Abstract
Antiseizure medications are the cornerstone pharmacotherapy for epilepsy. They are not devoid of side effects. In search for better-tolerated antiseizure agents, cannabinoid compounds and other N-acylethanolamines not directly binding cannabinoid receptors have drawn significant attention. Among these, palmitoylethanolamide (PEA) has shown neuroprotective, anti-inflammatory, and analgesic properties. All studies examining PEA’s role in epilepsy and acute seizures were systematically reviewed. Preclinical studies indicated a systematically reduced PEA tone accompanied by alterations of endocannabinoid levels. PEA supplementation reduced seizure frequency and severity in animal models of epilepsy and acute seizures, in some cases, similarly to available antiseizure medications but with a better safety profile. The peripheral-brain immune system seemed to be more effectively modulated by subchronic pretreatment with PEA, with positive consequences in terms of better responding to subsequent epileptogenic insults. PEA treatment restored the endocannabinoid level changes that occur in a seizure episode, with potential preventive implications in terms of neural damage. Neurobiological mechanisms for PEA antiseizure effect seemed to include the activation of the endocannabinoid system and the modulation of neuroinflammation and excitotoxicity. Although no human study was identified, there is ground for testing the antiseizure potential of PEA and its safety profile in human studies of epilepsy.
1. Introduction
According to the International League Against Epilepsy (ILAE), seizures can be described as the “transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain.” Epilepsy subsists when the patient’s brain shows an augmented tendency to the recurrence of seizures []. It is one of the most frequent neurological disorders, involving about 50 million people worldwide, mainly in developing countries. It shows a U-shaped distribution in terms of age, with a first peak during middle childhood (5–9 years) and a second one around 80 years of age, affecting all races and both genders []. While childhood-onset epilepsy is more likely to be idiopathic, late-onset epilepsy is likely to be due to an identifiable cause, including trauma, central nervous system (CNS) infections, space-occupying lesions, cerebrovascular accidents (CVA), metabolic disorders, and drugs []. Chronic use of antiseizure medications (ASMs) is still considered as the cornerstone pharmacotherapy for seizures even though it might expose the patient to major somatic adverse effects, such as fatigue, dizziness, sedation, headache, and nausea, as well as neuropsychiatric problems, such as anxiety, depression, or sleep disorders, especially when polytherapy is needed []. Benzodiazepine drugs (BDZ) represent a frequently used therapeutic option: seizures are often efficiently reduced in the early stages of treatment through the production of allosteric changes in GABA-A receptors, which lead to an increase of GABAergic neurotransmission and to a decreased neuronal excitability. BDZs are not devoid of causing unpleasant drowsiness and incoordination and may affect cognitive performance alongside the eventual development of physical dependence and tolerance [].
The search is on for novel antiseizure agents with fewer adverse effects. Cannabinoid compounds as ASMs via the cannabinoid receptor type 1 (CB1) have already shown promising results [,]. The endocannabinoids (eCBs) anandamide (AEA) and 2-arachidonoyl-glycerol (2-AG), as well as the N-acylethanolamines palmitoylethanolamide (PEA) and N-oleylethanolamine (OEA), which act as endogenous lipid signaling molecule analogues not directly binding CB1 receptor, are under investigation as possible therapeutic options for CNS diseases, including the control of epileptic seizures. PEA has firstly been identified in egg yolk, soy bean, and peanut oil and subsequently detected in mammalian tissues. It is an endogenous fatty acid amide, which exerts its biological effects through the activation of peroxisome proliferator-activated receptor-α (PPAR-α) and its related independent pathways, including ion channels involved in neuronal firing and the Transient Receptor Potential Vanilloid 1 (TRPV1) receptor [], whose role is considered crucial in the fulfilment of neuroprotective, anti-inflammatory, and analgesic properties []. PEA has been suggested as an effective treatment for inflammatory disorders and pain [,], together with possible therapeutic implications in depressive symptoms and autism spectrum disorder [,].
Objectives
PEA effects on glutamate signaling, ion channels and systemic inflammation, and/or peripheral immunity activation could represent promising mechanisms to reduce the likelihood for seizures’ occurrence and progression. This review aimed to bring together and discuss all available data generated by studies investigating the role of PEA in epilepsy by conducting a systematic literature search for all such data. All interventional and observational studies have been reviewed.
2. Experimental Procedures
2.1. Inclusion and Exclusion Criteria
In order to summarize previous research on the subject, inclusion criteria for studies were as follows: (1) human or animal studies; (2) studies assessing the effects of PEA administration in epilepsy and acute seizures; and (3) studies investigating PEA signaling-related molecular markers in epilepsy and acute seizures, including (a) blood serum levels, (b) brain tissue levels, (c) peripheral tissue levels, (d) enzyme activity, and (e) receptors. Exclusion criteria were (1) studies where PEA was not the intervention considered (studies evaluating only exogenous cannabinoid agonists or antagonists or any eCBs other than PEA), (2) studies where PEA’s neuroprotective role was not evaluated with reference to epilepsy or acute seizures, and (3) studies in which PEA effects were not directly reported on.
2.2. Search Strategy and Data Extraction
A literature search was conducted using electronic databases (PubMed, Web of Science and Scopus) for any published original study written in English, using a combination of search terms describing and/or concerning epilepsy (‘epilep*’, ‘seizure’, ‘convuls*’, ‘tonic’, ’clonic’, ‘myoclon*’, ‘attack’, ‘paroxysm’, ‘tremor’ and ‘antiepileptic’) and PEA (‘palmitoylethanolamide’, ‘palmitylethanolamide’, ‘(N-(2-hydroxyethyl)hexadecanamide)’, ‘(N-(2-hydroxyethyl)palmitate)’, ‘N-palmitoylethanolamine’), on 4 December 2021. No predefined duration of PEA exposure, default gender, stage of life/epilepsy, or therapeutic strategies for study search was adopted in order to be the most inclusive as possible. Reference lists of eligible studies were screened to identify additional eligible research. Publication data screening and extraction were performed following a 2-step selection process (conventional double-screening) conducted by 2 reviewers independently of each other (R.B. and M.C.).
2.3. Risk of Bias
The methodological heterogeneity of the studies (Table 1) included in this review necessitated a suitably inclusive and flexible approach to assess risk of bias and study quality. An adapted set of criteria suggested by the Agency for Healthcare Research and Quality (AHRQ) guidance was used [], amended as appropriate for interventional and observational studies in animals. To assess any factor that may account for similarities and differences between animal studies, information was extracted about study characteristics, including animal model (mouse or rat), seizure model (chemical stimulation, electrical stimulation, strain of idiopathic epilepsy), developmental stage (prenatal, postnatal, adult), gender, and PEA dosage and exposure (Table 2).

Table 1.
Summary of studies investigating palmitoylethanolamide and its correlations to epilepsy and acute seizures.

Table 2.
Methodological quality of animal studies investigating palmitoylethanolamide and its correlations to epilepsy and acute seizures.
3. Results
3.1. Study Selection
In summary, 52 records were retrieved. After excluding articles owing to article type (systematic and non-systematic reviews), by using a three-step screening approach, titles, abstracts, or full texts of all records were screened against the inclusion and exclusion criteria (Figure 1). A final list of eight studies was used for systematic analysis in this review (Table 1). No eligible human study was identified. In total, the eligible studies assessed different aspects of the palmitoylethanolamide (PEA) signaling pathway (Table 1). These included (1) in-vivo single/acute PEA exposure in animal models of epilepsy (two studies) and acute seizures (three studies); (2) in-vivo acute vs. subchronic PEA exposure in animal models of acute seizures (one study); (3) in-vivo PEA vs. other eCBs/antiseizure medications (ASMs) exposure in animal models of acute seizures (one study); (4) PEA quantitative brain assessment in animal models of epilepsy (one study) and acute seizures (one study); (5) PEA quantitative brain assessment in young vs. adult animal models of acute seizures (one study); (6) PEA quantitative peripheral tissues assessment in animal models of acute seizures (one study); (7) eCB and eicosanoid (eiC) quantitative brain assessment in PEA-treated animal models of acute seizures (one study); and (8) eCB and eiC quantitative blood assessment in PEA-treated animal models of acute seizures (one study). Additional data on methodological quality of studies are reported in Table 2. A brief synthesis of the main results is presented below and summarized in Table 1.
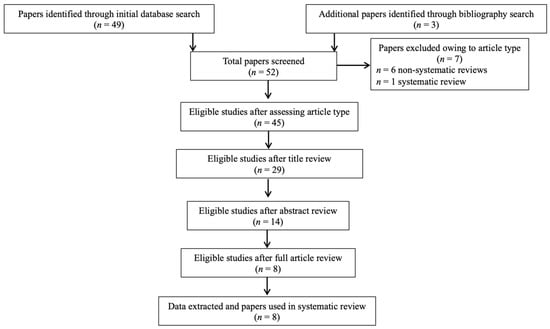
Figure 1.
PRISMA flowchart of search strategy for systematic review.
3.2. In Vivo Acute and Subchronic PEA Treatment Exposure and Comparison with Other Endocannabinoids (eCBs) and ASMs Exposure in Animal Models of Epilepsy and Acute Seizures
Most studies identified in this review addressed the effects of PEA exposure in animal models of epilepsy and acute seizures using similar but not overlapping methodologies in terms of animal type (mice [,,], rat [,,]), mode of administration (intraperitoneal [,,,,], intracerebroventricular [,]), period of exposure (from 14 days old to month 7 months old), dosage of PEA (0.5 to 250 mg/kg for intraperitoneal administration [,,,]; 0.5 to 10 µg/2 µL [] or 1 to 25 µg/kg [] for intracerebroventricular administration), and model of pathology (chemical stimulation [,,], electrical stimulation [,], genetic models [,]). The first of these studies estimated the superiority of PEA to placebo, indicating antiseizure properties of the compound, with an effective dose (ED50) comparable to that of the ASM phenytoin (PHT) and a higher protective index []. While being effective in controlling tonic seizures, PEA was not effective against clonic convulsions, where it performed worse than PHT []. Except for some modest activity of anandamide (AEA) and palmitamide (PAA), other compounds related to eCBs or to the palmitic acid structure were devoid of ASM activity in the experimental conditions []. A subsequent study confirmed and extended such findings, supporting the evidence that PEA suppresses the tonic component in animal models of tonic-clonic seizure, increasing latency to clonus, and prolonging the latency between convulsive episodes []. Another study found that PEA administration reduces the epileptic spike-wave discharges (SWDs) in a widely validated genetic animal model for generalized absence epilepsy, the Wistar Albino Glaxo from Rijswijk (WAG/Rij) rat []. Such antiseizure effect was completely blocked by pretreatment with a synthetic cannabinoid receptor type 1 (CB1) antagonist/inverse agonist and a nuclear peroxisome proliferator-activated receptors (PPAR-α) antagonist []. While the synthetic CB1 antagonist/inverse agonist had pro-epileptic effects and interfered with the antiseizure activity of AEA, the PPAR-α antagonist did not have any effect and did not modify the antiseizure properties of AEA []. Studies carried out in a genetic model of reflex audiogenic epilepsy, the DBA/2 mouse [], as well as by inducing seizures with a chemical kindling process [] confirmed that the antiseizure effect of PEA is diminished by antagonizing CB1 and CB2 receptors [,] and PPAR-α [], while the activation on the eCB system in the brain has ASM effects []. Co-administration of both PEA and CB1 and CB2 receptor agonists potentiated ASMs’ activity via pharmacodynamic mechanisms []. A more recent study was novel in indicating that the subchronic administration of PEA (double exposure at 7-h and 30-min prior to chemically induce the seizure) exerts larger beneficial effects as compared to single PEA injection (single exposure 30-min prior to chemically induce the seizure) in terms of attenuating both the behavioral (seizure intensity) and neurobiological (peripheral and hippocampal inflammatory responses to the excitotoxicity) seizure-related alterations []. Elevating PEA levels by inhibiting the fatty acid amide hydrolase (FAAH), which degrades it, resulted in similar effects than exogenously administering PEA []. Combinatorial administration of FAAH inhibitor with PEA did not produce any cumulative therapeutic effect in seizure alleviation []. Systemic pharmacological blockade of FAAH (occurring in the brain) rather than peripheral appeared to be required to successfully modulate inflammation and exert efficient antiseizure properties [].
3.3. PEA Quantitative Brain and Peripheral Tissue Assessment and Comparison as a Function of Age in Animal Models of Epilepsy and Acute Seizures
In total, three studies did not evaluate the direct effect of PEA exposure while analyzing PEA levels in the brain and peripheral tissues of animal models of epilepsy. A study found that PEA levels in the amygdala, cortex, and thalamus are reduced in the genetic animal model of epilepsy, WAG/Rij, as compared to control animals, possibly compensated by an increase of cortical PEA levels at a later age, with such early and persistent decrease in PEA tone being accompanied by alterations in the eCB levels []. Similar results were found in another report, indicating lower PEA levels in the striatum and cerebellum but not in cerebral cortex, thalamus, hypothalamus, and hippocampus of animals presenting with chemically induced epilepsy versus controls []. This study identified reduced PEA levels in the lung and plasma in the context of epilepsy. Brain region- and periphery tissue-specific alterations were observed for eCBs, acylethanolamines, phospholipids, and eiCs []. A study identified differential responses as a function of age in an animal model of epilepsy, indicating higher hippocampal PEA levels in young animals but lower levels in the same region in adult animals, with differential responses in terms of eCB levels [].
3.4. Endocannabinoid (eCB) and Eicosanoid (eiC) Quantitative Brain and Blood Assessment in PEA-Treated Animal Models of Acute Seizures
This systematic review identified a single study specifically investigating whether epileptic animals differ in terms of brain and blood levels of eCB and eiC levels as a function of PEA treatment. This study found that the acute seizure phase represents a turn-over time point in the dynamic of eCB and eiC level changes. Specifically, they expressed an increase in hippocampus accompanied by a decrease in periphery before the acute phase and normalization to basal hippocampal levels followed by augmentation in periphery after the acute phase []. PEA administration completely restored to basal such hippocampal increase, which occurs in early response to the chemically induced excitotoxicity, with subsequent reduction of seizure intensity. Similar normalizing effects of PEA were observed in periphery, supporting the notion of a modulation of eCB and eiC levels across the periphery-brain axis [].
4. Discussion
This is the first systematic review of all studies investigating the behavioral effects of palmitoylethanolamide (PEA) and their neurobiological underpinnings in seizures and epilepsy. All records identified consisted of animal studies, while no research conducted in humans was available. Previous reviews had mainly focused on the role of major phytocannabinoids, indicating that they may represent a complementary tool for the symptomatic management of refractory epilepsy []. Specifically, research findings converged on the efficacy of pure cannabidiol (CBD), the isolated chemical product, and CBD-enriched cannabis extracts, where the therapeutic effect is supposedly driven by the complex interactions between all the components of the cannabis plant and remains poorly understood []. Such evidence led to Epidiolex, a cannabis plant-derived oral CBD solution, becoming licensed in the United States and Europe for treatment-resistant severe forms of childhood epilepsy, such as Lennox–Gastaut syndrome and Dravet syndrome []. Research studies on its therapeutic properties indicate that CBD is a negative allosteric modulator of cannabinoid (CB) receptors [] and may modulate endogenous endocannabinoid (eCB) levels by indirect mechanisms involving the nuclear receptor peroxisome proliferator-activated receptor-α (PPAR-α) and PPAR-α-independent pathways, such as Transient Receptor Potential Vanilloid 1 (TRPV1) and G55 protein-coupled receptor (GPR55) []. Consistent with this, recent research highlighted the importance of widening the investigation to cannabinoid-related compounds whose actions depend on the interaction with non-CB receptors []. Overall, this review demonstrated that PEA, a naturally occurring N-acylethanolamine whose biological effects are related to indirect activation of CB1 receptors as well as PPAR-α and TRPV1 modulation [,], may be involved in seizures and epilepsy. Evidence was obtained from interventional studies of the positive behavioral and neurobiological effects of PEA supplementation, observational studies of aberrancies in the PEA tone, and studies of PEA-mediated modulation of eCB levels across the periphery-brain axis in the context of seizures and epilepsy.
PEA supplementation in animal models of epilepsy and acute seizures was found to reduce seizure frequency and severity [,,,,,], in some cases, similarly to available ASMs but with a better safety profile in terms of a higher therapeutic index as a ratio between the amount of drug that causes toxicity and the amount that causes the therapeutic effect []. Potential neurobiological mechanisms for such antiseizure effect included the activation of the eCB system [,,] and the modulation of neuroinflammation and excitotoxicity []. In particular, eCB system activation has been suggested to exert neuroprotective effects by modulating glutamate neurotransmission [,,,] and GABAergic tone [,,,]. The peripheral-brain immune system appeared to be more effectively modulated by subchronic pretreatment with PEA [], possibly because of a resulting state of ‘‘hypervigilance’’ capable of responding more adequately to subsequent negative stimuli, such as the epileptogenic insult [,]. PEA treatment was found to restore the eCB and eicosanoid (eiC) level changes that occur in acute and post-acute phases of an epileptic episode, with potential preventive implications in terms of neural damage [].
Another line of research identified lower brain levels of PEA and related alterations in the eCB levels in animal models of epilepsy and acute seizures [,], dependent on the disease progression []. Brain PEA levels were found to be reduced only in adult epileptic animals while paradoxically increased in young animals []. PEA levels also appeared to be systemically reduced, as indicated by the finding of lower levels in the lung and plasma of epileptic animals [].
The findings of this systematic review must be seen considering some limitations. Research in the field is still in its infancy, and animal experiments often do not translate into replications in human trials []. In the absence of human studies, no conclusions can be drawn about the relevance of PEA for the different clinical phenotypes of epilepsy. While limited evidence supports a neuroprotective effect of PEA against neuroinflammation and glutamate toxicity in the context of different neuropsychiatric conditions [], studies are needed to elucidate the exact mechanism of action of PEA in epilepsy. Caution is needed about the finding of reduced PEA tone in epilepsy. The limited available evidence calls for research about the potential role of PEA concentration profile as a biomarker for the prevention, assessment, and management of epilepsy. Apart from a single observational study conducted in animals of different age [] and a single study of subchronic pretreatment with PEA [], no longitudinal evidence was available about the longer-term clinical utility of such a treatment. Except for one study, all evidence was gathered from male animal models, and no data were available on gender-dependent PEA effect in epilepsy []. Single evidence that PEA co-administration potentiates ASMs’ activity via pharmacodynamic mechanisms [] requires replication. Clinical trials are needed to fully explore the efficacy and tolerability of PEA supplementation in epilepsy.
5. Conclusions
This review found a paucity of observational and experimental studies of PEA signaling in epilepsy. The eight investigations presented seemed to converge on the presence of PEA aberrancies in the brain and periphery, their role for the manifestations of different forms of seizures and epilepsy, and beneficial effects of PEA supplementation both in terms of antiseizure and antiepileptic effect. Subchronic pretreatment with PEA before the epileptogenic stimulus appeared to be a strategy to prevent its behavioral and neurobiological consequences, warranting investigation of PEA disease-modifying effect in epilepsy. It is time to test the antiseizure potential of PEA and its safety profile in human studies of epilepsy.
Author Contributions
Conceptualization, R.B., M.B., S.B. and M.C.; methodology, R.B., M.B., S.B. and M.C.; validation, R.B., M.B., S.B. and M.C.; investigation, R.B. and M.C.; resources, R.B., M.B., S.B. and M.C.; data curation, R.B., M.B., S.B. and M.C.; writing—original draft preparation, R.B. and M.C.; writing—review and editing, R.B., M.B., S.B. and M.C.; visualization, R.B., M.B., S.B. and M.C.; supervision, M.C.; project administration, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge infrastructure from the Integrated University Hospital of Verona and the University of Udine.
Conflicts of Interest
M.C. has been a consultant/advisor to GW Pharma Limited and F. Hoffmann-La Roche Limited, outside of this work. All the other authors declare no conflict of interest.
References
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Falco-Walter, J. Epilepsy-Definition, Classification, Pathophysiology, and Epidemiology. Semin. Neurol. 2020, 40, 617–623. [Google Scholar] [CrossRef]
- Kaur, S.; Garg, R.; Aggarwal, S.; Chawla, S.P.S.; Pal, R. Adult onset seizures: Clinical, etiological, and radiological profile. J. Fam. Med. Prim. Care 2018, 7, 191–197. [Google Scholar] [CrossRef]
- Carpay, J.A.; Aldenkamp, A.P.; van Donselaar, C.A. Complaints associated with the use of antiepileptic drugs: Results from a community-based study. Seizure 2005, 14, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, J.G.; Kilgo, W.A. The Role of Benzodiazepines in the Treatment of Epilepsy. Curr. Treat. Options Neurol. 2016, 18, 18. [Google Scholar] [CrossRef]
- Marsicano, G.; Goodenough, S.; Monory, K.; Hermann, H.; Eder, M.; Cannich, A.; Azad, S.C.; Cascio, M.G.; Gutiérrez, S.O.; van der Stelt, M.; et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003, 302, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monory, K.; Massa, F.; Egertová, M.; Eder, M.; Blaudzun, H.; Westenbroek, R.; Kelsch, W.; Jacob, W.; Marsch, R.; Ekker, M.; et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 2006, 51, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Rankin, L.; Fowler, C.J. The Basal Pharmacology of Palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21, 7942. [Google Scholar] [CrossRef] [PubMed]
- Lo Verme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Solorzano, C.; Zhu, C.; Battista, N.; Astarita, G.; Lodola, A.; Rivara, S.; Mor, M.; Russo, R.; Maccarrone, M.; Antonietti, F.; et al. Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc. Natl. Acad. Sci. USA 2009, 106, 20966–20971. [Google Scholar] [CrossRef] [Green Version]
- Jaggar, S.I.; Hasnie, F.S.; Sellaturay, S.; Rice, A.S. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain 1998, 76, 189–199. [Google Scholar] [CrossRef]
- Yu, H.L.; Deng, X.Q.; Li, Y.J.; Li, Y.C.; Quan, Z.S.; Sun, X.Y. N-palmitoylethanolamide, an endocannabinoid, exhibits antidepressant effects in the forced swim test and the tail suspension test in mice. Pharmacol. Rep. 2011, 63, 834–839. [Google Scholar] [CrossRef]
- Colizzi, M.; Bortoletto, R.; Costa, R.; Zoccante, L. Palmitoylethanolamide and Its Biobehavioral Correlates in Autism Spectrum Disorder: A Systematic Review of Human and Animal Evidence. Nutrients 2021, 13, 1346. [Google Scholar] [CrossRef]
- West, S.; King, V.; Carey, T.S.; Lohr, K.N.; McKoy, N.; Sutton, S.F.; Lux, L. Systems to rate the strength of scientific evidence. Evid. Rep./Technol. Assess. (Summ.) 2002, 1–11. Available online: https://europepmc.org/article/nbk/nbk33881 (accessed on 4 December 2021).
- Lambert, D.M.; Vandevoorde, S.; Diependaele, G.; Govaerts, S.J.; Robert, A.R. Anticonvulsant activity of N-palmitoylethanolamide, a putative endocannabinoid, in mice. Epilepsia 2001, 42, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citraro, R.; Russo, E.; Leo, A.; Russo, R.; Avagliano, C.; Navarra, M.; Calignano, A.; De Sarro, G. Pharmacokinetic-pharmacodynamic influence of N-palmitoylethanolamine, arachidonyl-2’-chloroethylamide and WIN 55,212-2 on the anticonvulsant activity of antiepileptic drugs against audiogenic seizures in DBA/2 mice. Eur. J. Pharmacol. 2016, 791, 523–534. [Google Scholar] [CrossRef] [Green Version]
- Post, J.M.; Loch, S.; Lerner, R.; Remmers, F.; Lomazzo, E.; Lutz, B.; Bindila, L. Antiepileptogenic Effect of Subchronic Palmitoylethanolamide Treatment in a Mouse Model of Acute Epilepsy. Front. Mol. Neurosci. 2018, 11, 67. [Google Scholar] [CrossRef] [Green Version]
- Sheerin, A.H.; Zhang, X.; Saucier, D.M.; Corcoran, M.E. Selective antiepileptic effects of N-palmitoylethanolamide, a putative endocannabinoid. Epilepsia 2004, 45, 1184–1188. [Google Scholar] [CrossRef]
- Citraro, R.; Russo, E.; Scicchitano, F.; van Rijn, C.M.; Cosco, D.; Avagliano, C.; Russo, R.; D’Agostino, G.; Petrosino, S.; Guida, F.; et al. Antiepileptic action of N-palmitoylethanolamine through CB1 and PPAR-α receptor activation in a genetic model of absence epilepsy. Neuropharmacology 2013, 69, 115–126. [Google Scholar] [CrossRef]
- Aghaei, I.; Rostampour, M.; Shabani, M.; Naderi, N.; Motamedi, F.; Babaei, P.; Khakpour-Taleghani, B. Palmitoylethanolamide attenuates PTZ-induced seizures through CB1 and CB2 receptors. Epilepsy Res. 2015, 117, 23–28. [Google Scholar] [CrossRef]
- Lerner, R.; Post, J.; Loch, S.; Lutz, B.; Bindila, L. Targeting brain and peripheral plasticity of the lipidome in acute kainic acid-induced epileptic seizures in mice via quantitative mass spectrometry. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Fezza, F.; Marrone, M.C.; Avvisati, R.; Di Tommaso, M.; Lanuti, M.; Rapino, C.; Mercuri, N.B.; Maccarrone, M.; Marinelli, S. Distinct modulation of the endocannabinoid system upon kainic acid-induced in vivo seizures and in vitro epileptiform bursting. Mol. Cell. Neurosci. 2014, 62, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, A.M.; Vlachou, S.; Grintzalis, K. Efficacy of Phytocannabinoids in Epilepsy Treatment: Novel Approaches and Recent Advances. Int. J. Environ. Res. Public Health 2021, 18, 3993. [Google Scholar] [CrossRef]
- Espinosa-Jovel, C. Cannabinoids in epilepsy: Clinical efficacy and pharmacological considerations. Neurologia 2021, (in press). [Google Scholar] [CrossRef] [PubMed]
- Colizzi, M.; Ruggeri, M.; Bhattacharyya, S. Unraveling the Intoxicating and Therapeutic Effects of Cannabis Ingredients on Psychosis and Cognition. Front. Psychol. 2020, 11, 833. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [Green Version]
- Filipiuc, L.E.; Ababei, D.C.; Alexa-Stratulat, T.; Pricope, C.V.; Bild, V.; Stefanescu, R.; Stanciu, G.D.; Tamba, B.I. Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics 2021, 13, 1823. [Google Scholar] [CrossRef]
- Maier, N.; Morris, G.; Schuchmann, S.; Korotkova, T.; Ponomarenko, A.; Böhm, C.; Wozny, C.; Schmitz, D. Cannabinoids disrupt hippocampal sharp wave-ripples via inhibition of glutamate release. Hippocampus 2012, 22, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Polissidis, A.; Galanopoulos, A.; Naxakis, G.; Papahatjis, D.; Papadopoulou-Daifoti, Z.; Antoniou, K. The cannabinoid CB1 receptor biphasically modulates motor activity and regulates dopamine and glutamate release region dependently. Int. J. Neuropsychopharmacol. 2013, 16, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Ruehle, S.; Remmers, F.; Romo-Parra, H.; Massa, F.; Wickert, M.; Wörtge, S.; Häring, M.; Kaiser, N.; Marsicano, G.; Pape, H.C.; et al. Cannabinoid CB1 receptor in dorsal telencephalic glutamatergic neurons: Distinctive sufficiency for hippocampus-dependent and amygdala-dependent synaptic and behavioral functions. J. Neurosci. 2013, 33, 10264–10277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Blázquez, P.; Rodríguez-Muñoz, M.; Garzón, J. The cannabinoid receptor 1 associates with NMDA receptors to produce glutamatergic hypofunction: Implications in psychosis and schizophrenia. Front. Pharmacol. 2014, 4, 169. [Google Scholar] [CrossRef] [Green Version]
- Albayram, O.; Alferink, J.; Pitsch, J.; Piyanova, A.; Neitzert, K.; Poppensieker, K.; Mauer, D.; Michel, K.; Legler, A.; Becker, A.; et al. Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proc. Natl. Acad. Sci. USA 2011, 108, 11256–11261. [Google Scholar] [CrossRef] [Green Version]
- Antonucci, F.; Alpár, A.; Kacza, J.; Caleo, M.; Verderio, C.; Giani, A.; Martens, H.; Chaudhry, F.A.; Allegra, M.; Grosche, J.; et al. Cracking down on inhibition: Selective removal of GABAergic interneurons from hippocampal networks. J. Neurosci. 2012, 32, 1989–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, R.E.; Deshpande, L.S.; Sombati, S.; Elphick, M.R.; Martin, B.R.; DeLorenzo, R.J. Prolonged exposure to WIN55,212-2 causes downregulation of the CB1 receptor and the development of tolerance to its anticonvulsant effects in the hippocampal neuronal culture model of acquired epilepsy. Neuropharmacology 2009, 57, 208–218. [Google Scholar] [CrossRef] [Green Version]
- Karlócai, M.R.; Tóth, K.; Watanabe, M.; Ledent, C.; Juhász, G.; Freund, T.F.; Maglóczky, Z. Redistribution of CB1 cannabinoid receptors in the acute and chronic phases of pilocarpine-induced epilepsy. PLoS ONE 2011, 6, e27196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skaper, S.D.; Facci, L.; Giusti, P. Mast cells, glia and neuroinflammation: Partners in crime? Immunology 2014, 141, 314–327. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L. Mast cell-glia axis in neuroinflammation and therapeutic potential of the anandamide congener palmitoylethanolamide. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3312–3325. [Google Scholar] [CrossRef]
- Bracken, M.B. Why animal studies are often poor predictors of human reactions to exposure. J. R. Soc. Med. 2009, 102, 120–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).