, 1.400]), speech, and language (estimate = 0.828, 95% CI = [0.133, 1.524]) could be observed in comparison from time T1 to time T4 in the control group.

Table 3.
Comparison between time points T1 to T4/T3 for the rTMS group and control group. SE = standard error, 95% CI = 95% confidence interval.
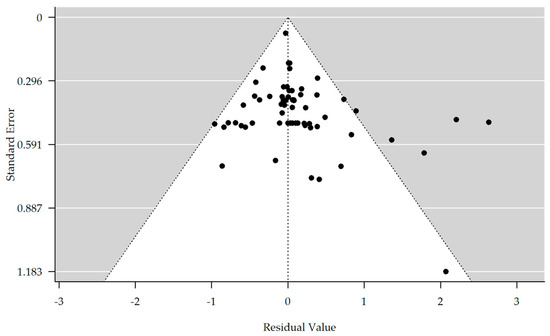
Figure 3.
Funnel plot showing no significant publication bias (rank correlation test for funnel plot asymmetry: Kendall’s tau = 0.148, p-value = 0.085).
3.2.3. Other Effects on Memory and Concentration in the rTMS and Control Group
In addition, we analyzed the possible effects of rTMS site, rTMS frequency, rTMS intensity, and number of given pulses on the category’s memory and concentration in the rTMS group and control group of T1 compared with T4. In the rTMS group, there was a significant improvement in the memory parameters with treatment over the left hemisphere (estimate = 0.796, 95% CI = [0.373, 1.218]), but no significant improvement over the right hemisphere (estimate = −0.223, 95% CI = [−1.248, 0.802]). Similar results were analyzed in the control group (Table 4). In the rTMS group, when treated over the left hemisphere, there was no significant improvement or deterioration in concentration compared with treatment over the right hemisphere. Similar results were seen in the control group (Table 5). When considering the different frequencies, there was no significant improvement or deterioration in memory, either in the rTMS group or in the control group of T1 compared with T4 (Table 6).

Table 4.
Comparison between time points for the category memory and stimulation site from T1 to T4/T3 for the rTMS and control groups.

Table 5.
Comparison between time points for the category concentration and stimulation site from T1 to T4/T3 for the rTMS and control groups.

Table 6.
Comparison between time points for the category memory and frequency from T1 to T4/T3 for the rTMS and control groups.
In the rTMS group, there was a significant worsening in concentration between T1 and T4 at a frequency of 5 Hz (estimate = −0.897, 95% CI = [−1.782, −0.012]). There were no differences at other stimulation frequencies in the rTMS or the control group (Table 7). Considering the different stimulation intensities, memory was significantly improved when stimulated at and above 100% in the rTMS group compared to the control groups at timepoint T1 compared with T4 (Table 8).

Table 7.
Comparison between time points for the category concentration and frequency from T1 to T4/T3 for the rTMS and control groups.

Table 8.
Comparison between time points for the category memory and intensity from T1 to T4/T3 for the rTMS and control groups.
The number of pulses impacted memory: The memory improved in the rTMS group after stimulation with 1500, 2000, and 3000 pulses compared with the control groups at timepoint T1 compared with T4 (Table 9).

Table 9.
Comparison between time points for the category memory and number of pulses from T1 to T4/T3 for the rTMS and control groups.
Also, for the number of treatment days, the memory category improved after treatment longer than ten days at T1 compared to T4 in both the rTMS and the control groups (Table 10). In the according funnel plots, no significant publication bias was recognized.

Table 10.
Comparison between time points for the category memory and days of pulses from T1 to T4/T3 for the rTMS and control groups.
4. Discussion
The conducted literature search has yielded a total of seven publications encompassing Caucasian patients diagnosed with MCI.
It is important to note that the foundation of this review rests upon a relatively modest base of seven papers.
For calculation basis, sufficient data sets were available for five publications with 60 Caucasian patients. The outcome indicators in the rTMS group were compared with those in the control group, who received only sham rTMS.
The analyses revealed that rTMS improved cognitive functioning, especially in the category of memory. This effect was more pronounced when applying rTMS over the left DLPFC. According to research evidence, it is known that the DLPFC is involved in regulating executive functions such as working memory and cognitive flexibility [33,34]. One possible explanation is that the left DLPFC, in particular, is connected to other regions, forming one of the essential areas in the central executive network. Evidence shows that by regulating brain networks, rTMS can improve working memory in patients [33]. Likewise, it is conceivable that stimulation of the DLPFC may improve emotional feelings in patients, indirectly allowing memory improvement. Probably, the same can be assumed for the category concentration [34,35].
In the present meta-analysis, no significant improvement in depressive symptoms was detected. However, this could be due to the heterogeneous test procedures and the small sample sizes in the included studies. Another reason may be the difficulty of distinguishing memory disorders in depressed patients (pseudodementia). Another bias could be associated with pharmacological therapy that could influence the cognition and the symptoms of depression. The diagnosis of depression should be made after a clinical interview, including the psychometric diagnosis. In studies included in our analysis, the depressive symptoms have been evaluated chiefly solely by questionnaires, which could also be a source of bias. Concentration and memory disturbances are present both in depressive disorders and in MCI.
The rTMS frequency is one of the main factors affecting cortical activity. However, studies suggest that the number of pulses may also influence the regulation of brain excitability [35,36,37]. However, there is no consensus on the optimal number of rTMS pulses required to achieve cortical excitability. According to our meta-analysis, memory improved after stimulation with pulse values of 1500 and above. Further studies should be conducted to determine the benefits of rTMS on cognition with different numbers of pulses in patients with MCI.
These effects may be due to the limited number of available studies, especially in the Caucasian population. Specifically, only five MCI studies were identified with Caucasians, compared to five studies analyzed in studies with Asians [9]. Second, the ceiling effect of the cognitive tests can potentially limit the ability to detect changes [37] in performance before and after rTMS, particularly in the MCI population. Lastly, not all studies examine MCI populations with precisely the same age and gender. A comparison of rTMS’s effect on global cognition between MCI patients and younger individuals with cognitive deficits of the same gender could be interesting. In line with this, the differential impact of rTMS on functional networks could affect organization and associative memory in young and older adults in the Caucasian population.
Also, a few limitations should be considered when interpreting our study’s results. Using different scales to measure global cognition across other papers likely contributes to the high heterogeneity. There were only a small number of studies on MCI patients. More rTMS studies, specifically in the MCI population, will be needed to confirm the efficacy of this method. Lastly, because our study focused on immediate outcomes, future studies will be required to investigate the long-term clinical utility of rTMS on various effects in the MCI population.
Further studies should be conducted to determine the benefits of rTMS on the emotion and cognition function, especially in patients with MCI. In the future, new research results could also address the question of individual differences between Caucasians and Asians regarding plasticity during brain stimulation. Although the effects of rTMS on cognition showed some sustained effects after treatment in the included articles, this meta-analysis cannot fully address the sustainability of the impact. Studies with larger sample sizes are required to determine the best stimulation targets for rTMS that yield optimal emotional and cognitive improvement.
Author Contributions
Conceptualization, C.L.; methodology, S.H., C.v.M. and C.L.; software, S.H. and C.v.M.; validation, S.H., C.v.M. and C.L.; formal analysis, S.H. and C.v.M.; investigation, S.H., C.v.M. and C.L.; resources, S.H., C.v.M. and C.L.; data curation, S.H. and C.v.M.; writing—original draft preparation, C.L.; writing—review and editing, C.L.; visualization, S.H., C.v.M. and C.L.; supervision, H.L., T.H. and K.R.; project administration, C.L.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Theo und Friedl Schöller-Stiftung, implementation as a special project, funder/coordinator: Beatrix Jauch, funding number: 972201.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luis, C.; Loewenstein, D.; Acevedo, A.; Barker, W.; Duara, R. Mild cognitive impairment: Directions for future research. Neurology 2003, 61, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Knopman, D.S. Classification and epidemiology of MCI. Clin. Geriatr. Med. 2013, 29, 753–772. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Lipnicki, D.M.; Kochan, N.A.; Crawford, J.D.; Thalamuthu, A.; Andrews, G.; Brayne, C.; Matthews, F.E.; Stephan, B.C.M.; Lipton, R.B.; et al. The Prevalence of Mild Cognitive Impairment in Diverse Geographical and Ethnocultural Regions: The COSMIC Collaboration. PLoS ONE 2015, 10, e0142388. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments in Alzheimer Disease: An Update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Bonnì, S.; Pellicciari, M.C.; Casula, E.P.; Mancini, M.; Esposito, R.; Ponzo, V.; Picazio, S.; Di Lorenzo, F.; Serra, L.; et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. NeuroImage 2018, 169, 302–311. [Google Scholar] [CrossRef]
- Cui, H.; Ren, R.; Lin, G.; Zou, Y.; Jiang, L.; Wei, Z.; Li, C.; Wang, G. Repetitive Transcranial Magnetic Stimulation Induced Hypoconnectivity Within the Default Mode Network Yields Cognitive Improvements in Amnestic Mild Cognitive Impairment: A Randomized Controlled Study. J. Alzheimer’s Dis. 2019, 69, 1137–1151. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, W.-J.; Shan, P.-Y.; Lu, M.; Wang, T.; Li, R.-H.; Zhang, N.; Ma, L. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer’s disease: A systematic review and meta-analysis. J. Neurol. Sci. 2019, 398, 184–191. [Google Scholar] [CrossRef]
- Jiang, L.; Cui, H.; Zhang, C.; Cao, X.; Gu, N.; Zhu, Y.; Wang, J.; Yang, Z.; Li, C. Repetitive Transcranial Magnetic Stimulation for Improving Cognitive Function in Patients with Mild Cognitive Impairment: A Systematic Review. Front. Aging Neurosci. 2021, 12, 593000. [Google Scholar] [CrossRef]
- Zhang, X.; Lan, X.; Chen, C.; Ren, H.; Guo, Y. Effects of Repetitive Transcranial Magnetic Stimulation in Patients with Mild Cognitive Impairment: A Meta-Analysis of Randomized Controlled Trials. Front. Hum. Neurosci. 2021, 15, 723715. [Google Scholar] [CrossRef]
- Moscatelli, F.; Toto, G.A.; Valenzano, A.; Cibelli, G.; Monda, V.; Limone, P.; Mancini, N.; Messina, A.; Marsala, G.; Messina, G.; et al. High frequencies repetitive transcranial magnetic stimulation (rTMS) increase motor coordination performances in volleyball players. BMC Neurosci. 2023, 24, 30. [Google Scholar] [CrossRef]
- Hao, Q.; Wong, L.K.; Lin, W.H.; Leung, T.W.; Kaps, M.; Rosengarten, B. Ethnic Influences on Neurovascular Coupling. Stroke 2010, 41, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Kochunov, P.; Fox, P.; Lancaster, J.; Tan, L.H.; Amunts, K.; Zilles, K.; Mazziotta, J.; Gao, J.H. Localized morphological brain differences between English-speaking Caucasians and Chinese-speaking Asians: New evidence of anatomical plasticity. NeuroReport 2003, 14, 961. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Fisher, K.M.; Lai, M.; Mansoor, K.; Bicker, R.; Baker, S.N. Differences between Han Chinese and Caucasians in transcranial magnetic stimulation parameters. Exp. Brain Res. 2014, 232, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Li, C.T.; Brunoni, A.R.; Yang, F.C.; Tseng, P.T.; Tu, Y.K.; Stubbs, B.; Carvalho, A.F.; Thompson, T.; Yeh, T.C.; et al. Cognitive effects and acceptability of non-invasive brain stimulation on Alzheimer’s disease and mild cognitive impairment: A component network meta-analysis. J. Neurol. Neurosurg. Psychiatry 2021, 92, 195–203. [Google Scholar] [CrossRef]
- Chou, Y.; Ton That, V.; Sundman, M. A systematic review and meta-analysis of rTMS effects on cognitive enhancement in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2020, 86, 1–10. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Y.; Nie, L.; Zhang, W.; Ke, Z.; Ku, Y. Cognitive Enhancement of Repetitive Transcranial Magnetic Stimulation in Patients With Mild Cognitive Impairment and Early Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Cell Dev. Biol. 2021, 9, 734046. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Kokmen, E.; Tangelos, E.G. Aging, memory, and mild cognitive impairment. Int. Psychogeriatr. 1997, 9 (Suppl. S1), 65–69. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- PICO: Model for Clinical Questions. Available online: https://www.cochrane.de/lit_vortrag_einf%C3%BChrung_literaturrecherche (accessed on 26 July 2023).
- Hedges, L.V. Distribution Theory for Glass’s Estimator of Effect size and Related Estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Kalaian, H.A.; Raudenbush, S.W. A multivariate mixed linear model for meta-analysis. Psychol. Methods 1996, 1, 227–235. [Google Scholar] [CrossRef]
- Berkey, C.S.; Hoaglin, D.C.; Antczak-Bouckoms, A.; Mosteller, F.; Colditz, G.A. Meta-analysis of multiple outcomes by regression with random effects. Stat. Med. 1998, 17, 2537–2550. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 7 May 2023).
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Roque Roque, G.Y.; Reyes-López, J.V.; Ricardo Garcell, J.; López Hidalgo, M.; Aguilar Fabré, L.; Trejo Cruz, G.; Cañizares Gómez, S.; Calderón Moctezuma, A.R.; Ortega Cruz, F.; Ortíz Baron, A.; et al. Effect of transcranial magnetic stimulation as an enhancer of cognitive stimulation sessions on mild cognitive impairment: Preliminary results. Psychiatry Res. 2021, 304, 114151. [Google Scholar] [CrossRef] [PubMed]
- Padala, P.R.; Padala, K.P.; Lensing, S.Y.; Jackson, A.N.; Hunter, C.R.; Parkes, C.M.; Dennis, R.A.; Bopp, M.M.; Caceda, R.; Mennemeier, M.S.; et al. Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: A double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res. 2018, 261, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Drumond Marra, H.L.; Myczkowski, M.L.; Maia Memória, C.; Arnaut, D.; Leite Ribeiro, P.; Sardinha Mansur, C.G.; Alberto, R.L.; Bellini, B.B.; da Silva, A.A.F.; Tortella, G.; et al. Transcranial magnetic stimulation to address mild cognitive impairment in the elderly: A randomized controlled study. Behav. Neurol. 2015, 2015, 287843. [Google Scholar] [CrossRef] [PubMed]
- Solé-Padullés, C.; Bartrés-Faz, D.; Junqué, C.; Clemente, I.C.; Molinuevo, J.L.; Bargalló, N.; Sánchez-Aldeguer, J.; Bosch, B.; Falcón, C.; Valls-Solé, J. Repetitive transcranial magnetic stimulation effects on brain function and cognition among elders with memory dysfunction. A randomized sham-controlled study. Cereb Cortex 2006, 16, 1487–1493. [Google Scholar] [CrossRef]
- Esposito, S.; Trojsi, F.; Cirillo, G.; de Stefano, M.; Di Nardo, F.; Siciliano, M.; Caiazzo, G.; Ippolito, D.; Ricciardi, D.; Buonanno, D.; et al. Repetitive Transcranial Magnetic Stimulation (rTMS) of Dorsolateral Prefrontal Cortex May Influence Semantic Fluency and Functional Connectivity in Fronto-Parietal Network in Mild Cognitive Impairment (MCI). Biomedicines 2022, 10, 994. [Google Scholar] [CrossRef]
- Cirillo, G.; Pepe, R.; Siciliano, M.; Ippolito, D.; Ricciardi, D.; de Stefano, M.; Buonanno, D.; Atripaldi, D.; Abbadessa, S.; Perfetto, B.; et al. Long-Term Neuromodulatory Effects of Repetitive Transcranial Magnetic Stimulation (rTMS) on Plasmatic Matrix Metalloproteinases (MMPs) Levels and Visuospatial Abilities in Mild Cognitive Impairment (MCI). Int. J. Mol. Sci. 2023, 24, 3231. [Google Scholar] [CrossRef]
- Eliasova, I.; Anderkova, L.; Marecek, R.; Rektorova, I. Non-invasive brain stimulation of the right inferior frontal gyrus may improve attention in early Alzheimer’s disease: A pilot study. J. Neurol. Sci. 2014, 346, 318–322. [Google Scholar] [CrossRef]
- Fox, M.D.; Buckner, R.L.; Liu, H.; Chakravarty, M.M.; Lozano, A.M.; Pascual-Leone, A. Resting-state networks link invasive and non-invasive brain stimulation across diverse psychiatric and neurological diseases. Proc. Natl. Acad. Sci. USA 2014, 111, E4367–E4375. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.G.; Jerskey, B.A.; Robertson, E.M.; Brenninkmeyer, C.; Ozdemir, E.; Leone, A.P. The effects of repetitive transcranial magnetic stimulation (rTMS) on procedural memory and dysphoric mood in patients with major depressive disorder. Cogn. Behav. Neurol. 2005, 18, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Maeda, F.; Keenan, J.P.; Tormos, J.M.; Topka, H.; Pascual-Leone, A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp. Brain Res. 2000, 133, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Peinemann, A.; Reimer, B.; Löer, C.; Quartarone, A.; Münchau, A.; Conrad, B.; Siebner, H.R. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin. Neurophysiol. 2004, 115, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.J.; Wendell, C.R.; Giggey, P.P.; Katzel, L.I.; Lefkowitz, D.M.; Siegel, E.L.; Waldstein, S.R. Psychometric limitations of the mini-mental state examination among nondemented older adults: An evaluation of neurocognitive and magnetic resonance imaging correlates. Exp. Aging Res. 2013, 39, 382–397. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).