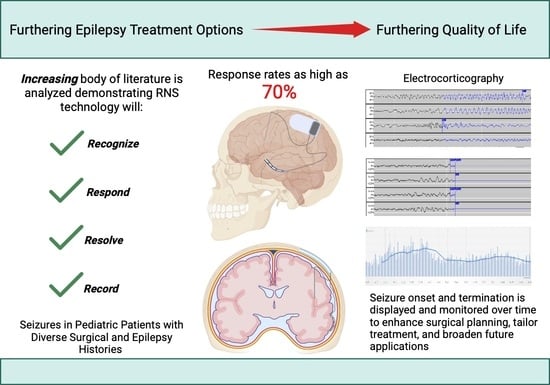
The Utility of Responsive Neurostimulation for the Treatment of Pediatric Drug-Resistant Epilepsy
Abstract
:1. Introduction
2. Common Neuromodulation Techniques
3. RNS Placement and Proposed Mechanism
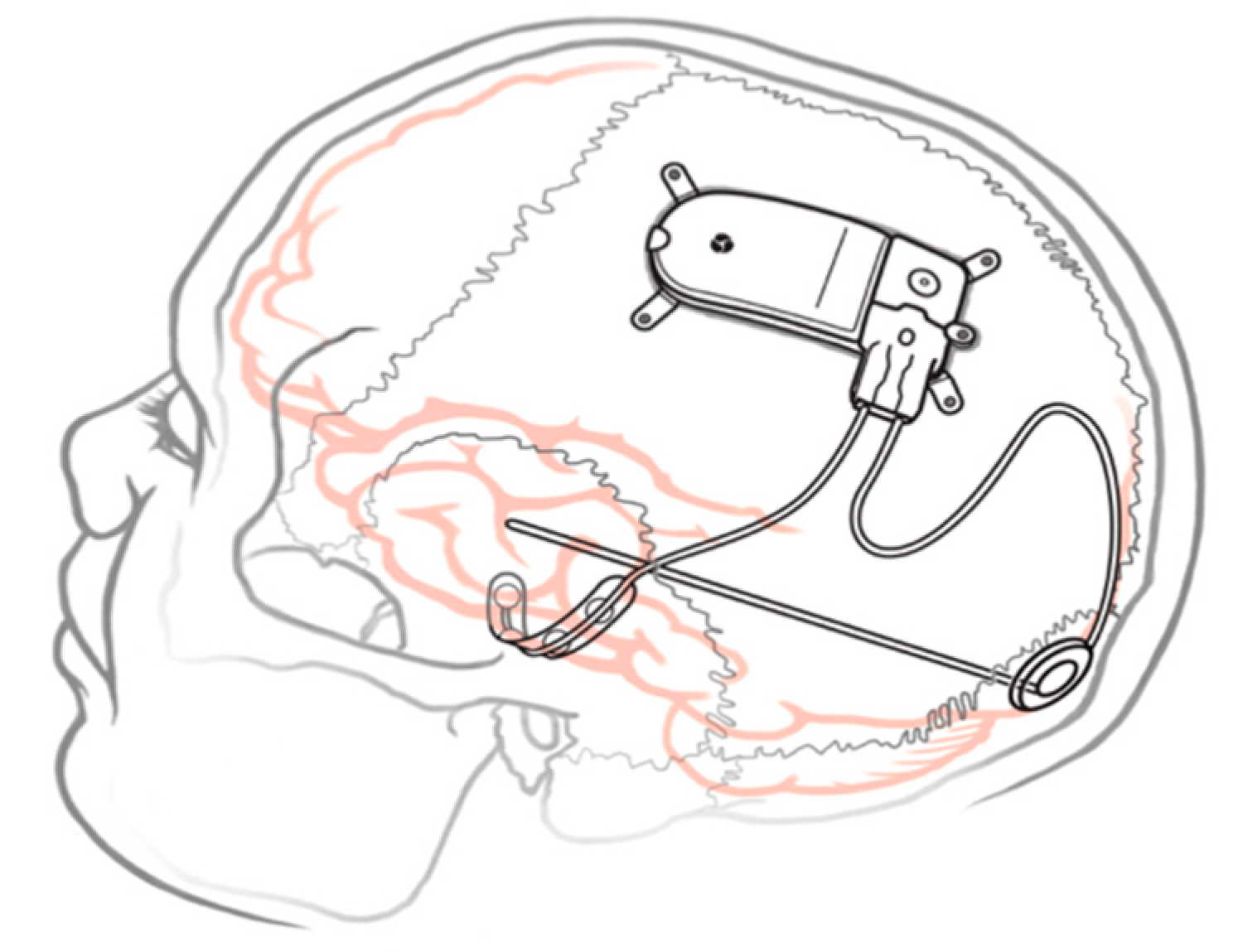
3.1. Cortical RNS
3.2. Subcortical RNS
4. Effectiveness and Clinical Indications
5. RNS in Pediatric Patients
6. Complication Rates and Pediatric Considerations
7. Future Uses for RNS at Large
8. Long-Term Quality of Life with RNS and Effects on the Developing Brain
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hatoum, R.; Nathoo-Khedri, N.; Shlobin, N.A.; Wang, A.; Weil, A.G.; Fallah, A. Barriers to Epilepsy Surgery in Pediatric Patients: A Scoping Review. Seizure 2022, 102, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Zack, M.; Kobau, R. National and State Estimates of the Numbers of Adults and Children with Active Epilepsy—United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.F.; Coffield, E.; Leroy, Z.; Wallin, R. Prevalence and Costs of Five Chronic Conditions in Children. J. Sch. Nurs. 2016, 32, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of Drug Resistant Epilepsy: Consensus Proposal by the Ad Hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2009, 51, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Brodie, M.J. Early Identification of Refractory Epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Kalilani, L.; Sun, X.; Pelgrims, B.; Noack-Rink, M.; Villanueva, V. The Epidemiology of Drug-Resistant Epilepsy: A Systematic Review and Meta-Analysis. Epilepsia 2018, 59, 2179–2193. [Google Scholar] [CrossRef]
- Yan, H.; Ibrahim, G.M. Resective Epilepsy Surgery Involving Eloquent Cortex in the Age of Responsive Neurostimulation: A Value-Based Decision-Making Framework. Epilepsy Behav. 2019, 99, 106479. [Google Scholar] [CrossRef]
- Brodie, M.J.; Barry, S.J.E.; Bamagous, G.A.; Norrie, J.D.; Kwan, P. Patterns of Treatment Response in Newly Diagnosed Epilepsy. Neurology 2012, 78, 1548–1554. [Google Scholar] [CrossRef]
- Anderson, M.; Egunsola, O.; Cherrill, J.; Millward, C.; Fakis, A.; Choonara, I. A Prospective Study of Adverse Drug Reactions to Antiepileptic Drugs in Children. BMJ Open 2015, 5, e008298. [Google Scholar] [CrossRef]
- Choonara, I. Anti-Epileptic Drug Toxicity in Children. Children 2018, 5, 57. [Google Scholar] [CrossRef]
- Dwivedi, R.; Ramanujam, B.; Chandra, P.S.; Sapra, S.; Gulati, S.; Kalaivani, M.; Garg, A.; Bal, C.S.; Tripathi, M.; Dwivedi, S.N.; et al. Surgery for Drug-Resistant Epilepsy in Children. N. Engl. J. Med. 2017, 377, 1639–1647. [Google Scholar] [CrossRef]
- Jehi, L.; Jette, N.; Kwon, C.-S.; Josephson, C.B.; Burneo, J.G.; Cendes, F.; Sperling, M.R.; Baxendale, S.; Busch, R.M.; Triki, C.C.; et al. Timing of Referral to Evaluate for Epilepsy Surgery: Expert Consensus Recommendations from the Surgical Therapies Commission of the International League Against Epilepsy. Epilepsia 2022, 63, 2491–2506. [Google Scholar] [CrossRef] [PubMed]
- Englot, D.J. Lesional Epilepsy in Children: Removing Doubt to Cut It Out. Epilepsy Curr. 2023, 23, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Piper, R.J.; Richardson, R.M.; Worrell, G.; Carmichael, D.W.; Baldeweg, T.; Litt, B.; Denison, T.; Tisdall, M.M. Towards Network-Guided Neuromodulation for Epilepsy. Brain 2022, 145, 3347–3362. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.T.; Morrell, M.J. The RNS System: Responsive Cortical Stimulation for the Treatment of Refractory Partial Epilepsy. Expert Rev. Med. Devices 2014, 11, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Starnes, K.; Miller, K.; Wong-Kisiel, L.; Lundstrom, B.N. A Review of Neurostimulation for Epilepsy in Pediatrics. Brain Sci. 2019, 9, 283. [Google Scholar] [CrossRef]
- Morris, G.L.; Mueller, W.M. Long-Term Treatment with Vagus Nerve Stimulation in Patients with Refractory Epilepsy. Neurology 1999, 53, 1731. [Google Scholar] [CrossRef]
- Fisher, R.; Salanova, V.; Witt, T.; Worth, R.; Henry, T.; Gross, R.; Oommen, K.; Osorio, I.; Nazzaro, J.; Labar, D.; et al. Electrical Stimulation of the Anterior Nucleus of Thalamus for Treatment of Refractory Epilepsy. Epilepsia 2010, 51, 899–908. [Google Scholar] [CrossRef]
- Lundstrom, B.N.; Osman, G.M.; Starnes, K.; Gregg, N.M.; Simpson, H.D. Emerging Approaches in Neurostimulation for Epilepsy. Curr. Opin. Neurol. 2023, 36, 69–76. [Google Scholar] [CrossRef]
- Touma, L.; Dansereau, B.; Chan, A.Y.; Jetté, N.; Kwon, C.; Braun, K.P.J.; Friedman, D.; Jehi, L.; Rolston, J.D.; Vadera, S.; et al. Neurostimulation in People with Drug-resistant Epilepsy: Systematic Review and Meta-analysis from the ILAE Surgical Therapies Commission. Epilepsia 2022, 63, 1314–1329. [Google Scholar] [CrossRef]
- Krahl, S.E.; Clark, K.B. Vagus Nerve Stimulation for Epilepsy: A Review of Central Mechanisms. Surg. Neurol. Int. 2012, 3 (Suppl. S4), S255. [Google Scholar] [CrossRef] [PubMed]
- Englot, D.J.; Chang, E.F.; Auguste, K.I. Vagus Nerve Stimulation for Epilepsy: A Meta-Analysis of Efficacy and Predictors of Response—A Review. J. Neurosurg. 2011, 115, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Toyota, E.; Anderson, M.; Abel, T.J.; Donner, E.; Kalia, S.K.; Drake, J.; Rutka, J.T.; Ibrahim, G.M. A Systematic Review of Deep Brain Stimulation for the Treatment of Drug-Resistant Epilepsy in Childhood. J. Neurosurg. Pediatr. 2018, 23, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Vetkas, A.; Fomenko, A.; Germann, J.; Sarica, C.; Iorio-Morin, C.; Samuel, N.; Yamamoto, K.; Milano, V.; Cheyuo, C.; Zemmar, A.; et al. Deep Brain Stimulation Targets in Epilepsy: Systematic Review and Meta-Analysis of Anterior and Centromedian Thalamic Nuclei and Hippocampus. Epilepsia 2022, 63, 513–524. [Google Scholar] [CrossRef]
- Penfield, W.; Jasper, H.H. Epilepsy and the Functional Anatomy of the Human Brain; Little, Brown & Co.: Oxford, UK, 1954; Available online: https://jamanetwork-com.pitt.idm.oclc.org/journals/jama/fullarticle/316575 (accessed on 9 September 2023).
- Psatta, D.M. Control of Chronic Experimental Focal Epilepsy by Feedback Caudatum Stimulations. Epilepsia 1983, 24, 444–454. [Google Scholar] [CrossRef]
- Lesser, R.P.; Kim, S.H.; Beyderman, L.; Miglioretti, D.L.; Webber, W.R.; Bare, M.; Cysyk, B.; Krauss, G.; Gordon, B. Brief Bursts of Pulse Stimulation Terminate Afterdischarges Caused by Cortical Stimulation. Neurology 1999, 53, 2073–2081. [Google Scholar] [CrossRef]
- Motamedi, G.K.; Lesser, R.P.; Miglioretti, D.L.; Mizuno-Matsumoto, Y.; Gordon, B.; Webber, W.R.S.; Jackson, D.C.; Sepkuty, J.P.; Crone, N.E. Optimizing Parameters for Terminating Cortical Afterdischarges with Pulse Stimulation. Epilepsia 2002, 43, 836–846. [Google Scholar] [CrossRef]
- Peters, T.E.; Bhavaraju, N.C.; Frei, M.G.; Osorio, I. Network System for Automated Seizure Detection and Contingent Delivery of Therapy. J. Clin. Neurophysiol. 2001, 18, 545–549. [Google Scholar] [CrossRef]
- NeuroPace, Inc. RNS (R) System Physician Manual for the RNS (R) Neurostimulator Model RNS-320; NeuroPace, Inc.: Mountain View, CA, USA, 2022. [Google Scholar]
- Ma, B.B.; Rao, V.R. Responsive Neurostimulation: Candidates and Considerations. Epilepsy Behav. 2018, 88, 388–395. [Google Scholar] [CrossRef]
- Kerezoudis, P.; Gyftopoulos, A.; Alexander, A.Y.; Keith Starnes, D.; Nickels, K.C.; Worrell, G.A.; Wirrell, E.C.; Lundstrom, B.N.; Van Gompel, J.J.; Miller, K.J. Safety and Efficacy of Responsive Neurostimulation in the Pediatric Population: Evidence from Institutional Review and Patient-Level Meta-Analysis. Epilepsy Behav. 2022, 129, 108646. [Google Scholar] [CrossRef]
- Geller, E.B.; Skarpaas, T.L.; Gross, R.E.; Goodman, R.R.; Barkley, G.L.; Bazil, C.W.; Berg, M.J.; Bergey, G.K.; Cash, S.S.; Cole, A.J.; et al. Brain-Responsive Neurostimulation in Patients with Medically Intractable Mesial Temporal Lobe Epilepsy. Epilepsia 2017, 58, 994–1004. [Google Scholar] [CrossRef]
- Hirsch, L.J.; Mirro, E.A.; Salanova, V.; Witt, T.C.; Drees, C.N.; Brown, M.; Lee, R.W.; Sadler, T.L.; Felton, E.A.; Rutecki, P.; et al. Mesial Temporal Resection Following Long-term Ambulatory Intracranial EEG Monitoring with a Direct Brain-responsive Neurostimulation System. Epilepsia 2020, 61, 408–420. [Google Scholar] [CrossRef]
- Heck, C.N.; King-Stephens, D.; Massey, A.D.; Nair, D.R.; Jobst, B.C.; Barkley, G.L.; Salanova, V.; Cole, A.J.; Smith, M.C.; Gwinn, R.P.; et al. Two-year Seizure Reduction in Adults with Medically Intractable Partial Onset Epilepsy Treated with Responsive Neurostimulation: Final Results of the RNS System Pivotal Trial. Epilepsia 2014, 55, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Boddeti, U.; McAfee, D.; Khan, A.; Bachani, M.; Ksendzovsky, A. Responsive Neurostimulation for Seizure Control: Current Status and Future Directions. Biomedicines 2022, 10, 2677. [Google Scholar] [CrossRef]
- Kokkinos, V.; Sisterson, N.D.; Wozny, T.A.; Richardson, R.M. Association of Closed-Loop Brain Stimulation Neurophysiological Features with Seizure Control among Patients with Focal Epilepsy. JAMA Neurol. 2019, 76, 800–808. [Google Scholar] [CrossRef]
- Morrell, M.J. Responsive Cortical Stimulation for the Treatment of Medically Intractable Partial Epilepsy. Neurology 2011, 77, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Sisterson, N.D.; Wozny, T.A.; Kokkinos, V.; Constantino, A.; Richardson, R.M. Closed-Loop Brain Stimulation for Drug-Resistant Epilepsy: Towards an Evidence-Based Approach to Personalized Medicine. Neurother. J. Am. Soc. Exp. Neurother. 2019, 16, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Stefan, H.; Lopes Da Silva, F. Epileptic Neuronal Networks: Methods of Identification and Clinical Relevance. Front. Neurol. 2013, 4, 8. [Google Scholar] [CrossRef]
- Mirski, M.A.; Rossell, L.A.; Terry, J.B.; Fisher, R.S. Anticonvulsant Effect of Anterior Thalamic High Frequency Electrical Stimulation in the Rat. Epilepsy Res. 1997, 28, 89–100. [Google Scholar] [CrossRef]
- Velasco, M.; Velasco, F.; Velasco, A.L.; Luján, M.; Vázquez del Mercado, J. Epileptiform EEG Activities of the Centromedian Thalamic Nuclei in Patients with Intractable Partial Motor, Complex Partial, and Generalized Seizures. Epilepsia 1989, 30, 295–306. [Google Scholar] [CrossRef]
- Beaudreault, C.P.; Muh, C.R.; Naftchi, A.; Spirollari, E.; Das, A.; Vazquez, S.; Sukul, V.V.; Overby, P.J.; Tobias, M.E.; McGoldrick, P.E.; et al. Responsive Neurostimulation Targeting the Anterior, Centromedian and Pulvinar Thalamic Nuclei and the Detection of Electrographic Seizures in Pediatric and Young Adult Patients. Front. Hum. Neurosci. 2022, 16, 876204. [Google Scholar] [CrossRef] [PubMed]
- Burdette, D.; Mirro, E.A.; Lawrence, M.; Patra, S.E. Brain-responsive Corticothalamic Stimulation in the Pulvinar Nucleus for the Treatment of Regional Neocortical Epilepsy: A Case Series. Epilepsia Open 2021, 6, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Gadot, R.; Korst, G.; Shofty, B.; Gavvala, J.R.; Sheth, S.A. Thalamic Stereoelectroencephalography in Epilepsy Surgery: A Scoping Literature Review. J. Neurosurg. 2022, 137, 1210–1225. [Google Scholar] [CrossRef] [PubMed]
- Caciagli, L.; Allen, L.A.; He, X.; Trimmel, K.; Vos, S.B.; Centeno, M.; Galovic, M.; Sidhu, M.K.; Thompson, P.J.; Bassett, D.S.; et al. Thalamus and Focal to Bilateral Seizures: A Multiscale Cognitive Imaging Study. Neurology 2020, 95, e2427–e2441. [Google Scholar] [CrossRef]
- Fisher, R.S. Deep Brain Stimulation of Thalamus for Epilepsy. Neurobiol. Dis. 2023, 179, 106045. [Google Scholar] [CrossRef]
- Birbeck, G.L.; Hays, R.D.; Cui, X.; Vickrey, B.G. Seizure Reduction and Quality of Life Improvements in People with Epilepsy. Epilepsia 2002, 43, 535–538. [Google Scholar] [CrossRef]
- Bergey, G.K.; Morrell, M.J.; Mizrahi, E.M.; Goldman, A.; King-Stephens, D.; Nair, D.; Srinivasan, S.; Jobst, B.; Gross, R.E.; Shields, D.C.; et al. Long-Term Treatment with Responsive Brain Stimulation in Adults with Refractory Partial Seizures. Neurology 2015, 84, 810–817. [Google Scholar] [CrossRef]
- Nair, D.R.; Laxer, K.D.; Weber, P.B.; Murro, A.M.; Park, Y.D.; Barkley, G.L.; Smith, B.J.; Gwinn, R.P.; Doherty, M.J.; Noe, K.H.; et al. Nine-Year Prospective Efficacy and Safety of Brain-Responsive Neurostimulation for Focal Epilepsy. Neurology 2020, 95, e1244–e1256. [Google Scholar] [CrossRef]
- Bondallaz, P.; Boëx, C.; Rossetti, A.O.; Foletti, G.; Spinelli, L.; Vulliemoz, S.; Seeck, M.; Pollo, C. Electrode Location and Clinical Outcome in Hippocampal Electrical Stimulation for Mesial Temporal Lobe Epilepsy. Seizure 2013, 22, 390–395. [Google Scholar] [CrossRef]
- Jobst, B.C.; Kapur, R.; Barkley, G.L.; Bazil, C.W.; Berg, M.J.; Bergey, G.K.; Boggs, J.G.; Cash, S.S.; Cole, A.J.; Duchowny, M.S.; et al. Brain-Responsive Neurostimulation in Patients with Medically Intractable Seizures Arising from Eloquent and Other Neocortical Areas. Epilepsia 2017, 58, 1005–1014. [Google Scholar] [CrossRef]
- Singh, R.K.; Eschbach, K.; Samanta, D.; Perry, M.S.; Liu, G.; Alexander, A.L.; Wong-Kisiel, L.; Ostendorf, A.; Tatachar, P.; Reddy, S.B.; et al. Responsive Neurostimulation in Drug-Resistant Pediatric Epilepsy: Findings From the Epilepsy Surgery Subgroup of the Pediatric Epilepsy Research Consortium. Pediatr. Neurol. 2023, 143, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Panov, F.; Ganaha, S.; Haskell, J.; Fields, M.; La Vega-Talbott, M.; Wolf, S.; McGoldrick, P.; Marcuse, L.; Ghatan, S. Safety of Responsive Neurostimulation in Pediatric Patients with Medically Refractory Epilepsy. J. Neurosurg. Pediatr. 2020, 26, 525–532. [Google Scholar] [CrossRef]
- Nagahama, Y.; Zervos, T.M.; Murata, K.K.; Holman, L.; Karsonovich, T.; Parker, J.J.; Chen, J.-S.; Phillips, H.W.; Fajardo, M.; Nariai, H.; et al. Real-World Preliminary Experience with Responsive Neurostimulation in Pediatric Epilepsy: A Multicenter Retrospective Observational Study. Neurosurgery 2021, 89, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Bercu, M.M.; Friedman, D.; Silverberg, A.; Drees, C.; Geller, E.B.; Dugan, P.C.; Devinsky, O.; Doyle, W.H. Responsive Neurostimulation for Refractory Epilepsy in the Pediatric Population: A Single-Center Experience. Epilepsy Behav. 2020, 112, 107389. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.S.; Numis, A.L.; Lee, M.B.; Chang, E.F.; Sullivan, J.E.; Auguste, K.I.; Rao, V.R. Responsive Neurostimulation for Treatment of Pediatric Drug-Resistant Epilepsy. Epilepsy Behav. Case Rep. 2018, 10, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.-S.; Schupper, A.J.; Fields, M.C.; Marcuse, L.V.; La Vega-Talbott, M.; Panov, F.; Ghatan, S. Centromedian Thalamic Responsive Neurostimulation for Lennox-Gastaut Epilepsy and Autism. Ann. Clin. Transl. Neurol. 2020, 7, 2035–2040. [Google Scholar] [CrossRef]
- Welch, W.P.; Hect, J.L.; Abel, T.J. Case Report: Responsive Neurostimulation of the Centromedian Thalamic Nucleus for the Detection and Treatment of Seizures in Pediatric Primary Generalized Epilepsy. Front. Neurol. 2021, 12, 656585. [Google Scholar] [CrossRef]
- Kokoszka, M.A.; Panov, F.; La Vega-Talbott, M.; McGoldrick, P.E.; Wolf, S.M.; Ghatan, S. Treatment of Medically Refractory Seizures with Responsive Neurostimulation: 2 Pediatric Cases. J. Neurosurg. Pediatr. 2018, 21, 421–427. [Google Scholar] [CrossRef]
- Curtis, K.; Hect, J.L.; Harford, E.; Welch, W.P.; Abel, T.J. Responsive Neurostimulation for Pediatric Patients with Drug-Resistant Epilepsy: A Case Series and Review of the Literature. Neurosurg. Focus 2022, 53, E10. [Google Scholar] [CrossRef]
- Hartnett, S.M.; Greiner, H.M.; Arya, R.; Tenney, J.R.; Aungaroon, G.; Holland, K.; Leach, J.L.; Air, E.L.; Skoch, J.; Mangano, F.T. Responsive Neurostimulation Device Therapy in Pediatric Patients with Complex Medically Refractory Epilepsy. J. Neurosurg. Pediatr. 2022, 30, 499–506. [Google Scholar] [CrossRef]
- Herlopian, A.; Cash, S.S.; Eskandar, E.M.; Jennings, T.; Cole, A.J. Responsive Neurostimulation Targeting Anterior Thalamic Nucleus in Generalized Epilepsy. Ann. Clin. Transl. Neurol. 2019, 6, 2104–2109. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, V.; Urban, A.; Sisterson, N.D.; Li, N.; Corson, D.; Richardson, R.M. Responsive Neurostimulation of the Thalamus Improves Seizure Control in Idiopathic Generalized Epilepsy: A Case Report. Neurosurgery 2020, 87, E578–E583. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.; McDermot, M.P.; Wiebe, S.; Langfitt, J.T.; Stern, J.M.; Dewar, S.; Sperling, M.R.; Gardiner, I.; Erba, G.; Fried, I.; et al. Early Surgical Therapy for Drug-Resistant Temporal Lobe Epilepsy. JAMA 2012, 307, 922. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, F.; Wyllie, E.; Kashden, J.; Faught, E.; Kotagal, P.; Bebin, M.; Wise, M.; Comair, Y.; Morawetz, R.; Kuzniecky, R. Epilepsy Surgery Outcome: Comprehensive Assessment in Children. Neurology 1997, 48, 1368–1374. [Google Scholar] [CrossRef]
- Edelvik, A.; Rydenhag, B.; Olsson, I.; Flink, R.; Kumlien, E.; Kallen, K.; Malmgren, K. Long-Term Outcomes of Epilepsy Surgery in Sweden: A National Prospective and Longitudinal Study. Neurology 2013, 81, 1244–1251. [Google Scholar] [CrossRef]
- DiLorenzo, D.J.; Mangubat, E.Z.; Rossi, M.A.; Byrne, R.W. Chronic Unlimited Recording Electrocorticography–Guided Resective Epilepsy Surgery: Technology-Enabled Enhanced Fidelity in Seizure Focus Localization with Improved Surgical Efficacy. J. Neurosurg. 2014, 120, 1402–1414. [Google Scholar] [CrossRef]
- Chandrabhatla, A.S.; Pomeraniec, I.J.; Horgan, T.M.; Wat, E.K.; Ksendzovsky, A. Landscape and Future Directions of Machine Learning Applications in Closed-Loop Brain Stimulation. Npj Digit. Med. 2023, 6, 79. [Google Scholar] [CrossRef]
- Roth, J.; Olasunkanmi, A.; MacAllister, W.S.; Weil, E.; Uy, C.C.; Devinsky, O.; Weiner, H.L. Quality of Life Following Epilepsy Surgery for Children with Tuberous Sclerosis Complex. Epilepsy Behav. 2011, 20, 561–565. [Google Scholar] [CrossRef]
- Khambhati, A.N.; Shafi, A.; Rao, V.R.; Chang, E.F. Long-Term Brain Network Reorganization Predicts Responsive Neurostimulation Outcomes for Focal Epilepsy. Sci. Transl. Med. 2021, 13, eabf6588. [Google Scholar] [CrossRef]
- Kolb, B.; Gibb, R. Brain Plasticity and Behaviour in the Developing Brain. J. Can. Acad. Child Adolesc. Psychiatry 2011, 20, 265–276. [Google Scholar]
| Authors | Study Type | Title | Summary Points |
|---|---|---|---|
| Singh (2023) [53] | Case series | Responsive neurostimulation in drug-resistant pediatric epilepsy findings from the Epilepsy Surgery Subgroup of the Pediatric Epilepsy Research Consortium | Largest multicenter pediatric sample (n = 56). 67% with >50% reduction in seizures; 10% seizure-free at 1 year. |
| Kerezoudis (2022) [32] | Meta-analysis | Safety and efficacy of responsive neurostimulation in the pediatric population: evidence from institutional review and patient level meta-analysis | 8 studies (n = 49) reviewed with 80% responders and 75% median seizure reduction. Most common locations for implantation were frontal and mesial temporal lobe; 8% infection rate. |
| Curtis (2022) [61] | Case series | Responsive neurostimulation for pediatric patients with drug-resistant epilepsy: a case series and review of the literature | n = 20. Cohort with varied semiology and both eloquent and thalamic electrode implantation. Similar complication profile to the adult literature. |
| Hartnett (2022) [62] | Case series | Responsive neurostimulation device therapy in pediatric patients with complex medically refractory epilepsy | n = 8, 50% with previous surgery for epilepsy. All achieved >50% seizure reduction. |
| Beaudreault (2022) [43] | Case series | Responsive neurostimulation targeting the anterior, centromedian, and pulvinar thalamic nuclei and the detection of electrographic seizures in pediatric and young adult patients | n = 17 (mean 16.5 years old) underwent thalamic depth electrode placement with or without cortical strip leads. Thalamic leads alone were able to detect and prevent propagation similarly to combined thalamic and cortical strip setup. |
| Nagahama (2021) [55] | Case series | Real-world preliminary experience with responsive neurostimulation in pediatric epilepsy: a multicenter retrospective observational study | n = 35 identified from 5 centers (age 3–25 years old). 50% had >50% reduction in seizures. No complications in pediatric patients, 3 complications in young adults. RNS can be used in patients as young as 3 years old. |
| Welch (2021) [59] | Case report | Responsive neurostimulation of the centromedian thalamic nucleus for the detection and treatment of seizures in pediatric primary generalized epilepsy | 16 year old male with primary generalized epilepsy with 75% seizure reduction following bilateral CMT RNS placement and complete resolution of absence seizures at 6 months. |
| Panov (2020) [54] | Retrospective review | Safety of responsive neurostimulation in pediatric patients with medically refractory epilepsy | Among 27 consecutive pediatric RNS placements. Three patients had infections, but no other complications at 22 months. Seizure frequency improved for all patients. |
| Kwon (2020) [58] | Case series | Centromedian thalamic responsive neurostimulation for Lennox-Gastaut epilepsy and autism | Two cases of Lennox-Gastaut patients who experienced 75–99% seizure reduction at 1 year after CMT RNS placement. |
| Bercu (2020) [56] | Case series | Responsive neurostimulation for refractory epilepsy in the pediatric population: a single-center experience | Six patients with focal epilepsy who underwent RNS experienced improvement in seizure frequency and changes in semiology as well. |
| Kokoszka (2018) [60] | Case report | Treatment of medically refractory seizures with responsive neurostimulation: 2 pediatric cases | 14 year old with bilateral cortical dysplasia with 80–90% reduction in seizure frequency with cortical strip RNS. Corticothalamic treatment reduced seizures another 50%; 9 year old with cortical leads placed at focus resulted in behavioral improvement and >80% seizure reduction. |
| Singhal (2018) [57] | Case report | Single report of successful RNS placement in pediatric patient | 16 year old male with simultaneous subtotal resection of cortical dysplasia and RNS placement; 100% reduction in impairing seizures at 6 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piazza, M.G.; Varga, G.; Welch, W.; Abel, T.J. The Utility of Responsive Neurostimulation for the Treatment of Pediatric Drug-Resistant Epilepsy. Brain Sci. 2023, 13, 1455. https://doi.org/10.3390/brainsci13101455
Piazza MG, Varga G, Welch W, Abel TJ. The Utility of Responsive Neurostimulation for the Treatment of Pediatric Drug-Resistant Epilepsy. Brain Sciences. 2023; 13(10):1455. https://doi.org/10.3390/brainsci13101455
Chicago/Turabian StylePiazza, Martin G., Gregory Varga, William Welch, and Taylor J. Abel. 2023. "The Utility of Responsive Neurostimulation for the Treatment of Pediatric Drug-Resistant Epilepsy" Brain Sciences 13, no. 10: 1455. https://doi.org/10.3390/brainsci13101455
APA StylePiazza, M. G., Varga, G., Welch, W., & Abel, T. J. (2023). The Utility of Responsive Neurostimulation for the Treatment of Pediatric Drug-Resistant Epilepsy. Brain Sciences, 13(10), 1455. https://doi.org/10.3390/brainsci13101455